Abstract
Studies evaluating the association between human immunodeficiency virus (HIV) infection continuum of care outcomes [antiretroviral (ART) adherence, retention in care, viral suppression] and health literacy have yielded conflicting results. Moreover, studies from the southern United States, a region of the country disproportionately affected by the HIV epidemic and low health literacy, are lacking. We conducted an observational cohort study among 575 people living with HIV (PLWH) at the Vanderbilt Comprehensive Care Clinic (Nashville, Tennessee). Health literacy was measured using the brief health literacy screen, a short tool which can be administered verbally by trained clinical personnel. Low health literacy was associated with a lack of viral suppression, but not with poor ART adherence or poor retention. Age and racial disparities in continuum of care outcomes persisted after accounting for health literacy, suggesting that factors in addition to health literacy must be addressed in order to improve outcomes for PLWH.
Keywords: Human immunodeficiency virus, Health literacy, ART adherence, Retention in care, Viral suppression
Introduction
Human immunodeficiency virus (HIV) infection remains an important public health problem both globally and in the United States (US) with over 900,000 persons aged ≥ 13 years of age estimated to be living with diagnosed HIV infection in the US [1]. In the US, Black people are more likely to be infected with HIV [2, 3] and once infected are less likely to adhere to medical appointments and achieve suppression of HIV replication with antiretroviral therapy (ART) [4, 5]. In addition to racial disparities there are also regional disparities in HIV health outcomes, with the highest rate of HIV diagnosis in the southern US (South) compared to other regions of the country [1, 4].
Low health literacy has been shown to be a barrier to improved linkage, retention in care, and uptake of ART, even during global scaleup of ART programs, particularly in sub-Saharan Africa [6]. In the US, low health literacy disproportionately affects populations and regions similarly to the HIV epidemic [7]. In the US, low health literacy is more common among Black persons, compared to White persons. Moreover, a higher proportion of persons in the South are estimated to have lower health literacy compared to persons in other regions of the US [8]. It is possible that HIV-related health disparities by race or other factors may be mediated in part by health literacy as is seen in other chronic illnesses including diabetes [9, 10]. In one previous study among people living with HIV (PLWH), the effect of race on antiretroviral adherence was reduced 25% after adjustment for health literacy skills [11].
Studies evaluating the association of health literacy with important HIV continuum of care outcomes, including ART adherence [11–24], medical appointment adherence [25], and suppression of HIV viral replication [15, 23, 24], have yielded conflicting results. These studies of the association of health literacy and HIV continuum of care outcomes are difficult to compare due to differences in study settings, patient populations, tools used to measure health literacy, cutoffs used to categorize or define health literacy, as well as definitions for the HIV continuum of care outcomes of interest. None of these studies have utilized the Centers for Disease Control and Prevention (CDC) surveillance definitions for retention in care or viral suppression [1]. Additionally, none of these studies have used tools to measure health literacy that are brief, can be administered verbally, and have been validated when administered by clinical personnel during routine clinical care.
Our objective was to measure the health literacy of PLWH in care at the Vanderbilt Comprehensive Care Clinic (VCCC), a primary medical home for HIV care located in the South—a region of the country disproportionately affected by both the HIV epidemic and low health literacy. We sought to overcome the limitations of previous studies by utilizing a brief, validated tool to assess health literacy administered during routine clinical care and by using definitions for HIV continuum of care outcomes commonly utilized by the CDC. Additionally, we aimed to evaluate the association of health literacy levels with important HIV continuum of care outcomes in order to determine if interventions targeted to improve health literacy might improve the health of PLWH.
Methods
Patient Population
We conducted an observational cohort study among adult PLWH with ≥ 1 healthcare provider appointment at the VCCC in Nashville, Tennessee between 1 January 1998 and 31 December 2012 (the study period). Patients were excluded if health literacy data, collected in inpatient and outpatient clinical settings between 1 October 2010 and 31 December 2013, were not available in the electronic health record. Person-time was contributed from the date of the first HIV healthcare provider visit during the study period until date of death, date of loss to follow-up, or the date of the last HIV healthcare provider visit during the study period. Loss to follow-up was defined as the lack of a laboratory or healthcare provider visit during a calendar year period without any subsequent laboratory or healthcare provider visits during the study period. Conversely, patients who re-entered care with observations in a later year were considered “not retained in care” during gaps in care and were included in population denominators during those years. All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Data Collection
Demographic, laboratory, appointment, health literacy/education, and medication adherence data were extracted from the electronic health record. Demographic data included age at enrollment (years), birth sex, patient self-reported race/ethnicity, HIV transmission risk factor, year of enrollment into HIV care, and socioeconomic status (SES, categorized: very low, low, not low, or unknown based on utilization of health insurance services with distinct income eligibilities). Patients were categorized “low SES” if receiving Ryan White services [income eligibility threshold in TN of ≤ 400% of the federal poverty level (FPL)] [26]. Patients were categorized “very low SES” if receiving Medicare or Medicaid (income eligibility threshold in TN of ≤ 250% of the FPL) [27]. Patients were categorized “not low SES” if using private or commercial insurance or with self-pay status. Prior work has noted a relationship between SES and poor HIV-related outcomes when using insurance data as a proxy for SES [28, 29]. SES was assessed the year prior to the year in which outcomes were determined (i.e., lagged) to avoid reverse causation. HIV transmission risk was categorized as male-to-male sexual contact (MSM), injection drug use (IDU), heterosexual contact (Hetero), or other/unknown (including perinatal infection); these categories were mutually exclusive, and multiple risks were collapsed according to a hierarchy in which IDU took precedence over MSM, and MSM took precedence over other risk factors. This hierarchy was based on the putative strengths of potential confounding effects of the dominant behavioral exposures, with IDU at greater risk of poorer ART adherence and therefore poorer virologic control than MSM in prior studies [5, 25]. Laboratory data included CD4+ lymphocyte (CD4) values and dates, HIV-1 RNA viral load (VL) values and dates. Laboratory measures were collected as part of routine clinical care. Appointment data included dates of completed HIV healthcare provider primary care visits.
