Abstract
For decades, chemical cross-linking of proteins has been an established method to study protein interaction partners. The chemical cross-linking approach has recently been revived by mass spectrometric analysis of the cross-linking reaction products. Chemical cross-linking and mass spectrometric analysis (CXMS) enables the identification of residues that are close in three-dimensional (3D) space but not necessarily close in primary sequence. Therefore, this approach provides medium resolution information to guide de novo structure prediction, protein interface mapping and protein complex model building. The robustness and compatibility of the CXMS approach with multiple biochemical methods have made it especially appealing for challenging systems with multiple biochemical compositions and conformation states. This review provides an overview of the CXMS approach, describing general procedures in sample processing, data acquisition and analysis. Selection of proper chemical cross-linking reagents, strategies for cross-linked peptide identification, and successful application of CXMS in structural characterization of proteins and protein complexes are discussed.
Keywords: chemical cross-linking, mass spectrometry, integrative modeling
1. Introduction
The enzymatic, scaffolding and regulatory functions of proteins are carried out on the platform of protein three-dimensional (3D) structure, which is intrinsically dynamic [1, 2]. The structure of a protein is highly contextual, sensitive towards multiple factors including cellular localization [3], ligand-binding [4], posttranslational modifications and oligomerization state [5]. Subtle changes in primary structure can cause substantial changes in secondary structures [6] or overall fold of a protein [7, 8]. Furthermore, moonlighting proteins can populate distinct conformational states for unrelated functions [9, 10]. Such structural multiplexity provides the underlying mechanism for multi-specificity of antibodies [11], promoter-binding plasticity [12], chameleon sequences in protein folding [13], protein misfolding and diseases [14]. Therefore, biochemical and biophysical measurements to elucidate protein structures under various cellular contexts are essential to mechanistic understandings of complex cellular processes.
Proteins function in macromolecular assemblies, which further connect and organize into intricate networks [15, 16]. In eukaryotes, almost 90% of soluble proteins are observed to be part of at least one protein complex [17]. In a genome-reduced bacterium, about a third of the heteromultimeric complexes show higher levels of proteome organization, suggesting sequential steps in biological processes and extensive sharing of components [18]. Therefore, structural elucidation of dynamic protein complexes provides additional insights on the function and regulation of proteins [19].
2. Structural characterization of proteins and protein complexes
Nuclear magnetic resonance spectroscopy (NMR) and X-ray crystallography provide protein structures at an atomic-level (<3Å resolution), thus considered as the ‘gold standard’ for structural characterization. However, both NMR and crystallography require in vitro expression and purification of proteins sufficient quantity (usually in milligrams), purity and concentration. In addition, effective analysis of NMR spectra is limited by the size of proteins; while protein crystals need to diffract well to generate informative X-ray crystal structures.
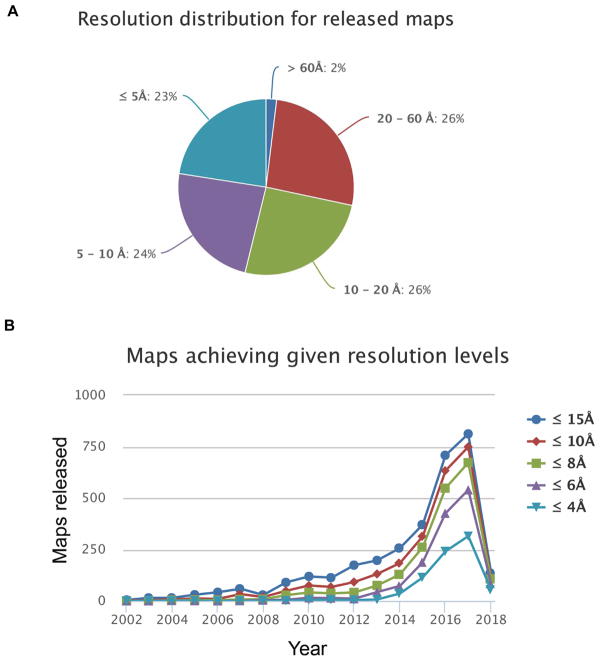
Single particle cryogenic electron microscopy (cryo-EM) provides an attractive alternative, recently generating near-atomic resolution protein structures some with the ability to extract different conformations of the same biomolecules [20, 21]. However, proper processing of cryo-EM data is lengthy and computationally challenging. As expected, high-resolution (<5Å) EM maps constitute only 23% of the total maps; while medium-resolution (5Å – 20Å) maps constitute ~50% of the total released maps (Fig. 1, data obtained from EMStates (pdbe.org/emstats)). For a medium-resolution EM map, crystal or NMR structures of individual domains or subunits can be fitted into the electron density map. This process is often accompanied by ambiguity, ideally to be complemented by experimental results from other structural analyses [22].
Figure 1.
Resolution distribution of EM maps, released up to February 2018 in the Electron Microscopy Data Bank (EMDB) (data obtained from EMStates (pdbe.org/emstats)).
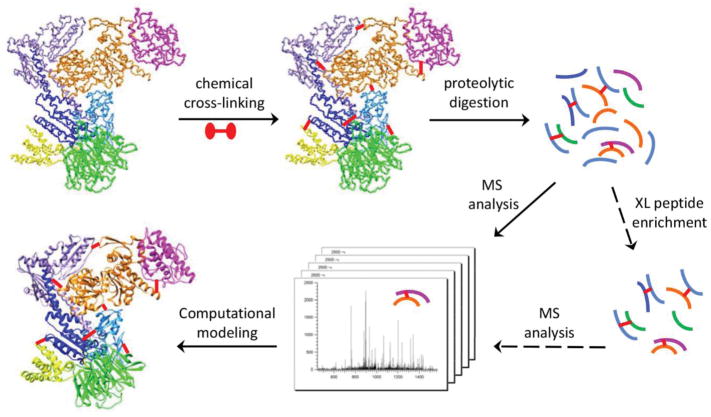
For decades, chemical cross-linking of proteins has been used to stabilize transient interactions and to reveal protein interaction partners [23, 24]. The following mass spectrometric (MS) analysis can identify cross-linked residues that are in spatial proximity but not necessarily close in primary sequences, providing valuable spatial restraints that can be employed to provide low-to-medium resolution structural information on proteins and protein complexes (Fig. 2). Chemical cross-linking and mass spectrometry (CXMS) analysis has recently become a powerful method in protein structural elucidation, due to its high sensitivity and throughput, its ability to handle protein complexes of various sizes or heterogeneity, and its compatibility with biochemistry and cell biology approaches to reveal protein actions inside the cell [25–29]. This article reviews the effectiveness of CXMS in structural elucidation and describes general experimental procedures in CXMS approach. Special considerations on the selection of cross-linking reagents, strategies to facilitate the identification of cross-linked peptides, recent developments and future directions for the CXMS method are also discussed.
Figure 2.
General work-flow of chemical cross-linking and mass spectrometric analysis (CXMS) for structural characterization of proteins and protein complexes.
3. Chemical cross-linking and mass spectrometric analysis
3.1. Cross-linkers do not significantly alter the structure of proteins and complexes
Early CXMS studies of highly purified protein complexes utilized biochemical assays to monitor and ensure the structural integrity of protein complexes during chemical cross-linking reactions. Such biochemical assays include tryptophan fluorescence profile [30], enzymatic activity [31, 32], ligand/peptide/protein-binding activity [33, 34], and direct electron microscopic profile assays [35, 36]. To minimize potential structural perturbation, some CXMS studies employed conditions that allowed each protein molecule to be cross-linked once (single-hit conditions) [37]. Nevertheless, single-hit conditions tend to produce lower cross-linking yield and less informative results due to vast differences in solvent accessibility and the reactivity of cross-linked residues [38].
Recently, a few studies have investigated possible structural perturbations that chemical cross-linking might introduce to proteins and protein complexes. Results from these studies validate empirical CXMS practice in the selection of cross-linker concentration and protein interaction affinity. When the molar excess of cross-linkers was equal to the number of reactive residues of a protein, minimal perturbations in enzymatic activity and NMR chemical shifts were detected in human carbonic anhydrase I [32]. Even at a high cross-linker concentration, no perturbation was observed in secondary structures, indicating a broad preservation of the protein structure [32]. In a separate study, a few protein complexes with various affinities were used to assess the effects of binding affinity on cross-linking specificity [39]. A direct correlation between protein interaction affinity and the fraction of cross-linked complex was observed in mid-affinity range complexes (KD from 10−4 to 35 μM), suggesting that cross-linking does not substantially shift the protein interaction equilibrium in mid-affinity complexes [39].
