Abstract
Background
During left-sided cardiac valve surgery in patients with mild or no tricuspid regurgitation (TR), the tricuspid valve exploration plays a vital role in the decision-making of concomitant tricuspid annuloplasty. Currently lack of an appropriate standard to define tricuspid annular dilatation is a challenge. The aim of this study is to introduce and evaluate an alternative intraoperative method for Tricuspid Valve annuloplasty based on annular circumference that could trace the extent of annular dilatation and predict TR-progression, which can also guide the surgeons to intraoperative decision.
Methods
From January 2011 to December 2011, 131 patients (73 females, 58 males, and mean age 54±10.8) were selected for the study underwent left-sided cardiac valve surgery at Anzhen Hospital, all the subjects preoperatively diagnosed with non-significant TR. The enrolled subjects were followed-up over 5years for TR-progression. Tricuspid annular circumference (TAC) measurement was performed via special sizer for each patient, and the obtained values divided on the subject’s body surface area (BSA) to achieve the tricuspid annular circumference index (TACI). The endpoint was set as the TR-progression by more than two grades, or a final TR grade ≥3+.
Results
The mean follow up period was 68±3.8 months (range, 60–77 months). In univariate, multivariate and logistic regression analysis three variables were noticed to be associated with TR progression, female gender (P<0.002), body mass index (BMI) (P<0.021), and intraoperatively measured TACI (P<0.001). But in multiple regression the TACI (OR 0.812; 95% CI: 0.748–0.883; P<0.001) was the single parameter which significantly related to TR-progression. Based on the receiver-operator curve (ROC), it was likely to derive an optimal cut-off 80.2 mm/m2 which could predict the postoperative development of TR with acceptable sensitivity and specificity 69%, 89%.
Conclusions
Patients with mild or no TR undergoing left-sided valve surgery, intraoperative measurement of the TAC proved to be an ideal method to judge the tricuspid-annulus, it is capable to predict TR-progression. For prophylactic tricuspid repair a presumed TACI of 80.2 mm/m2 is recommended as an indicative threshold.
Keywords: Tricuspid regurgitation (TR), tricuspid annular dilatation (TAD), prospective study
Introduction
Secondary tricuspid regurgitation (TR) is frequently observed in the patients assessed for left-sided cardiac valve surgery (1,2). Decisions for the interventional approach on appropriate time is critical, because significant TR can influence the long-term clinical outcomes of surgery in patients with such condition (3-5). Even though currently many research articles suggested that subjects undergoing left-sided cardiac valve surgery can concomitantly undergo tricuspid valve annuloplasty if the TR is at moderate to severe stage (1,2,6), but in clinical practice patients with mild or less TR are being overlooked.
Previously some clinical researches have been revealed that, a significant number of patients accessed for isolated left-sided cardiac valve surgery with mild or no TR at the initial stage needed to undergo reoperation for significant TR (7,8). Therefore, to prevent the reoperation for a selective number of patients, the concomitant tricuspid annuloplasty is required. Tricuspid annular dilatation is generally accepted indicator for tricuspid valve intervention irrespective of the TR grading (1,8-12). However, due to the limitations of conventional echocardiography and the declining admiration of three-dimensional (3D) echocardiography, intraoperative exploration remains an essential procedure for the tricuspid valve to be performed (13). Lack of a certain standard for dilated tricuspid annulus is challenging intraoperatively, though, many surgeons largely relied on their personal experiences. The aim of this study was to introduce an alternative intraoperative method for Tricuspid Valve annuloplasty based on the annular circumference that could judge the extent of annular dilatation and guide the surgeons to a decision-making stage during the surgery. Besides that, its capability to predict the progression of postoperative TR.
Methods
Study population
From January 2011 to December 2011, at the author’s institution 255 patients with an isolated mitral valve or mitral and aortic valve disease underwent mitral valve replacement or double-valve replacement procedures. For concomitant tricuspid valve repair the implementation of widely accepted indications were strictly followed: a preoperative significant TR (grade 3+/4+) and an echocardiographically measured tricuspid annular diameter (TAD) >40 mm or 21 mm/m2 (apical four-chamber view, adjusted to the patient’s body surface area; BSA), regardless of the TR grading. Exclusion criteria that were required for the Patients enrollment excluded patients with organic tricuspid valve lesions, acute endocarditis, dilated cardiomyopathy, congenital heart disease, concomitant coronary artery bypass procedure, and acute emergency cases. Pace-makers were not implanted in the enrolled patients previously. Eventually, 131 patients with TR grading <3+ and normal echocardiographic TAD underwent isolated left-sided cardiac valve procedures, and these patients were enrolled for the study prospectively. The author’s institutional Medical Ethics Committee provided permission to conduct the study, and asked the author to obtain written consents from the enrolled patients.
