Abstract
Background
The incidence of postoperative complications and the in-hospital mortality rate of infective endocarditis (IE) complicated with renal insufficiency are relatively high. This study aimed to analyze the clinical features, etiological characteristics, diagnosis and treatment, and prognosis of IE with renal insufficiency and to explore the risk factors for renal damage.
Methods
IE patients undergoing valvular surgery between 2008 and 2017 in two cardiac centers were retrospectively analyzed. They were divided into renal insufficiency (RI) [endogenous creatinine clearance rate (Ccr) <60 mL/min/1.73 m2] and normal renal function (NRF) (Ccr ≥60 mL/min/1.73 m2) groups. The disease conditions at admission, etiology, treatment, and prognosis were compared between the two groups. Multivariate regression analysis was performed for the related factors.
Results
A total of 8,055 cases of valvular surgery was performed during the study period. We analyzed 401 IE patients [average age 43.9±15 years; RI, n=56 (14%); NRF, n=345 (86%)], after the exclusion of 2 patients with primary glomerulonephritis. RI patients showed higher perioperative mortality (14.3% vs. 4.5%, P=0.042) and streptococcal infection (71.4% vs. 43.8%, P=0.001) rates. The RI group was also older and had worse heart function, greater decreases in hemoglobin and platelet levels, a higher rate of prosthetic valve involvement, more cases of postoperative dialysis, and worse prognosis (all P<0.05). Binary logistic multivariate regression analysis showed that the incidence of streptococcal infection [odds ratio (OR) =4.271, 95% confidence interval (CI), 1.846–9.884; P=0.001], age ≥51 years (OR =5.138, 95% CI, 2.258–11.694; P<0.001), and New York Heart Association (NYHA) functional class III–IV (OR =10.768, 95% CI, 2.417–47.972; P=0.002) were independent risk factors for preoperative renal insufficiency.
Conclusions
IE patients with preoperative renal insufficiency had a high mortality rate and poor prognosis, with streptococcal infection predisposing to a higher risk of renal insufficiency. Moreover, older the age and worse heart function in IE resulted in a greater risk for renal insufficiency.
Keywords: Infective endocarditis, renal insufficiency, etiology, cardiac surgery
Introduction
Although the prevention and treatment of infective endocarditis (IE) has greatly improved in recent years, the incidence of IE in the past few decades has not decreased (1), with an overall incidence of approximately 3–10 per 100,000 (2). IE has a high mortality rate and poor prognosis. According to survey data of the International Council on Endocarditis, the in-hospital mortality of IE patients is as high as 17.7% (3). Renal insufficiency caused by IE is very common and preoperative renal insufficiency in IE patients may greatly affect prognosis (4). Tamura et al. reported that the hospitalization and long-term mortality rates of IE patients with renal insufficiency were higher than those of patients with normal renal function (5). However, the relationship between IE and renal insufficiency has not been fully elucidated. This study retrospectively analyzed the clinical features, treatment, and prognosis of IE patients with renal damage and explored the risk factors for preoperative renal insufficiency.
Methods
Subjects
Adult IE patients undergoing surgery from January 2008 to January 2017 in First People’s Hospital, affiliated with Shanghai Jiao Tong University, and Changhai Hospital, affiliated with the Second Military Medical University, were included in this study. The patients were diagnosed according to the modified Duke criteria (6,7), and those with other primary kidney diseases were excluded. This study was approved by the ethics committees of the universities (No. SMMUEC2017-81).
Study design and data analysis
The preoperative endogenous creatinine clearance rate (Ccr) of the included cases was calculated based on the Cockcroft-Gault equation (8). A total of 401 IE patients were divided into two groups: a renal insufficiency group (n=56, Ccr <60 mL/min/1.73 m2) and a normal renal function group (n=345, Ccr ≥60 mL/min/1.73 m2) (9). The demographic data, results of laboratory tests at admission (including routine blood indexes and renal function), preoperative complications and medications, and results of pathogen detection were analyzed and compared between the two groups. Outcome indexes including surgical data and prognosis were propensity score-matched and compared to adjust for baseline differences between the two groups. One patient underwent renal biopsy and pathological examination.