Health literacy was assessed with the brief health literacy screen (BHLS) [30]. The three-item measure asks patients to report their level of confidence filling out medical forms, their need for assistance in reading hospital materials, and their understanding of written medical information, each on a five-point response scale. Scores for this measure can range from 3 to 15, with higher scores representing higher health literacy. The BHLS was administered orally by nursing staff during any admission to Vanderbilt University Hospital or at any visit to Vanderbilt Internal Medicine Primary Care Clinics as a part of the standard intake process beginning in 2011 [31] BHLS scores were assessed in this study as the average value of within-individual scores for each patient with ≥ 1 available BHLS score. We have validated this measure as a reliable indicator of health literacy in inpatient and outpatient settings at Vanderbilt University [30, 31]. There is also evidence that BHLS scores are stable in adulthood, allowing us to explore the relationship between a single average score per patient and the outcome during the entire study period [30–34]. The responses were recorded in the electronic health record and the mean score during the period of data collection was used in the analysis.
Antiretroviral adherence was measured using a brief questionnaire [35] modified from previously validated questionnaires [36, 37]. Using consecutive sampling at routine clinic visits, with the questionnaire administered once per patient per visit, patients were first asked, “Are you on antiretroviral therapy (ART)?” and secondly, “In the past week (7 days), how many times have you missed it?” Responses to the first question were recorded in the electronic health record categorically as “yes” or “no”. Responses to the second question were recorded in the electronic health record as a continuous measure from 0 to 7, as the responses naturally followed this pattern.
Study Outcome Definitions
Healthcare provider visit dates were used to determine retention in care and retention was defined as ≥ 2 healthcare provider visits in the calendar year of interest > 90 days apart [5, 38, 39]. Viral suppression was defined as ≥ 1 VL, with the last VL in the calendar year of interest < 200 copies/mL [5, 38, 39]. Patients with missing VL data during the calendar year of interest were not included in the viral suppression denominator in that respective calendar year. For the purposes of this study, ART adherence was assessed among those receiving ART during the year and was categorized as “None missed” if the answer was 0 and “Any missed” if the answer was ≥ 1. This coding was consistent with prior work published in this cohort [35] and maximized the contrast between maximally adherent patients and their comparator group. To simplify interpretation of relative risks (RRs) in regression modeling, with values > 1 implying adverse associations and < 1 implying protective associations, outcomes were coded in the negative; that is, the regression outcomes were properly lack of ART adherence, lack of retention in care, and lack of viral suppression.
In sensitivity analyses, lack of ART adherence was alternately defined as missing ≥ 2 doses in the prior 7 days (vs. missing ≤ 1 dose), and lack of retention in care was alternately defined as the incidence of missed visits in each year, defined using billing data available between 2003 and 2012 and treating “No Show” appointments as missed visits while excluding “Cancelled” appointments.
Statistical Analysis
Chi square and Wilcoxon rank sum tests were used to compare categorical and continuous variables, respectively. Modified Poisson regression was used to estimate adjusted RRs and 95% confidence intervals (95% CIs) for lack of ART adherence, lack of retention in care, and lack of viral suppression [40]. A generalized estimating equation was used to account for multiple outcomes per individual over time. In sensitivity analysis for the incidence of missed visits, Poisson regression with robust standard errors was used to estimate adjusted incidence rate ratios (IRR)s. The multivariate regression models were adjusted for age at enrollment (years), birth sex, race/ethnicity, HIV transmission risk factor, year of enrollment into HIV care, educational attainment (years), SES, calendar year of analysis, and time from enrollment (in years). Continuous variables, including BHLS scores, were modeled using restricted cubic splines with three or four knots [41]. All tests were two-tailed and considered statistically significant if p < 0.05. Statistical analyses were conducted using Stata 14.0 software (Stata-Corp, College Station, TX).
Results
There were 575 individuals with BHLS scores measured during the study period. The average BHLS score was 12.5 (standard deviation 3.00), and the median was 13.5 [inter-quartile range (IQR) 11, 15] (Fig. 1). Those with below-average BHLS scores were significantly older (median age of 40, IQR 34, 46 years old) than those with above-average BHLS scores (median age of 38, IQR 32, 45 years old). They were also disproportionately more likely to have heterosexual transmission risk (45%) and less likely to be MSM (39%) compared to those with above-average scores (32 and 52%, respectively), more likely to be of Black race (51 vs. 41%) and less likely to be White (42 vs. 54%), and were more likely to have very low (36 vs. 27%) and less likely to have high (48 vs. 55%) SES (p < 0.05 each). They also had significantly fewer median years of education (12, IQR 12, 14) compared to those with above-average scores (13, IQR 12, 15). The two groups did not significantly differ by sex, year of enrollment at clinic, median baseline CD4 or log10 VL (Table 1).
Fig. 1.
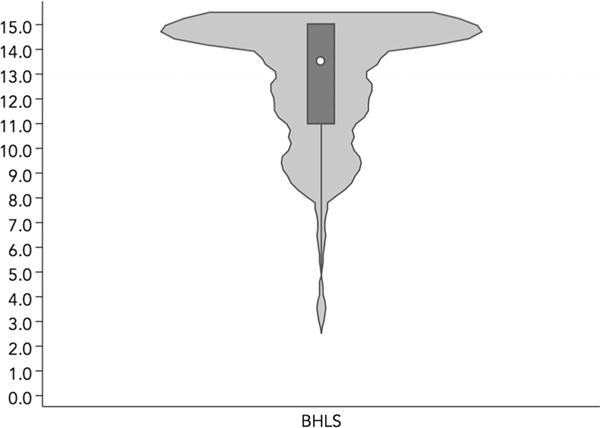
Violin plot for distribution of BHLS scores of the study population
Table 1.
