Abstract
Several chemotherapeutic agents used for cancer treatment induce dose-limiting peripheral neuropathy that compromises patients’ quality of life and limits cancer treatment. Recently, mitochondrial dysfunction has been shown to be involved in the mechanism of chemotherapy-induced peripheral neuropathy. SS-20 is a mitochondria-targeted peptide that promotes mitochondrial respiration and restores mitochondrial bioenergetics. In the present study, we examined the protective effect of SS-20 against the development of chemotherapy-induced peripheral neuropathy utilizing a murine model of peripheral neuropathy induced by oxaliplatin, a first-line chemotherapy agent for colon cancer. Weekly administrations of oxaliplatin induced peripheral neuropathy as demonstrated by the development of neuropathic pain and loss of intraepidermal nerve fibers in the hind paw. Continuous administration of SS-20 protected against the development of oxaliplatin-induced neuropathic pain and mitigated the loss of intraepidermal nerve fibers to normal levels. Our findings suggest that SS-20 may be a drug candidate for the prevention of chemotherapy-induced peripheral neuropathy.
Keywords: Oxaliplatin, chemotherapy, chemotherapy-induced peripheral neuropathy, pain, neuropathic pain, mitochondria
Graphical Abstract
Chemotherapy agents such as paclitaxel, oxaliplatin, and vincristine cause dose-dependent peripheral neuropathy, i.e., chemotherapy-induced peripheral neuropathy (CIPN) that compromises patients’ quality of life during cancer treatment and may lead to changes in treatment to lower doses or non-neurotoxic agents with obvious negative implications for disease outcomes. Pain due to CIPN is often difficult to treat, and duloxetine, a serotonin-norepinephrine reuptake inhibitor, has been shown to have some effect in relieving pain due to CIPN.1 It is the sole treatment that has been recommended by the American Society of Clinical Oncology (ASCO) for the treatment of CIPN with a moderate strength of recommendation.2 On the other hand, the ASCO guideline states that there is no established agent recommended for the prevention of CIPN. Thus, the development of innovative strategies to prevent CIPN is warranted.
In order to study the mechanism and develop treatment of CIPN, we developed a murine model of CIPN using oxaliplatin, a first-line chemotherapy agent against colon cancer.3 This model was carefully designed to closely mimic clinical conditions. The mice gradually developed neuropathic pain which was assessed by the presentation of hypersensitivity to mechanical stimuli in the hind paw, a symptom similar to what is observed in patients. Morphological studies showed that while there were no overt changes in the DRG neurons and peripheral nerves innervating the hind paw, there was loss of intraepidermal nerve fibers (IENFs) of the skin, which has been reported in other animal models of CIPN and in patients with CIPN.4,5
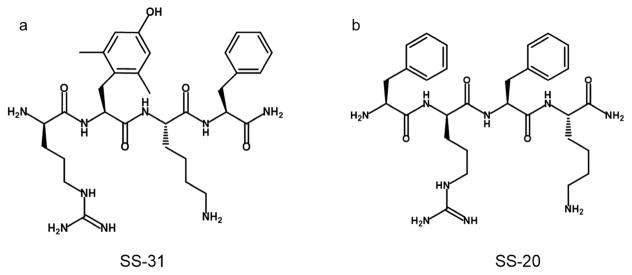
Although the mechanism of CIPN is still unclear, it has been shown that mitochondrial dysfunction caused by mitotoxic effects of cancer chemotherapeutic agents is an important component of the dysregulation in primary afferent sensory neurons.3,4,6,7 Studies with animal models suggest that several drugs that prevent mitochondrial injury or improve mitochondrial function may be effective in the treatment of CIPN.3,4,6 In our animal model of oxaliplatin-induced neuropathic pain,3 a tetrapeptide, SS-31 (Figure 1a), which targets and concentrates on the inner mitochondrial membrane, the site of the electron transport chain and reactive oxygen species (ROS) production,8 was shown to protect against the development of neuropathic pain. In addition, SS-31 prevented the loss of IENFs in our study. IENFs are likely to have high energy consumption due to frequent turnover of the skin and to be vulnerable to energy deficiency. SS-31 is a ROS scavenger and has also been shown to improve mitochondrial bioenergetics. Thus, it is unclear whether the protective effect of SS-31 against the development of CIPN was due to the ability to promote mitochondrial respiration or the ability to scavenge ROS. Another tetrapeptide, SS-20 (Figure 1b), is an analogue of SS-31, which has the same property of promoting mitochondrial bioenergetics but does not have ROS scavenging capacity.9 In the present study we examined the protective effect of SS-20 against the development of CIPN using our murine model in order to determine whether oxaliplatin-induced peripheral neuropathy involves mitochondrial bioenergetics deficit and to examine whether SS-20 may be a drug candidate for the prevention of CIPN.