Statistical methods
SPSS 19.0 software was used to perform the statistical analysis of the included data. Categorical data are presented as the numbers and percentages, and the chi-square test or Fisher exact test was used for comparisons. The measurement data are presented as mean ± SD or as medians (25th to 75th percentile). The unpaired t-test was performed for data with normal distribution, while a non-parametric Mann-Whitney U-test was performed for data without normal distribution. All statistically significant factors were included in the two groups based on clinical characteristics. The cutoff value for continuous variables was obtained using a receiver operating characteristic (ROC) curve. Binary logistic regression analysis was performed in both univariable and multivariable analyses to identify risk factors for preoperative renal insufficiency. Significant variables associated with preoperative renal insufficiency by univariable analysis were entered into the multivariable analysis and were used to match the renal insufficiency group with the normal renal function group via propensity-matching analysis (1:5). The multivariable analysis used a Forward Stepwise (Conditional) procedure to determine independent significant prognostic factors. Matched patients were included to compare outcomes. Differences with P<0.05 were considered statistically significant.
Results
During the study period, a total of 8,055 cases underwent valve surgery (Figure 1) and a total of 403 patients were diagnosed with IE. However, two patients with primary glomerulonephritis were excluded and 401 IE patients were included in the study. Among them, 119 (29.7%) had leukocytosis, 281 (70.1%) had anemia, 34 (8.5%) had thrombocytopenia, and 34 (8.5%) had increased creatinine.
Figure 1.
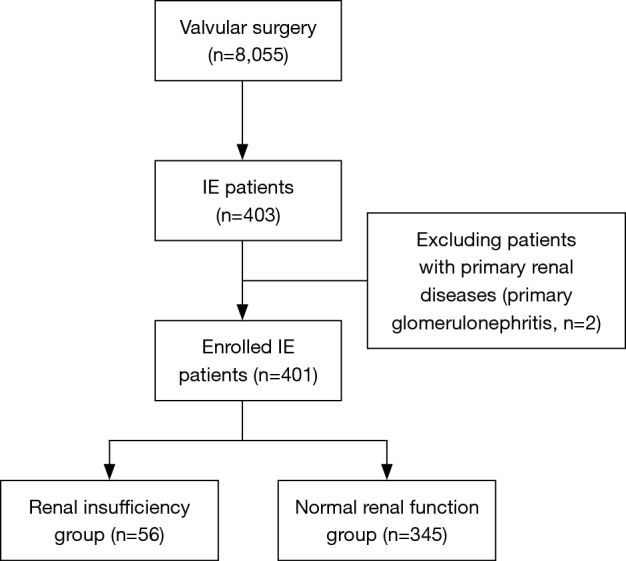
Flowchart of the study selection.
The general characteristics of both groups are shown in Table 1. Compared with patients with normal renal function, those with renal insufficiency had a greater age (56.6 vs. 43.0 years, P<0.001), longer pre-hospitalization disease duration (8.0 vs. 4.0 days, P=0.011), and worse cardiac function (10.7% vs. 40.9%, P<0.001). There were no significant differences between the two groups regarding other general parameters. For valvular involvement, the renal insufficiency group had a higher incidence of prosthetic valve infection (8.9% vs. 2.6%, P=0.017). Regarding laboratory parameters, the hemoglobin and platelet indexes of the patients in the renal insufficiency group were lower than those in the normal group (99.5 vs. 109.0 g·L−1 and 174.6×109 vs. 207.4×109·L−1, respectively, P=0.001 both). Concerning preoperative complications, the incidences of hypertension (19.6% vs. 8.1%, P=0.007) and heart failure (8.9% vs. 2.3%, P=0.01) were higher in the renal insufficiency group. For preoperative medication, the administration rates of digitalis (71.4% vs. 48.4%, P=0.001) and dopamine (12.5% vs. 5.2%, P=0.037) were higher in the renal insufficiency group, while the administration rates of other drugs showed no significant differences (all P>0.05).
Table 1. Comparison of the general characteristics of 401 IE patients at admission.