Demographic characteristics of the study population, by person-years contributed in each category (among N = 575 individuals)
| Characteristics | High health literacy (BHLS ≥ 12.5) N = 2378 p-y |
Low health literacy (BHLS < 12.5) N = 1629 p-y |
P values* |
|---|---|---|---|
| Age (years), median (IQR) | 38 (32, 45) | 40 (34, 46) | 0.03 |
| Male sex, number (%) | 1776 (75) | 1120 (69) | 0.28 |
| Race/ethnicity, number (%) | |||
| White, non-Hispanic | 1292 (54) | 679 (42) | Ref. |
| Black, non-Hispanic | 985 (41) | 835 (51) | 0.05 |
| Hispanic | 56 (2) | 79 (5) | 0.07 |
| Other/unknown | 45 (2) | 36 (2) | 0.99 |
| Years of education, median (IQR) | 13 (12, 15) | 12 (12, 14) | <0.01 |
| HIV transmission risk factor, number (%) | |||
| MSM | 1225 (52) | 633 (39) | Ref. |
| IDU | 328 (14) | 210(13) | 0.47 |
| Hetero | 756 (32) | 725 (45) | < 0.01 |
| Other/unknown | 69 (3) | 61 (4) | 0.22 |
| Socioeconomic status | |||
| Not low | 1296 (55) | 788 (48) | Ref. |
| Low | 83 (3) | 49 (3) | 0.44 |
| Very low | 649 (27) | 579 (36) | < 0.01 |
| Unknown | 350 (15) | 213 (13) | 0.13 |
| Year of enrollment into HIV care at the VCCC, median (IQR) | 2002 (2000, 2006) | 2003 (2000, 2006) | 0.50 |
| Baseline CD4 + lymphocyte count (cells/mm3), median (IQR) | 309 (135, 510) | 321 (133, 544) | 0.41 |
| Baseline HIV-1 viral load (log10 copies/mL), median (IQR) | 4.45 (3.53, 4.94) | 4.24 (3.46, 4.89) | 0.15 |
Percents may not sum to 100 due to rounding
p-y Person-years, IQR interquartile range, MSM men who have sex with men, IDU injection drug use, Hetero heterosexual contact, VCCC Vanderbilt Comprehensive Care Clinic
P-values from bivariate modified Poisson regression with a generalized estimating equation to account for multiple outcomes per individual (a test for trend across categories for categorical variables)
Some individuals were missing data: N = 17 individuals had missing education data (n = 8 among those with above-average BHLS scores, and n = 9 among those with below-average BHLS scores), N = 1 individual had missing CD4+ lymphocyte count, and N = 1 individual had missing HIV-1 viral load
Among these 575 individuals with measured BHLS scores, 524 individuals (contributing 1598 person-years) were eligible for the ART adherence outcome, 508 (contributing 3463 person-years) for the retention outcome, and 574 (contributing 3618 person-years) for the viral suppression outcome (Fig. 2). In the fully adjusted models, BHLS score was significantly associated with neither poor ART adherence nor with lack of retention in care (Tables 2, 3), though lower BHLS score was indeed associated with higher risk of poor viral suppression (RR = 1.09, 95% CI 1.01–1.19, for score of 9 vs. 13.5; Table 4).
Fig. 2.
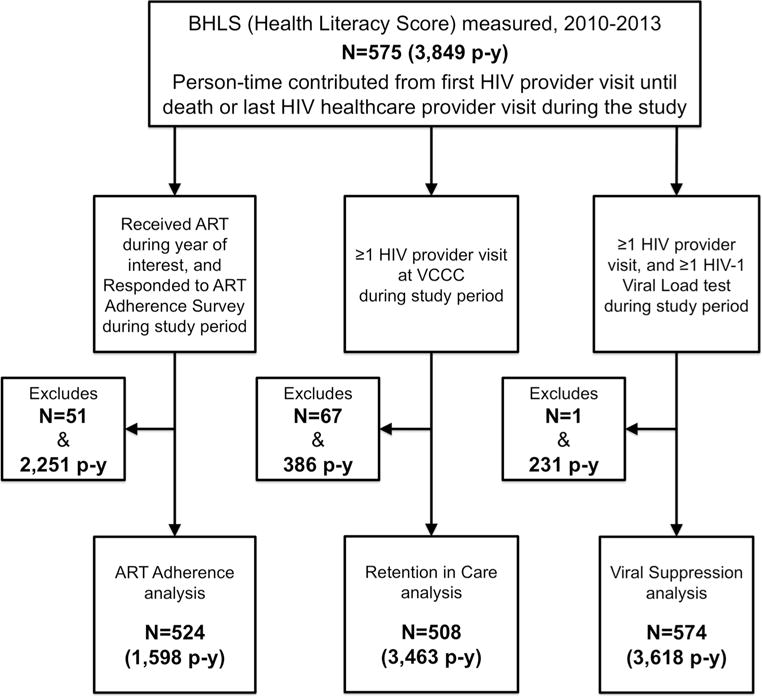
Population selection diagram for analyses of ART adherence, retention in care, and viral suppression outcomes
Table 2.