3.2. CXMS results consistent with other biochemical and cell biology studies
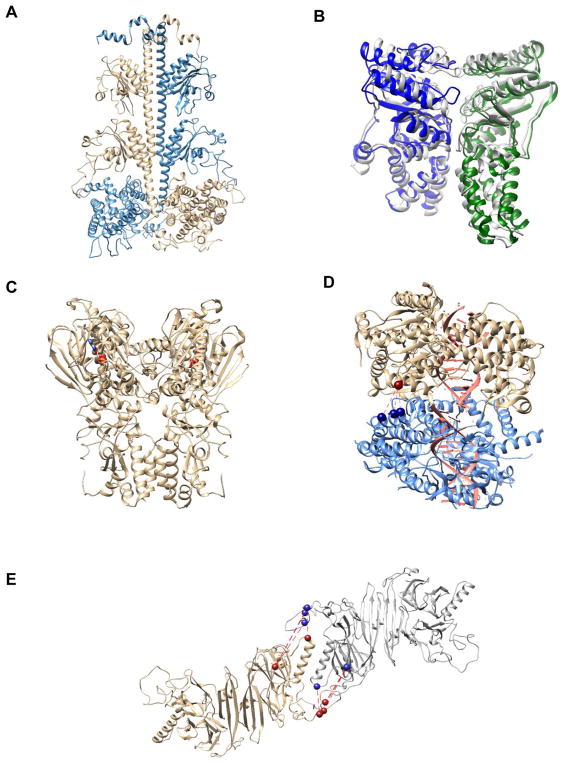
Consistent with aforementioned model systems, many CXMS studies have generated results consistent with that obtained from conventional biochemical and biophysical methods. For instance, a computation model of phosphodiesterase 6 (PED6) that integrated CXMS data with negative-staining EM density is in excellent agreement with biochemical data collected for decades, and it provides mechanistic insights on PDE6 catalysis and regulation (Fig. 3A) [31]. Furthermore, the computational model built from CXMS results of bacterial signal recognition particle (SRP) and SRP receptor (SR) (Fig. 3B, in grey) [30] was in excellent agreement with the crystal structure (Fig. 3B, in green and blue) solved shortly after [40]. The CXMS study suggested a rearrangement of the M-domain during SRP•SR complex formation, which was later detected and reported in other studies [41, 42]. Similarly, a CXMS study on GRP94, an endoplasmic reticulum ortholog of heat shock protein 90 (Hsp90), revealed a novel quaternary structure [33], which was observed later in the ADP-binding state of bacterial Hsp90 (HtpG) (Fig. 3C) [43]. Besides elucidating structures consistent with conventional biophysical and biochemical methods, functional assays using mutants that disrupted protein interaction surfaces have further shown biological relevance and proven CXMS as a reliable method for structural elucidation. For instance, mutations introduced at the oligomerization interface of a viral double-stranded RNA receptor (MDA5), identified by a CXMS study, abolished both MDA5 filament formation in vitro and the IFN-γ production in vivo (Fig. 3D) [44]. Similarly, the mutation of a hydrophobic patch at the oligomerization interface of human IRE1 identified by a CXMS study abolished the cellular function of IRE1 in unfolded protein response without perturbing its hydrophobic peptide binding activity (Fig. 3E) [34].
Figure 3.
Structure models of some protein complexes: A) computational model of catalytic dimer in PDE6 holoenzyme; B) computational models of SRP•SR NG complex (colored in blue and green) superimposed with crystal structure of SRP•SR NG complex (colored in gray); C) crystal structure of HtpG, a bacterial Hsp90 homology (ADP shown as stick & ball with heteroatom color); D) computational models of MDA5 dimer in complex with viral dsRNA (cross-linked residues shown as spheres); E) computational models of human IRE1 tetramer (cross-linked residues in oligomer formation shown as spheres).
Increasingly, CXMS is contributing important information to the structural and functional understanding of proteins and protein complexes [25, 45–48]. Identification of cross-linked residues by mass spectrometric analysis provides medium resolution information to guide de novo structure prediction [49], and to reveal the architectural organization of protein complexes and the molecular recognition among their subunits [30, 33, 44]. CXMS is not only reliable for structural elucidation, but also highly complementary with other structural characterization methods to significantly improve the accuracy of integrative structure prediction [28, 50].
3.3. CXMS analysis of large protein complexes
The robustness and compatibility of CXMS analysis with multiple biochemical methods have made it especially appealing for challenging systems with multiple biochemical compositions and conformation states. Recent developments in bioinformatics have further enhanced the throughput of CXMS data processing and analysis [51–58], enabling its application to large multi-subunit protein complexes, such as the complete 26S proteasome assembly [59, 60], the RNA polymerase II-TFIIF complex [61], the RNA polymerase II initiation complex [62, 63], the mediator complex [64] and the mediator-RNA polymerase II pre-initiation complex [65], the chromatin remodeling INO80 [66] and SWR1 complex [67], the transcription regulation SAGA [68] and polycomb repressive complex [69], and the bacterial photosystem [70] and secretion system [71]. In addition, CXMS analysis has been used to characterize proteome-wide protein complexes and generate a few thousand cross-linked peptides [72–75]. Considering the extensive interactions in a mammalian proteome, the scope of interactome revealed by CXMS analysis thus far remains limited. Current challenges include a large dynamic range in protein complex abundance, a drastic difference in the solvent accessibility and cross-linking reactivity of reactive residues in a proteome, as well as difficulties in an effective enrichment and sensitive identification of cross-linked peptides at the whole proteome level.
4. General procedures for CXMS analysis
4.1. Chemical cross-linking reactions
Proteins and protein complexes are usually cross-linked under native biochemical conditions with the appropriate buffer composition, pH value and concentration of proteins and cross-linking reagents. The cross-linking buffer should not contain any component that reacts with the cross-linker and interferes with cross-linking reactions. Different cross-linking reagents are active within different pH ranges. Therefore, the cross-linking buffer should be set at a pH where the cross-linker is reactive and the protein or protein complex remains in a native, relevant conformation. The concentration of proteins should be aligned with the affinity of protein-protein interactions, so that protein complexes are formed effectively without nonspecific oligomerization. The concentration of cross-linking reagents probably bears the largest variability. Empirically, the molar excess of the cross-linker is nearly equivalent to the number of targeted amino acid residues in the complex. Cross-linking reactions should be quenched before down-stream biochemical manipulation to ensure that chemical cross-linking reactions take place only when proteins and protein complexes exist in native conformations.
To optimize cross-linking conditions, various cross-linker concentrations can be screened in small scale reactions. The cross-linked products can be monitored and visualized by SDS-PAGE to select conditions where desired covalently linked complexes are formed without noticeable aggregate formation. In addition, biochemical assays that are specific to the protein or protein complex can be carried out after the cross-linking reaction to ensure that the broad conformation of the protein or protein complex remains intact after the reaction. For instance, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to detect nonspecific oligomerization products, and fold-specific fluorescence signals were used to monitor cross-linker induced protein denaturation [30]. After chemical cross-linking reactions, the covalently linked complexes can be separated from cross-linker treated monomers, which can be used as negative controls to both distinguish inter-subunit cross-links from intra-subunit cross-links and to increase the confidence of cross-linked peptide assignment [33, 34, 44].
4.2. Direct mass spectrometric analysis of cross-linked products
Cross-linked proteins can be directly fragmented in the gas-phase and analyzed by high resolution, high mass accuracy Fourier-transform ion cyclotron resonance (FTICR) mass spectrometers. Data analysis based on the tandem mass spectrometric (MSMS) fragment ions can be used to localize the cross-links [37]. This approach bypasses enzymatic digestion of the protein mixture after cross-linking reaction. Thus, one can specifically pre-select a subgroup of the protein population for MSMS analysis, e.g. proteins with only one cross-link. Moreover, gas-phase fragmentation of the whole protein averts some inherent bias in conventional proteolytic digestion methods, such as under-representation of short peptides, long stretches of sequence without basic residues etc. However, the assignment of a whole protein fragmentation pattern is complex, which is especially true after cross-linking reactions since most cross-linking reagents lead to additional sequence heterogeneity by reactions at various cross-linking sites. Employing complementary ion activation methods, such as ultraviolet photodissociation in conjunction with high energy collision dissociation, can provide additional sequence coverage and potentially address the ambiguity in cross-linking site assignment [76].