Echocardiographic evaluation
Preoperative and postoperative echocardiography was performed, the purpose was to analysis the patients based on preoperative and final follow up examinations. Doppler color flow imaging and two dimensional (2D) echocardiography were performed in all patients. The TR severity was assessed via the maximal TR jet area, utilizing the apical four chamber and right ventricular inflow views, with a TR area to right atrial area ratio, based on this criterion the patients were placed into four groups, 1; a TR area to right atrial area ratio of <10% counted as grade 1+, 2; TR area to right atrial area ratio 10–20% as grade 2+, 3; TR area to right atrial area ratio 20-40% as grade 3+, 4; and TR area to right atrial area ratio >40% as grade 4+ (10,14). The systolic flow reversal in the hepatic veins was also implemented as a standard of grade 4+ TR. A maximal TR jet velocity was obtained to calculate the systolic pulmonary artery pressure. The apical four chamber view, where a maximal TAD during diastole was measured from the point of insertion of the septal tricuspid leaflet to insertion of the anterior leaflet (15). The obtained diameter value (mm) was divided by the patient’s BSA (in m2) as the tricuspid annular diameter index (TADI). Some related indices, such as tricuspid annular plane systolic excursion (TAPSE) and right ventricular fractional area change (RVFAC) were calculated to evaluate the right ventricular function accurately (16).
Surgical approach and intraoperative measurements
During the study all 131 surgical procedures were performed by a single surgeon (Xu Meng). The procedure was open heart surgery, the patients operated via conventional median sternotomy under cardiopulmonary bypass (CPB), during cardioplegic arrest with antegrade cold blood cardioplegia at moderate systemic hypothermia.
Trans-septal approach was preferred during mitral valve replacement due to its convenience to explore the tricuspid valve, in some cases if the maze procedure was intended, then the patient was operated through the trans-atrial route. After completion of the left-sided cardiac valve surgery and radiofrequency ablation for atrial fibrillation (AF), the surgeon directly explored the tricuspid valve. During the assessment of tricuspid valve the surgeon utilized both the commercial Edwards tricuspid sizers (Model 1175, 26#–36#; Edwards Lifesciences) and a set of self-made tricuspid sizers (36#–60#) were used (Figure 1). While measuring the TV annulus, the sizer should pass through the orifice smoothly, neither loosely nor with much tension. The circumference of the tricuspid annular orifice should be just equal to that of the sizer. The annulus values obtained (in mm) were divided by the patient’s BSA (in m2), the outcome was expressed as the tricuspid annular circumference index (TACI = mm/m2).
Figure 1.
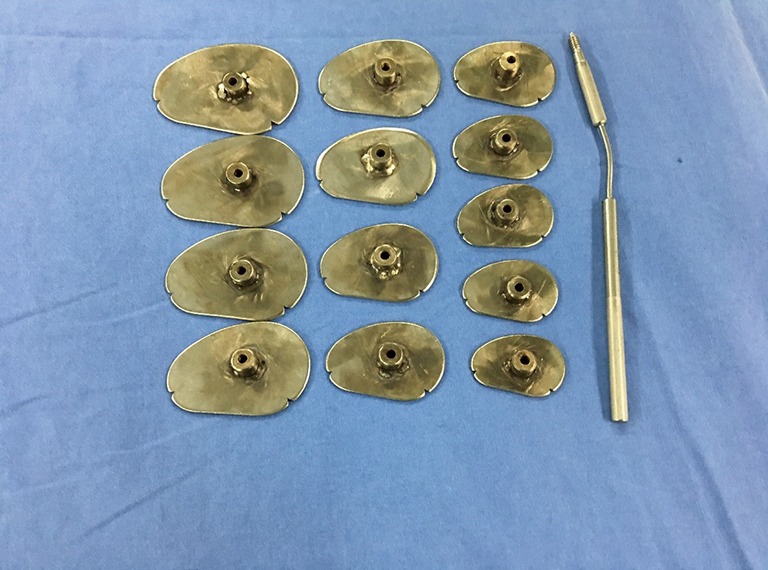
Tricuspid sizers. A set of metal, kidney-shaped TV sizers; the sizes range from 36# to 60#, with intervals of 2 mm. The circumference is calculated as 3× the size number, and the difference between two adjacent sizers is 6 mm.