Modified Poisson regression model for lack of ART adherence among N = 524 individuals contributing 1598 person-years
| Characteristics | Unadjusted relative risk (95% confidence interval) | Adjusted relative risk (95% confidence interval) |
|---|---|---|
| BHLS score | 1.15 (0.87, 1.52) | 1.01 (0.73, 1.40) |
| 7 | 1.15 (0.96, 1.37) | 1.05 (0.86, 1.28) |
| 9 | 1.14 (1.00, 1.29) | 1.08 (0.96, 1.23) |
| 11 | Ref. | Ref. |
| 13.5 | ||
| Age (years) | 2.04 (1.29, 3.21) | 1.84 (1.15, 2.94) |
| 20 | 1.19 (0.91, 1.54) | 1.16 (0.88, 1.53) |
| 30 | Ref. | Ref. |
| 40 | 1.02 (0.83, 1.25) | 0.99 (0.80, 1.23) |
| 50 | 1.04 (0.62, 1.74) | 0.97 (0.57, 1.65) |
| 60 | ||
| Female sex (vs. male) | 1.26 (0.95, 1.67) | 0.89 (0.61, 1.29) |
| Race/ethnicity | Ref. | Ref. |
| White, non-Hispanic | 1.46 (1.12, 1.92) | 1.23 (0.93, 1.63) |
| Black, non-Hispanic | 1.40 (0.79, 2.49) | 1.51 (0.85, 2.70) |
| Hispanic | 1.06 (0.54, 2.09) | 1.19 (0.61, 2.32) |
| Other/unknown | ||
| Education (years)a | 1.02 (0.70, 1.48) | 0.97 (0.64, 1.46) |
| 8 | Ref. | Ref. |
| 12 | 0.74 (0.57, 0.98) | 0.79 (0.60, 1.04) |
| 14 | 0.57 (0.42, 0.78) | 0.64 (0.46, 0.88) |
| 16 | 0.36 (0.17, 0.75) | 0.44 (0.21, 0.90) |
| 20 | ||
| HIV transmission risk factor | Ref. | Ref. |
| MSM | 1.55 (1.04, 2.30) | 1.16 (0.76, 1.75) |
| IDU | 1.33 (0.99, 1.77) | 1.01 (0.70, 1.47) |
| Hetero | 1.69 (1.01, 2.84) | 1.58 (0.91, 2.72) |
| Other/unknown | ||
| Socioeconomic status | Ref. | Ref. |
| Not low | 0.88 (0.50, 1.52) | 0.91 (0.54, 1.54) |
| Low | 1.65 (1.30, 2.09) | 1.47 (1.14, 1.90) |
| Very low | 1.75 (1.03, 2.99) | 1.79 (1.02, 3.13) |
| Unknown | ||
| Year of enrollment at VCCC | 1.14 (0.94, 1.38) | 1.10 (0.91, 1.34) |
| 2000 | Ref. | Ref. |
| 2003 | 0.98 (0.80, 1.19) | 0.98 (0.79, 1.21) |
| 2005 | 0.83 (0.59, 1.17) | 0.86 (0.60, 1.23) |
| 2010 | ||
| Baseline CD4+ lymphocyte count (per 100 cells/mm3)a | 1.01 (0.97, 1.06) | 1.01 (0.96, 1.06) |
| Baseline HIV-1 viral load (log10 copies/ mL)a | 0.98 (0.89, 1.09) | 0.98 (0.87, 1.10) |
Bold estimates are statistically significant (p < 0.05). BHLS score, age, education, and year of enrollment were continuous variables modeled using restricted cubic splines with four knots. As these numbers comprised < 5% of the total study sample, missing data were not imputed in the adjusted model, as they were not seen to be a source of significant bias
MSM men who have sex with men, IDU injection drug use, Hetero heterosexual contact
Some individuals were missing data: N = 16 individuals had missing education data, N = 1 individual had missing CD4+ lymphocyte count, and N = 1 individual had missing HIV-1 viral load
Table 3.
Modified Poisson regression model for lack of retention in care among N = 508 individuals contributing 3463 person-years
| Characteristics | Unadjusted relative risk (95% confidence interval) | Adjusted relative risk (95% confidence interval) |
|---|---|---|
| BHLS score | 0.99 (0.78, 1.26) | 1.10 (0.84, 1.43) |
| 7 | 1.00 (0.86, 1.16) | 1.08 (0.92, 1.27) |
| 9 | 1.01 (0.91, 1.13) | 1.06 (0.96, 1.17) |
| 11 | Ref. | Ref. |
| 13.5 | ||
| Age (years) | 1.94 (1.38, 2.72) | 1.77 (1.26, 2.47) |
| 20 | 1.39 (1.10, 1.76) | 1.33 (1.06, 1.67) |
| 30 | Ref. | Ref. |
| 40 | 0.70 (0.57, 0.85) | 0.65 (0.53, 0.80) |
| 50 | 0.48 (0.28, 0.81) | 0.39 (0.22, 0.68) |
| 60 | ||
| Female sex (vs. male) | 1.01 (0.80, 1.29) | 0.67 (0.52, 0.88) |
| Race/ethnicity | Ref. | Ref. |
| White, non-Hispanic | 1.40 (1.11, 1.77) | 1.34 (1.06, 1.68) |
| Black, non-Hispanic | 0.77 (0.37, 1.61) | 0.72 (0.37, 1.42) |
| Hispanic | 0.70 (0.31, 1.54) | 0.74 (0.31, 1.74) |
| Other/unknown | ||
| Education (years)a | 0.91 (0.67, 1.24) | 0.95 (0.71, 1.27) |
| 8 | Ref. | Ref. |
| 12 | 0.97 (0.76, 1.24) | 1.06 (0.85, 1.33) |
| 14 | 0.85 (0.65, 1.11) | 1.01 (0.78, 1.31) |
| 16 | 0.62 (0.26, 1.46) | 0.86 (0.44, 1.69) |
| 20 | ||
| HIV transmission risk factor | Ref. | Ref. |
| MSM | 1.41 (1.02, 1.95) | 1.65 (1.19, 2.28) |
| IDU | 1.34 (1.05, 1.73) | 1.46 (1.10, 1.94) |
| Hetero | 1.66 (0.95, 2.92) | 1.93 (1.21, 3.08) |
| Other/unknown | ||
| Socioeconomic status | Ref. | Ref. |
| Not low | 0.60 (0.37, 0.98) | 0.67 (0.42, 1.05) |
| Low | 0.97 (0.82, 1.15) | 0.98 (0.82, 1.17) |
| Very low | 1.85 (1.52, 2.25) | 1.80 (1.47, 2.19) |
| Unknown | ||
| Year of enrollment at VCCC | 0.98 (0.82, 1.16) | 0.84 (0.72, 0.99) |
| 2000 | Ref. | Ref. |
| 2003 | 0.96 (0.80, 1.14) | 1.01 (0.87, 1.18) |
| 2005 | 0.72 (0.51, 1.03) | 0.82 (0.58, 1.17) |
| 2010 | ||
| Baseline CD4+ lymphocyte count (per 100 cells/mm3)a | 1.03 (1.00, 1.07) | 1.02 (0.98, 1.05) |
| Baseline HIV-1 viral load (copies/mL)a | 1.02 (0.93, 1.11) | 0.97 (0.89, 1.07) |
Bold estimates are statistically significant (p < 0.05). BHLS score, age, education, and year of enrollment were continuous variables modeled using restricted cubic splines with four knots. As these numbers comprised < 5% of the total study sample, missing data were not imputed in the adjusted model, as they were not seen to be a source of significant bias
MSM men who have sex with men, IDU injection drug use, Hetero heterosexual contact
Some individuals were missing data: N = 16 individuals had missing education data, N = 1 individual had missing CD4+ lymphocyte count, and N = 1 individual had missing HIV-1 viral load
Table 4.