Alternatively, a limited proteolysis step followed by liquid chromatographic separation could be added upstream to FTICR mass spectrometric analysis to facilitate the generation of informative fragment ions. Limited proteolysis and cross-linking experiments in conjunction with mass spectrometric analysis were employed to study the calcium-calmodulin-melittin ternary complex [77]. Two zero-length pairs of cross-links mediated by 1-ethyl-3-(3- dimethylaminopropyl)carbodiimide hydrochloride (EDC) were observed, joining Glu83/Glu84calmodulin with N-terminusmelittin, and Glu11/Glu14calmodulin with Lys23melittin. Consistent with the proteolysis experiment, the cross-linking results suggest an antiparallel binding mode between calmodulin and melittin, which is inverse as compared to the binding mode adopted by peptides derived from MLCL, CaMKI and CaMKII [77]. A more recent study revisited the calcium-calmodulin-melittin ternary complex, employing three chemical cross-linking reagents with different reaction specificities and spacer lengths in combination with high-resolution FTICR-MS [78]. The distance restraints derived from chemical cross-linking data were utilized in computational conjoined rigid body/torsion angle simulated annealing to generate low-resolution structure models of the complex. Interestingly, two opposing peptide binding orientations are identified simultaneously, implicating a more flexible and complex calmodulin target recognition [78].
4.3. Analyzing the proteolytic digestion mixtures of cross-linked products
Most of the high-density, informative CXMS data has been generated from proteolytic digestion of cross-linked proteins or protein complexes (Fig. 2). Like other proteomics studies, trypsin remains most effective in producing informative cross-linking data. Tryptic peptides carry a basic residue, Lys or Arg, at the C-terminus of tryptic peptides, thus generating relatively even sequence ions in most MS instrumentation platforms. In addition, bioinformatic algorithms often assign higher scores on C-terminal fragment ions (y ions) than N-terminal fragment ions (b ions) in database search, further favoring the identification of tryptic peptides [79]. To increase the sequence coverage of proteins and protein complexes, additional proteases (Glu-C, Asp-N, chymotrypsin etc.) have been employed to complement results from tryptic digestion [31, 80].
Tryptic digestion mixtures of the cross-linked product are highly heterogenous, containing normal peptides, cross-linked peptides, peptides with miscleavages or nonspecific cleavages, and peptides modified by cross-linkers, molecular oxygen or other reagents during sample handling. Therefore, sensitive detection of individual cross-linked pairs from heterogenous cross-linking products through routine high-pressure liquid chromatography and tandem mass spectrometric (HPLC-MS/MSMS) analysis becomes more challenging. Conceivably, up-stream fractionations that target the physiochemical properties of cross-linked peptides have the potential to enrich the lower abundance cross-linked peptides and facilitate their detection (Fig. 2). For instance, most cross-linked peptides contain two N-terminal ends and two C-terminal ends that can be protonated, thus likely carrying +4 charges instead of +2 charges for typical tryptic peptides [81]. Therefore, strong-cation exchange (SCX) can partially enrich for cross-linked peptides together with highly basic peptides as contaminants [51]. Similarly, cross-linked peptides tend to be bigger than typical tryptic peptides. Therefore, size-exclusion chromatography (SEC) can also partially enrich cross-linked peptides [80]. In addition to distinct properties of cross-linked peptides in charge and size, incorporation of special functionalities in cross-linking reagents provide additional ways for the enrichment and detection of cross-linked peptides, which will be discussed in detail in the next cross-linking reagents section 5.5.
Because most cross-linking reagents possess reactivity at multiple sites of a protein, the confident identification of cross-linked pairs by peptide mass mapping alone will be problematic due to the mass degeneracy of putatively cross-linked species. This situation becomes more severe as the size of proteins and protein assemblies or the number of cross-linker reactive residues increases. Therefore, sequence information on cross-linked peptide moieties becomes indispensable for a reliable assignment of the cross-linked sites. For example, a cross-linking study on the Op18-tublin complex using isotope-tagged cross-linking reagents and tandem mass spectrometry revealed a “tubulin capping” activity of the N-terminal domain of Op18 [82], which is inconsistent with the model derived from another cross-linking study on the complex [83]. Whether the discrepancy reflects various conformations of the complex or stems from erroneous interpretation of the mass spectrometric data remains unknown; however, the binding orientation derived from tandem mass spectrometric results are consistent with that observed in X-ray crystal structure of the tubulin-stathmin-like domain complex [84].
4.4. Bioinformatics in cross-linked peptide identification
In addition to the quality of mass spectrometric analysis, bioinformatic approaches in the identification of cross-linked peptides and the assessment of false discovery rate (FDR) are also essential to the accuracy of deduced structural models. A few commonly used, public available software tools for high throughput CXMS analysis are listed in Table 1. Compared to database searches of linear peptides, the number of possible cross-linked products that match any given putative peptide increases quadratically with the size of the cross-linkable peptide pool. When a generic functional group is targeted in a cross-linking reaction, the high throughput database search of LC-MS/MSMS data using bioinformatic tools developed for regular, linear peptide searches becomes substantially more challenging [58]. Conceivably, additional constraints, such as predetermined protein subunit sequences [54, 56, 85-87] or particular fragment ion patterns [51, 88], have been imposed to reduce the number of permutations at MS level or to reduce the complexity of MS/MS spectra. An alternative approach is to treat one cross-linked peptide moiety and the cross-linker as an undefined, large mass modification to the other peptide moiety in a cross-linked peptide. Therefore, the favorably fragmented peptide moiety in a cross-linked peptide can be identified as a regular, linear peptide [53, 57, 58, 75, 89]. This strategy can also be applied to search against a restricted database, constituted by a list of proteins identified by a regular peptide search [53], providing an unbiased way to improve the sensitivity of database search for cross-linked peptides [58].
Table 1.
Public available, high-throughput CXMS software tools
| Software | Website & Feature | Reference |
|---|---|---|
| Crux | http://crux.ms/ regular database search approach within defined protein components | McIlwain et al.[86] |
| DXMSMS | http://creativemolecules.com/CM_Software.htm isotope-coded, cleavable cross-linkers used | Petrotchenko et al. [88] |
| Kojak | http://www.kojak-ms.org/ mass modification search, scable for large database | Hoopman et al. [57] |
| pLink | http://pfind.ict.ac.cn/software/pLink/ mass modification search, scable for large database | Yang et al. [75] |
| Protein Prospector | http://prospector.ucsf.edu/prospector/mshome.htm mass modification search, scable for large database | Chu et al. [53], Trnka et al. [58] |
| StavroX | https://www.stavrox.com/ regular database search approach within defined protein components | Gotze et al. [54, 87] |
| xComb | http://goodlettlab.org/xcomb.php regular database search approach within defined protein components | Panchaud et al. [54] |
| xQuest/xProphet | https://omictools.com/xquest-tool regular database search approach with isotope-coded cross-linkers | Rinner et al. [51] |
Several methods are currently utilized to assess the database search results of a cross-linking dataset, including manual inspections of cross-linked peptide MSMS spectra, FDR calculation with a decoy cross-linker mass [61] and FDR calculation with a target-decoy concatenated database [75, 90]. Manual inspection of MSMS spectra tends to be low throughput, thus less attainable for large datasets. The distribution of nominal mass and mass defects of amino acids is not even, thus FDR calculation with a decoy cross-linker mass can be biased sometimes. False discovery rate calculation might be more accurate using a target-decoy concatenated database, even when one of the cross-linked peptide moieties is identified correctly. These random hits do not identify the correct cross-linked pairs, providing no additional structural information on proteins or protein complexes. To address the issue of limited fragmentation for one of the peptide moieties, some software set an arbitrary minimal number of fragments from the less confidently identified peptide [61, 75]; while some suggest manual inspection of less confident spectra [90]. The bioinformatic search software, Protein Prospector, take a different approach. It not only identifies two peptide moieties for a cross-linked pair, but independently assess the reliability of the less confident peptide identification. Hence, it offers more sensitive and accurate identification of cross-linked peptides, especially for large protein complex structural characterization [58]. Besides software for cross-linked peptide identification, software tools have been developed to integrate CXMS data, to facilitate structural visualization and protein complex model building [91–93].
