Abstract
Background
Pulmonary rehabilitation can be effective in perioperative condition. Our aim was to examine whether the changes of functional markers are significant and search connections between these values and the severity of postoperative complications.
Methods
A total of 238 chronic obstructive pulmonary disease (COPD) patients underwent perioperative pulmonary rehabilitation with thoracic surgery. Health status and the following parameters were examined: lung function (FEV1, FVC), chest kinematics [chest wall expansion (CWE)], 6-minute walking test (6MWT), breath holding time (BHT), grip strength (GS) and exercise capacity. Patients were separated into three groups: 72 patients had preoperative rehabilitation only (PRE group), 80 had only postoperative rehabilitation (POS group), and 86 patients underwent pre- and postoperative rehabilitation as well (PPO group). Postoperative complications were classed as “severe” and “not severe”. We evaluated the changes in functional parameters. Significance was recognized at P<0.05. Connections in between variables and severity of complications were analyzed.
Results
Pulmonary rehabilitation resulted significant changes of all examined parameters in all three groups. The direction of changes were favourable, so all of the changes can be considered to be improvement [PRE: CWE: 4.2±2.3 vs. 5.8±2.2 cm; FEV1: 63.2±15.6 vs. 70.1±16.6%pred; 6-minute walking distance (6MWD): 392.9±93.5 vs. 443.2±86.6 m; FVC: 83.1±15.9 vs. 90.9±15.6%pred; POS: CWE: 2.9±1.4 vs. 5.0±2.0 cm; FEV1: 56.4±15.6 vs. 64.6±16.0%pred; 6MWD: 354.7±90.7 vs. 437.0±96.0 m; FVC: 66.2±18.7 vs. 76.1±17.7%pred; PPO: preoperatively: CWE: 4.0±2.1 vs. 5.6±2.6 cm; FEV1: 58.2±15.1 vs. 67.0±14.6%pred; 6MWD: 378.3±90.5 vs. 441.3±86.4 m; FVC: 82.4±16.7 vs. 93.3±16.7%pred; postoperatively: CWE: 2.7±1.5 vs. 4.4±2.2 cm; FEV1: 47.4±13.0 vs. 53.4±14.7%pred; 6MWD: 341.4±115.9 vs. 403.3±98.4 m; FVC: 63.6±16.9 vs. 72.6±18.6%pred; P<0.05]. BHT, GS, dyspnoea and health status were also improved significantly. By discriminant analysis 5 of the variables proved to have discriminative value: kilometers travelled via cycle ergometer at the onset of the preoperative rehabilitation, gender, FEV1 after preoperative rehabilitation, extent of the operation and 6MWD before preoperative rehabilitation. These 5 parameters can predict severe complications correctly in 72.5% of all cases.
Conclusions
Pulmonary rehabilitation can reduce the functional depletion caused by the thoracic surgical operation. Identification of more predictive factors of severe complications can help making preoperative risk stratification more precisely.
Keywords: Chronic obstructive pulmonary disease (COPD), lung cancer, thoracic operation, pulmonary rehabilitation, risk stratification, postoperative complications
Introduction
In recent years, the field of thoracic surgery became more sophisticated (1-3). Video assisted thoracic surgery is widely accepted, many advanced surgical interventions became routine procedure. However, new intra- and post-operative complications have been appeared (1,2). Keeping the delicate balance between the risks and advantages of the operations needed to develop the practice of pre-operative risk stratification; the creation of predictive algorithms meant to maximise surgical safety and enhance the efficiency of post-surgical care planning (1,2).
Preoperative assessment in the field of thoracic surgery remains in a state of flux, with many of its elements being constantly revised or contested (4). A great number of biological markers with potential significance for risk stratification have been collected; however, the subject of their individual inclusion or exclusion in a predictive algorithm is still debated (4).
Amongst the debated biological markers, a particular set of parameters appears to be prominent in relating literature (5-8). In accordance, the parameters of forced expiratory volume in the first second (FEV1), diffusion capacity (DLCO), oxygen uptake (VO2/kg) during exercise, cardiovascular function and arterial blood gas values have been consistently found in and referred to by relating literature and have been proven to play a significant role in predicting the risk, outcome and postoperative planning of thoracic interventions (5-8).
Pulmonary rehabilitation is a procedure that capable to ameliorate the post-surgical deterioration of some physiologic values. We believe that observation of the benefits brought upon by pulmonary rehabilitation and their relationship to the severity of the patients’ post-operative complications can help to identify the individual importance of each physiologic parameter in risk evaluation. Identifying high risk patients before thoracic surgery and indicating preoperative pulmonary rehabilitation for them may can reduce the rate of severe complications.
Our aim was to investigate the effectiveness of pulmonary rehabilitation by measuring the changes of functional parameters and the analysing the correlations of the changes of the variables. Furthermore, we examined the connections in between these variables and severity of postoperative complications for a better risk stratification before thoracic surgery. We would like to identify the variables, which have discriminating value in point of severity of postoperative complications.