Modified Poisson regression model for lack of viral suppression among N = 574 individuals contributing 3618 person-years
| Characteristics | Unadjusted relative risk (95% confidence interval) | Adjusted relative risk (95% confidence interval) |
|---|---|---|
| BHLS score | 1.17 (1.02, 1.33) | 1.14 (0.99, 1.30) |
| 7 | 1.11 (1.03, 1.21) | 1.09 (1.01, 1.19) |
| 9 | 1.06 (1.00, 1.12) | 1.05 (1.00, 1.11) |
| 11 | Ref. | Ref. |
| 13.5 | ||
| Age (years) | 1.62 (1.32, 1.98) | 1.28 (1.05, 1.57) |
| 20 | 1.19 (1.05, 1.36) | 1.14 (1.01, 1.28) |
| 30 | Ref. | Ref. |
| 40 | 0.85 (0.76, 0.94) | 0.83 (0.75, 0.92) |
| 50 | 0.70 (0.52, 0.94) | 0.67 (0.52, 0.87) |
| 60 | ||
| Female sex (vs. male) | 1.27 (1.12, 1.44) | 1.00 (0.86, 1.17) |
| Race/ethnicity | Ref. | Ref. |
| White, non-Hispanic | 1.38 (1.21, 1.56) | 1.27 (1.13, 1.43) |
| Black, non-Hispanic | 1.09 (0.80, 1.48) | 1.14 (0.86, 1.51) |
| Hispanic | 1.17 (0.76, 1.80) | 1.46 (1.00, 2.12) |
| Other/unknown | ||
| Education (years)a | 1.08 (0.94, 1.24) | 1.11 (0.96, 1.28) |
| 8 | Ref. | Ref. |
| 12 | 0.90 (0.78, 1.04) | 0.94 (0.84, 1.06) |
| 14 | 0.83 (0.71, 0.97) | 0.92 (0.80, 1.05) |
| 16 | 0.72 (0.42, 1.23) | 0.88 (0.63, 1.24) |
| 20 | ||
| HIV transmission risk factor | Ref. | Ref. |
| MSM | 1.37 (1.15, 1.64) | 1.15 (0.95, 1.39) |
| IDU | 1.27 (1.10, 1.45) | 0.99 (0.84, 1.16) |
| Hetero | 0.94 (0.68, 1.30) | 0.90 (0.68, 1.20) |
| Other/unknown | ||
| Socioeconomic status | Ref. | Ref. |
| Not low | 0.59 (0.41, 0.86) | 0.62 (0.44, 0.88) |
| Low | 1.32 (1.18, 1.47) | 1.30 (1.16, 1.45) |
| Very low | 2.01 (1.79, 2.26) | 1.96 (1.72, 2.24) |
| Unknown | ||
| Year of enrollment at VCCC | 1.18 (1.08, 1.28) | 1.04 (0.96, 1.13) |
| 2000 | Ref. | Ref. |
| 2003 | 0.94 (0.86, 1.04) | 1.02 (0.93, 1.11) |
| 2005 | 0.88 (0.75, 1.03) | 1.05 (0.90, 1.24) |
| 2010 | ||
| Baseline CD4+ lymphocyte count (per 100 cells/mm3)a | 1.05 (1.04, 1.07) | 1.07 (1.05, 1.09) |
| Baseline HIV-1 viral load (log10 copies/mL)a | 1.12 (1.06, 1.18) | 1.18 (1.11, 1.25) |
Bold estimates are statistically significant (p < 0.05). BHLS score, age, education, and year of enrollment were continuous variables modeled using restricted cubic splines with four knots. As these numbers comprised < 5% of the total study sample, missing data were not imputed in the adjusted model, as they were not seen to be a source of significant bias
MSM men who have sex with men, IDU injection drug use, Hetero: heterosexual contact
Some individuals were missing data: N = 17 individuals had missing education data, N = 1 individual had missing CD4+ lymphocyte count, and N = 1 individual had missing HIV-1 viral load
For each outcome, younger age was significantly associated with poorer outcomes compared to older age (RR = 1.84, 95% CI 1.15–2.94; RR = 1.77, 95% CI 1.26–2.47; RR = 1.28, 95% CI 1.05–1.57 for 20 vs. 40 year-olds at risk of poor ART adherence, retention in care and viral suppression, respectively; Tables 2, 3, 4). Female sex was significantly associated with a decreased probability of poor retention compared to males (RR = 0.67, 95% CI 0.52–0.88; Table 3), though sex was associated with neither poor ART adherence nor poor viral suppression (Tables 2, 4). Both those with IDU and heterosexual transmission risk had greater likelihood of poor retention compared to MSM individuals (RR = 1.65, 95% CI 1.19–2.28; RR = 1.46, 95% CI 1.10–1.94 for IDU and Hetero risk, respectively; Table 3), though, as with sex, there was no significant difference by transmission risk with respect to poor ART adherence or viral suppression outcomes (Table 4).
In turn, those of Black race were at increased risk of poor retention in care (RR = 1.34, 95% CI 1.06–1.68), and poor viral suppression compared to Whites (RR = 1.28, 95% CI 1.14–1.44; Tables 3, 4), though not of poor ART adherence. Those with higher baseline CD4 and log10 VL were at increased risk of poor viral suppression alone (RR = 1.07, 95% CI 1.05–1.09; RR = 1.18, 95% CI 1.11–1.25; Table 4), but not of poor ART adherence or poor retention in care (Tables 2, 3).