5. The selection of chemical cross-linking reagents
5.1. Amine-reactive cross-linkers
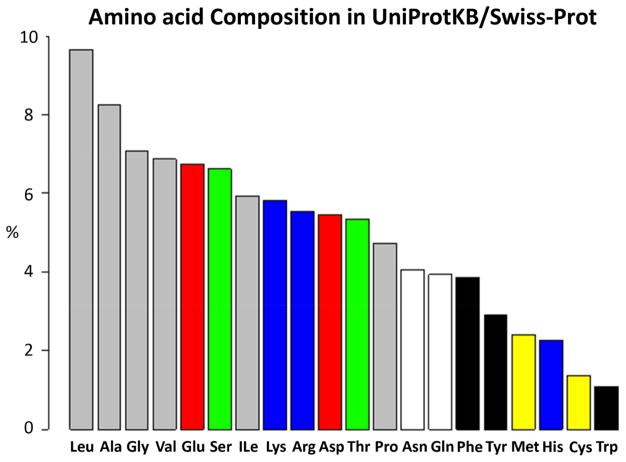
Thus far, bifunctional N-hydroxysuccinimidyl (NHS) ester (Fig.4A) and its water soluble analog sulfo-NHS ester (Fig. 4B) cross-linkers have been the most widely utilized and successful cross-linking reagents in CXMS studies, generating important and validated results on the structural characterization of protein and protein complexes. NHS and sulfo-NHS ester cross-linkers are reactive under basic conditions, targeting the ε-amino group of Lys residues and the α-amino group of polypeptide chains. The high prevalence (5.8% of all amino acids in the UniProt protein database (Fig. 5)) and solvent accessibility of lysine residues make amine-reactive cross-linkers widely applicable and successful. Hydrolysis is a major competing side-reaction, leading to the production of dead-end cross-links after the initial formation of α-aminolysyl function.
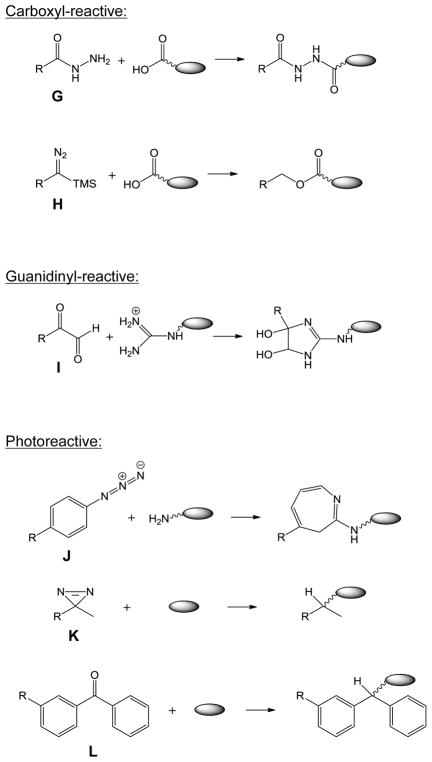
Figure 4.
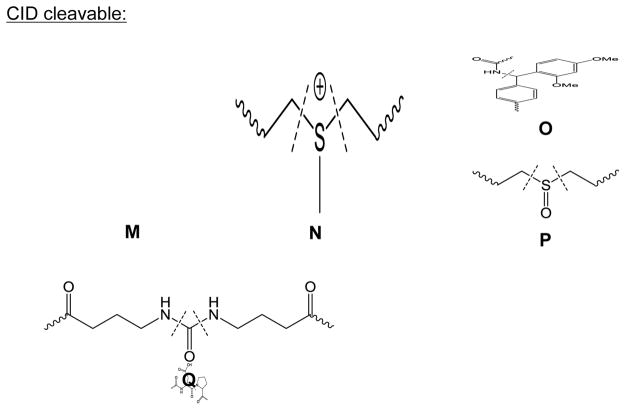
Reactive functionalities of some commonly used cross-linkers, including amine-reactive A) NHS-ester, B) sulfo-NHS-ester, C) imidoester (X=O) and thioimidoester (X=S), D) aldehyde; sulfhydryl-reactive E) maleimide, F) haloacetyl; carboxyl-reactive G) hydrazide, H) diazo-containing; guanidinyl-reactive I) glyoxal; photoreactive J) azide, K) diazirine, L) benzophenone; CID cleavable M) RINK group, N) Asp-Pro peptide bond; O) sulfonium, P) sulfoxide, Q) urea-linked butyrate groups.
Figure 5.
The composition of amino acids in the UniProtKB/Swiss-Prot protein knowledgebase released on February 28th, 2018, which includes 556,825 protein sequence entries (https://web.expasy.org/docs/relnotes/relstat.html).
Chemical cross-linking with an NHS ester reagent forms a neutral amide bond, masking a positive charge at the Lys side-chain after the reaction. Therefore, NHS cross-linking reactions can potentially change the local electrostatic environment of a protein, although changes in secondary structures were not detected in a model protein [32]. Imidoester (Fig. 4C, X=O) or thioimidoester (Fig. 4C, X=S) cross-linkers produce a amidate bond, which retains the original positive charge from the lysyl ε-amino group, preserving the electrostatic microenvironment of the protein after cross-linking reactions. The preservation of Lys basicity after cross-linking further increase the charge states of cross-linked peptides (+6 vs +4 for NHS-ester cross-linked peptides), which is beneficial for the enrichment of cross-linked peptides through SCX chromatography [94]. By installing a better leaving group, thioimidoester cross-linkers improve the reaction yield and specificity under physiological pH [95].
Aldehyde cross-linkers (Fig. 4D) form Schiff bases between lysyl ε-amino functions, which can be readily reduced by cyanoborohydride to generate stable secondary amino linkages. Like imidoester reagents, aldehyde cross-linkers preserve Lys native basicity, thus maintain the electrostatic microenvironment of a protein and facilitate cross-linked peptide separation through SCX chromatography [96]. Unlike NHS ester or imidoester reagents, aldehyde cross-linkers are stable under physiological conditions. They are widely used for cell or tissue fixation in immunohistochemistry and immunofluorescence.
5.2. Sulfhydryl-reactive cross-linkers
Cross-linkers that target the sulfhydryl group of cysteine residues can form either permanent or reversible linkages. The reversible disulfide linkages are susceptible to cleavage by reducing agents and to sulfur scrambling by free thiol groups, thus less used in CXMS studies. The sulfhydryl can add to the double bond of maleimide (Fig. 4E) or react with a haloacetyl functional group (Fig. 4F) in a cross-linker to form a stable thioether linkage [31]. Cysteine is one of the least abundant natural amino acids, constituting ~1.4% of all amino acids in the UniProt protein database (Fig. 5). The percentages of proteins lacking a cysteine residue are 14.6% in E. Coli, 8.7% in yeast and 4.4% in human. Due to its natural low abundance, cysteine residues can be engineered at specific sites and screened for cross-linked products. CXMS identification of cross-linked peptides provide medium resolution structural information of a protein complex [44].
5.3. Carboxyl-reactive cross-linkers
Acidic amino acids, glutamate (Glu) and aspartate (Asp), are the 5th and 10th most abundant residues in the proteome, constituting 6.7% (for Glu) and 5.5% (for Asp) of all amino acids in the UniProt protein database (Fig. 5). The carboxyl group of Glu and Asp can be activated by a coupling reagent, such as EDC to form an active O-acylisourea intermediate that can react with a hydrazide in a cross-linker (Fig. 4G) to yield a stable imide linkage [97]. However, relatively low pH (pH < 5.8) needs to be employed with EDC to obtain sufficient cross-linking yield. When 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) was employed as the couple reagent, numerous cross-linked pairs were detected under native physiological pH [98]. Recently, a class of diazo-containing cross-linkers (Fig. 4H) were developed to target the carboxylate groups, without using a coupling reagent [99].
5.4. Guanidinyl-reactive cross-linkers
Arginine (Arg) is the 9th most abundant residues in the proteome, constituting 5.5% of all amino acids in the UniProt protein database (Fig. 5). The guanidinyl group of Arg, often localized to protein surface and solvent accessible, can be specifically targeted by glyoxal cross-linkers under alkaline conditions to form a Schiff base-like complex (Fig. 4I) [100]. In addition, diglyoxal compounds has been used to identify protein-DNA or protein-RNA interactions that organize ribosome molecular architecture [101].