Clinical evaluation and follow up
All enrolled Patients were required to visit the outpatient department regularly, the postoperative clinical checkups performed to conduct the follow up. For each individual, the echocardiographic follow-up, and clinical evaluation was performed at 6 months interval after discharge from hospital. All the follow up data were obtained based on outpatient department regular consultation. Recently the follow up data collected was in October 2017. The mean period of follow up was 68±3.8 months (range, 60–77 months). The key end-point was defined as the progression of TR by more than two grades, or a final TR grade ≥3+ on echocardiographic follow up.
Statistical analysis
All the obtained data were expressed as either mean ± SD or number (percentage). To evaluate differences between groups, Student’s t-test was used for continuous variables that displayed approximately normal distributions, but if the data was not normally distributed the Mann Whitney U-test was used, and a chi-square test was used for categorical variables. A P value <0.05 was considered to be significant statistically. According to the primary endpoint, risk factors for postoperative TR progression were investigated via univariate, multivariate, logistic regression, and multiple regression analysis. Via receiver-operator characteristic (ROC) curves, examination of the specificities and sensitivities and area under the curve at the cut-off points were conducted and these would reliably predict the progression of postoperative TR. Statistical Package for the Social Sciences, v. 21 (SPSS, Inc., Chicago, IL, USA) was utilized for statistical analysis.
Results
In-patients data
For this study 131 patients (73 females, 58 males; mean age 54±10.8 years) were selected as appropriate candidates within one year enrollment period. Preoperative placement of the individuals in the TR grading revealed that, 35 had no TR, 45 had grade 1+ TR, and 51 had grade 2+ TR. The preoperative and operative data of the patients are recorded in Table 1. Majority of the patients had chronic AF, and rheumatic etiology. Echocardiographic data of the enrolled patients exposed that left ventricle and left atrium were both slightly dilated, while the mean values indicated that the right ventricular function was largely in normal position.
Table 1. Preoperative & operative characteristics of the enrolled patients.
| Variables | Values |
|---|---|
| Age (year) | 54±10.8 |
| Follow-up (months) | 68±3.8 |
| BSA (m2) | 1.73±0.16 |
| BMI | 23.8±3 |
| Chronic AF | 103 (78.6%) |
| Euro-score II | 1.12±0.33 |
| Gender | |
| Female | 73 (55.7%) |
| Male | 58 (44.3%) |
| NYHA | 2.5±0.5 |
| Class II | 64 (48.9%) |
| Class III | 67 (51.1%) |
| Etiology | |
| Rheumatic etiology | 94 (71.8%) |
| Degenerative etiology | 37 (28.2%) |
| LVEF (%) | 58.8±6.8 |
| ≥50% | 120 (91.6%) |
| <50% | 11 (8.4%) |
| Preoperative PASP (mmHg) | 42.1±9.1 |
| ≥40 | 85 (64.9%) |
| <40 | 46 (35.1%) |
| Preoperative TR grade | |
| None | 35 (26.7%) |
| 1+ | 45 (34.4%) |
| 2+ | 51 (38.9%) |
| LVEDD (mm) | 48.9±6.9 |
| LAD (mm) | 51.5±4.3 |
| ≥60 | 7 (5.3%) |
| <60 | 124 (94.7%) |
| RVFAC (mm) | 46.4±4.2 |
| TAPSE (mm) | 17.8±2.4 |
| TADI (mm/m2) | 18.5±0.5 |
| Maze procedure | 75 (57.3%) |
| Isolated MVR | 87 (66.4%) |
| AVR + MVR | 44 (33.6%) |
| TAC (mm) | 125.9±10.3 |
| Special-sizer | 41.9±3.4 |
Values are mean ± SD. Values in brackets represent percentages. AF, atrial fibrillation; BSA, body surface area; LAD, left atrial dimension; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; MVR, mitral valve replacement; AVR: aortic valve replacement; PASP, pulmonary artery systolic pressure; RVFAC, right ventricular fractional area change; TADI, tricuspid annular diameter index; TAPSE, tricuspid annular plane systolic excursion; TAC, tricuspid annular circumference.
