Abstract
Objective
Age-associated skeletal muscle weakness is a major contributing factor to an increased late life mortality and morbidity, but its neurobiology is poorly understood. Previously, we provided histological evidence of dying-back axonal degeneration of motor neurons and denervation of neuromuscular junctions (NMJ) in age-associated muscle weakness. Given this, we aim to evaluate the relation between impaired neuromuscular transmission and various aspects of age-associated muscle weakness.
Design
We compared two electrophysiological measures, single fiber jitter and compound motor action potential (CMAP) in mice of different age groups, and correlated them with various physical performance measures, such as grip strength, standing and walking time and treadmill performance.
Results
Consistent with our previous histological data, single fiber jitter, a measure of NMJ transmission, was significantly increased in older animals, while CMAP shows no difference between young and old age groups. Neither jitter nor CMAP correlated with any of physical performance measures, except for jitter and standing activity.
Conclusion
Impaired neuromuscular transmission – represented as increase in SFEMG jitter level – reflects decline in motor function with aging.
Keywords: aging, neuromuscular junction, single fiber electromyography, dying-back, axon, sarcopenia, electrophysiology, frailty
INTRODUCTION
Decline in skeletal muscle function with aging predicts an increased morbidity and mortality in late life1–7, however, to date, the pathogenesis of the age-associated muscle weakness is poorly characterized. Reduction of muscle size and function, also known as sarcopenia, has been extensively studied in the context of aging, but recent clinical studies have shown that decline in muscle strength occurs much earlier than decline in muscle size, suggesting the important roles of neurogenic decline earlier in the course of age-associated skeletal muscle weakness1,8–10. Consistent with this, degeneration of neuromuscular junction (NMJ), the most distal part of motor neuron axon, is found to precede muscle atrophy in both animal and human11,12, providing further evidence that peripheral neurodegeneration is an important contributing factor to age-associated muscle weakness, likely early in the stage. Our lab has recently provided histological evidence of dying-back axonal degeneration of aging motor neurons in spinal cords, a form of neurodegeneration under a systematic metabolic stress13. Taken together, it is possible that degeneration of NMJ is one of the earliest neurological events in age-associated muscle weakness, and as dying-back axonopathy progresses, sarcopenia, as defined by reduction of muscle size and function, occurs later in the stage. However, there is a lack of electrophysiological studies in sarcopenia.
In this study, we aimed to characterize electrophysiological aspects of age-associated muscle weakness, and correlated them with skeletal muscle functions of wild type C57BL/6J mice. Given the histological evidence of NMJ denervation, we were particularly interested in the role of impaired neuromuscular transmission in age-associated weakness, and therefore, compared two electrodiagnostic measures - single fiber electromyography (SFEMG) jitter and compound motor action potential (CMAP) between young and old age groups of mice. SFEMG is a special electrophysiological technique to evaluate neuromuscular transmission at NMJ, and allows comparing of a few action potentials (AP) arising from individual muscle fibers. The variability of interpotential interval (IPI) at consecutive discharges of AP is defined as jittter, and an increase in jitter level sensitively represents impaired neuromuscular transmission14–16. CMAP is one of the most commonly used electrophysiological measures, and CMAP amplitude is known to accurately represent the total number of motor axons that depolarize all the muscle fibers innervated by them. Therefore, a decrease in CMAP amplitudes suggests impaired axonal integrity17. Reduction of CMAP can also reflect loss of myofibers due to muscle degeneration and/or severe atrophy, which can be seen in sarcopenia.
There are different aspects of skeletal muscle function, and it is not known which aspects of skeletal muscle function are most affected by aging. Therefore, we performed various physical performance tests, in an attempt to best capture the decline of skeletal muscle function with aging. We employed the physical tests that have been used to evaluate frailty in aging mice, such as treadmill performance, grip strength, and standing/traveling time. Each physical performance test reflects different aspects of skeletal muscle functions; for example, grip strength likely reflects maximal voluntary contraction (MVC) of skeletal muscle, whereas treadmill performance represents cardiovascular capacity and endurance quality. Once all the physical performance tests were completed, we correlated the results with the above electrophysiological data to examine their relations.