Methods
Study subjects
A total of 238 COPD patients participated in the perioperative pulmonary rehabilitation program in connection with thoracic surgery at the Department of Thoracic Surgery in the National Koranyi Institute for Pulmonology, Budapest, Hungary. Indication of the operation was primary lung cancer in 179 (75.2%) cases, pulmonary metastasis in 11 (4.6%), benign disease in 10 (4.2%), infection in 16 (6.7%) and other causes in 22 cases (9.2%). The physical parameters of all patients are presented in Table 1. There were no significant differences between the groups in terms of patient characteristics (Table 1). All the patients confirmed consent to the study as part of the general informed consent form for patients at the Department of Pulmonary Rehabilitation and at the Department of Thoracic Surgery. The study was primarily observational, overseeing the general management of the patients. Local ethics approval was done on 20/Nov/2016 with registration number of 36/2016. The study was registered in the ISRCTN international registry with ID ISRCTN97596271. The enrolment of the patients was started after getting the IRB approval. The study period was between 21/Nov/2016 and 05/Jan/2018. Inclusion criteria were patients waiting for thoracic operation with functional impairment including reduced lung function (FEV1 <1.3 L), lower physical activity and significant comorbidities. Exclusion criteria were joint disease, mental disorder, cardiac disease which is not allowed the patients to be involved into the training program. Primary outcome of the study was functional improvement. Secondary outcomes were change in exercise tolerance, lung function, lung mechanics, chest kinematics, health status and dyspnoea score (CONSORT checklist in Table S1).
Table 1. Patients’ characteristics (n=238).
| Variables | Group PRE (n=72) | Group POS (n=80) | Group PPO (n=86) | Significance |
|---|---|---|---|---|
| Age, mean ± SD (years) | 65±7 | 61±10 | 65±6 | n.s. |
| Male:female | 47:24 | 43:38 | 42:44 | n.s. |
| BMI, mean ± SD (kg/m2) | 27±5 | 25±5 | 27±6 | n.s. |
| FEV1, mean ± SD (%pred) | 62±17 | 79±20 | 57±15 | n.s. |
| Hypertension, n [%] | 40 [56] | 42 [53] | 45 [52] | n.s. |
| Diabetes, n [%] | 22 [31] | 22 [28] | 23 [27] | n.s. |
| Atherosclerosis, n [%] | 20 [28] | 21 [26] | 24 [28] | n.s. |
| Pulmonary hypertension, n [%] | 9 [13] | 9 [11] | 8 [9] | n.s. |
| Quitting rate of smoking cessation, n [%] | 53 [74] | 57 [71] | 60 [70] | n.s. |
BMI, body mass index; FEV1, forced expiratory volume in the first second; n.s., not significant.
The patients were randomly assigned into three groups by an independent committee working at the institute. Randomization was performed by assigning the random numbers from random number tables to the treatment conditions. The first group of 72 patients performed only preoperative pulmonary rehabilitation (PRE). The second group comprised of 80 patients who performed both pre- and postoperative rehabilitation procedures (PPO). The third group consisted of 86 patients who performed postoperative rehabilitation only (POS) (Figure 1). The enrolment was stopped, when it reached the target number of the patients. All of the patients finished the study after randomisation and enrolment. We started the recruitment of the patients in Nov/2016 and stopped the recruitment in Jan/2018. None of the participants reported any side effect during the rehabilitation period.
Figure 1.
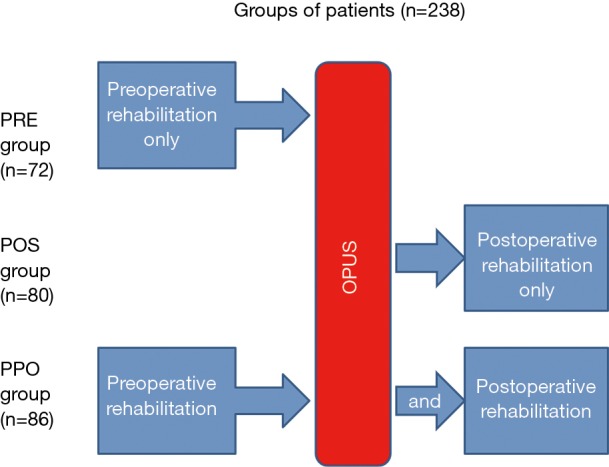
Flow chart of participants of the perioperative rehabilitation study and risk stratification. PRE group, preoperative rehabilitation only; POS group, postoperative rehabilitation only; PPO group, pre- and postoperative rehabilitation as well.
Functional follow-up and health condition questionnaire
Preoperative check-up and functional follow-up included complex assessment which includes measuring of lung function (FEV1, FVC), chest wall expansion (CWE), 6-minute walking test (6MWT) (9) and quality of life tests such as Modified Medical Research Council Dyspnoea Scale (mMRC) (10) and COPD Assessment Test (CAT) (11). Breath holding time (BHT) and grip strength (GS) was also measured. Power, distance and performance time was examined during cycle ergometry. We examined the 11 variables above. Blood sample analysis, activity monitoring, measuring maximal inspiratory pressure (MIP) can also be part of the routine check-up.
Pulmonary function
In accordance to ATS/ERS guidelines all patients underwent post-bronchodilator pulmonary function testing (Vmax 229 and Autobox 6200, Sensormedics) including spirometry measurements (12). All COPD patients were administered 400 µg of inhaled salbutamol 20 minutes before testing.
CWE
Chest wall expansion (CWE) was calculated as the difference between the side values of chest circumference measured in maximal inspiration phase and in maximal expiration phase—at the level of the xiphoid process (13).