Figure 1.
Chemical structures of SS-31 and SS-20.
RESULTS AND DISCUSSION
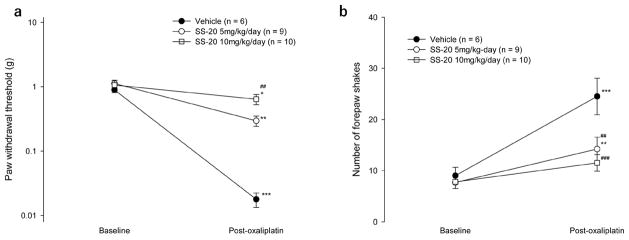
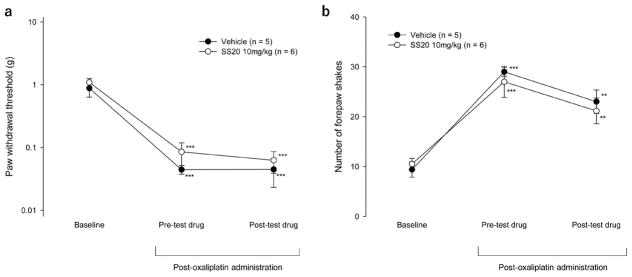
CIPN was induced in mice by three weekly administrations of oxaliplatin at 10 mg/kg and was assessed behaviorally by the development of neuropathic pain and morphologically by the loss of IENFs. The protective effect of SS-20 was examined by continuously administering the compound subcutaneously throughout the course of CIPN development and comparing the degree of CIPN developed with that in mice that received continuous administration of vehicle. Neuropathic pain was manifested by hypersensitivity of the paws to non-noxious mechanical and cold stimuli. The mechanical hypersensitivity was assessed by the decrease in mechanical threshold to withdraw the hind paw when touched by von Frey filaments and the cold hypersensitivity was assessed by the increased pain-related behavior (shaking of the forepaw) in response to 15 °C stimulation, a temperature that does not normally cause pain. Mice were administered SS-20 (5 mg/kg/day or 10 mg/ kg/day) or saline via subcutaneously implanted Alzet pumps. Three weekly administrations of oxaliplatin decreased paw withdrawal mechanical thresholds compared to baseline in all mice, demonstrating the development of mechanical hypersensitivity (0.02 ± 0.01 vs 0.90 ± 0.21 g, p = 0.001 for vehicle group; 0.30 ± 0.16 vs 1.12 ± 0.42 g, p < 0.001 for mice given SS-20 at 5 mg/kg/day; 0.64 ± 0.40 vs 1.06 ± 0.61 g, p = 0.032 for mice given SS-20 at 10 mg/kg/day) (Figure 2a). However, the paw withdrawal mechanical thresholds after oxaliplatin administrations were significantly higher in mice that were administered SS-20 at 10 mg/kg/day compared to the vehicle group (0.64 ± 0.40 vs 0.02 ± 0.01 g, p = 0.009) (Figure 2a). In the mice that were administered vehicle continuously, three weekly administrations of oxaliplatin significantly increased the number of forepaw shakes induced by 15 °C stimulation as compared to baseline, demonstrating the development of cold hypersensitivity (post-oxaliplatin vs baseline: 25 ± 9 vs 9 ± 4/ 2.5 min, p < 0.001) (Figure 2b). The continuous administration of SS-20 inhibited the increase in forepaw shakes (SS-20 at 5 mg/kg/day vs vehicle, 14 ± 7 vs 25 ± 9/2.5 min, p = 0.002; SS-20 at 10 mg/kg/day vs vehicle, 12 ± 5 vs 25 ± 9/2.5 min, p < 0.001) (Figure 2b). In mice that were continuously administered SS-20 at 10 mg/kg/day, the number of forepaw shakes after 3 weekly administrations of oxaliplatin was not different from baseline values (post-oxaliplatin vs baseline: 12 ± 5 vs 8 ± 1/2.5 min, p = 0.058) (Figure 2b). These results indicate that continuous SS-20 treatment dose-dependently attenuates the development of mechanical and cold hypersensitivity induced by repeated oxaliplatin administrations.