| Patients’ characteristics | IE patients (n=401) | Renal insufficiency group (n=56) | Normal renal function group (n=345) | P |
|---|---|---|---|---|
| Male, case (%) | 295 (73.6) | 40 (71.4) | 255 (73.9) | 0.696 |
| Age (years) | 46.0 (30.4,56.0) | 56.6 (47.3, 62.2) | 43.0 (29.7, 54.0) | 0.000 |
| Age ≥51 years case (%) | 143 (35.7) | 39 (69.6) | 104 (30.1) | 0.000 |
| Disease duration (days) | 4.0 (2.0, 20.5) | 8.0 (3.0, 30.0) | 4.0 (2.0, 18.0) | 0.011 |
| Hospitalization (days) | 23.0 (16.0, 35.0) | 22.5 (17.0, 35.8) | 23.0 (16.0, 35.0) | 0.666 |
| LVEF (%) | 62.0 (57.0, 67.0) | 58.0 (52.5, 64.5) | 63.0 (58.0, 67.0) | 0.000 |
| Valve involved | 0.148 | |||
| Mitral valve | 124 (30.9) | 19 (33.9) | 105 (30.4) | |
| Aortic valve | 107 (26.7) | 10 (17.9) | 97 (28.1) | |
| Tricuspid valve | 27 (6.7) | 4 (7.1) | 23 (6.7) | |
| Multiple valves | 103 (25.7) | 16 (28.6) | 87 (25.1) | |
| Congenital disease | 26 (6.5) | 2 (3.6) | 24 (7.0) | |
| Prosthetic valve | 14 (3.5) | 5 (8.9) | 9 (2.6) | |
| NYHA class | 0.000 | |||
| I–II, case (%) | 147 (36.7) | 6 (10.7) | 141 (40.9) | |
| III–IV, case (%) | 254 (63.3) | 50 (89.3) | 204 (59.1) | |
| Valve involved, case (%) | 0.017 | |||
| Native valve | 387 (96.5) | 51 (91.1) | 336 (97.4) | |
| Prosthetic valve | 14 (3.5) | 5 (8.9) | 9 (2.6) | |
| Laboratory test | ||||
| Leukocytes (×109L−1) | 7.7 (5.9,10.5) | 7.2 (6.2,10.4) | 7.8 (5.9,10.5) | 0.666 |
| Hemoglobin (g·L–1) | 108.0 (97.0, 124.0) | 99.5 (85.8, 113.8) | 109.0 (98.0, 125.0) | 0.001 |
| Platelets (×109·L–1) | 202.8±3.9 | 174.6±8.2 | 207.4±4.3 | 0.001 |
| Creatinine (ìmoL) | 73.0 (58.0, 87.0) | 123.5 (102.5, 165.0) | 68.0 (55.5, 80.0) | 0.000 |
| Ccr (mL·min–1) | 94.0 (74.3, 112.9) | 47.0 (38.7, 55.6) | 98.1 (83.0, 117.4) | 0.000 |
| Complication, case (%) | ||||
| Hypertension | 39 (9.7) | 11 (19.6) | 28 (8.1) | 0.007 |
| Diabetes | 13 (3.2) | 3 (5.4) | 10 (2.9) | 0.335 |
| Heart failure | 13 (3.2) | 5 (8.9) | 8 (2.3) | 0.01 |
| Arrhythmia | 51 (12.7) | 8 (14.3) | 43 (12.5) | 0.704 |
| History of embolism | 39 (9.7) | 5 (8.9) | 34 (9.9) | 0.828 |
| COPD | 4 (1.0) | 0 (0) | 4 (1.2) | 0.547 |
| Cerebrovascular accident | 22 (5.5) | 6 (10.7) | 16 (4.6) | 0.064 |
| Preoperative medication, case (%) | ||||
| Digitalis | 207 (51.6) | 40 (71.4) | 167 (48.4) | 0.001 |
| Dopamine | 25 (6.2) | 7 (12.5) | 18 (5.2) | 0.037 |
| Receptor β blockers | 37 (9.2) | 4 (7.1) | 33 (9.6) | 0.740 |
| Nitric acid lipids | 19 (4.7) | 3 (5.4) | 14 (4.1) | 0.928 |
| Intravenous drug abuse, case (%) | 13 (3.2) | 3 (5.4) | 10 (2.9) | 0.578 |
| Surgery, case (%) | 0.197 | |||
| Emergency | 19 (4.7) | 4 (7.1) | 15 (4.3) | |
| Urgency | 31 (7.7) | 6 (10.7) | 25 (7.2) | |
| Election | 351 (87.5) | 46 (82.1) | 305 (88.4) | |
The variables are presented as n (%) or mean or median. LVEF, left ventricular ejection fraction; NYHA, New York Heart association; Ccr, creatinine clearance; COPD, chronic obstructive pulmonary disease.
Pathogens
Among the 401 patients in this study, 121 (30.2%) had positive blood cultures, 90 (22.4%) had positive intraoperative neoplasm cultures, and 202 (50.4%) had positive microbial cultures (Table 2). The infection incidences of Streptococcus (49.5%) and Staphylococcus (28.7%) were the highest among all of the pathogenic bacteria, followed by Gram-negative bacilli (10.9%) and Enterococcus (5.0%). In this study, Candida glabrata, Micrococcus kristinae, and other rare pathogens were also detected. The positive microbial culture rate of the patients in the renal insufficiency group was higher than that in the normal renal function group (75% vs. 46%, P<0.001). The incidence of streptococcal infection in the renal insufficiency group was higher than that in the normal renal function group (71.4% vs. 43.8%, P=0.001) but there were no significant differences in the infection rates with Staphylococcus, Enterococcus, and Gram-negative bacilli between the two groups (P>0.05).