Each patient’s tricuspid annular circumference (TAC) was measured by special sizers at the surgical intervention. TACI in the relatively normal tricuspid functional group (no-TR) was calculated from 53.9 to 72.7 mm/m2 (mean 65.96±5.3 mm/m2), and patients presenting with TR had a larger annular circumference, ranging from 58.2 to 81.8 mm/m2 (mean 72.5±6.7 mm/m2) in the grade 1+ TR group, and patients in grade 2+ group had a range from 61.9 to 97 mm/m2 (mean 79.2±9.3 mm/m2).
There was no in-patient mortality after the surgery. Early postoperative morbidity was noticed in 33 patients (25.2%) included internal bleeding in 4 patients (3%), low cardiac output in 6 cases (4.6%), transient paroxysmal AF in 14 cases (10.7%), neurologic complication in 7 patients (5.3%), and pericardial effusion in 2 cases (1.5%). After a proper medical approach all patients recovered completely and discharged from hospital in a stable condition.
Postoperative echocardiographic follow-up
The echocardiographic follow up was successfully accomplished for 131 patients (100%) in October 2017. The primary endpoint set as the progression of postoperative TR by more than two grades or a final TR grade ≥3+, which was met in 38 patients (29%), while the remaining 93 (71%) patients revealed stable TR or had not reached the description of postoperative TR progression in the follow up period. The patients in the TR progression group were asymptomatic. Detailed changes in TR severity among patients meeting the primary endpoint at follow up are summarized in Table 2. Seven patients out of 35 patients who had no TR preoperatively developed grade 2+ TR during the follow up period; in contrast, patients with preoperative grade 1+ TR, 17 patients out of 45 patients developed significant TR (grade 3+ TR in 15 cases, and grade 4+ TR in 2 cases), patients with preoperative grade 2+ TR, 14 patients out of 51 patients developed significant TR (8 patients grade 3+ TR, and 6 patients grade 4+ TR).
Table 2. Endpoints met in different preoperative TR-grades.
| Preoperative groups (n=131) | Intraoperative-TACI (mean TACI), mm/m2 | TR-endpoint met (n=38) | 2+ (n=7) | 3+ (n=23) | 4+ (n=8) |
|---|---|---|---|---|---|
| No-TR [35] | 65.96±5.3 | 7 | 7 | 0 | 0 |
| Grade 1+ [45] | 72.5±6.7 | 17 | 0 | 15 | 2 |
| Grade 2+ [51] | 79.2±9.3 | 14 | 0 | 8 | 6 |
Detailed changes of tricuspid regurgitation (TR) grading and its average tricuspid annular circumference index (TACI) in patients meeting the primary endpoint during follow up period.
Evaluation of TR progression via SPSS
The associated potential predictors of postoperative TR progression are arranged in Table 3. In univariate and multivariate analysis revealed that, the obtained data suggested three variables are relevant with TR progression, including female gender (P<0.002), BMI (P<0.021), and an intraoperatively measured TACI (P<0.001). When these three variables were inserted into a logistic regression analysis, all three values were noticed to be significantly associated with TR progression, and the noticed values for each risk factor were; TACI (OR 1.481; 95% CI: 1.289–1.702; P<0.001), female gender (OR 0.279; 95% CI: 0.119–0.653; P<0.003) and BMI (OR 0.845; 95% CI: 0.731–0.978; P<0.024) to serve as risk factors. But in multiple regression only TACI was significantly relevant with TR progression (OR 0.812; 95% CI: 0.748–0.883; P<0.001), while female gender (OR 1.426; 95% CI: 0.494–4.116; P=0.512) and BMI (OR 0.947; 95% CI: 0.775–1.158; P=0.596) failed to be significantly associated with TR progression. As the TACI was noticed to be a strong independent predictor of postoperative TR progression, then ROC-curve was developed to define the cut-off value of this specific TAC index (Figure 2). Achieved value for the area under the curve (AUC) was 0.848 (95% CI: 0.766–0.929), and the optimal TACI cut-off value recommended was 80.2 mm/m2, with acceptable sensitivity and specificity 69% and 89%.