5.5. Photoreactive cross-linkers
Some cross-linkers contain photolabile functional groups that upon photolysis can indiscriminately react with various residues in vicinity. For instance, UV light activation of an aryl azide (Fig. 4J) at wavelength <280 nm can generate a nitrene that reacts preferentially with primary amines [102]; while UV activation of a diazirine (Fig. 4K) at wavelength 360 nm will generate a carbene that can insert into a C-H or heteroatom-H bond [103]. UV activation of a benzophenone (Fig. 4L) at wavelength 365 nm generates a triple state intermediate, which can either insert in a C-H bond or deactivate back to the benzophenone. Therefore, a benzophenone cross-linker can be activated for multiple cycles to produce sufficient cross-linking yield. When installed as an unnatural amino acid in a protein [104], p-benzoyl-L-phenylalanine (pBpa) can be employed to screen for local interactions using CXMS analysis [31]. The indiscriminate reactivity of intermediates after photolysis prevents the formation of a few dominant products, which brings difficulties in MS identification of cross-linked peptides. A much shorter linker of pBpa limits its cross-linking multiplexity, which might have partly contributed to its wide success in CXMS analysis.
5.6. CID-cleavable cross-linkers
To reduce the database search dimension of cross-linked peptides, cross-linkers with cleavable bonds under low-energy CID conditions were designed and applied on various protein complexes. These cleavable reagents incorporate a low-energy cleavable bond. The structure of a few CID-cleavable bonds are illustrated in Fig. 4M–4Q, including an amine bond between two phenyl groups in a RINK linker [105] (Fig. 4M), an Asp-Pro peptide bond [106] (Fig. 4N), a fixed charge sulfonium ion [107] (Fig. 4O), a sulfoxide [108] (Fig. 4P), or a urea-linked butyrate moiety [109] (Fig. 4Q). Cleavage of labile bonds in these cross-linkers separates cross-linked peptide moieties, each carrying expected mass additions introduced by the cross-linker. Therefore, further fragmentation of these peptide moieties in MS3 analysis will elucidate the sequence of cross-linked peptide moieties. Since the peptide moieties in MS3 spectra can be searched as linear peptides with expected modifications, this approach reduces the database search dimension from a quadratic space to a linear space. Using CID-cleavable cross-linkers, more than two thousand cross-linked pairs were identified in a CXMS study of the human proteome, demonstrating its utility and throughput [73]. However, besides special cross-linkers, dedicated software tools in data acquisition and data analysis are required in this approach. In addition, the mandatory acquisition of MS3 spectra for both cross-linked peptides and dead-end cross-links can reduce the effective duty cycle of CXMS analysis, potentially preventing the identification of low abundance cross-linked peptides.
6. Strategies to facilitate cross-linked peptide identification
CXMS constitutes a powerful method to analyze the structure of proteins and protein complexes for its speed, sensitivity, and capability to handle large protein assemblies. However, except a site-specific incorporation of reactive groups, most generic cross-linking reagents react at multiple sites and undergo competing side reactions simultaneously, e.g. hydrolysis of the reactive functionality leading to dead-end cross-links. Therefore, an accurate analysis of a cross-linked protein digestion mixture is often challenging, complicated by the high heterogeneity of the peptide mixture and low stoichiometry of the cross-linked products.
To facilitate the detection and separation of cross-linked species, various functionalities have been introduced into cross-linking reagents. For instance, the application of 1:1 mixtures of isotopically labeled cross-linking reagents can incorporate a distinctive 1:1 doublet isotopic pattern on cross-linked peptides after enzymatic digestion [51, 82]. Moreover, cross-linkers containing fluorophores provide a mean for a multi-channel readout of cross-linked peptides [110, 111]. In addition, the incorporation of an affinity handle in cross-linkers [112–115] can greatly simplify the analytes, thus facilitating the separation and detection of cross-linked peptides. Nevertheless, the addition of an affinity tag in a cross-linker usually increases the size of cross-linkers, placing steric hindrance in cross-linking reactions. To overcome this limitation, azide-containing, clickable cross-linkers were designed, synthesized and applied on model proteins. The azide functional group barely increase the size of a cross-linker, yet providing a handle to enrich cross-linked peptides through solid-phase separation [116, 117] or biotin-avidin interactions [118]. Furthermore, unique physiochemical properties of cross-linked peptides, such as a higher charge state (for SCX) and a larger size (for SEC), can be utilized together with cross-linker functionalities, such as an azide group in trifunctional cross-linkers [96], to facilitate the detection and identification of cross-linked peptides.
7. Summary and future perspectives
While obtaining qualitative information on the spatial proximity of amino acids from CXMS has become nearly routine, the more challenging quantitative analysis has the potential to locate structural elements that distinguish different conformational states [119]. Strategies utilizing isotope-labeled cross-linkers [120–122] and label-free quantitation of cross-linked peptides [36] have been utilized to elucidate conformational changes in proteins and protein complexes. Such approaches can be fairly accurate for present/absent situations, where a cluster of cross-linked peptides is present in one conformation while absent in another conformation. However, direct utilization of quantitative MS signals of cross-linked peptides to infer large-scale domain displacement or rearrangements can be misleading. The overall yield of a cross-linked pair is highly convoluted, being affected by multiple factors including the distance of cross-linked pairs, solvent accessibility, pKa value [38, 123, 124] and protein surface electrostatic topology [125]. Conceivably, proper normalization of quantitative MS data might allow deconvolution of various aforementioned factors and elucidate conformational changes with additional detail and accuracy. Such measurements might be especially informative, providing molecular understanding in the function and regulation of proteins and protein complexes.
Recently, a study through CASP11 (the 11th critical assessment of methods of protein structure prediction) assessed the effectiveness of CXMS in protein structure prediction, and suggested only slight improvements from CXMS data [126]. More importantly, the study revealed current limitations of CXMS, including uneven distribution of target residues, lack of cross-links around beta-sheets and weak restraints from CXMS for structural prediction. Conceivably, expanding targeted residue using carboxyl-reactive [98, 99] and short photoreactive cross-linkers [103] will help increase the sequence coverage of cross-linked residues and close the gap in secondary structures.
Recent developments in the purification and structural characterization of large protein complexes [72, 127] have poised the application of CXMS in in vivo studies. Special sample handling procedures might be necessary to maximally preserve cellular structures. In vivo cross-linking of living cells provides an attractive approach [128, 129] to covalently stabilize protein structure and interactions under native conditions inside the cell. Cross-linkers are introduced either through membrane permeation [128] or through amber suppression [129]. The spatial gradient of cross-linker and the large dynamic range of protein abundance are the major challenges for in vivo cross-linking using membrane permeable, generic cross-linkers. Meanwhile, using photo-crosslinkable amino acids together with affinity tagging can enrich the protein of interest and reveal direct interactions. However, the photo-crosslinking yield tends to be low. Further developments to improve the cross-linking yield and the genetic manipulation of unnatural amino acids will facilitate the wide applicability of these techniques of the enabling CXMS approach.
Highlights.
Chemical cross-linking and mass spectrometric (CXMS) analysis identified amino acid residues in spatial proximity.
When used at low concentrations, cross-linkers do not substantially alter protein structures.
CXMS analysis can elucidate the molecular architecture of large, heterogeneous protein assemblies in a sensitive, high throughput manner.
Quantitative CXMS analysis can reveal conformation changes of proteins.