Despite the increasing interests in sarcopenia and age-associated muscle weakness, there are only few studies that evaluated electrophysiological aspects of aging skeletal muscle function. To our knowledge, our study first examined the electrophysiological aspects of the neuromuscular transmission in aging skeletal muscle, and its relation to various skeletal muscle function.
METHODS
Animal Use
All the behavioral and surgical procedures were carried out under the approval of the Johns Hopkins University Animal Care and Use Committee. Male C57BL/6J mice were divided into two age groups: young (3–5 months old; N=6) and old (20–24 months old; N=9). Weight was measured using a Mettler PE 2000 (Columbus, OH) with a sensor ranging 0–210 g and accuracy of 0.15%.
Grip Strength
The Chatillon force measurement device (Ametek, Largo/FL, USA) was used for grip strength measurements. The mice were held by the tail and allowed to grasp the bars on the device. The tails were pulled at a 45-degree angle, and the maximum force generated was recorded in kilogram-force (KgF), which was divided by the body weight (g) of each mouse. Grip strength (KgF/g) of forelimbs and all four limbs were measured separately. Forelimb grip strength is measured when a mouse is holding the grasp bars only using the forelimbs; all four limb strength was measured when the mouse uses all four limbs to hang on to the grasp bars. All the measurements were done three times, and the average scores were recorded.
Traveling and Standing Activity
For this behavioral evaluation, mice were placed in a new cage and acclimated to the new environment for 3 minutes. The number of times each mouse traveled across the midline of the cage during five-minute observation was counted as “traveling activity”. The number of times each mouse balanced itself on two hind paws with or without cage support with forelimbs during five-minute observation was counted as “standing activity”. Both traveling and standing activities were measured three consecutive days, and the average scores from the three different observations were used for analysis.
Treadmill test
An exercise treadmill with an electronic shock grid (Columbus Instruments, Columbus/OH, USA) was used. Mice were placed in the respective lanes with the shock grid turned off and allowed to adjust to the surroundings for 10 minutes. The treadmill was then started, and set at 15m/min speed for a total of 30 minutes. A small electric shock was automatically administered if a mouse got tired and came in contact with the electric grid at the end of the treadmill. The total number of electric shocks were measured and used for final analysis. When mice reached exhaustion as measured by > 60 total shocks or failed to re-engage on the treadmill despite electrical stimuli > 15 seconds, the amount of time was recorded as total treadmill time. The treadmill test was also done over the three consecutive days, and the average numbers were used for analysis.
Compound Motor Action Potential (CMAP)
Compound Motor Action Potential (CMAP) was measured on the right sciatic nerve at the right gastrocnemius muscle. The mice were anesthetized with isoflurane, and a recording needle electrode was placed in the medial head of gastrocnemius muscles, with a reference needle inserted near the Achilles tendon. A stimulating needle was inserted near the right sciatic nerve with a reference needle electrode placed about 1cm proximal to the stimulating needle. Stimulation was delivered incrementally until supra-maximal amplitude was obtained. The needle placements were adjusted to obtain a biphasic waveform, and the maximal amplitude of the negative deflection was measured as peak amplitude. Distal latency was also calculated.
Stimulated single fiber electromyography (SFEMG)
Stimulated single fiber electromyography (SFEMG) was performed using a Cadwell electromyoprahy (EMG) unit (Sierra Wave, Kennewick/Washington, USA). The mice were first anesthetized using isoflurane delivered via a nose cone (2.5L/min for induction and maintenance). The mice were carefully observed for vitality during the anesthesia, and a heat lamp was used to prevent hypothermia. After anesthesia was induced, a 27-gauge, 25-mm single fiber EMG needle electrode with a platinum-iridium recording surface (Technomed Europe, Maastricht-Airport, Netherlands) was inserted into the right gastrocnemius, and a stimulating needle was inserted near the right sciatic nerve with a reference needle electrode placed about 1cm proximal to the stimulating needle. Motor axons were stimulated at a rate of 10 Hz. The filter was set at 500Hz to 10kHz, and the gain ranged from 100 μV to 1mV. Sweep speed ranged from 1 to 2 ms/div, and stimulation intensity was adjusted within a range from 10–30mA to avoid visible muscle contraction. A single fiber action potential (SFAP) was defined by a potential that arose within 2 msec from stimulation potential, and was > 200 μV in amplitude with rise time < 200 μs. The SFAPs were recorded in response to needle stimulation of intramuscular nerves by moving the needle to at least 10 different locations. The average mean consecutive difference (MCD) from the 10 different locations was then calculated.