Table 2. Etiology comparison of the IE etiology of the renal insufficiency group and the normal renal function groups.
| Variable | IE positive (n=202) | Renal insufficiency group (n=42) | Normal renal function group (n=160) | P |
|---|---|---|---|---|
| Positive in microbial culture etiology, cases (%) | 0.025 | |||
| Streptococcus | 100 (49.5) | 30 (71.4) | 70 (43.8) | |
| Oral streptococci | 44 (21.8) | 15 (35.7) | 29 (18.1) | |
| Pyogenic streptococci1 | 25 (12.4) | 8 (19.0) | 17 (10.6) | |
| S. bovis/equinus | 12 (5.9) | 3 (7.1) | 9 (5.6) | |
| Other streptococcaceae2 | 19 (9.4) | 4 (9.5) | 15 (9.4) | |
| Staphylococcus | 58 (28.7) | 8 (19.0) | 50 (31.3) | |
| S. aureus | 42 (20.8) | 5 (11.9) | 37 (23.1) | |
| Coagulase-negative staphylococci (CNS) | 16 (7.9) | 3 (7.1) | 13 (8.1) | |
| Enterococcus | 10 (5.0) | 1 (2.4) | 9 (5.6) | |
| Gram-negative bacilli | 22 (10.9) | 3 (7.1) | 19 (11.9) | |
| Other3 | 12 (5.9) | 0 (0) | 12 (7.5) | |
| Streptococcus infection, case (%) | 0.001 | |||
| Yes | – | 30 (71.4) | 70 (43.8) | |
| No | – | 12 (28.6) | 90 (56.2) | |
| Staphylococcus infection, case (%) | 0.120 | |||
| Yes | – | 8 (19.0) | 50 (31.3) | |
| No | – | 34 (81.0) | 110 (68.7) | |
1, Beta-hemolytic streptococci (group A, B, C and G); 2, S. pneumonia (n=9), S. acidominimus (n=3), S. anginosus (n=2), S. gallolyticus (n=2), S. anginosus pasteurianus (n=1), Streptococcus spp. (n=2); 3, There were seven cases of Gram-positive bacilli, 1 case of Bacillus subtilis, one case of Candida glabrata, one case of Leuconostoc, one case of Pseudomonas maltophilia, and one case of Micrococcus kristinae.
Treatment and prognosis
All 401 patients received open heart surgery under general anesthesia with a cardiopulmonary bypass. Among them, 375 underwent valve replacement and 26 underwent radical surgery for congenital heart disease. Propensity-matching analysis was used to adjust for baseline differences. Age, NYHA class III–IV, left ventricular ejection fraction, duration of disease, prosthetic valve infection, hemoglobin, platelets, hypertension, heart failure, digitalis, and dopamine were used to match the renal insufficiency and normal renal function groups. After matching, 204 IE patients were included for outcome analysis and comparison. The perioperative data of the two groups are shown in Table 3. The average cardiopulmonary bypass duration, ascending aorta blocking time, ICU stay, and mechanical ventilation time were 115.3 minutes, 77.5 minutes, 73.6 hours, and 33.2 hours, respectively. Perioperative death occurred in 14 (6.9%) patients, of which nine died of low cardiac output syndrome, three died of ventricular fibrillation, one died of severe infection, and one died of cerebral hemorrhage. Compared with the normal renal function group, the renal insufficiency group had a higher mortality (14.3% vs. 4.5%, P=0.042), more postoperative dialysis (8.2% vs. 0%, P=0.001), and a higher probability of low cardiac output syndrome (10.2% vs. 2.6%, P=0.006).
Table 3. Comparison of the intraoperative data and postoperative complications between the renal insufficiency group and the normal renal function group (after propensity matching).