6MWT
The 6MWT was performed in the corridors of our department. The patients were instructed to walk as fast as possible during 6 minutes, the covered distance was measured [as 6-minute walking distance (6MWD)] (9). Before, during and after the test, oxygen saturation and heart rate measurements were recorded. A modified Borg-scale was then evaluated.
Health condition markers and dyspnoea scores
Health condition was evaluated using the COPD Assessment Test (CAT) markers (11). The severity of patients’ dyspnoea was measured via use of the mMRC (10).
Quality of life tests
The mMRC stratifies severity of dyspnoea in respiratory diseases, particularly COPD. CAT test is a patient-completed instrument that can quantify the impact of COPD on the patient’s health. These tests were completed before and after pulmonary rehabilitation.
BHT
BHT was used as a measure of the severity of COPD. After a maximal inhalation the patients were asked to hold their breath as long as possible with closed nose and mouth and the time was measured in seconds (14).
GS measurement
A Kern hand grip dynamometer (2016 Kern & Sohn GmbH, Germany) was used to identify the peripheral muscle force (15). Three measurements were made and the average was calculated. This was used to determine the level of peripheral muscle atrophy accompanying the primary respiratory disease.
Cycle ergometry
Maximal intensity was measured by cycle training, time distance and power were recorded. Pulse rate, blood pressure and electrocardiogram (ECG) of the patients was monitored during the examination.
MIP
To evaluate MIP a specialized digital instrument was utilized. The instrument in question is referred to as the Power Breathe K1 (POWERbreathe International Limited, Southam, UK). The calculation of diaphragmatic force was based on the patient’s height, weight, age and sex. The results were categorized as “very poor”, “poor”, “average”, “fair”, “good” and “very good”. Patients were asked to abruptly inhale with maximal force after maximal expiration (16).
Our pulmonary rehabilitation program
Our pulmonary rehabilitation program included 30 minutes of respiratory training in the morning, chest wall mobilization, learning controlled breathing techniques, inhalation, expectoration, psychological support, smoking cessation and a session of further personalized training for each patient.
Personalized training
The patients participated in an individualized continuous or interval type of cycle and/or treadmill training lasting for 10–30 minutes, 2–3 times a day at a level of 60–80% of maximal intensity (17,18). The total duration of the rehabilitation program was 3 weeks. The intensity of the training was incremental, progressing from 60% to 80% of peak work rate based on the Borg dyspnoea scale breathlessness and leg fatigue, with aim of maintaining its value at Grade No. 7.
Smoking cessation
Smoking cessation was an important part of our perioperative rehabilitation program. With the help of psychologists, our institute held special anti-smoking sessions for the patients once per week for a duration of 45 minutes (19).
Postoperative complications
Postoperative complications were classified into two groups as “severe” and “not severe”. Prolonged surgical treatment, negative-pressure wound therapy, need of reoperation, postoperative re-intubation and/or prolonged invasive respiration, intensive care treatment lasting further than 4 days, reanimation and death considered to be “severe”. The “not severe” postoperative complication group referred to patients that experienced no or mild complications such as wound-revision, successful re-drainage, sputum retention, temporary atelectasis, or other problem that could be solved by conservative treatment.
Statistical analysis
Analysis was preformed through a combination of t-tests, non-parametric sign tests on patient characteristics, functional markers and health condition scores. 3D paired T probe (Sign test and Wilcoxon test) was made for start and post-rehabilitation values. We used Pearson chi-square analysis (χ2-probe) for discrete variables. The continuous variables were utilised to determine the continuous distribution. The distribution around the mean was expressed as ± SD. We used ANOVA statistics for the three groups and asserted that the minimum clinically important differences between groups the required standard deviation of change in 6MWT among subjects was 40 meters, and utilized a power of 0.8 and α=0.05. This analysis indicated that 72 subjects in each group is required.
We analysed the correlations between improvements of variables. The level of connection is represented by the Pearson correlation coefficient.
By classifying the postoperative complications into “severe” and “not severe” group we performed discriminant analysis to get know whether there are independent variables that responsible for the patients to get into the severe complication group. First discriminant analysis was performed on the functional parameters.
In the second discriminant analysis some new (surgeon- and operation-dependent) variables were involved into the examination such as the person of the surgeon, the experience of the surgeon in years after graduation and the extent of the operation.