Statistical Analysis
GraphPad Prism 7 (La Jolla/CA, USA) software was used for statistical analysis and display of the results. Wilcoxon test was used for group comparison between young and old mice (two-tailed). P value less than 0.05 was considered significantly different between two groups. Spearman correlation statistics was used to measure the linear dependence between two variables (two-tailed).
RESULTS
Various physical performance tests show reduction in muscle function with aging
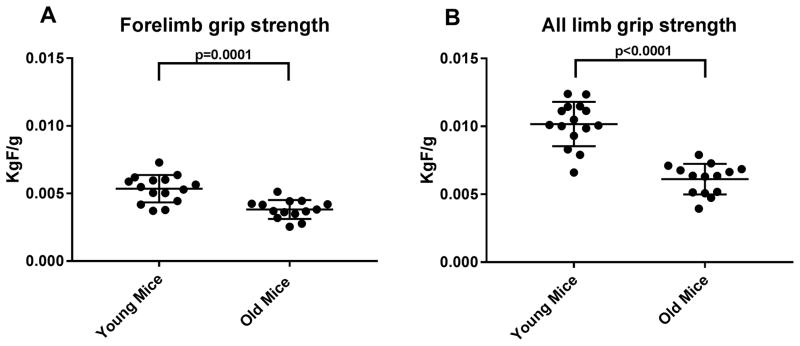
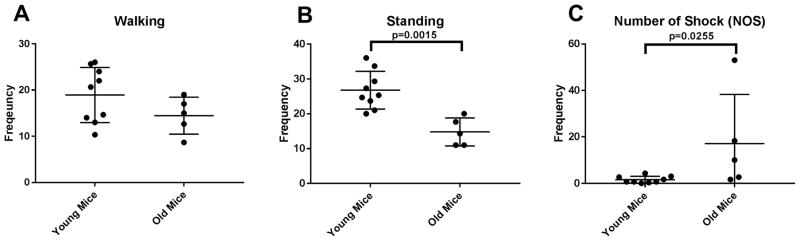
We examined the effects of aging on various behavioral tests. We found significant reduction in grip strength of both forelimb and all limbs [figure 1]. We also measured walking and standing activities of mice in cage, and found that standing activities were significantly reduced in aged mice. While walking activity was reduced in the old mice group, it didn’t reach the statistical significance [figure 2]. Lastly, we measured number of shocks (NOS) on a treadmill; mice were placed on a treadmill machine for 30 minutes, and as described above, the higher number of shocks correlate with poor gait function and reduced cardiovascular capacity. As seen in the figure 2, NOS is significantly higher in the old mice group.
Figure 1. Grip strength of forelimbs (A) and all limbs (B).
Both grip strength measures were significantly reduced in old mice group (N=14), as compared to young mice group (N=15)
Figure 2. Various physical performance tests for frailty.