| Intraoperative and postoperative variables | IE patients (n=204) | Renal insufficiency group (n=49) | Normal renal function group (n=155) | P |
|---|---|---|---|---|
| Cardiopulmonary bypass (min) | 107.0 (88.3, 131.8) | 111.0 (84.5, 145.0) | 105.0 (90.0, 128.0) | 0.258 |
| ACC time (min) | 73.0 (58.0, 91.5) | 71.0 (57.5, 101.5) | 73.0 (58.0, 89.0) | 0.717 |
| ICU stay (hr) | 41.0 (26.2, 82.0) | 43.5 (26.0, 94.0) | 43.5 (26.0, 94.0) | 0.547 |
| Mechanical ventilation (hr) | 18.0 (15.0, 21.0) | 18.0 (14.3, 21.5) | 18.2 (15.0, 21.0) | 0.961 |
| In-hospital death, case (%) | 14 (6.9) | 7 (14.3) | 7 (4.5) | 0.042 |
| CRRT, case (%) | 4 (2.0) | 4 (8.2) | 0 (0) | 0.001 |
| Low cardiac output syndrome, case (%) | 9 (4.4) | 5 (10.2) | 4 (2.6) | 0.006 |
| Gastrointestinal bleeding, case (%) | 1 (0.5) | 1 (2.0) | 0 (0) | 0.090 |
| Cerebrovascular accident, case (%) | 2 (1.0) | 0 (0) | 2 (1.3) | 0.293 |
| Hepatic insufficiency, case (%)1 | 6 (2.9) | 1 (2.0) | 5 (3.2) | 1.000 |
| Pulmonary infection, case (%)2 | 8 (3.9) | 2 (4.1) | 6 (3.9) | 1.000 |
| Reoperation for bleeding | 12 (5.9) | 1 (2.0) | 11 (7.1) | 0.336 |
The variables are presented as n (%) or median (interquartile). Age, NYHA class III–IV, left ventricular ejection fraction, duration of disease, prosthetic valve infection, hemoglobin, platelets, hypertension, streptococcus infection, heart failure, digitalis and dopamine were used to match the renal insufficiency group with the normal renal function group. 1, an increase of ALT (≥80 U/L) and bilirubin (≥12 mg/dL) in blood, with abnormal function of synthesis and metabolism; 2, a new or infiltrating shadow on X-ray, with a fever >38.5 °C or leucocyte increase (WBC >11.0×109/L) or decrease (WBC <3.5×109/L). ACC, aortic cross-clamp; ICU, intensive care unit; CRRT, continuous renal replacement therapy.
Kidney pathology
One patient was hospitalized due to acute left heart failure and acute renal failure. Echocardiography revealed severe mitral regurgitation, aortic regurgitation, and a serum creatinine of 1,695 µmol/L at admission. The blood culture showed positive Streptococcus sanguis. Renal biopsy was performed and light microscopy showed the proliferation of glomerular endothelial cells and mesangial cells, capillary necrosis, adhesion to the capsule wall, and the proliferation of epithelial cells on the capsule wall, with the formation of a crescent. The case was diagnosed as crescentic nephritis (Figure 2). Small-dose immunosuppressive therapy on the basis of adequate antibiotics was applied, with continuous hemodialysis. Valve replacement was performed after the patient’s disease condition was stabilized, with continuous hemodialysis after the surgery. Immunosuppression and symptomatic treatment were provided. The patient was discharged after 4 weeks of anti-infective treatment. The patient showed improved cardiac function and increased urine output, with the serum creatinine restored to 150–190 µmol/L at discharge.
Figure 2.
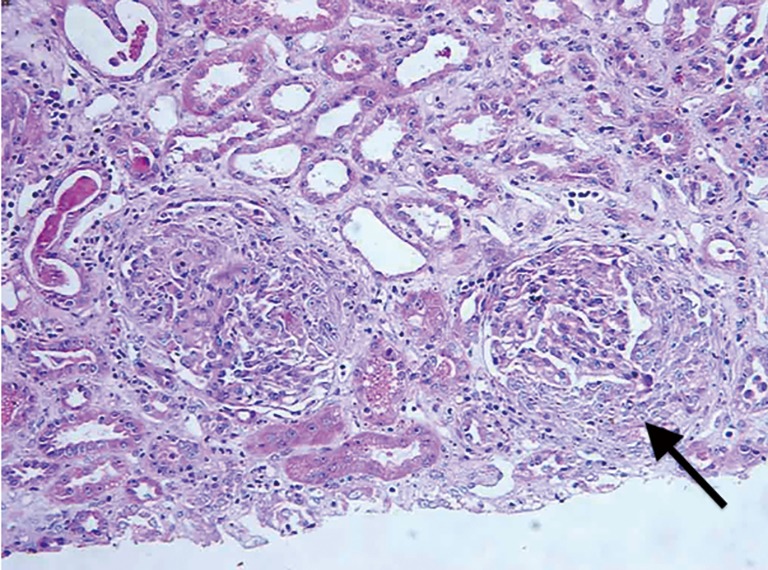
Histopathology of the renal tissue [arrow, formation of the glomerular cell type crescent, hematoxylin-eosin staining (200×)].