Table 3. Postoperative TR progression and its related variables.
| Variables | TR-progression | P values | |
|---|---|---|---|
| Yes (n=38) | No (n=93) | ||
| Age (years) | 55.8±8.4 | 53.2±11.7 | 0.263 |
| Follow-up (months) | 67.8±3.9 | 68.1±3.8 | 0.144 |
| Gender | 0.990 | ||
| Female | 29 (76.3%) | 44 (47.3%) | |
| Male | 9 (23.7%) | 49 (52.7%) | |
| Chronic AF | 0.602 | ||
| Yes | 31 (81.6%) | 72 (77.4%) | |
| No | 7 (18.4%) | 21 (22.6%) | |
| Etiology | 0.462 | ||
| Rheumatic | 24 (63.2%) | 70 (75.3%) | |
| Degenerative | 14 (36.8%) | 23 (24.7%) | |
| LAD (mm) | 46.9±9.9 | 45.2±9.2 | 0.615 |
| ≥60 | 4 (10.5%) | 3 (3.2%) | |
| <60 | 34 (89.5%) | 90 (96.8%) | |
| LVEF (%) | 58.8±4.9 | 61±4.9 | 0.223 |
| ≥50% | 34 (89.5%) | 86 (92.5%) | |
| <50% | 4 (10.5%) | 7 (7.5%) | |
| PASP (mmHg) | 31.9±4.5 | 31.8±5.9 | 0.267 |
| ≥40 | 24 (63.2%) | 61 (65.6%) | |
| <40 | 14 (36.8%) | 32 (34.4%) | |
| LVEDD (mm) | 47.8±3 | 48.3±2.9 | 0.453 |
| TACI (mm/m2) | 81.8±8.9 | 69.9±6.8 | <0.001 |
| BMI | 22.9±3.1 | 24.2±2.9 | 0.596 |
| TADI (mm/m2) | 18.5±0.4 | 18.5±0.5 | 0.987 |
| TAPSE (mm) | 17.7±2.3 | 17.8±2.5 | 0.900 |
| RVFAC (%) | 47±4.4 | 46.1±4.1 | 0.268 |
Values are mean ± SD. Values in brackets represent percentage.
Figure 2.
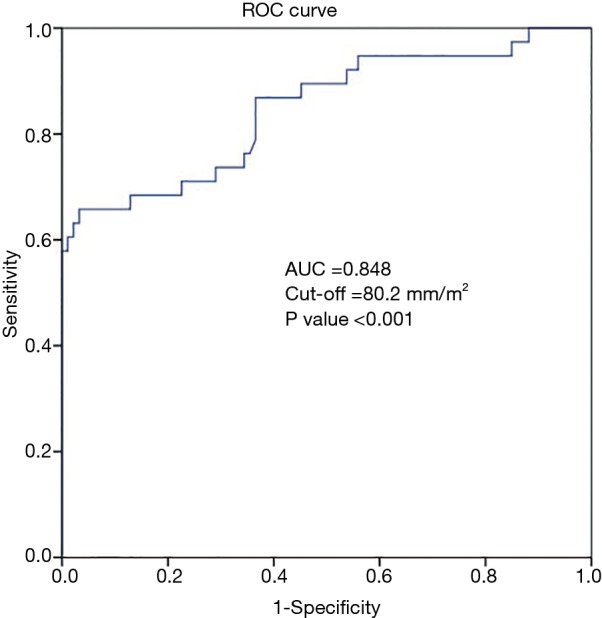
Characteristics of Receiver-operator curve (ROC) for determining cut-off values to predict the postoperative progression of tricuspid regurgitation (TR).
Discussion
In this study, an intraoperative quantitative method was presented to evaluate the extent of tricuspid annular dilatation, whereas the tricuspid annular circumference index (TACI) was perceived to be an independent factor for predicting postoperative TR progression among initial non-significant TR patients. Besides that, female gender and body mass index (BMI) were also perceptible during the progression of TR, a recommended cut-off TACI value 80.2 mm/m2 was significant according to the ROC curve result, and this can be suggested as the threshold for concomitant tricuspid annuloplasty at the time of left side cardiac valve surgery.