Finally, individuals with very low SES (either Medicare or Medicaid insurance) were more likely to have poor ART adherence responses (RR = 1.47, 95% CI 1.14–1.90; Table 2) and poor viral suppression (RR = 1.30, 95% CI 1.16–1.45; Table 4) than those with not low SES; those with low SES (receiving Ryan White services) were actually at lower risk of poor viral suppression (RR = 0.62, 95% CI 0.44–0.88; Table 4).
In sensitivity analysis restricting the definition of poor ART adherence to those missing ≥ 2 doses in the prior 7 days, multivariable regression yielded substantively similar results to the primary analysis, though in this case, Black race (RR = 1.51, 95% CI 1.00–2.27) and IDU (RR = 1.74, 95% CI 1.02–2.97) were significantly associated with poorer ART adherence. In sensitivity analysis relying on missed visits to define poor retention in care, covering a shorter span of the study period than the primary analysis, results were again substantively similar to the primary analysis, with the notable exception of estimates for BHLS score. Lower literacy scores were in fact associated with higher adjusted incidences of missed visits at scores just below the median (IRR = 1.06, 95% CI 1.00–1.13, for score of 11 vs. 13.5). More recent year of care was also associated with an increased likelihood of missed visits (IRR = 1.38, 95% CI 1.14–1.68, comparing 2010–2003).
Discussion
In this population of PLWH in the South, lower health literacy significantly increased the likelihood of not achieving viral suppression; further, age and racial disparities in viral suppression persisted even after accounting for health literacy differences. Though other demographic and clinical factors were again associated with poor ART adherence and poor retention in care, similar to prior work in this and other high-income settings [42–48], health literacy was apparently only associated with viral suppression in this population.
One strength of this study is the use of the BHLS tool to measure health literacy. This tool is brief, can be administered verbally, and has been validated for administration by clinical personnel during routine clinical care [30]. In 2004, the National Academy of Medicine recommended the assessment of health literacy be incorporated into health care information systems and data collection [49]. Our medical center is relatively unique in having accomplished this, enabling population based studies in a variety of areas [50–52]. The use of the BHLS tool for this purpose allows for larger-scale studies of associations of health literacy with important health outcomes as well as the evaluation of system-level interventions designed to address health literacy-related barriers.
Studies of interventions to improve health literacy among PLWH are lacking. A recent systematic review of health literacy interventions for PLWH identified several studies evaluating interventions aimed to improve ART adherence, although none had a significant impact potentially due to methodologic issues. However, there were significant improvements in knowledge, behavioral skills, and e-Health literacy following interventions. Additional research assessing the efficacy of interventions aimed to improve health literacy among PLWH are needed [53].
Although we did find an association of low health literacy with a lack of viral suppression, we did not see similar associations with poor ART adherence or a lack of retention in clinical care. It is possible that our definitions of these outcomes may have impacted our findings. Review of pharmacy refill records, Medical Equipment Management Systems caps, pill counts, or monitoring of serum drug levels would have allowed a more objective assessment of adherence. However, these types of adherence measurement tools are either costly or not easily conducted as part of routine clinical care. When our definition of self-reported ART adherence was made less stringent, our findings with respect to health literacy were unaltered, though racial and transmission risk factor disparities became statistically significant.
Similarly, a previous study examined the use of a less stringent measure of retention in care and instead utilized missed visits as the retention in care outcome of interest. This study found that despite being classified as retained in care using the definition recommended by the CDC, that PLWH who missed even one visit had a higher risk of all-cause mortality [54]. When we utilized missed visits in place of the CDC’s retention in care outcome, we observed similar inferences as in our primary analysis. Even so, the magnitude and pattern of the point estimates in the adjusted model between health literacy and lack of retention were in the same direction as those between health literacy and the lack of viral suppression, even if not statistically significant. This, despite the fact that retention and viral suppression metrics are not always strongly correlated, and retention measures may not discriminate the presence of viral suppression very well [55]. This pattern in our findings lends credence to the relationship between health literacy and success at accessing and remaining in care while realizing the benefits of proper HIV care, even if our study was not adequately powered to detect all of the nuances of these relationships.
There were several limitations in our analysis. First, we may have misclassified our retention outcome, as it is possible that individuals we classified as not retained actually received clinical care at a site outside of our cohort, however briefly. This is an inherent limitation in observational data unlinked to surveillance sources with complete reporting, though our overall proportion retained in this cohort has, in general, been higher than populations living with HIV across the state of Tennessee, perhaps mitigating this concern [43, 56].
Second, our ART adherence outcome was based on self-report in a nurse-administered survey, which may lead our measure to suffer from social desirability bias, wherein those actually missing ART doses may have been less likely to report missed doses due to the presence of healthcare workers [57, 58]. If this bias were equally distributed across health literacy levels, the resulting non-differential misclassification of the outcome might explain the apparent lack of association between BHLS score and poor ART adherence, coincident with the significant association between BHLS score and poor viral suppression (which would not suffer from the same biases as the ART adherence outcome).
Third, it is possible that our inferences were biased due to unmeasured confounding. There are numerous other potential confounders that may be associated with health literacy and HIV continuum of care outcomes including mental illness, substance use, characteristics of one’s community, or socioeconomic factors such as employment, income, or housing. For example, a previous study of the association between literacy and medical adherence among PLWH found that perceived social stigma mediated this relationship [18]. Further, there were no HIV-specific aspects of health literacy captured in the literacy measure that we used. Though the BHLS has been previously validated in both inpatient and outpatient populations at Vanderbilt University [30, 31], there may be unique factors present among PLWH in the same setting which rendered the measure less able to capture potential confounders of the health literacy–HIV outcome relationship. Therefore, future studies of the association between health literacy and HIV outcomes as well as strategies to improve health literacy may need to address additional factors, such as perceived stigma, and capture data on disease-specific aspects of health literacy. That said, it appears unlikely that the significant associations detected between health literacy and lack of viral suppression are merely artefactual, as that particular outcome is less prone to measurement error than the others, and the association between health literacy and lack of viral suppression was similar in direction, magnitude, and significance both in univariate and in multivariable models accounting for demographic, educational, clinical, socioeconomic, and temporal factors.