Results
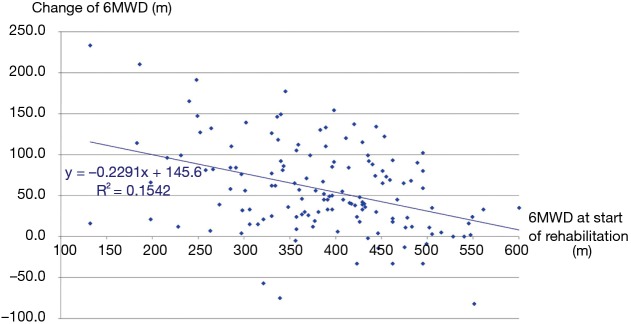
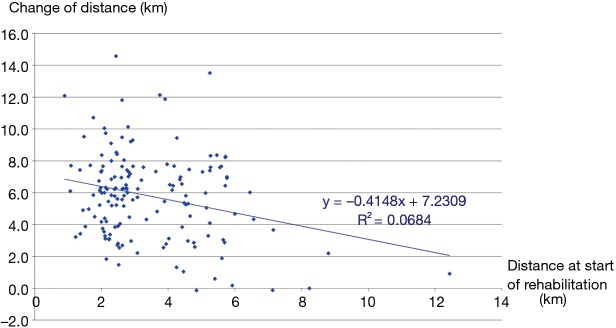
The three groups of patients proved to be comparable. 3D paired T probe (Sign test and Wilcoxon test) was made on the start and post-rehabilitation variables in all 3 groups: our rehabilitation program resulted significant changes in all investigated functional parameters and health status. Significance level was defined as P<0.05. All of the 11 post-rehabilitation parameters were significantly better than the start values so the changes can be considered to be improvement in PRE, POS and PPO group as well. In PPO group—after the favourable effect of preoperative rehabilitation and function depleting effect of the operation—postoperative rehabilitation resulted additional improvement (Tables 2-4). No strong correlations were found between the starting values and the degree of improvement after rehabilitation in all of the examined variables. Correlation diagram of starting value and degree of improvements following rehabilitation of 6MWD, FEV1%pred and the Km travelled on the cycle ergometer can be seen on Figures 2-4. It should be noted that out of the three patient groups under observation, the POS group underwent the most substantial upturn of values. In this group, the improvements in exercise capacity, lung function, chest kinematics, lung mechanics and health condition scoring were more prominent, whilst the dyspnoea score was lower than the corresponding value recorded amongst the other two groups (Tables 2-4).
Table 2. Changes in functional parameters demonstrating the effects of preoperative pulmonary rehabilitation.
| Parameters | PRE (preoperative rehabilitation only) (n=72) | ||
|---|---|---|---|
| Before rehabilitation | After rehabilitation | Change, significance (P value) | |
| FEV1 (%pred) | 63.2±15.6 | 70.1±16.6 | <0.0001 |
| FVC (%pred) | 83.1±15.9 | 90.9±15.6 | 0.0001 |
| Chest wall movement (cm) | 4.2±2.3 | 5.8±2.2 | <0.0001 |
| 6MWD (m) | 392.9±93.5 | 443.2±86.6 | <0.0001 |
| mMRC | 0.93±0.70 | 0.61±0.58 | 0.0005 |
| Breath holding time (s) | 29.7±11.3 | 33.4±13.8 | 0.0177 |
| Grip strength (kg) | 29.8±9.8 | 31.7±9.3 | <0.0001 |
| CAT | 8.3±5.2 | 5.3±4.6 | 0.0001 |
| Cycle ergometry—time (minute) | 6.9±2.5 | 16.8±4.7 | <0.0001 |
| Cycle ergometry—power (watt) | 31.5±7.9 | 46.5±14.4 | <0.0001 |
| Cycle ergometry—distance (km) | 3.3±1.4 | 9.1±2.7 | <0.0001 |
Data are presented as mean value ± SD. FEV1, force expiratory volume in the first second; FVC, forced expiratory volume; 6MWD, 6-minute walking distance; mMRC, modified Medical Research Council dyspnoea scale; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease.
Table 3. Changes in functional parameters demonstrating the effects of postoperative pulmonary rehabilitation (n=238).
| Parameters | POS (postoperative rehabilitation only) (n=80) | ||
|---|---|---|---|
| Before rehabilitation | After rehabilitation | Change, significance (P value) | |
| FEV1 (%pred) | 56.4±15.6 | 64.6±16.0 | <0.0001 |
| FVC (%pred) | 66.2±18.7 | 76.1±17.7 | <0.0001 |
| Chest wall movement (cm) | 2.9±1.4 | 5.0±2.0 | <0.0001 |
| 6MWD (m) | 354.7±90.7 | 437.0±96.0 | <0.0001 |
| mMRC | 1.5±1.0 | 1.0±0.8 | <0.0001 |
| Breath holding time (s) | 26.4±12.2 | 32.1±14.7 | <0.0001 |
| Grip strength (kg) | 25.8±7.7 | 28.1±7.6 | <0.0001 |
| CAT | 16.9±8.1 | 11.4±8.1 | <0.0001 |
| Cycle ergometry—time (minute) | 6.2±2.8 | 14.6±4.9 | <0.0001 |
| Cycle ergometry—power (watt) | 30.0±8.2 | 40.8±10.2 | <0.0001 |
| Cycle ergometry—distance (km) | 2.8±1.8 | 7.8±3.4 | <0.0001 |
Data are presented as mean value ± SD. FEV1, force expiratory volume in the first second; FVC, forced expiratory volume; 6MWD, 6-minute walking distance; mMRC, modified Medical Research Council dyspnoea scale; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease.