Multivariate analysis of factors influencing renal insufficiency
To predict the risk factors for preoperative renal insufficiency combined with the clinical characteristics, the 11 variables with significant differences in the univariate analysis, including age, duration of disease, streptococcal infection, NYHA class III–IV, hemoglobin level, platelet level, left ventricular ejection fraction, prosthetic valve infection, heart failure, digitalis, and dopamine were analyzed using binary logistic multivariate regression analysis. Among them, age, disease duration, and hemoglobin and platelet levels were continuous variables, showing a statistical difference at baseline. The cutoff values of age (51 years), duration of disease (5 days), hemoglobin (100 g·L−1), and platelets (192×109·L−1) were identified by ROC analysis. The results showed that age >51 years, NYHA class III–IV, and streptococcal infection were independent risk factors for preoperative renal dysfunction (Table 4).
Table 4. Multivariate binary logistic regression analysis of renal insufficiency.
| Factor | OR | 95% CI | P |
|---|---|---|---|
| Age ≥51 years | 5.138 | 2.258–11.694 | <0.001 |
| NYHA class III–IV | 10.768 | 2.417–47.972 | 0.002 |
| Streptococcal infection | 4.271 | 1.846–9.884 | 0.001 |
OR, odds ratio; CI, confidence interval; NYHA, New York Heart association.
Discussion
The main finding of this study was that patients with streptococcal infection had a higher chance of renal dysfunction than those without streptococcal infection. The older the patient and the worse the cardiac function, the greater the risk for renal dysfunction.
IE with renal dysfunction is very common and the most common pathological changes in the kidney are diffuse proliferative glomerulonephritis and exudative glomerulonephritis, with mesangial and endothelial cell proliferation associated with the infiltration of inflammatory cells. However, crescentic glomerulonephritis is very rare and is seldom reported (10-12). Herein, we reported a case of crescentic glomerulonephritis caused by infection with Streptococcus sanguis (Figure 1). In our study, the positive microbial culture rate was 50.4%, which was lower than the rate of 64% reported in a related study (13). This difference may be related to the use of antibiotics before admission in some patients, the timing and method of blood sampling, and the techniques used for the tests. Streptococcus and Staphylococcus are the most common IE-causing pathogens. In recent years, with the changes in the etiology of IE, the incidence of staphylococcal infection has continued to rise (14). Data from the United States and Europe indicates that in IE patients, staphylococcal infection is predominant (15). However, our data are consistent with those reported in Japan (13,16), showing the predominance of streptococcal infection (49.5%). A possible reason for this difference is the increase in intravenous drug abuse and invasive medical procedures in Western countries, which are not common in China.
Based on our analysis, we found that patients with streptococcal infection were more likely to have renal impairment. The causes of renal impairment are often multifactorial (2), including immune complex and vasculitic glomerulonephritis, renal infraction, antibiotic toxicity and nephrotoxic contrast agent used. However, the result showed a great significance difference on the streptococcal infection and streptococcal infection was the independent risk factors of renal impairment. We infer that this outcome may chiefly result from immune related. The occurrence of acute renal impairment in patients with IE is due to a series of immune processes, such as the formation of circulating immune complexes, hypocomplementemia, and complement deposition in the glomerular basement membrane (17). The pathogenic antigens of Streptococcus include the cytoplasmic components or secretory proteins. At present, the most commonly studied pathogenic antigens are nephritis-associated plasmin receptor (NAPlr) of Streptococcus and streptococcal pyrogenic exotoxin B (SPE B), which were both confirmed by Oda et al. as the pathogenic factors for glomerulonephritis after streptococcal infection (18,19). Streptococcal antigens can induce an immune response and can then be deposited in the glomeruli via circulating or in situ immune complexes, thereby, causing disease. Autoimmune and abnormal complement activation may also be involved in the pathogenesis. The proliferation of glomerular endothelial and mesangial cells and inflammatory cell infiltration may lead to a decrease in the glomerular filtration rate, resulting in renal dysfunction.
This study found that age and heart function were also risk factors for renal dysfunction. The onset age of IE patients has been increasing. In an IE case report, as early as 1980, the average onset age was 20–30 years (10). The average onset age of IE reported by Legrand et al. (16) was 42 years, and the average onset age in our study was 46 years, showing agreement. The average onset age reported by Koeda et al. (13) was even higher. Their statistical analysis of IE patients before and after 2005 showed that the average onset age of IE after 2005 was significantly higher (54 vs. 60 years, P<0.05). These results all suggest that although the onset ages of IE patients in different regions differed, the overall onset ages showed an increasing trend. Our results showed that IE patients with impaired renal function were generally older and that those over 51 years old had an increased risk for renal dysfunction, which may be related to the increase in the overall onset age of IE and the aging of the population.