Finally, our study inferences may not be as generalizable to populations outside of Tennessee, in populations that are not clinically engaged, in culturally distinct populations, or in resource-limited settings such as sub-Saharan Africa. Though our clinical population does exhibit diversity by age, race, HIV transmission risk, and rural versus urban residence, our measure of health literacy should be validated in other populations before inferences may be presumed to be externally valid.
Despite these potential limitations, our study made use of a carefully followed clinical population with rich longitudinal data on critical HIV care continuum benchmarks in a region of great concern—the South. While findings from our cohort may not be fully generalizable to PLWH residing in southern states outside of Tennessee, we do believe that examining these relationships in a population that is receiving care within the South is of value and provides a substantive contribution to the literature. We leveraged the longitudinal nature of these data by using appropriate regression techniques and modeled the relationship between health literacy and these outcomes as flexibly and rigorously as possible, making every attempt to minimize confounding and secular trends by including demographics, years of formal education, SES, and year of cohort entry. We also suspect that our inferences are not subject to large selection biases, as the only outcome which suffered attrition from > 10% of the eligible exposed population was retention in care, and even so, the attrition was only 12% (Fig. 2).
Conclusions
Based on the findings from this population, addressing health literacy alone may not be sufficient to narrow particular demographic and clinical disparities in retention and viral suppression outcomes, which remain stubbornly associated with inferior outcomes in North America. However, addressing health literacy may yield marginal benefits among all groups, and may yet improve overall patient engagement in care and long-term individual- and population-level health outcomes- a worthy goal indeed.
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health: K01 AI131895 (Rebeiro), K08 AI104352 (Pettit), and P30 AI110527 (Tennessee Center for AIDS Research).
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was waived in accordance with standards for “on the shelf data,” and the waiver was approved by the Vanderbilt University School of Medicine Institutional Review Board (IRB).
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report. 2014;26 http://www.cdc.gov/hiv/library/reports/surveillance/. Published Nov 2015. Accessed 1 May 2017. [Google Scholar]
- 2.Johnson AS, Beer L, Sionean C, et al. HIV infection— United States, 2008 and 2010. Morb Mortal Wkly Rep Suppl. 2013;62(3):112–9. [PubMed] [Google Scholar]
- 3.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall HI, An Q, Tang T, et al. Prevalence of diagnosed and undiagnosed HIV infection-United States, 2008–2012. Morb Mortal Wkly Rep. 2015;64(24):657–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2011. HIV surveillance supplemental report. 2013;18(5) http://www.cdc.gov/hiv/library/reports/surveillance/. Published Oct 2013. Accessed 1 May 2017. [Google Scholar]
- 6.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;16:2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 7.Baumann KE, Phillips AL, Arya M. Overlap of HIV and low health literacy in the southern USA. Lancet HIV. 2015;2(7):e269–70. doi: 10.1016/S2352-3018(15)00121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The National Center for Education Statistics. The health literacy of America’s adults: results from the 2003national assessment of adult literacy. https://nces.ed.gov/pubs2006/2006483.pdf. Published Sep 2006.Accessed 1 May 2017.
- 9.Mantwill S, Monestel-Umana S, Schulz PJ. The relationship between health literacy and health disparities: a systematic review. PLoS ONE. 2015;10(12):e0145455. doi: 10.1371/journal.pone.0145455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(Suppl 3):268–78. doi: 10.1080/10810730.2011.604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn CY, Paasche-Orlow MK, Davis TC, Wolf MS. Health literacy: an overlooked factor in understanding HIV health disparities. Am J Prev Med. 2007;33(5):374–8. doi: 10.1016/j.amepre.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Graham J, Bennett IM, Holmes WC, Gross R. Medication beliefs as mediators of the health literacy–antiretroviral adherence relationship in HIV-infected individuals. AIDS Behav. 2007;11(3):385–92. doi: 10.1007/s10461-006-9164-9. [DOI] [PubMed] [Google Scholar]
- 13.Kalichman SC, Cherry C, Kalichman MO, et al. Randomized clinical trial of HIV treatment adherence counseling interventions for people living with HIV and limited health literacy. J Acquir Immune Defic Syndr. 2013;63(1):42–50. doi: 10.1097/QAI.0b013e318286ce49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ownby RL, Waldrop-Valverde D, Caballero J, Jacobs RJ. Baseline medication adherence and response to an electronically delivered health literacy intervention targeting adherence. Neurobehav HIV Med. 2012;4:113–21. doi: 10.2147/NBHIV.S36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalichman SC, Rompa D. Functional health literacy is associated with health status and health-related knowledge in people living with HIV-AIDS. J Acquir Immune Defic Syndr. 2000;25(4):337–44. doi: 10.1097/00042560-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14(5):267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalichman SC, Pope H, White D, et al. Association between health literacy and HIV treatment adherence: further evidence from objectively measured medication adherence. J Int Assoc Physicians AIDS Care (Chic) 2008;7(6):317–23. doi: 10.1177/1545109708328130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waite KR, Paasche-Orlow M, Rintamaki LS, Davis TC, Wolf MS. Literacy, social stigma, and HIV medication adherence. J Gen Intern Med. 2008;23(9):1367–72. doi: 10.1007/s11606-008-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf MS, Davis TC, Osborn CY, Skripkauskas S, Bennett CL, Makoul G. Literacy, self-efficacy, and HIV medication adherence. Patient Educ Couns. 2007;65(2):253–60. doi: 10.1016/j.pec.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Nelsen A, Gupta S, Trautner BW, et al. Intention to adhere to HIV treatment: a patient-centred predictor of antiretroviral adherence. HIV Med. 2013;14(8):472–80. doi: 10.1111/hiv.12032. [DOI] [PubMed] [Google Scholar]
- 21.Navarra AM, Neu N, Toussi S, Nelson J, Larson EL. Health literacy and adherence to antiretroviral therapy among HIV-infected youth. J Assoc Nurses AIDS Care. 2014;25(3):203–13. doi: 10.1016/j.jana.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbert AM, Sereika SM, Erlen JA. Functional health literacy, medication-taking self-efficacy and adherence to antiretroviral therapy. J Adv Nurs. 2013;69(2):295–304. doi: 10.1111/j.1365-2648.2012.06007.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy DA, Lam P, Naar-King S, et al. Health literacy and antiretroviral adherence among HIV-infected adolescents. Patient Educ Couns. 2010;79(1):25–9. doi: 10.1016/j.pec.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paasche-Orlow MK, Cheng DM, Palepu A, Meli S, Faber V, Samet JH. Health literacy, antiretroviral adherence, and HIV-RNA suppression: a longitudinal perspective. J Gen Intern Med. 2006;21(8):835–40. doi: 10.1111/j.1525-1497.2006.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldrop-Valverde D, Guo Y, Ownby RL, Rodriguez A, Jones DL. Risk and protective factors for retention in HIV care. AIDS Behav. 2014;18(8):1483–91. doi: 10.1007/s10461-013-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tennessee Department of Health. Ryan White Program. 2016 https://www.tn.gov/health/topic/STD-ryanwhite. Accessed Nov 2016.