Figure 2.
Effects of continuous administration of SS-20 on mechanical hypersensitivity (a) and cold hypersensitivity (b) induced by repeated administrations of oxaliplatin. Mechanical hypersensitivity was assessed by determining paw withdrawal mechanical thresholds using the von Frey hair test. Cold hypersensitivity was assessed by counting the number of forepaw-shaking behavior of mice placed on a cold plate set at 15 °C during a 2.5 min test period. Baseline measurements were made prior to the implantation of Alzet pumps filled with SS-20 at 5 mg/kg/day (n = 9), SS-20 at 10 mg/kg/day (n = 10) or vehicle (normal saline, n = 6). The Alzet pumps were implanted 2 days before the first oxaliplatin injection. Oxaliplatin (10 mg/kg) was administered intraperitoneally in mice, once per week for 3 weeks. Post-oxaliplatin measurements were made 5 days after the last administration of oxaliplatin. ##p < 0.01, ###p < 0.001 compared to the corresponding time point of the vehicle control group; *p < 0.05, **p < 0.01, ***p < 0.001 compared to baseline of the same group. Data were analyzed by two-way repeated ANOVA followed by the Bonferroni method.
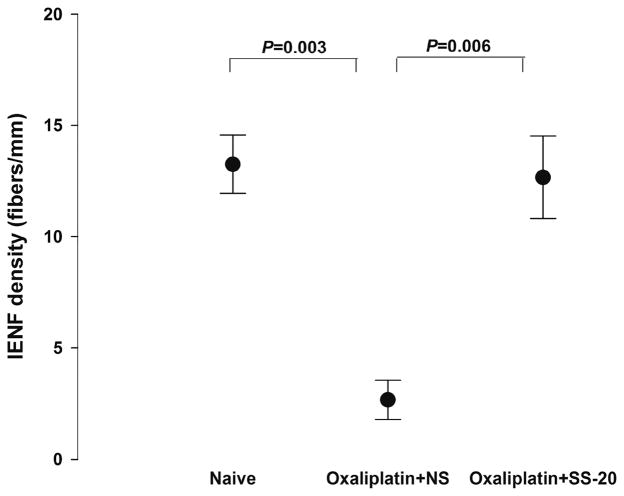
The effect of continuous SS-20 treatment on the changes in IENF density induced by three weekly administrations of oxaliplatin were also examined (Figures 3 and 4). In mice that were administered vehicle, the density of IENF was markedly decreased compared to naïve mice 7 days after the last injection of oxaliplatin (2.7 ± 1.5 vs 13.3 ± 2.6 fibers/mm, p = 0.003). The decrease in IENF density was mitigated in mice that were continuously administered SS-20 at 10 mg/kg/day and IENF density in these mice was not different from that in naïve mice (SS-20 treatment vs vehicle treatment: 12.7 ± 3.2 vs 2.7 ± 1.5 fibers/mm, p = 0.006; mice with SS-20 treatment vs naïve mice: 12.7 ± 3.2 vs 13.3 ± 2.6 fibers/mm, p = 1.000).