Walking activity (A) didn’t show significant difference between young and old mice groups (young N=9; old N=5). However, there was significant decline in standing activity and NOS in old mice group (B,C)
Jitter level, but not CMAP, inversely correlates with grip strength
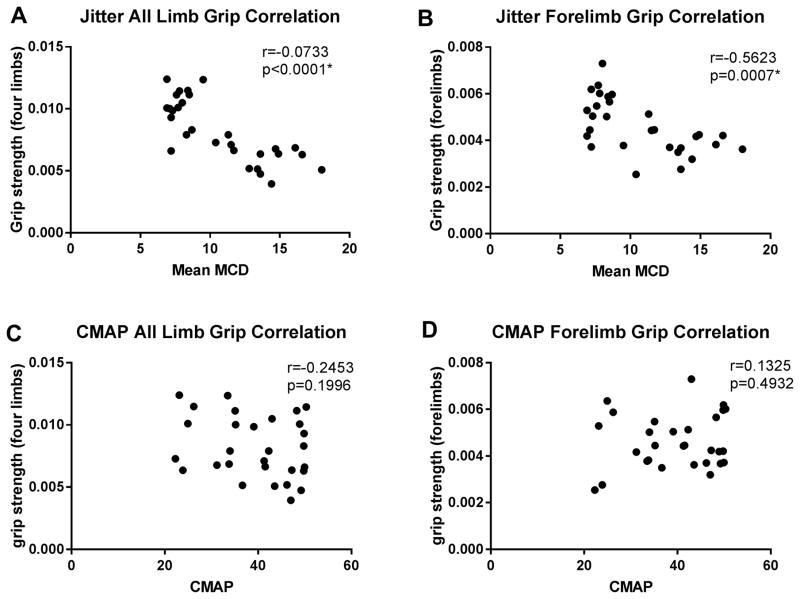
We performed two electrophysiological measures, single fiber EMG jitter and CMAP, and correlated these measures with grip strength of forelimbs and all limbs. Interestingly, as can be seen in figure 3, there was a significant, inverse correlation between single fiber EMG jitter level and the grip strength of both forelimbs and all limbs (forelimb grip: Spearman correlation r=−0.5623, p=0.0015; all limb grip: Pearson correlation, r=−0.7333, p<0.0001). However, CMAP did not correlate with any of grip strength measures (forelimb grip p=0.4932; all limbs p=0.1996, both Spearman correlation).
Figure 3. Correlation between grip strength, jitter, and CMAP.
There was a significant correlation between jitter levels and both grip strength (all limbs A; forelimbs B), while no correlation was found between CMAP and both grip strength (all limbs C; forelimbs D).
We also performed multiple regression analyses to test if the jitter significantly predicted both grip strength. The results of the regression indicated that the model explained 63% of variance for forelimb strength (R2, F (2,12)=10.51, p=0.0023), and 69% of variance for all limb strength (R2, F(2,12)=13.75, p=0.0079) [supplement table]. However, when age was included in the covariate analysis, jitter did not predict grip strength (p=0.89 for forelimb strength, p=0.69 for all limb strength).
Jitter level, but not CMAP, inversely correlates with standing activity
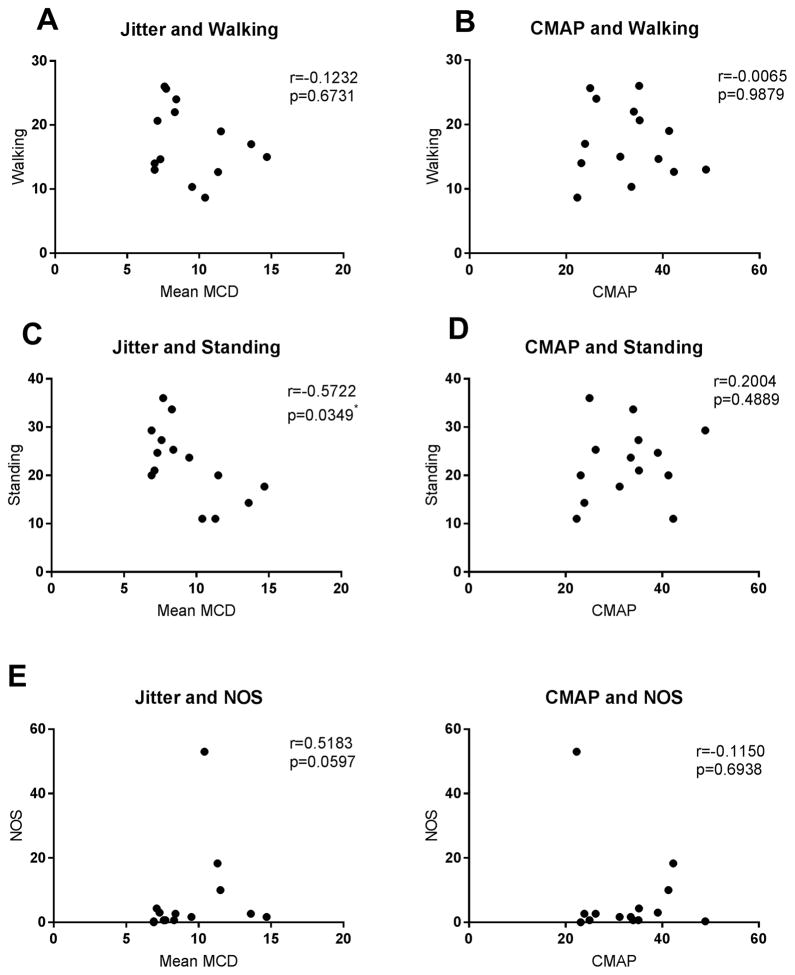
We also investigated correlation between the eletrophysiological measures, single fiber EMG jitter and CMAP, and other physical performance tests that evaluate age-related frailty in mice. Similar to the data on grip strength, there was a significant, inverse correlation between jitter level and standing activity (Spearman correlation, r=−0.5722, p=0.0349), while CMAP did not (p=0.4889). Jitter levels didn’t correlate with walking or NOS; CMAP didn’t correlate with any of the above measures [figure 4].
Figure 4. Correlation between walking activity, standing activity, NOS, jitter, and CMAP.
Except for jitter and standing activity (C), there was no significant correlation between all the physical performance measure and jitter (A,E) or CMAP (B,D,F).
DISCUSSION