At admission, IE patients with impaired renal function had poorer cardiac function. Combined with the indicators of poor left ventricular ejection fraction, preoperative heart failure, and preoperative administration of digitalis, it was not difficult to determine that most of the IE patients had cardiac insufficiency at admission. Infection with pathogens will cause a decrease in the glomerular filtration rate, with water and sodium retention and systemic circulation congestion. Most IE patients have underlying heart disease, and cardiac insufficiency can aggravate systemic circulation congestion and cause inadequate renal perfusion, which, in turn, aggravates renal dysfunction. Therefore, we believe that the renal dysfunction and cardiac insufficiency in IE patients are mutually reinforcing, forming a vicious circle.
Anemia caused by IE is very common and is mostly related to the inhibition of hematopoiesis in the bone marrow and hypersplenism due to the infection. For the 401 patients in this study, the incidence of anemia was 66.1%, higher than in previous reports (54.2%) (20). We found that the anemia and thrombocytopenia were more severe in the patients with renal dysfunction than in those with normal renal function. This difference in severity may have been due to the insufficient production of erythropoietin and the inhibition of hematopoietic function in the bone marrow caused by hemodilution and the in vivo accumulation of toxins, which affects the life spans of erythrocytes and platelets. Therefore, in IE patients with renal dysfunction at admission, who have anemia and thrombocytopenia, a preoperative correction of the anemia should be considered. However, multivariate logistic regression analysis showed that anemia and thrombocytopenia were not independent risk factors for preoperative renal insufficiency. This may be because anemia and thrombocytopenia are preoperative pathological changes probably caused by renal dysfunction. Therefore, they may not be predictive factors of preoperative renal insufficiency.
There was a significant difference between the two groups in the incidence of prosthetic valve infection, with the rate of prosthetic valve infection increasing yearly (21). The rate of prosthetic valve infection in this study was 3.5%. Regression analysis showed that prosthetic valve infection was not a risk factor for renal damage. We speculate that this result may be related to the small number of included cases; more case-control studies on prosthetic valve endocarditis can be conducted in the future.
Previous studies have shown that in IE, early surgical intervention had a better prognosis than medical therapy (22,23). In this study, the 401 diagnosed patients all received surgical treatment. The overall in-hospital mortality rate was 4.7%, with most patients (63.2%) dying due to a postoperative low cardiac output syndrome. Perioperative mortality and complication rates were higher in the renal insufficiency group than in the normal renal function group, in agreement with the study by Tamura et al. (5). They also conducted the long-term follow-up of both groups and the results showed that the long-term mortality and complication rates were higher in the renal insufficiency group than in the normal renal function group; the most important factor affecting the mortality rate was cardiac events. Thus, the pathophysiological changes caused by IE with renal dysfunction are more severe and renal dysfunction is an important factor affecting IE patients (13). The mortality rate in our study was lower than previously reported (3,16), possibly due to our timely diagnosis and surgery and our careful postoperative care. The keys for the postoperative care of IE patients with renal dysfunction include the following: (I) discontinue antibiotics that cause renal damage; (II) closely monitor any changes in urine output and urine color; (III) maintain a balance of intake and output; (IV) maintain in vivo electrolytes and the stability of the internal environment; and (V) closely monitor any changes in serum creatinine and urea nitrogen, and perform hemodialysis if necessary.
In summary, pathogenic bacteria in IE can trigger an immune response, reducing the glomerular filtration rate and leading to the retention of water and sodium. With the deterioration of cardiac function, systemic circulation congestion, and insufficient renal perfusion caused by the original basic heart disease, renal function can also deteriorate, forming a vicious circle. In this study, IE patients with renal dysfunction exhibited a high mortality rate and poor prognosis. Renal impairment is more likely to occur in patients with streptococcal infection. In IE patients, older age and worse heart function predispose to a greater risk of renal damage.
Limitations of this study
The limitations of the study include, first, its retrospective nature. All patient information was obtained by reviewing the medical records and the quality of the data was not high. Second, the renal insufficiency group comprised only 56 patients (14% of the population) and patients in both groups may have had important differences that are not comparable. Third, we did not have any long-term follow-up data on the patients. The long-term prognosis of the patients with renal dysfunction was unclear. Fourth, the positive rate of our microbial culture was low (50.4%) because some diagnosed patients had a history of antibiotic use at admission. The diagnosis of patients whose microbial culture was negative was made according to the modified Duke criteria when combinations of clinical criteria were met. Despite these limitations, this multicenter large-scale study, for the first time, illustrated the relationship between IE and renal dysfunction and its etiology in China.
Acknowledgements
Funding: This work was sponsored by Health System Foundation of Shanghai (2014ZYJB0401, Zhiyun Xu) and Natural Science Foundation of Shanghai (16ZR1400900) and National Natural Science Foundation of China (81300094).