Acknowledgments
This work was supported by grants from the UNH Collaborative Research Excellence (CoRE), National Science Foundation (CLF 1307367) and the National Institute of Health (R01 HD093783). H.T.N. acknowledges UNH Hamel Center for SURF support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busenlehner LS, Armstrong RN. Insights into enzyme structure and dynamics elucidated by amide H/D exchange mass spectrometry. Arch Biochem Biophys. 2005;433(1):34–46. doi: 10.1016/j.abb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Karplus M. Dynamics of proteins. Adv Biophys. 1984;18:165–90. doi: 10.1016/0065-227x(84)90011-x. [DOI] [PubMed] [Google Scholar]
- 3.Lee AY, Gulnik SV, Erickson JW. Conformational switching in an aspartic proteinase. Nat Struct Biol. 1998;5(10):866–71. doi: 10.1038/2306. [DOI] [PubMed] [Google Scholar]
- 4.Vetter IR, Wittinghofer A. Nucleoside triphosphate-binding proteins: different scaffolds to achieve phosphoryl transfer. Q Rev Biophys. 1999;32(1):1–56. doi: 10.1017/s0033583599003480. [DOI] [PubMed] [Google Scholar]
- 5.Barford D, Hu SH, Johnson LN. Structural mechanism for glycogen phosphorylase control by phosphorylation and AMP. J Mol Biol. 1991;218(1):233–60. doi: 10.1016/0022-2836(91)90887-c. [DOI] [PubMed] [Google Scholar]
- 6.Yang WZ, et al. Conversion of a beta-strand to an alpha-helix induced by a single-site mutation observed in the crystal structure of Fis mutant Pro26Ala. Protein Sci. 1998;7(9):1875–83. doi: 10.1002/pro.5560070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander PA, et al. A minimal sequence code for switching protein structure and function. Proc Natl Acad Sci U S A. 2009;106(50):21149–54. doi: 10.1073/pnas.0906408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pokkuluri PR, et al. A domain flip as a result of a single amino-acid substitution. Structure. 1998;6(8):1067–73. doi: 10.1016/s0969-2126(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery CJ. Molecular mechanisms for multitasking: recent crystal structures of moonlighting proteins. Curr Opin Struct Biol. 2004;14(6):663–8. doi: 10.1016/j.sbi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Chapple CE, et al. Extreme multifunctional proteins identified from a human protein interaction network. Nat Commun. 2015;6:7412. doi: 10.1038/ncomms8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299(5611):1362–7. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 12.Tan S, Richmond TJ. Crystal structure of the yeast MATalpha2/MCM1/DNA ternary complex. Nature. 1998;391(6668):660–6. doi: 10.1038/35563. [DOI] [PubMed] [Google Scholar]
- 13.Minor DL, Jr, Kim PS. Context-dependent secondary structure formation of a designed protein sequence. Nature. 1996;380(6576):730–4. doi: 10.1038/380730a0. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc E, et al. Immobilized prion protein undergoes spontaneous rearrangement to a conformation having features in common with the infectious form. EMBO J. 2001;20(7):1547–54. doi: 10.1093/emboj/20.7.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavin AC, et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440(7084):631–6. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 16.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440(7084):637–43. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 17.Collins SR, et al. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol Cell Proteomics. 2007;6(3):439–50. doi: 10.1074/mcp.M600381-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Kuhner S, et al. Proteome organization in a genome-reduced bacterium. Science. 2009;326(5957):1235–40. doi: 10.1126/science.1176343. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CV, Sali A, Baumeister W. The molecular sociology of the cell. Nature. 2007;450(7172):973–82. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]
- 20.Elmlund D, Elmlund H. Cryogenic electron microscopy and single-particle analysis. Annu Rev Biochem. 2015;84:499–517. doi: 10.1146/annurev-biochem-060614-034226. [DOI] [PubMed] [Google Scholar]
- 21.Bai XC, McMullan G, Scheres SH. How cryo-EM is revolutionizing structural biology. Trends Biochem Sci. 2015;40(1):49–57. doi: 10.1016/j.tibs.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Zeng-Elmore X, et al. Molecular architecture of photoreceptor phosphodiesterase elucidated by chemical cross-linking and integrative modeling. J Mol Biol. 2014;426(22):3713–28. doi: 10.1016/j.jmb.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gunzburg J, Riehl R, Weinberg RA. Identification of a protein associated with p21ras by chemical crosslinking. Proc Natl Acad Sci U S A. 1989;86(11):4007–11. doi: 10.1073/pnas.86.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt-Ulms G, et al. Time-controlled transcardiac perfusion cross-linking for the study of protein interactions in complex tissues. Nat Biotechnol. 2004;22(6):724–31. doi: 10.1038/nbt969. [DOI] [PubMed] [Google Scholar]
- 25.Rappsilber J. The beginning of a beautiful friendship: cross-linking/mass spectrometry and modelling of proteins and multi-protein complexes. J Struct Biol. 2011;173(3):530–40. doi: 10.1016/j.jsb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitner A, et al. Crosslinking and Mass Spectrometry: An Integrated Technology to Understand the Structure and Function of Molecular Machines. Trends Biochem Sci. 2016;41(1):20–32. doi: 10.1016/j.tibs.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Sinz A. Cross-Linking/Mass Spectrometry for Studying Protein Structures and Protein-Protein Interactions: Where Are We Now and Where Should We Go from Here? Angew Chem Int Ed Engl. 2018 doi: 10.1002/anie.201709559. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt C, Urlaub H. Combining cryo-electron microscopy (cryo-EM) and cross-linking mass spectrometry (CX-MS) for structural elucidation of large protein assemblies. Curr Opin Struct Biol. 2017;46:157–168. doi: 10.1016/j.sbi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Panchaud A, Goodlett DR. Chemical cross-linking and mass spectrometry as a low-resolution protein structure determination technique. Anal Chem. 2010;82(7):2636–42. doi: 10.1021/ac1000724. [DOI] [PubMed] [Google Scholar]
- 30.Chu F, et al. Unraveling the interface of signal recognition particle and its receptor by using chemical cross-linking and tandem mass spectrometry. Proc Natl Acad Sci U S A. 2004;101(47):16454–9. doi: 10.1073/pnas.0407456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng-Elmore X, et al. Molecular Architecture of Photoreceptor Phosphodiesterase Elucidated by Chemical Cross-Linking and Integrative Modeling. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozbesky D, et al. Impact of Chemical Cross-Linking on Protein Structure and Function. Anal Chem. 2018;90(2):1104–1113. doi: 10.1021/acs.analchem.7b02863. [DOI] [PubMed] [Google Scholar]
- 33.Chu F, et al. Identification of novel quaternary domain interactions in the Hsp90 chaperone, GRP94. Protein Sci. 2006;15(6):1260–9. doi: 10.1110/ps.052065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karagoz GE, et al. An unfolded protein-induced conformational switch activates mammalian IRE1. Elife. 2017;6 doi: 10.7554/eLife.30700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32(5):631–40. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Street TO, et al. Elucidating the mechanism of substrate recognition by the bacterial Hsp90 molecular chaperone. J Mol Biol. 2014;426(12):2393–404. doi: 10.1016/j.jmb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruppa GH, Schoeniger J, Young MM. A top down approach to protein structural studies using chemical cross-linking and Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2003;17(2):155–62. doi: 10.1002/rcm.885. [DOI] [PubMed] [Google Scholar]
- 38.Guo X, et al. Partial acetylation of lysine residues improves intraprotein cross-linking. Anal Chem. 2008;80(4):951–60. doi: 10.1021/ac701636w. [DOI] [PubMed] [Google Scholar]
- 39.Madler S, et al. Does chemical cross-linking with NHS esters reflect the chemical equilibrium of protein-protein noncovalent interactions in solution? J Am Soc Mass Spectrom. 2010;21(10):1775–83. doi: 10.1016/j.jasms.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Egea PF, et al. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004;427(6971):215–21. doi: 10.1038/nature02250. [DOI] [PubMed] [Google Scholar]
- 41.Spanggord RJ, et al. RNA-mediated interaction between the peptide-binding and GTPase domains of the signal recognition particle. Nat Struct Mol Biol. 2005;12(12):1116–22. doi: 10.1038/nsmb1025. [DOI] [PubMed] [Google Scholar]