- 27.TennCare Division of Health Care Finance and Administration. TennCare eligibility. 2016 https://www.tn.gov/tenncare/topic/eligibility. Accessed Nov 2016.
- 28.Jain S, Schwarcz S, Katz M, Gulati R, McFarland W. Elevated risk of death for African Americans with AIDS, San Francisco, 1996– 2002. J Health Care Poor Underserved. 2006;17(3):493–503. doi: 10.1353/hpu.2006.0106. [DOI] [PubMed] [Google Scholar]
- 29.Chen SY, Moss WJ, Pipkin SS, McFarland W. A novel use of AIDS surveillance data to assess the impact of initial treatment regimen on survival. Int J STD AIDS. 2009;20(5):330–5. doi: 10.1258/ijsa.2008.008381. [DOI] [PubMed] [Google Scholar]
- 30.Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med. 2014;29(1):119–26. doi: 10.1007/s11606-013-2568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon C, Mion LC, Willens DE, Roumie CL, Kripalani S. Implementing routine health literacy assessment in hospital and primary care patients. Jt Comm J Qual Patient Saf. 2014;40(2):68–76. doi: 10.1016/s1553-7250(14)40008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker DW, Gazmararian JA, Sudano J, Patterson M. The association between age and health literacy among elderly persons. J Gerontol B. 2000;55:S368–74. doi: 10.1093/geronb/55.6.s368. [DOI] [PubMed] [Google Scholar]
- 33.Wångdahl JM, Mårtensson LI. The communicative and critical health literacy scale—Swedish version. Scand J Public Health. 2014;42(1):25–31. doi: 10.1177/1403494813500592. [DOI] [PubMed] [Google Scholar]
- 34.Baker DW. The meaning and the measure of health literacy. J Gen Intern Med. 2006;21(8):878–83. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasbach DA, Desruisseau AJ, Kipp AM, et al. Active cocaine use is associated with lack of HIV-1 virologic suppression independent of nonadherence to antiretroviral therapy: use of a rapid screening tool during routine clinic visits. AIDS Care. 2013;25(1):109–17. doi: 10.1080/09540121.2012.687814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 37.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 38.White House Office of AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020. https://www.AIDS.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. Accessed 21 nov 2017.
- 39.Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep. 2013;5:354–9. doi: 10.1177/003335491312800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd BE, Rebeiro PF, for the Caribbean Central and South America Network for HIV Epidemiology Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr. 2017;74(3):e60–3. doi: 10.1097/QAI.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebeiro PF, Abraham AG, Horberg MA, et al. Sex, race, and HIV risk disparities in discontinuity of HIV care after antiretroviral therapy initiation in the United States and Canada. AIDS Patient Care STDs. 2017;31(3):129–44. doi: 10.1089/apc.2016.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wester C, Rebeiro PF, Shavor TJ, et al. The 2013 HIV continuum of care in Tennessee: progress made, but disparities persist. Public Health Rep. 2016;131(5):695–703. doi: 10.1177/0033354916660082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rebeiro PF, Gange SJ, Horberg MA, et al. Geographic variations in retention in care among HIV-infected adults in the United States. PLoS ONE. 2016;11(1):e0146119. doi: 10.1371/journal.pone.0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodward B, Person A, Rebeiro P, Kheshti A, Raffanti S, Pettit A. Risk prediction tool for medical appointment attendance among HIV-infected persons with unsuppressed viremia. AIDS Patient Care STDs. 2015;29(5):240–7. doi: 10.1089/apc.2014.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yehia BR, Rebeiro P, Althoff KN, et al. Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. 2015;68(4):413–9. doi: 10.1097/QAI.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis. 2014;58(8):1185–9. doi: 10.1093/cid/ciu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62(3):356–62. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Academy of Medicine. Health literacy: a prescription to end confusion. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 50.Willens DE, Kripalani S, Schildcrout JS, et al. Association of brief health literacy screening and blood pressure in primary care. J Health Commun. 2013;18(Suppl 1):129–42. doi: 10.1080/10810730.2013.825663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNaughton CD, Kripalani S, Cawthon C, Mion LC, Wallston KA, Roumie CL. Association of health literacy with elevated blood pressure: a cohort study of hospitalized patients. Med Care. 2014;52(4):346–53. doi: 10.1097/MLR.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA.115.001799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perazzo J, Reyes D, Webel A. A systematic review of health literacy interventions for people living with HIV. AIDS Behav. 2017;21(3):812–21. doi: 10.1007/s10461-016-1329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–9. doi: 10.1093/cid/ciu603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;5:574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rebeiro PF, Althoff KN, Lau B, et al. Laboratory measures as proxies for primary care encounters: implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol. 2015;182(11):952–60. doi: 10.1093/aje/kwv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–52. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- 58.Jiamsakul A, Kumarasamy N, Ditangco R, et al. Factors associated with suboptimal adherence to antiretroviral therapy in Asia. J Int AIDS Soc. 2014;17:18911. doi: 10.7448/IAS.17.1.18911. [DOI] [PMC free article] [PubMed] [Google Scholar]