Table 4. Changes in functional parameters demonstrating the effects of combined pre- and postoperative rehabilitation.
| Parameters | PPO (pre- and postoperative rehabilitation as well) (n=86) | ||||||
|---|---|---|---|---|---|---|---|
| Before surgery | After surgery | ||||||
| Before rehabilitation | After rehabilitation | Change, significance (P value) | Before rehabilitation | After rehabilitation | Change, significance (P value) | ||
| FEV1 (%pred) | 58.2±15.1 | 67.0±14.6 | <0.0001 | 47.4±13.0 | 53.4±14.7 | 0.0003 | |
| FVC (%pred) | 82.4±16.7 | 93.3±16.7 | <0.0001 | 63.6±16.9 | 72.6±18.6 | 0.0001 | |
| Chest wall movement (cm) | 4.0±2.1 | 5.6±2.6 | <0.0001 | 2.7±1.5 | 4.4±2.2* | <0.0001 | |
| 6MWD (m) | 378.3±90.5 | 441.3±86.4 | <0.0001 | 341.4±115.9 | 403.3±98.4* | <0.0001 | |
| mMRC | 1.2±1.0 | 0.8±0.8 | <0.0001 | 1.8±0.9 | 1.4±0.8 | 0.0001 | |
| Breath holding time (s) | 29.3±11.8 | 33.7±11.8 | <0.0001 | 23.3±10.4 | 28.1±10.1 | <0.0001 | |
| Grip strength (kg) | 27.5±7.7 | 29.6±7.9 | <0.0001 | 26.9±8.4 | 27.7±9.2* | 0.0376 | |
| CAT | 11.4±6.8 | 7.7±5.8 | <0.0001 | 15.4±6.9 | 9.9±4.7* | <0.0001 | |
| Cycle ergometry—time (minutes) | 7.2±3.2 | 17.8±6.3 | <0.0001 | 7.1±3.3 | 14.5±4.5* | <0.0001 | |
| Cycle ergometry—power (watt) | 31.1±8.7 | 44.1±10.8 | <0.0001 | 30.4±10.1 | 39.7±9.5* | <0.0001 | |
| Cycle ergometry—distance (km) | 3.6±1.9 | 9.3±2.9 | <0.0001 | 3.3±1.8 | 7.5±2.9* | <0.0001 | |
Data are presented as mean value ± SD. *, these marked values of postoperative and post-rehabilitation parameters are better than its preoperative (start-value) was, so at these variables the preoperative and postoperative rehabilitations were able to compensate the functional-depleting effect of the operation. FEV1, force expiratory volume in the first second; FVC, forced expiratory volume; 6MWD, 6-minute walking distance; mMRC, modified Medical Research Council dyspnoea scale; CAT, COPD assessment test; COPD, chronic obstructive pulmonary.
Figure 2.
Correlation between the start-value of exercise tolerance in 6MWT and the change of 6MWD during rehabilitation. 6MWT, 6-minute walking test; 6MWD, 6-minute walking distance.
Figure 3.
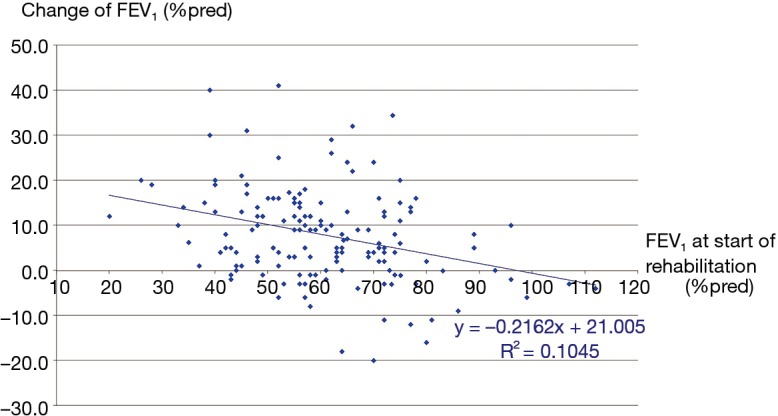
Correlation between the start-value of FEV1 and the change of FEV1 in %pred during rehabilitation. FEV1, forced expiratory volume in the first second.
Figure 4.
Correlation between the start-value of km achieved in distance via treadmill ergometry and the improvement of the km value after preoperative pulmonary rehabilitation.
By analysing the correlations between improvements of the functional variables, the following four variable-pairs’ improvements showed correlation: (I) CWE and BHT; (II) FEV1 and FVC; (III) 6MWD and CAT; (IV) minutes and kilometers travelled via cycle ergometer.
The correlation matrix of the 10 variables is shown on Table 5. According to Guilford from the correlation coefficients we can conclude that all of these four correlations are moderate and the connection is significant.
Table 5. Correlation matrix for the changes (Δ) of ten functional values as a result of preoperative pulmonary rehabilitation.
| Variables | ÄChest wall extensions (cm) | ÄFEV1 (%pred) | Ä6MWD (m) | ÄFVC (%pred) | ÄBreath holding time (s) | ÄGrip strength (kg) | ÄCAT | Cycle ergometry—Ätime (minutes) | Cycle ergometry—Äpower (watt) | Cycle ergometry—Ädistance (km) |
|---|---|---|---|---|---|---|---|---|---|---|
| ÄChest wall extensions (cm) | 1.0000 | – | – | – | – | – | – | – | – | – |
| ÄFEV1 (%pred) | −0.1116 | 1.0000 | – | – | – | – | – | – | – | – |
| Ä6MWD (m) | 0.1589 | −0.0053 | 1.0000 | – | – | – | – | – | – | – |
| ÄFVC (%pred) | −0.1668 | 0.6484* | −0.1066 | 1.0000 | – | – | – | – | – | – |
| ÄBreath holding time (s) | 0.4271* | 0.0355 | 0.1005 | −0.1141 | 1.0000 | – | – | – | – | – |
| ÄGrip strength (kg) | 0.2148 | −0.0615 | −0.0494 | −0.1439 | 0.1584 | 1.0000 | – | – | – | – |
| ÄCAT | −0.1991 | −0.0128 | −0.4021* | −0.0245 | −0.1288 | 0.0012 | 1.0000 | – | – | – |
| Cycle ergometry—Ätime (minutes) | 0.1631 | −0.1808 | −0.0099 | −0.1921 | −0.0815 | −0.1420 | −0.0045 | 1.0000 | – | – |
| Cycle ergometry—Äpower (watt) | −0.1312 | −0.0772 | 0.0198 | −0.1402 | 0.1233 | −0.0853 | 0.0882 | 0.0493 | 1.0000 | – |
| Cycle ergometry—Ädistance (km) | −0.0939 | −0.2340 | 0.0690 | −0.0968 | −0.1150 | −0.1781 | 0.0548 | 0.6742* | 0.1993 | 1.0000 |
*, correlation coefficients that reach significance level. FEV1, force expiratory volume in the first second; FVC, forced expiratory volume; 6MWD, 6-minute walking distance; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease.