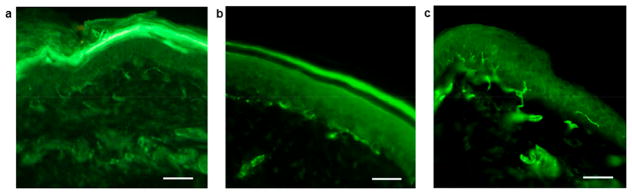
Figure 3.
Changes in intraepidermal nerve fiber density by repeated administrations of oxaliplatin with or without concomitant SS-20 administration. Fluorescence microscopic photograph of IENFs in the planter skin harvested from a naïve mouse (a), a mouse that received three weekly administrations of oxaliplatin (10 mg/kg) with continuous administration of vehicle (normal saline) (b), and a mouse that received three weekly administrations of oxaliplatin (10 mg/kg) with continuous administration of SS-20 (10 mg/kg/day) (c). Scale bars are 50 μm.
Figure 4.
Intraepidermal nerve fiber (IENF) densities of plantar skin (hind paw) in naïve mice (n = 4), mice given repeated administrations of oxaliplatin with continuous administration of vehicle (normal saline: NS) (oxaliplatin + NS, n = 3), and mice given repeated administrations of oxaliplatin with continuous administration of SS-20 at 10 mg/kg/day (oxaliplatin + SS-20, n = 3). Oxaliplatin at 10 mg/ kg was administered once a week for 3 weeks. IENFs counts were determined per 1 mm of epidermal border. The skin specimens were harvested 7 days after the last administration of oxaliplatin. Data were analyzed by one-way ANOVA followed by the Bonferroni method.
In addition, the effect of acute administration of SS-20 on established oxaliplatin-induced neuropathic pain was examined. The acute administration of SS-20 at 10 mg/kg did not affect mechanical (Figure 5a) and cold hypersensitivity (Figure 5b) that had been induced by 3 weekly administrations of oxaliplatin (SS-20 treatment vs vehicle treatment: 0.06 ± 0.06 vs 0.04 ± 0.05 g, p = 0.917 for paw withdrawal mechanical threshold; SS-20 treatment vs vehicle treatment, 21 ± 6 vs 23 ± 5/2.5 min, p = 0.559 for paw shakes induced by 15 °C stimulation).
Figure 5.
Effects of acute administration of SS-20 on mechanical hypersensitivity (a) and cold hypersensitivity (b) induced by repeated administrations of oxaliplatin. Mechanical hypersensitivity was assessed by determining paw withdrawal mechanical thresholds using the von Frey hair test. Cold hypersensitivity was assessed by counting the number of forepaw-shaking behavior of mice placed on a cold plate set at 15 °C during a 2.5 min test period (cold plate test). Baseline measurements were made prior to the first administration of oxaliplatin. Oxaliplatin (10 mg/kg) was administered intraperitoneally in mice, once per week for 3 weeks. Drug tests were performed on the 5th day after the last administration of oxaliplatin. The von Frey tests and the cold plate tests were performed before and 1 h after acute subcutaneous administration of SS-20 (10 mg/kg, n = 6) or vehicle (n = 5). **p < 0.01, ***p < 0.001 compared to baseline of the same group. Data were analyzed by two-way repeated ANOVA followed by the Bonferroni method.
These results demonstrated that continuous administration of SS-20 prevented the development of neuropathic pain and the loss of IENFs induced by repeated administration of oxaliplatin, but SS-20 did not affect neuropathic pain symptoms after they were established. Thus, it is indicated that SS-20 is capable of preventing oxaliplatin-induced peripheral neuropathy.
Recently, mitochondrial dysfunction and oxidative stress have been shown to be involved in neuropathic pain10,11 and in chemotherapy-induced neuropathies.3,4,6,7 Chemotherapeutic agents, such as oxaliplatin and paclitaxel, cause functional impairment of peripheral nerve mitochondria, with significant decrease in state 3 respiration and adenosine triphosphate (ATP) production,4,7 and the mitochondrial dysfunction leads to electron leak and increased production of ROS.4,6,7 The resultant bioenergetic deficiency likely causes abnormal spontaneous discharge in the axons of somatosensory primary neurons due to ion-pumping deficiency.12 The specific mechanism by which oxaliplatin and paclitaxel inhibit mitochondrial bioenergetics and promote ROS production is not known, but cisplatin, another agent that causes CIPN, is known to interact with cardiolipin present in the inner mitochondrial membrane, resulting in cardiolipin peroxidation, loss of cardiolipin, respiratory inhibition, glutathione depletion, and apoptosis.7 Cardiolipin plays important roles in cristae formation as well as in the organization and function of respiratory supercomplexes.