Our study showed that single fiber jitter level is increased in aged animals, correlating with their grip strength, while CMAP shows no difference between two age groups. This suggests that reduction in motor strength with aging is associated more with neuromuscular transmission than axonal loss or muscle atrophy. Our results are also supported by the histological evidence of dying-back axonal degeneration. Therefore, combined, NMJ degeneration appears to be an early neurological event of age-associated muscle weakness, leading to axonal loss and myofiber atrophy (sarcopenia) later in the stage.
Although we showed an inverse correlation between jitter and grip strength, our regression analysis showed that jitter does not predict grip strength independently of age. While it was not the primary aim of our study to establish a causal relationship between jitter and grip strength, this could be due to the insufficient number of animals in each age group. In addition, there are multiple factors that determine grip strength, such as nutritional status, comorbidities, and muscle mass. Given that the genetic background of all the mice we used is identical, the effects of NMJ denervation to age-associated weakness have been influenced by those other factors. In the future studies, we will need multiple age points with larger number of animals per age group.
In line with our previous study that proved dying-back axonopathy spinal motor neurons with aging, it will be of interest to perform motor unit assessments. Motor unit number estimation (MUNE) is a validated, well-established electrophysiological technique that has been shown to reflect loss of anterior horn motor neurons in various health conditions, including normal aging18–21. Unfortunately, our electrophysiology set up was not compatible with MUNE evaluation, and we were not able to perform MUNE on our animals. By comparing MUNE, CMAP, and SFEMG, it is possible to evaluate electrophysiological properties of entire peripheral motor neuraxis, from motor neuron, motor axon, to NMJ. Given the results from our study, a future study is needed to longitudinally track MUNE, CMAP, and SFEMG throughout the entire lifespan of mice, and correlate them with grip strength and other physical performance measures, in vivo.
It is also noteworthy that among all the physical performance tests, grip strength has the best correlation with jitter level. It is likely because grip strength best reflects pure motor function, minimally affected by sensory function. While all the other physical performance tests were previously shown to reflect increasing frailty with aging, most of them didn’t correlate with SFEMG jitter measures, except for standing activity. In fact, frailty is a composite scale and involved in decline of multiple systems, such as motor, sensory, cognitive, and cardiovascular systems. It appears that increased SFEMG jitter level is associated with decline of motor function with aging, but not generalized frailty.