A prospective study conducted by Zhu et al. (14), with comparatively shorter period of follow up revealed different risk factors besides the TACI, the author reported that a cut-off value of TACI 83 mm/m2 was a potential predictor for the progression of TR with good AUC value, but the sensitivity and specificity values reported for 83 mm/m2 cut-off were highest sensitivity and specificity values, the study didn’t report the required sensitivity and specificity values for the cut-off point. The study also mentioned the significance of concomitant AF and preoperative left atrial diameter as other two predictors of TR progression in univariate analysis, in contrast to our obtained result. Our other two risk factors of postoperative progression of TR were, female gender and BMI. Our findings suggested a cut-off value 80.2 mm/m2, its sensitivity and specificity values were 69% and 89% at the required cut-off point, the AUC we obtained in our result was smaller (AUC =0.848).The previous consensus attained is instructing that, surgical intervention should be considered for moderate and severe TR at the initial open heart surgery for left-sided cardiac valve diseases, the persistence of controversy as to what path should be chosen when the TR is mild at the initial operation (7,17).
Among the patients who have undergone successful left side cardiac valve surgery developing a significant secondary TR in initially with mild-TR or no-TR is not a rare experience, previously some studies reported the occurrence of TR between 7.7% and 33.7% in the initial non-significant TR patients (8,10,18,19). Many studies have reported the risk factors of late TR as female gender, old age, AF, rheumatic etiology, right ventricular dysfunction, enlargement of the left atrium and tricuspid annular size (1,18,19).
Over five years follow-up in this study we found that the incidence rate of postoperative TR progression was 29%, while female gender, BMI, and TACI were each predictor of TR progression according to univariate, multivariate and logistic regression analysis, but in multiple regression only TACI revealed significance, female gender and BMI failed to express significance, and that failure might be due to small number of patients. However, other risk factors failed to express its significance during the follow up period too, and it is also most likely due to the relatively small number of patients. The intraoperative application of Maze procedure directly affected the postoperative AF result (20).
Annular dilatation and increased tricuspid leaflet tethering in relation to the right ventricular pressure and/or volume overload are causing secondary TR (12). According to contemporary evidence, tricuspid annular dilatation was broadly accepted as an indication for prophylactic tricuspid repair, irrespective of the severity of TR. Hereafter, the selection of patients with enlarged tricuspid annulus who presented with non-significant TR would be meaningful. Available guidelines are based on preoperative echocardiographic measurements, while a similar negligence of the tricuspid valve exists among echocardiography practitioners, and with the addition of conventional echocardiographic limitations and a de-popularization of 3D echocardiography, the intraoperative investigation still plays an important role for the tricuspid valve exploration.
The assessment of tricuspid dilatation intraoperatively can be outlined back to the digital palpation technique introduced by Carpentier and colleagues during the 1970s (21). Another approach introduced by Dreyfus et al. (8) was to measure the maximal distance between the anteroseptal commissure and the anteroposterior commissure in the flaccid heart; a value >70 mm indicated a necessity for tricuspid annuloplasty. Unfortunately, the digital evaluation lacked accuracy and due to racial discrepancies, the recommended value of 70 mm was observed to be too large for the East Asian population.
Today, the definition of tricuspid annular dilatation during surgery remains a challenging clinical subject, and there is only one study conducted by Zhu et al. which reported a threshold of 83 mm/m2 intraoperatively with good clinical result. Therefore, a set of special sizers for the accurate measurement of tricuspid valve was developed, the purpose was to utilize these special sizers to measure the annular circumference intraoperatively. As the sizer is of a similar shape to the native annulus, its accuracy rate is higher than using a ruler or surgical sutures when making such measurements. In this case, the circumference of the tricuspid annular orifice is used as a potential indicator for two reasons: firstly the total tricuspid orifice circumference is the direct and true size for an individual patient; secondly the dilatation of the tricuspid annulus is irregular, and therefore any diameter may lead to an underestimation. Based on the results of previous studies, all circumferences are corrected by indexing to the patient’s BSA, and this has been proven to associate well with the intracardiac structures (22).