Discriminant analysis revealed that 4 parameters as gender, FEV1 after preoperative rehabilitation, start value of 6MWD and distance travelled via cycle ergometer at the onset of the rehabilitation could accurately predict the severeness of postoperative complications in 67.0% of all cases (Table 6) (It discriminates “not severe” group in 67.1% correctly, “severe” group in 66.7%). According to jacknifed classification this hit ratio is 63.5%.
Table 6. Classification matrix of discriminant analysis I and jackknifed classification.
| Type of classification | Group | Percent correct (%) |
|---|---|---|
| Classification matrix | Not severe | 67.1* |
| Severe | 66.7 | |
| Total | 67.0 | |
| Jackknifed classification | Not severe | 63.4 |
| Severe | 63.6* | |
| Total | 63.5 |
*, the best discriminating values (in percent of hit ratios).
At the second (extended) discriminant analysis the extent of the operation proved to have discriminating value, but not at the first place in row. Five values altogether can discriminate between the two severity groups in 66.4%— “severe” group can be discriminated in 72.5%, “not severe” in 64.2%. With a little bit more strict jackknifed classification these values are 67.5% and 62.3% in order (altogether 63.7%) (Table 7). The variables that has discriminating value are the following, according to descending of its discriminating force:
Table 7. Classification matrix of the (extended) discriminant analysis II and jackknifed classification.
| Type of classification | Group | Percent correct (%) |
|---|---|---|
| Classification matrix | Not severe | 64.2 |
| Severe | 72.5* | |
| Total | 66.4 | |
| Jackknifed classification | Not severe | 62.3 |
| Severe | 67.5* | |
| Total | 63.7 |
*, the best discriminating values (in percent of hit ratios).
❖ Gender;
❖ Distance travelled via cycle ergometer at the onset of the rehabilitation;
❖ FEV1 after preoperative rehabilitation;
❖ Extent of the operation;
❖ Start value of 6MWD.
Discussion
Preoperative pulmonary risk assessment was conducted though monitoring of lung function, exercise capacity, chest kinematics, post-operative complications, lung mechanics and health condition markers in 238 patients suffering of lung cancer and COPD. The subjects were separated into three groups according to the form of rehabilitation they experienced. Consequently, the aforementioned groups were classified as the pre-operative, post-operative and combined pulmonary rehabilitation groups. At the end of our rehabilitation program significant improvements were prominent in all of the investigated parameters of the three groups. Furthermore, we have discovered a successful algorithm for the prediction of severe postoperative complications in the form of a composite score utilising the parameters of gender, FEV1, 6MWD and km-distance travelled by cycle ergometer. By involving some more surgery-specific parameters—such as person of the surgeon, experience of the surgeon in years after graduation, and extent of the operation—the discriminant analysis resulted even higher discriminating value. We feel the need to note that throughout the duration of our study our team managed to accumulate a set of additional observations that we believe to hold value for further consideration.
Use of perfusion lung scintigraphy as an element of preoperative risk stratification preceding thoracic surgery has also promise for the approximation postoperative pulmonary function. We believe that these pursuits will help for risk assessment of postoperative respiratory failure and other cardiopulmonary complications (8). In clinical practice, observations were made of a relationship between %ppoFEV1 (% predicted postoperative FEV1) and the development of respiratory insufficiency. It has been stated that amongst patients with %ppoFEV1 higher than 30% of the predicted FEV1, as calculated in respect of sex, weight, height and age, the chance of respiratory insufficiency following resection was very low (8,20). We have observed the same phenomenon during our clinical practice.
Cardiopulmonary exercise testing (CPET) permitted measurement of oxygen uptake (VO2); an indicator of overall cardiopulmonary fitness and a useful measurement in the assessment of operative risk for lung cancer patients. The evidence supporting the use of CPET in pre-operative assessment of the lung cancer surgery patient was examined. CPET methodology and limitations, as well as alternatives to CPET for risk assessment are discussed (6,20,21). In our practice, CPET is an element of the preoperative risk stratification, especially before pneumonectomy and patients with severe comorbidities.
Elder age (>70 years) was not found to represent a contraindication to lung cancer surgery and an age limit for the operation could not be determined. However, patients of this nature were required to undergo a careful preoperative evaluation (22,23). In cases where complex perioperative management was applied, low mortality and morbidity following the operation could be anticipated (22). Where such care was not exercised, a considerable risk of death or major complication within the first 30 postoperative days was noted (22,23).
