Mortality from sepsis remains high with at least 270,000 deaths annually in the United States and over 5 million deaths worldwide.1,2 Despite increasing understanding of the pathophysiology of the sepsis, outside of targeted antibiotic therapy and source control (when appropriate), treatment is generally supportive.3 As such, improved understanding of mechanisms that lead to mortality as well as development of new therapeutics are of paramount importance to treat sepsis and septic shock, which have mortality rates of 20-40%. In this light, a study by Fang and colleagues in this issue of Anesthesiology examining macrophage pyroptosis in sepsis from intraperitoneal infection of E. Coli, identifying a new pathway of mortality from sepsis, has potential therapeutic implications.4
It has long been known that cell death plays a critical role in mortality from sepsis. However, there are multiple forms of cell death including apoptosis, necroptosis (and necrosis) as well as pyroptosis (Fig. 1). An extensive literature exists on the importance of apoptosis – mostly occurring in rapidly dividing cells such as lymphocytes and the gut epithelium – in sepsis.5,6 However, much less is known about the role of other forms of cell death. Pyroptosis is a lytic form of programmed cell death mediated by inflammatory caspases and characterized by the formation of large pores on the plasma membrane. Pyroptosis is induced via the inflammasome, a cytosolic multiprotein oligomer responsible for activation of inflammation. The canonical (or classical) inflammasome induces pyroptosis by activation of caspase-1 while the non-canonical inflammasome involves murine caspase-11 or its human orthologues caspase- 4/5.7 Pyroptosis functions as a method to remove intracellular bacteria and release damage-associated molecular pattern molecules and inflammatory mediators such as interleukin (IL)-1β, thereby protecting the host from invading pathogens. However, excessive activation of pyroptosis can lead to widespread cell death, causing extensive inflammation and tissue damage and organ failure, in turn inducing lethal septic shock.8 While it is known that activated caspases can cleave downstream gasdermin D which mediates membrane damage,9 the upstream signals of pyroptosis remain a subject of intense investigation.
Figure 1. Pathways of programmed cell death.
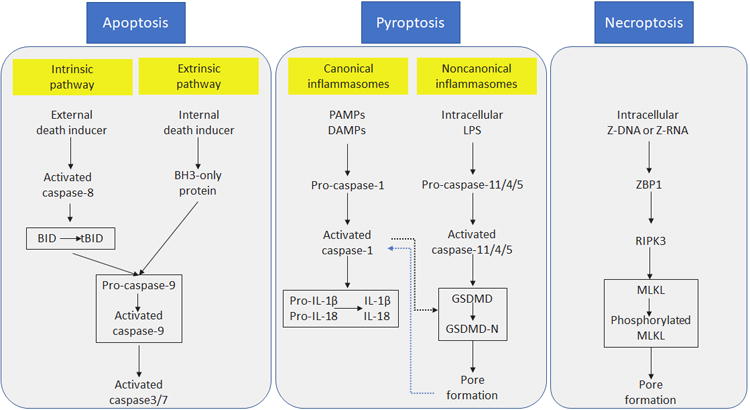
Apoptosis can be triggered through intrinsic or extrinsic pathways, which can induce caspase 9 activation. As a result, caspase 3 and caspase 7 are cleaved into their active forms and lead to apoptosis. In pyroptosis, canonical inflammasome complexes activate caspase-1 which in turn converts pro-IL-1β and pro-IL-18 into their active forms. Sensing of intracellular LPS can activate caspase-11, triggering noncanonical inflammasome. Activated caspase-11 then cleaves GSDMD and releases the N-terminal p30 domain, which induces membrane pores. External death inducers can activate RIPK3 which phosphorylates MLKL. Phosphorylated MLKL binds to the plasma membrane and forms the necroptotic pore. Both necroptosis and pyroptosis induce cell membrane rupture, releasing cellular components which may trigger inflammation. BID, BH3-interacting domain death agonist; tBID, truncated BID; DAMPs, damage associated molecular patterns; GSDMD, Gasdermin D; MLKL, mixed lineage kinase domain-like; PAMPs, pathogen associated molecular patterns; RIPK3, receptor-interacting protein kinase 3; ZBP1, Z-DNA binding protein 1.