The annular diameter is always used for the evaluation of tricuspid annular dilatation, and to date only one study has reported the relationship between TR development and annular dilatation based on TAC (14), this is most likely because traditional 2D echocardiography cannot establish the comprehensive tricuspid annulus in one sonographic view, while theoretically it is possible to see the whole tricuspid annulus with 3D echocardiography, a clear delineation could be found in only about half of the patients (23,24). In contrast, the self-made sizers, when combined with the Edwards commercial tricuspid sizers, were able to provide relatively quantitative data of the TAC, and this can assist surgeons to judge the extent of annular dilatation rather than relying on personal experience. Other than that, this method consumes minimal time intraoperatively, plus it is both feasible and reproducible. Patients enrolled in this study all had a relatively normal echocardiographic tricuspid diameter that did not support an indication for surgery, but the reason why they presented with various extents of annular circumference and eventually developed almost 29% TR progression in the mid-term follow up period. The answer to this question might derive from an underestimation of the annular size by 2D echocardiography. Anwar et al. (25) inspected the tricuspid annular morphology in 40 patients with real-time 3D echocardiography, and reported that 2D echocardiographic measurements of maximal TAD is significantly smaller than the major TAD measured by 3D echocardiography; besides that, half of the patients with a normal TAD had an actual dilated TAD with 3D imaging. Ton-Nu et al. (26) also observed the tricuspid annulus annular shape using 3D echocardiography in 75 patients, and the research found that the tricuspid annulus became more planar and larger with functional TR. When the tricuspid annulus dilates, enlargement often begins at the mural part of the annulus, and the extent of dilatation is more obvious in the posterior portion than both anterior portion and septal region. Therefore, there is a greater enlargement of the antero-posterior distance than of the septal-lateral distance (27). The potential dilated annulus that could not be detected by traditional 2D echocardiography would possibly be reflected by intraoperative measurements of the whole circumference.
Study limitations
During the study, few limitations appeared and the first one was that the choice of optimal sizer utilized by the surgeon was somewhat subjective, since human tissue has favorable elasticity and the extent where the tension is zero can’t be judged easily at that specific socket. Still, with good exposure of the surgical field several adjacent sizers could be used, eventually, the most appropriate sizer for each orifice was found. The second limitation was that the circumference acquired with the sizer was not a continuous value, as all adjacent sizers have an interval of 6 mm, the intrinsic error of measurement is 6 mm, although, compared to the >100 mm perimeter this had a relatively small influence on the final result. A third limitation was that lack of comparative group in the study, because to perform tricuspid valve repair at random in less severe TR patients would be unethical. The preoperative mitral valve lesions were variable and included rheumatic and degenerative etiologies, but multiple analysis showed this not to be a significant factor. Likewise, the current patient population had predominantly rheumatic left side cardiac valve diseases, and whether the findings can be applied to other etiologies requires further investigation. The final limitations were the study’s single-center design; and relatively small population. Further prospective studies with large numbers of patients and multi-center based can confirm the existing data.
Conclusions
In patients with predominantly rheumatic left-sided cardiac valve disease, the TAC, when evaluated utilizing special sizers, proved to be an ideal method to prospectively judge the tricuspid annulus would dilate, or not. A TACI value 80.2 mm/m2 suggested performing the prophylactic tricuspid annuloplasty in order to refrain from postoperative TR progression. The TAC assessed by the echocardiographic tricuspid diameter might be underestimated for a subset of patients, while intraoperative measurement of the TACI can prevent such disadvantages.
Acknowledgements
Funding: This study was supported by Beijing Natural Science Foundation of China (7174294).
Ethical Statement: This study was approved by the Ethic Committee of Beijing Anzhen Hospital of Capital Medical University (No. 2011007X).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Shinn SH, Schaff HV. Evidence-based surgical management of acquired tricuspid valve disease. Nat Rev Cardiol 2013;10:190-203 10.1038/nrcardio.2013.5 [DOI] [PubMed] [Google Scholar]
- 2.Porter A, Shapira Y, Wurzel M, et al. Tricuspid regurgitation late after mitral valve replacement: Clinical and echocardiographic evaluation. J Heart Valve Dis 1999;8:57-62. [PubMed] [Google Scholar]