- 42.Buskiewicz I, et al. Domain rearrangement of SRP protein Ffh upon binding 4.5S RNA and the SRP receptor FtsY. Rna. 2005;11(6):947–57. doi: 10.1261/rna.7242305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiau AK, et al. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127(2):329–40. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Wu B, et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152(1–2):276–89. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 45.Walzthoeni T, et al. Mass spectrometry supported determination of protein complex structure. Curr Opin Struct Biol. 2013;23(2):252–60. doi: 10.1016/j.sbi.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Merkley ED, Cort JR, Adkins JN. Cross-linking and mass spectrometry methodologies to facilitate structural biology: finding a path through the maze. J Struct Funct Genomics. 2013;14(3):77–90. doi: 10.1007/s10969-013-9160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran BQ, Goodlett DR, Goo YA. Advances in protein complex analysis by chemical cross-linking coupled with mass spectrometry (CXMS) and bioinformatics. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbapap.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Sinz A, et al. Chemical cross-linking and native mass spectrometry: A fruitful combination for structural biology. Protein Sci. 2015;24(8):1193–209. doi: 10.1002/pro.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young MM, et al. High throughput protein fold identification by using experimental constraints derived from intramolecular cross-links and mass spectrometry. Proc Natl Acad Sci U S A. 2000;97(11):5802–6. doi: 10.1073/pnas.090099097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneidman-Duhovny D, et al. A method for integrative structure determination of protein-protein complexes. Bioinformatics. 2012;28(24):3282–3289. doi: 10.1093/bioinformatics/bts628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinner O, et al. Identification of cross-linked peptides from large sequence databases. Nat Methods. 2008;5(4):315–8. doi: 10.1038/nmeth.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YJ. Probability-based shotgun cross-linking sites analysis. J Am Soc Mass Spectrom. 2009;20(10):1896–9. doi: 10.1016/j.jasms.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Chu F, et al. Finding chimeras: a bioinformatics strategy for identification of cross-linked peptides. Mol Cell Proteomics. 2010;9(1):25–31. doi: 10.1074/mcp.M800555-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panchaud A, et al. xComb: a cross-linked peptide database approach to protein-protein interaction analysis. J Proteome Res. 2010;9(5):2508–15. doi: 10.1021/pr9011816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du X, et al. Xlink-identifier: an automated data analysis platform for confident identifications of chemically cross-linked peptides using tandem mass spectrometry. J Proteome Res. 2011;10(3):923–31. doi: 10.1021/pr100848a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, et al. Database search algorithm for identification of intact cross-links in proteins and peptides using tandem mass spectrometry. J Proteome Res. 2010;9(7):3384–93. doi: 10.1021/pr100369y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoopmann MR, et al. Kojak: efficient analysis of chemically cross-linked protein complexes. J Proteome Res. 2015;14(5):2190–8. doi: 10.1021/pr501321h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trnka MJ, et al. Matching Cross-linked Peptide Spectra: Only as Good as the Worse Identification. Mol Cell Proteomics. 2014;13(2):420–34. doi: 10.1074/mcp.M113.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohn S, et al. Structure of the 26S proteasome from Schizosaccharomyces pombe at subnanometer resolution. Proc Natl Acad Sci U S A. 2010;107(49):20992–7. doi: 10.1073/pnas.1015530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lasker K, et al. Integrative structure modeling of macromolecular assemblies from proteomics data. Mol Cell Proteomics. 2010;9(8):1689–702. doi: 10.1074/mcp.R110.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ZA, et al. Architecture of the RNA polymerase II-TFIIF complex revealed by cross-linking and mass spectrometry. Embo J. 2010 doi: 10.1038/emboj.2009.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami K, et al. Architecture of an RNA polymerase II transcription pre-initiation complex. Science. 2013;342(6159):1238724. doi: 10.1126/science.1238724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muhlbacher W, et al. Conserved architecture of the core RNA polymerase II initiation complex. Nat Commun. 2014;5:4310. doi: 10.1038/ncomms5310. [DOI] [PubMed] [Google Scholar]
- 64.Robinson PJ, et al. Molecular architecture of the yeast Mediator complex. Elife. 2015;4 doi: 10.7554/eLife.08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson PJ, et al. Structure of a Complete Mediator-RNA Polymerase II PreInitiation Complex. Cell. 2016;166(6):1411–1422e16. doi: 10.1016/j.cell.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tosi A, et al. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell. 2013;154(6):1207–19. doi: 10.1016/j.cell.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen VQ, et al. Molecular architecture of the ATP-dependent chromatin-remodeling complex SWR1. Cell. 2013;154(6):1220–31. doi: 10.1016/j.cell.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han Y, et al. Architecture of the Saccharomyces cerevisiae SAGA transcription coactivator complex. EMBO J. 2014;33(21):2534–46. doi: 10.15252/embj.201488638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciferri C, et al. Molecular architecture of human polycomb repressive complex 2. Elife. 2012;1:e00005. doi: 10.7554/eLife.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H, et al. MS-based cross-linking analysis reveals the location of the PsbQ protein in cyanobacterial photosystem II. Proc Natl Acad Sci U S A. 2014;111(12):4638–43. doi: 10.1073/pnas.1323063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanowar S, et al. Interactions of the Transmembrane Polymeric Rings of the Salmonella enterica Serovar Typhimurium Type III Secretion System. MBio. 2010;1(3) doi: 10.1128/mBio.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Y, et al. A strategy for dissecting the architectures of native macromolecular assemblies. Nat Methods. 2015;12(12):1135–8. doi: 10.1038/nmeth.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu F, et al. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat Methods. 2015;12(12):1179–84. doi: 10.1038/nmeth.3603. [DOI] [PubMed] [Google Scholar]
- 74.Wu X, et al. In vivo protein interaction network analysis reveals porin-localized antibiotic inactivation in Acinetobacter baumannii strain AB5075. Nat Commun. 2016;7:13414. doi: 10.1038/ncomms13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang B, et al. Identification of cross-linked peptides from complex samples. Nat Methods. 2012;9(9):904–6. doi: 10.1038/nmeth.2099. [DOI] [PubMed] [Google Scholar]
- 76.Cammarata MB, Brodbelt JS. Characterization of Intra- and Intermolecular Protein Crosslinking by Top Down Ultraviolet Photodissociation Mass Spectrometry. ChemistrySelect. 2016;1(3):590–593. [Google Scholar]
- 77.Scaloni A, et al. Topology of the calmodulin-melittin complex. J Mol Biol. 1998;277(4):945–58. doi: 10.1006/jmbi.1998.1629. [DOI] [PubMed] [Google Scholar]
- 78.Schulz DM, et al. Mapping the topology and determination of a low-resolution three-dimensional structure of the calmodulin-melittin complex by chemical cross-linking and high-resolution FTICRMS: direct demonstration of multiple binding modes. Biochemistry. 2004;43(16):4703–15. doi: 10.1021/bi036149f. [DOI] [PubMed] [Google Scholar]
- 79.Chalkley RJ, et al. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: I. How much of the data is theoretically interpretable by search engines? Mol Cell Proteomics. 2005;4(8):1189–93. doi: 10.1074/mcp.D500001-MCP200. [DOI] [PubMed] [Google Scholar]
- 80.Leitner A, et al. Expanding the chemical cross-linking toolbox by the use of multiple proteases and enrichment by size exclusion chromatography. Mol Cell Proteomics. 2012;11(3):M111014126. doi: 10.1074/mcp.M111.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beausoleil SA, et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101(33):12130–5. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller DR, et al. Isotope-tagged cross-linking reagents. A new tool in mass spectrometric protein interaction analysis. Anal Chem. 2001;73(9):1927–34. doi: 10.1021/ac001379a. [DOI] [PubMed] [Google Scholar]
- 83.Wallon G, et al. Model for stathmin/OP18 binding to tubulin. Embo J. 2000;19(2):213–22. doi: 10.1093/emboj/19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gigant B, et al. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102(6):809–16. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 85.Maiolica A, et al. Structural analysis of multiprotein complexes by cross-linking, mass spectrometry, and database searching. Mol Cell Proteomics. 2007;6(12):2200–11. doi: 10.1074/mcp.M700274-MCP200. [DOI] [PubMed] [Google Scholar]
- 86.McIlwain S, et al. Detecting cross-linked peptides by searching against a database of cross-linked peptide pairs. J Proteome Res. 2010;9(5):2488–95. doi: 10.1021/pr901163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gotze M, et al. StavroX--a software for analyzing crosslinked products in protein interaction studies. J Am Soc Mass Spectrom. 2012;23(1):76–87. doi: 10.1007/s13361-011-0261-2. [DOI] [PubMed] [Google Scholar]
- 88.Petrotchenko EV, Makepeace KA, Borchers CH. DXMSMS Match Program for Automated Analysis of LC-MS/MS Data Obtained Using Isotopically Coded CID-Cleavable Cross-Linking Reagents. Curr Protoc Bioinformatics. 2014;48:8 18 1–19. doi: 10.1002/0471250953.bi0818s48. [DOI] [PubMed] [Google Scholar]