Finally, our study results can potentially improve the way we define age-associated muscle weakness and sarcopenia. In rodents, it has been shown that denervation causes myofiber atrophy in aging skeletal muscle, which is consistent with our electrophysiological results22. Currently, the European Working Group on Sarcopenia in Older People (EWGSOP) criteria is most commonly used to define age-related sarcopenia for clinical and research purposes23,24. The criteria are mainly based on the population study, and while it is validated for research studies, it has not been widely accepted for clinical use, mainly due to the lack of pathophysiological understanding of sarcopenia. The electrophysiological measures used in our study – SFEMG and CMAP – are well-validated and commonly used in neuromuscular clinics, and hold a great potential to be used as a clinical tool to investigate sarcopenia. Therefore, more studies are needed to understand the electrophysiological aspects of sarcopenia.
CONCLUSION
Increase in SFEMG jitter level, not reduction in CMAP amplitudes, is associated with reduction in motor function with aging, suggesting that failure in neuromuscular junction plays an important role in early stage of sarcopenia.
Supplementary Material
Acknowledgments
This work was supported by RMSTP K12 grant (5K12HD001097-19), the Dr. Miriam and Sheldon G Adelson Medical Research Foundation, the National Institutes on Aging (NIA) (R21-AG025143) and the Johns Hopkins Older Americans Independence Center (P30-AG021334).
References
- 1.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 6.Sabia S, Dumurgier J, Tavernier B, Head J, Tzourio C, Elbaz A. Change in fast walking speed preceding death: Results from a prospective longitudinal cohort study. J Gerontol A Biol Sci Med Sci. 2014;69(3):354–362. doi: 10.1093/gerona/glt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrucci L, de Cabo R, Knuth ND, Studenski S. Of greek heroes, wiggling worms, mighty mice, and old body builders. J Gerontol A Biol Sci Med Sci. 2012;67(1):13–16. doi: 10.1093/gerona/glr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. current consensus definition: Prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6(12):e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7(1):e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung T, Park JS, Kim S, Montes N, Walston J, Hoke A. Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline. Muscle Nerve. 2016 doi: 10.1002/mus.25267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gooch CL, Mosier DR. Stimulated single fiber electromyography in the mouse: Techniques and normative data. Muscle Nerve. 2001;24(7):941–945. doi: 10.1002/mus.1092. [DOI] [PubMed] [Google Scholar]
- 15.Sanders DB, Stalberg EV. AAEM minimonograph #25: Single-fiber electromyography. Muscle Nerve. 1996;19(9):1069–1083. doi: 10.1002/(SICI)1097-4598(199609)19:9<1069::AID-MUS1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DB. Clinical impact of single-fiber electromyography. Muscle Nerve Suppl. 2002;11:S15–20. doi: 10.1002/mus.10141. [DOI] [PubMed] [Google Scholar]
- 17.Crone C, Krarup C. Neurophysiological approach to disorders of peripheral nerve. Handb Clin Neurol. 2013;115:81–114. doi: 10.1016/B978-0-444-52902-2.00006-0. [DOI] [PubMed] [Google Scholar]
- 18.Gooch CL, Doherty TJ, Chan KM, et al. Motor unit number estimation: A technology and literature review. Muscle Nerve. 2014;50(6):884–893. doi: 10.1002/mus.24442. [DOI] [PubMed] [Google Scholar]
- 19.Bromberg MB, Swoboda KJ, Lawson VH. Counting motor units in chronic motor neuropathies. Exp Neurol. 2003;184(Suppl 1):S53–7. doi: 10.1016/j.expneurol.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31(4):461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 22.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7(1):e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the european working group on sarcopenia in older people (EWGSOP) definition: Findings from the hertfordshire cohort study (HCS) Age Ageing. 2013;42(3):378–384. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.