- 3.Yajima S, Yoshioka D, Toda K, et al. Definitive Determinant of Late Significant Tricuspid Regurgitation After Aortic Valve Replacement. Circ J 2018;82:886-94. 10.1253/circj.CJ-17-0996 [DOI] [PubMed] [Google Scholar]
- 4.Di Mauro M, Bivona A, Iaco AL, et al. Mitral valve surgery for functional mitral regurgitation: Prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009;35:635-9. 10.1016/j.ejcts.2008.12.040 [DOI] [PubMed] [Google Scholar]
- 5.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. 10.1016/j.jacc.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 6.Calafiore AM, Gallina S, Iaco AL, et al. Mitral valve surgery for functional mitral regurgitation: Should moderate-or-more tricuspid regurgitation be treated? A propensity score analysis. Ann Thorac Surg 2009;87:698-703. 10.1016/j.athoracsur.2008.11.028 [DOI] [PubMed] [Google Scholar]
- 7.Kim JB, Yoo DG, Kim GS, et al. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: The influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart 2012;98:24-30. 10.1136/heartjnl-2011-300403 [DOI] [PubMed] [Google Scholar]
- 8.Dreyfus GD, Corbi PJ, Chan KMJ, et al. Secondary tricuspid regurgitation or dilatation: Which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127-32. 10.1016/j.athoracsur.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 9.Kusajima K, Fujita T, Hata H, et al. Long-term echocardiographic follow-up of untreated 2+ functional tricuspid regurgitation in patients undergoing mitral valve surgery. Interact Cardiovasc Thorac Surg 2016;23:96-103. 10.1093/icvts/ivw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012;143:632-8. 10.1016/j.jtcvs.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 11.Colombo T, Russo C, Cilliberto GR, et al. Tricuspid regurgitation secondary to mitral valve disease: Tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg 2001;9:369-77. 10.1016/S0967-2109(00)00147-2 [DOI] [PubMed] [Google Scholar]
- 12.Vahanian A, Alfieri O, Andreotti F, et al. Guideline on the management of valvular heart disease (version 2012). Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC); European Association for Cardio-thoracic Surgery (EACTS). Eur Heart J 2012;33:2451-96. [DOI] [PubMed] [Google Scholar]
- 13.Chikwe J, Anyanwu AC. Surgical strategies for functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg 2010;22:90-6. 10.1053/j.semtcvs.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Zhu TY, Meng X, Han J, et al. An Alternative Intraoperative Method Based on Annular Circumference for the Decision-Making of Prophylactic Tricuspid Annuloplasty. J Heart Valve Dis 2014;23:370-6. [PubMed] [Google Scholar]
- 15.Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. 10.1093/ejechocard/jeq031 [DOI] [PubMed] [Google Scholar]
- 16.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease. Part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436-48. 10.1161/CIRCULATIONAHA.107.653576 [DOI] [PubMed] [Google Scholar]
- 17.Ro SK, Kim JB, Jung SH, et al. Mild-to-moderate functional tricuspid regurgitation in patients undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013;146:1092-7. 10.1016/j.jtcvs.2012.07.100 [DOI] [PubMed] [Google Scholar]
- 18.Song H, Kim MJ, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009;95:931-6. 10.1136/hrt.2008.152793 [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama K, Matsumoto M, Sugita T, et al. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826-8. 10.1016/S0003-4975(03)00028-6 [DOI] [PubMed] [Google Scholar]
- 20.Je HG, Song H, Choo SJ, et al. Impact of the Maze operation on the progression of mild functional tricuspid regurgitation. J Thorac Cardiovasc Surg 2008;136:1187-92. 10.1016/j.jtcvs.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Carpentier A, Deloche A, Hanania G, et al. Surgical management of acquired tricuspid valve disease. J Thorac Cardiovasc Surg 1974;67:53-5. [PubMed] [Google Scholar]
- 22.Daubeney PEF, Blackstone EH, Weintraub RG, et al. Relationship of the dimension of cardiac structures to body size: An echocardiographic study in normal infants and children. Cardiol Young 1999;9:402-10. 10.1017/S1047951100005217 [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46. 10.1016/j.echo.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 24.Badano LP, Agricola E, Perez de Isla L, et al. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three dimensional echocardiography. Eur J Echocardiogr 2009;10:477-84. 10.1093/ejechocard/jep044 [DOI] [PubMed] [Google Scholar]
- 25.Anwar AM, Geleijnse ML, Ten Cate FJ, et al. Assessment of tricuspid valve annulus size, shape and function using real-time three dimensional echocardiography. Interact Cardiovasc Thorac Surg 2006;5:683-7. 10.1510/icvts.2006.132381 [DOI] [PubMed] [Google Scholar]
- 26.Ton-Nu TT, Levine RA, Hand Schumacher MD, et al. Geometric determinants of functional tricuspid regurgitation: Insights from 3-dimensional echocardiography. Circulation 2006;114:143-9. 10.1161/CIRCULATIONAHA.106.611889 [DOI] [PubMed] [Google Scholar]
- 27.Deloche A, Guerinon J, Fabiani JN, et al. Anatomical study of rheumatic tricuspid valve diseases: Application to the study of various valvuloplasties (in French). Ann Chir Thorac Cardiovasc 1973;12:343-9. [PubMed] [Google Scholar]