Within this context, Fang and colleagues examined septic Sphingosine 1-phosphate Receptor 2 (S1PR2) genetically deficient mice. Sphingosine 1-phosphate is a biologically active lipid, generated from metabolites of membrane sphingolipids which exerts its effects by binding to five different receptors, of which S1PR2 is the dominant one in macrophages. S1PR2-/- mice had decreased peritoneal macrophage pyroptosis and pro-inflammatory cytokine production, and this was associated with improved survival. Furthermore, caspase-11 activation was lower in macrophages from S1PR2-/- mice and higher in S1PR2 over-expressing macrophages whereas it was unaffected in NLRP3-/- mice, demonstrating that pyroptosis occurred through the non-canonical pathway. This was mediated by Rho-A signaling as a RhoA inhibitor prevented the amplified caspase-11 activation in both WT and S1PR-2 overexpressing cells in vitro. To increase the clinical relevance of their results, the authors also examined expression of caspase-4 and S1PR2 in human monocytes taken from patients with gram negative sepsis and from critically ill non-septic controls. Levels of both caspase-4 and S1PR2 were higher in septic subjects, and increased caspase-4 expression positively correlated with S1PR2 levels. This suggests that increasing macrophage pyroptosis is maladaptive in sepsis and targeting this process of cell death could potentially have therapeutic promise in patients.
While the mechanisms identified are novel, the potential for translation of the findings must be understood within the context of this preclinical study. Due to rapid lysis of bacteria by complement, a model of live bacterial injection is more similar to endotoxemia than actual infection.10 This is highlighted by the 80% mortality within 24 hours of bacterial injection in this study, a mortality and time course far more severe than human sepsis. Previous data from the same group demonstrated that mortality is improved in S1PR2-/- mice following cecal ligation and puncture (a more commonly used preclinical model of sepsis) by inhibiting bacterial phagocytosis, although this was also in the setting of 100% lethality.11 This is directly relevant because numerous interventions that are effective in highly lethal of pre-clinical models of sepsis lose their efficacy or even become harmful when given in models of sepsis with lower mortality, more representative of the human syndrome. In addition, while inhibiting S1PR2 led to similar findings as knockout animals, the inhibitor was given either as a pre-treatment prior to the onset of sepsis or immediately after. Given the difficult in identifying “time zero” in septic patients (who almost assuredly have sepsis prior to the onset of clinical diagnosis), the benefit of S1PR2 inhibition in a patient population that is likely to have a mixed inflammatory picture with immunosuppression is unclear.
It is also unclear what the specific downstream effects of decreasing macrophage pyroptosis are that improve survival. While inflammation is decreased in S1PR2-/- mice, it is unclear if that is the mechanism through which these animals have higher survival. This, of course, is a critical question in light of the failure of multiple studies of anti-inflammatory agents in human sepsis. Further, as a G protein associated receptor, S1PR2 has diverse functions in multiple organ systems,12 and its inhibition would thus be expected to have numerous effects beyond blocking macrophage pyroptosis, and off target effects on other cell types could lead to unexpected side effects. Additionally, functional differences between human caspase-4 and murine caspase-11 have recently been described13 and thus the significance of caspase-11 changes in mice need to be replicated in patients above and beyond demonstration of elevated caspase-4 levels.
When these and related questions are answered, the potential for targeting S1PR2 or caspase-4 in patients will be better framed. Until then, the elegant mechanistic studies demonstrate a new role for pyroptosis in mortality in a highly lethal model of sepsis. While the majority of host cells are viable following an infection, increased death (apoptosis, necroptosis, pyroptosis) of multiple cell types increasingly appear to be important in the dysregulated host response14 that causes so many patients with sepsis to die.
Footnotes
Conflicts of interest: None
References
- 1.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M Program CDCPE. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318:1241–9. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K International Forum of Acute Care T. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 4.Song F, Hou J, Chen Z, Chen B, Lei RY, Cui P, Sun Y, Wang H, Fang X. Sphingosine-1-Phosphate receptor 2 signaling promotes Caspase-11 -dependent macrophage pyroptosis and worsens Eshcerichia coli sepsis outcome. Anesthesiology. 2018 doi: 10.1097/ALN.0000000000002196. in press. [DOI] [PubMed] [Google Scholar]
- 5.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–40. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM, Burd EM, Farris AB, Ford ML, Coopersmith CM. Murine Lung Cancer Increases CD4+ T Cell Apoptosis and Decreases Gut Proliferative Capacity in Sepsis. PLoS One. 2016;11:e0149069. doi: 10.1371/journal.pone.0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 8.Aglietti RA, Dueber EC. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends Immunol. 2017;38:261–71. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 10.Fink MP. Animal models of sepsis. Virulence. 2014;5:143–53. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Chen Q, Zhang K, Cheng B, Xie G, Wu X, Luo C, Chen L, Liu H, Zhao B, Dai K, Fang X. Sphingosine 1-phosphate Receptor 2 Signaling Suppresses Macrophage Phagocytosis and Impairs Host Defense against Sepsis. Anesthesiology. 2015;123:409–22. doi: 10.1097/ALN.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 12.Adada M, Canals D, Hannun YA, Obeid LM. Sphingosine-1-phosphate receptor 2. FEBS J. 2013;280:6354–66. doi: 10.1111/febs.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, Martin A, Di Lorenzo F, Py BF, Molinaro A, Henry T. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun. 2018;9:242. doi: 10.1038/s41467-017-02682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]