Ethical Statement: This study was approved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, Shanghai (No. SMMUEC2017-81).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bashore TM, Cabell C, Fowler V., Jr Update on infective endocarditis. Curr Probl Cardiol 2006;31:274-352. 10.1016/j.cpcardiol.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. 10.1093/eurheartj/ehp285 [DOI] [PubMed] [Google Scholar]
- 3.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neragi-Miandoab S, Skripochnik E, Michler R, et al. Risk factors predicting the postoperative outcome in 134 patients with active endocarditis. Heart Surg Forum 2014;17:E35-41. 10.1532/HSF98.2013270 [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Arai H, Yoshizaki T. Long-term outcome of active infective endocarditis with renal insufficiency in cardiac surgery. Ann Thorac Cardiovasc Surg 2012;18:216-21. 10.5761/atcs.oa.11.01748 [DOI] [PubMed] [Google Scholar]
- 6.Hoen B, Duval X. Clinical practice. Infective endocarditis. N Engl J Med 2013;368:1425-33. 10.1056/NEJMcp1206782 [DOI] [PubMed] [Google Scholar]
- 7.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 8.Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy 2012;32:604-12. 10.1002/j.1875-9114.2012.01098.x [DOI] [PubMed] [Google Scholar]
- 9.Xinwei J, Xianghua F, Jing Z, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol 2009;104:519-24. 10.1016/j.amjcard.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 10.Ades L, Akposso K, Costa de Beauregard MA, et al. Bacterial endocarditis associated with crescentic glomerulonephritis in a kidney transplant patient: first case report. Transplantation 1998;66:653-4. 10.1097/00007890-199809150-00019 [DOI] [PubMed] [Google Scholar]
- 11.Daimon S, Mizuno Y, Fujii S, et al. Infective endocarditis-induced crescentic glomerulonephritis dramatically improved by plasmapheresis. Am J Kidney Dis 1998;32:309-13. 10.1053/ajkd.1998.v32.pm9708618 [DOI] [PubMed] [Google Scholar]
- 12.Haseyama T, Imai H, Komatsuda A, et al. Proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) positive crescentic glomerulonephritis in a patient with Down's syndrome and infectious endocarditis. Nephrol Dial Transplant 1998;13:2142-6. 10.1093/ndt/13.8.2142 [DOI] [PubMed] [Google Scholar]
- 13.Koeda C, Tashiro A, Itoh T, et al. Mild renal dysfunction on admission is an important prognostic predictor in patients with infective endocarditis: a retrospective single-center study. Intern Med 2013;52:1013-8. 10.2169/internalmedicine.52.9305 [DOI] [PubMed] [Google Scholar]
- 14.Korem M, Israel S, Gilon D, et al. Epidemiology of infective endocarditis in a tertiary-center in Jerusalem: a 3-year prospective survey. Eur J Intern Med 2014;25:550-5. 10.1016/j.ejim.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 15.Wallace SM, Walton BI, Kharbanda RK, et al. Mortality from infective endocarditis: clinical predictors of outcome. Heart 2002;88:53-60. 10.1136/heart.88.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand M, Pirracchio R, Rosa A, et al. Incidence, risk factors and prediction of post-operative acute kidney injury following cardiac surgery for active infective endocarditis: an observational study. Crit Care 2013;17:R220. 10.1186/cc13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutman RA, Striker GE, Gilliland BC, et al. The immune complex glomerulonephritis of bacterial endocarditis. Medicine (Baltimore) 1972;51:1-25. 10.1097/00005792-197201000-00001 [DOI] [PubMed] [Google Scholar]
- 18.Oda T, Yoshizawa N, Yamakami K, et al. The role of nephritis-associated plasmin receptor (NAPlr) in glomerulonephritis associated with streptococcal infection. J Biomed Biotechnol 2012;2012:417675. 10.1155/2012/417675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda T, Yoshizawa N, Yamakami K, et al. Localization of nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Hum Pathol 2010;41:1276-85. 10.1016/j.humpath.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Lou XF, Yang DY, Liu ZY, et al. Clinical analysis of 120 cases of infective endocarditis. Zhonghua Nei Ke Za Zhi 2009;48:35-8. [PubMed] [Google Scholar]
- 21.Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025-35. 10.1378/chest.06-2048 [DOI] [PubMed] [Google Scholar]
- 22.Netzer RO, Altwegg SC, Zollinger E, et al. Infective endocarditis: determinants of long term outcome. Heart 2002;88:61-6. 10.1136/heart.88.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlessis AA, Hovaguimian H, Jaggers J, et al. Infective endocarditis: ten-year review of medical and surgical therapy. Ann Thorac Surg 1996;61:1217-22. 10.1016/0003-4975(96)00029-X [DOI] [PubMed] [Google Scholar]