- 89.Singh P, et al. Characterization of protein cross-links via mass spectrometry and an open-modification search strategy. Anal Chem. 2008;80(22):8799–806. doi: 10.1021/ac801646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walzthoeni T, et al. False discovery rate estimation for cross-linked peptides identified by mass spectrometry. Nat Methods. 2012;9(9):901–3. doi: 10.1038/nmeth.2103. [DOI] [PubMed] [Google Scholar]
- 91.Zheng C, et al. XLink-DB: database and software tools for storing and visualizing protein interaction topology data. J Proteome Res. 2013;12(4):1989–95. doi: 10.1021/pr301162j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courcelles M, et al. CLMSVault: A Software Suite for Protein Cross-Linking Mass-Spectrometry Data Analysis and Visualization. J Proteome Res. 2017;16(7):2645–2652. doi: 10.1021/acs.jproteome.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kosinski J, et al. Xlink Analyzer: software for analysis and visualization of cross-linking data in the context of three-dimensional structures. J Struct Biol. 2015;189(3):177–83. doi: 10.1016/j.jsb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lauber MA, Reilly JP. Novel amidinating cross-linker for facilitating analyses of protein structures and interactions. Anal Chem. 2010;82(18):7736–43. doi: 10.1021/ac101586z. [DOI] [PubMed] [Google Scholar]
- 95.Thumm M, Hoenes J, Pfleiderer G. S-Methylthioacetimidate is a new reagent for the amidination of proteins at low pH. Biochimica et Biophysica Acta, General Subjects. 1987;923(2):263–267. [Google Scholar]
- 96.Trnka MJ, Burlingame AL. Topographic studies of the GroEL-GroES chaperonin complex by chemical cross-linking using diformyl ethynylbenzene: the power of high resolution electron transfer dissociation for determination of both peptide sequences and their attachment sites. Mol Cell Proteomics. 2010;9(10):2306–17. doi: 10.1074/mcp.M110.003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novak P, Kruppa GH. Intra-molecular cross-linking of acidic residues for protein structure studies. Eur J Mass Spectrom (Chichester) 2008;14(6):355–65. doi: 10.1255/ejms.963. [DOI] [PubMed] [Google Scholar]
- 98.Leitner A, et al. Chemical cross-linking/mass spectrometry targeting acidic residues in proteins and protein complexes. Proc Natl Acad Sci U S A. 2014;111(26):9455–60. doi: 10.1073/pnas.1320298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, et al. Carboxylate-Selective Chemical Cross-Linkers for Mass Spectrometric Analysis of Protein Structures. Anal Chem. 2018;90(2):1195–1201. doi: 10.1021/acs.analchem.7b03789. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Q, Crosland E, Fabris D. Nested Arg-specific bifunctional crosslinkers for MS-based structural analysis of proteins and protein assemblies. Anal Chim Acta. 2008;627(1):117–28. doi: 10.1016/j.aca.2008.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noller HF, Chaires JB. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972;69(11):3115–8. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sinz A, Kalkhof S, Ihling C. Mapping protein interfaces by a trifunctional cross-linker combined with MALDI-TOF and ESI-FTICR mass spectrometry. J Am Soc Mass Spectrom. 2005;16(12):1921–31. doi: 10.1016/j.jasms.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 103.Giese SH, Belsom A, Rappsilber J. Optimized Fragmentation Regime for Diazirine Photo-Cross-Linked Peptides. Anal Chem. 2016;88(16):8239–47. doi: 10.1021/acs.analchem.6b02082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chin JW, et al. Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc Natl Acad Sci U S A. 2002;99(17):11020–4. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang X, et al. Mass spectrometry identifiable cross-linking strategy for studying protein-protein interactions. Anal Chem. 2005;77(1):311–8. doi: 10.1021/ac0488762. [DOI] [PubMed] [Google Scholar]
- 106.Soderblom EJ, Goshe MB. Collision-induced dissociative chemical cross-linking reagents and methodology: Applications to protein structural characterization using tandem mass spectrometry analysis. Anal Chem. 2006;78(23):8059–68. doi: 10.1021/ac0613840. [DOI] [PubMed] [Google Scholar]
- 107.Lu Y, et al. Ionic reagent for controlling the gas-phase fragmentation reactions of cross-linked peptides. Anal Chem. 2008;80(23):9279–87. doi: 10.1021/ac801625e. [DOI] [PubMed] [Google Scholar]
- 108.Kao A, et al. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol Cell Proteomics. 2011;10(1):M110002212. doi: 10.1074/mcp.M110.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muller MQ, et al. Cleavable cross-linker for protein structure analysis: reliable identification of cross-linking products by tandem MS. Anal Chem. 2010;82(16):6958–68. doi: 10.1021/ac101241t. [DOI] [PubMed] [Google Scholar]
- 110.Sinz A, Wang K. Mapping protein interfaces with a fluorogenic cross-linker and mass spectrometry: application to nebulin-calmodulin complexes. Biochemistry. 2001;40(26):7903–13. doi: 10.1021/bi010259+. [DOI] [PubMed] [Google Scholar]
- 111.Wine RN, et al. Identification of components of protein complexes using a fluorescent photo-cross-linker and mass spectrometry. Anal Chem. 2002;74(9):1939–45. doi: 10.1021/ac011041w. [DOI] [PubMed] [Google Scholar]
- 112.Alley SC, et al. Mapping protein-protein interactions in the bacteriophage T4 DNA polymerase holoenzyme using a novel trifunctional photo-cross-linking and affinity reagent. Journal of the American Chemical Society. 2000;122(25):6126–6127. [Google Scholar]
- 113.Trester-Zedlitz M, et al. A modular cross-linking approach for exploring protein interactions. J Am Chem Soc. 2003;125(9):2416–25. doi: 10.1021/ja026917a. [DOI] [PubMed] [Google Scholar]
- 114.Hurst GB, Lankford TK, Kennel SJ. Mass spectrometric detection of affinity purified crosslinked peptides. J Am Soc Mass Spectrom. 2004;15(6):832–9. doi: 10.1016/j.jasms.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 115.Chu F, et al. Isotope-coded and affinity-tagged cross-linking (ICATXL): an efficient strategy to probe protein interaction surfaces. J Am Chem Soc. 2006;128(32):10362–3. doi: 10.1021/ja0614159. [DOI] [PubMed] [Google Scholar]
- 116.Nessen MA, et al. Selective enrichment of azide-containing peptides from complex mixtures. J Proteome Res. 2009;8(7):3702–11. doi: 10.1021/pr900257z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buncherd H, et al. Selective enrichment and identification of cross-linked peptides to study 3-D structures of protein complexes by mass spectrometry. J Proteomics. 2012;75(7):2205–15. doi: 10.1016/j.jprot.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 118.Sohn CH, et al. Designer reagents for mass spectrometry-based proteomics: clickable cross-linkers for elucidation of protein structures and interactions. Anal Chem. 2012;84(6):2662–9. doi: 10.1021/ac202637n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fischer L, Chen ZA, Rappsilber J. Quantitative cross-linking/mass spectrometry using isotope-labelled cross-linkers. J Proteomics. 2013;88:120–8. doi: 10.1016/j.jprot.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmidt C, et al. Comparative cross-linking and mass spectrometry of an intact F-type ATPase suggest a role for phosphorylation. Nat Commun. 2013;4:1985. doi: 10.1038/ncomms2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walzthoeni T, et al. xTract: software for characterizing conformational changes of protein complexes by quantitative cross-linking mass spectrometry. Nat Methods. 2015;12(12):1185–90. doi: 10.1038/nmeth.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu C, et al. Gln40 deamidation blocks structural reconfiguration and activation of SCF ubiquitin ligase complex by Nedd8. Nat Commun. 2015;6:10053. doi: 10.1038/ncomms10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bandyopadhyay P, I, Kuntz D. Computational investigation of kinetics of cross-linking reactions in proteins: importance in structure prediction. Biopolymers. 2009;91(1):68–77. doi: 10.1002/bip.21083. [DOI] [PubMed] [Google Scholar]
- 124.Toews J, et al. Mass spectrometric identification of formaldehyde-induced peptide modifications under in vivo protein cross-linking conditions. Anal Chim Acta. 2008;618(2):168–83. doi: 10.1016/j.aca.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 125.Liu F, Goshe MB. Combinatorial electrostatic collision-induced dissociative chemical cross-linking reagents for probing protein surface topology. Anal Chem. 2010;82(14):6215–23. doi: 10.1021/ac101030w. [DOI] [PubMed] [Google Scholar]
- 126.Belsom A, et al. Blind testing cross-linking/mass spectrometry under the auspices of the 11(th) critical assessment of methods of protein structure prediction (CASP11) Wellcome Open Res. 2016;1:24. doi: 10.12688/wellcomeopenres.10046.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herzog F, et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science. 2012;337(6100):1348–52. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- 128.Weisbrod CR, et al. In vivo protein interaction network identified with a novel real-time cross-linked peptide identification strategy. J Proteome Res. 2013;12(4):1569–79. doi: 10.1021/pr3011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleiner RE, et al. A Chemical Proteomics Approach to Reveal Direct Protein-Protein Interactions in Living Cells. Cell Chem Biol. 2018;25(1):110–120e3. doi: 10.1016/j.chembiol.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]