The following protocol for preoperative physiologic assessment is to be recommended. Assessment can begin with a cardiovascular evaluation and spirometry for evaluation of the FEV1. If diffuse parenchymal lung disease is present, based on dyspnoea upon exertion FEV1, DLCO should subsequently be measured (1,8). If FEV1 or DLCO <80% predicted, the likely postoperative pulmonary reserve needs to be estimated by either the perfusion scan method for pneumonectomy or the anatomic method for lobectomy based on the number of segments to be removed (1,8). Consequently, an estimated postoperative FEV1 or DLCO <40% of the predicted value signifies an increased risk for perioperative complications including death during lobectomy or greater resection. CPET was found to be valuable for the estimation of perioperative risk when used to obtain values of maximal oxygen consumption (VO2max). A VO2max value of <15 mL/kg/min was found to associate to increased risk of perioperative complications (1,8). As rule, VO2max of <10 mL/kg/min has been established as a contraindication of operation. Alternative measurements of exercise tolerance, such as stair climbing, the shuttle walk, and the 6MWD can be considered as well. Desaturation during an exercise test has not clearly been associated with an increased risk for perioperative complications (1,8).
During our research we discovered mention of a previously formulated test, capable of predicting the occurrence of post-operatively mortality. This test is a scoring system known as the Charlson comorbidity index or CCI (22). We believe that incorporation of this test in predictive algorithms pertaining to the appearance of post-operative complications can potentially enhance their power. During our study the CCI index was not calculated. However, the potential value of CCI in post-operative risk assessment was noted in a large cohort study conducted in Norway in 2007 by the Cancer Registry of Norway where values of 26,665 patients were evaluated. A total of 4,395 patients, who underwent surgical resection were included in the analysis (22). A subset of 1,844 patients was scored according to the CCI (22). The overall postoperative mortality rate was 4.4% within 30 days with a declining trend in the period. Male sex, older age (between 70–79 years), right-sided tumours and extensive procedures were identified as risk factors for postoperative mortality via multivariate analysis (22). The CCI was identified as an independent risk factor for postoperative mortality (P=0.017).
Furthermore, in 2003 Birim et al. conducted a clinical study for validation of the CCI in patients with operated primary non-small cell lung cancer (24). In the aforementioned study, 205 consecutive resections for non-small cell lung cancer were performed. In a retrospective of the study, each patient was scaled according to the CCI and the complications of surgery were determined (24). The hospital mortality was found to be 2.4% with 32.7% patients experiencing minor complications and 15.6% major complications. Following a univariate analysis, gender, grades 3–4 of the CCI, any prior tumour treated in the last 5 years and chronic pulmonary disease were determined as significant predictors of an adverse outcome (24). However, further multivariate analysis displayed that only grades 3–4 of the CCI were a significant predictor. Despite this, every increase of the comorbidity grade was found to be correlated to a slight increase in the relative risk of an adverse outcome (24). In conclusion, the CCI was strongly linked with higher risk of surgery in primary non-small cell lung cancer patients and was determined to be a better predictor than individual risk factors (24).
Overall, it is our belief that use of the CCI for improvement of postoperative risk assessment algorithms should be further researched. In the future, our group hopes to integrate the CCI in prognostic models that may help in preemptively identifying patients in need of intensive postoperative care (22).
During our study, we noticed a considerable variability in the methods used for reporting postoperative mortality and risk factors for mortality after lung cancer surgery. The lack of a standardised system in reporting data of this nature represents a limitation in our scientific field. Population-based data help provide unbiased estimates and may aid in treatment selection.
Finally, the following limitations in our study are to be noted. Our group did not collect enough exercise physiologic parameters. We believe that this presents a limiting factor for our work at this point of time. In addition, while the calculation of the CCI score could potentially increase the power of our predictive algorithm, CCI scoring was not included in this study. Furthermore, we did not focus on the implications of psychological factors such as depression and anxiety, which in the future can be accounted for via use of the HADS score. In prospective work we hope to incorporate these parameters into a more comprehensive composite score.
We conducted a clinical research to evaluate the effectiveness of pulmonary rehabilitation in patients with COPD and lung cancer. There was no side effect during the rehabilitation program. The study was met with success significant improvements were detected in lung function, chest kinematics, lung mechanics, dyspnoea scores, respiratory and peripheral muscle function and health condition markers in both of our pre- and postoperative pulmonary rehabilitation protocols. Subsequently, we try to find the elements of a composite score capable of predicting the risk of severe postoperative complications amongst patients through the assessment of the parameters of gender, exercise distance travelled in km by cycle ergometer, FEV1, extent of the operation and 6MWD.
Conclusions
In conclusion, the complex assessment conducted on our selection of functional parameters helps shed light on the inner workings of comorbidity and prognostic risk factors. It is our belief that this study and all others of such similar content will help clinicians to promptly identify cases of poor prognosis and establish a more appropriate treatment strategy (2). For example, the assessment of prognostic factors is an area of active investigation and a promising field of research in optimising therapy of non-small cell lung cancer (NSCLC) patients (2).
Table S1. CONSORT 2010 checklist of information to include when reporting a randomised trial*.
| Section/topic | Item No. | Checklist item | Reported on page No. |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | Page 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | Page 4–5 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | Page 2-5 |
| 2b | Specific objectives or hypotheses | Page 2-3 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | Page 3 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | No important changes after trial commencement | |
| Participants | 4a | Eligibility criteria for participants | Page 2 |
| 4b | Settings and locations where the data were collected | Page 2 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | Pages 3-4 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | Pages 2-5 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | There was no change after the trial commence | |
| Sample size | 7a | How sample size was determined | Page 5 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | We analysed the data when we achieved the target number of the patient. Page 3 | |
| Randomisation | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | Page 3 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | Page 2-3, there was no restriction | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | Page 3 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | Page 2-3 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | After the randomisation it was not blinded |
| 11b | If relevant, description of the similarity of interventions | Pages 3-5 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | Page 5 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | Page 5 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | Page 3 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | Page 3 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | Page 3 |
| 14b | Why the trial ended or was stopped | Page 3 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | Page 3 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | Page 3 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | Pages 5-8 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Page 5 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | Page 5-8 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | Page 5 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | Page 11 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | Page 11 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | Page 11 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | Page 2 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | It is in Hungarian, it is available at site |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | Page 1 |
*, we strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement.org.
Acknowledgements
The authors would like to thank Zsuzsanna Balogh to work with the patients and measure functional parameters as a physiotherapist, Csaba Feher and Miklos Molnar thoracic surgeons to help about the thoracic surgical database management, Istvan Gaudi for the statistical analysis, Zsofia Hodovan to work with the patients as a psychologist and Erika Pataki for coordinating the smoking cessation program.
Ethical Statement: All the patients confirmed consent to the study as part of the general informed consent form for patients at the Department of Pulmonary Rehabilitation and at the Department of Thoracic Surgery. Local ethics approval was done on 20/Nov/2016 with registration number of 36/2016.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S. [DOI] [PubMed] [Google Scholar]
- 2.Birim O, Kappetein AP, Waleboer M, et al. Long-term survival after non-small cell lung cancer surgery: development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491-8. 10.1016/j.jtcvs.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 3.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. 10.1016/j.athoracsur.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 4.Myrdal G, Gustafsson G, Lambe M, et al. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg 2001;20:694-9. 10.1016/S1010-7940(01)00875-2 [DOI] [PubMed] [Google Scholar]
- 5.Liptay MJ, Basu S, Hoaglin MC, et al. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J Surg Oncol 2009;100:703-7. 10.1002/jso.21407 [DOI] [PubMed] [Google Scholar]
- 6.Voduc N. Physiology and clinical applications of cardiopulmonary exercise testing in lung cancer surgery. Thorac Surg Clin 2013;23:233-45. 10.1016/j.thorsurg.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. 10.1183/09031936.00184308 [DOI] [PubMed] [Google Scholar]
- 8.Charloux A, Brunelli A, Bolliger CT, et al. Lung function evaluation before surgery in lung cancer patients: how are recent advances put into practice? A survey among members of the European Society of Thoracic Surgeons (ESTS) and of the Thoracic Oncology Section of the European Respiratory Society (ERS). Interact Cardiovasc Thorac Surg 2009;9:925-31. 10.1510/icvts.2009.211219 [DOI] [PubMed] [Google Scholar]
- 9.Balke B. A simple field test for the assessment of physical fitness. Rep 63-6. Rep CivAeromed Res Inst US 1963:1-8. [PubMed]
- 10.Fletcher CM, Elmes PC, Fairbairn MB, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257-66. 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006;27:822-32. 10.1183/09031936.06.00145104 [DOI] [PubMed] [Google Scholar]
- 12.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40. 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 13.Debouche S, Pitance L, Robert A, et al. Reliability and Reproducibility of Chest Wall Expansion Measurement in Young Healthy Adults. J Manipulative Physiol Ther 2016;39:443-9. 10.1016/j.jmpt.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Mirsky IA, Lipman E, Grinker RR. Breath holding time in anxiety states. Fed Proc 1946;5:74. [PubMed] [Google Scholar]
- 15.An KN, Chao NY, Askew LJ. Hand strength measurement instruments. Arch Phys Med Rehabil 1980;61;366-8. [PubMed] [Google Scholar]
- 16.Neumeister W, Rasche K, Maas P, et al. Reproducibility of computer-assisted mouth occlusion pressure measurements. Med Klin (Munich) 1996;91 Suppl 2:73-5. [PubMed] [Google Scholar]
- 17.Varga J, Porszasz J, Boda K, et al. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007;101:2297-304. 10.1016/j.rmed.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 18.Corhay JL, Dang DN, Van Cauwenberge H, et al. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis 2014;9:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control 2006;15:352-8. 10.1136/tc.2005.015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cukic V. Preoperative prediction of lung function in pneumonectomy by spirometry and lung perfusion scintigraphy. Acta Inform Med 2012;20:221-5. 10.5455/aim.2012.20.221-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cukic V, Lovre V. Changes of arterial blood gases after different ranges of surgical lung resection. Mater Sociomed 2012;24:165-70. 10.5455/msm.2012.24.165-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strand TE, Rostad H, Damhuis RA, et al. Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude. Thorax 2007;62:991-7. 10.1136/thx.2007.079145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salati M, Brunelli A. Risk Stratification in Lung Resection. Curr Surg Rep 2016;4:37. 10.1007/s40137-016-0158-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. 10.1016/S1010-7940(02)00721-2 [DOI] [PubMed] [Google Scholar]