SS (Szeto–Schiller) peptides are cell-permeable tetrapeptides that target mitochondrial cardiolipin. SS-20 has been shown to bind to and interact with cardiolipin and modulate cytochrome c/cardiolipin complex activity resulting in promotion of oxidative phosphorylation, reduced ROS production, and prevention of the conversion of cytochrome c from an electron carrier to a peroxidase, thereby preventing cardiolipin peroxidation.13 By protecting cardiolipin, SS-20 protects cristae membranes and improves mitochondrial respiration and oxidative phosphorylation coupling during ischemia.14 Unlike SS-31, SS-20 cannot scavenge electrons, but it is still a very effective mitochondria-targeted antioxidant because it can reduce ROS production and prevent cytochrome c peroxidase activity. SS-20 is as effective as SS-31 in protecting striatal dopamine neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydrophridine in mice.15 In particular, both SS-20 and SS-31 prevented mitochondrial swelling and inhibition of mitochondrial respiration and ATP production induced by 1-methyl-4-phenylpyridium ion in isolated mitochondria and neuronal cell cultures.
The central nervous system has also been shown to be involved in oxaliplatin-induced neuropathy. Glial activation in the spinal cord and different areas of the brain was observed in rats given repeated administration of oxaliplatin.16 Furthermore, N-palmitoylethanolamine, an endogenous amide, was shown to protect against glial activation in the central nervous system as well as against the development of neuropathic pain in rats that were administered oxaliplatin repeatedly.17 In an in vitro study, oxaliplatin was suggested to cause damage to mitochondria of astrocytes,18 which may be associated with astrocyte activation induced by oxaliplatin in vivo. SS-20 crosses the blood brain barrier, and has been shown to have protective effects in a murine model of Parkinson’s disease which involve mitochondrial dysfuction.15 Thus, the effects of SS-20 on neuropathic pain symptoms observed in the present study may also involve its protective effects on astrocyte mitochondria in the central nervous system.
The present findings with SS-20 are similar to our earlier report that prophylactic administration of SS-31 can attenuate the pain due to oxaliplatin-induced neuropathy and mitigate the loss of IENFs.3 It should be noted that information on the effect of SS-20 on tumor growth is lacking and would be important for SS-20 to have therapeutic use. SS-31, on the other hand, had no effect on tumor growth in mice with and without oxaliplatin treatment (personal communication, Fabio Penna, University of Turin, Italy). Future studies are needed to address this point. Nonetheless, our results suggest that mitochondrial bioenergetics deficit is involved in the mechanism of CIPN and that SS-20 may be a promising drug candidate for the prevention of CIPN.
CONCLUSIONS
In a murine model of oxaliplatin-induced peripheral neuropathy, continuous administration of SS-20 protected against the development of oxaliplatin-induced neuropathic pain and mitigated the loss of IENFs. Our findings suggest that promotion of mitochondrial respiration is a high-potential strategy to prevent CIPN, and SS-20 may be a promising drug candidate for its prevention.
METHODS
Animals and Drugs
Male BALB/c mice, 8 weeks old at the time of first oxaliplatin administration, were used in the experiments. All mice were housed on a 12:12 h dark–light cycle with food and water ad libitum. Experiments were approved by the Institutional Animal Use Committee of Jikei University (Tokyo, Japan), and conducted in accordance with the National Institutes of Health guidelines and the International Association for the Study of Pain Committee for Research and Ethical Issues guidelines for animal research.19 Oxaliplatin was obtained from Yakult Co., Ltd. (Tokyo, Japan) and was dissolved in distilled water for intraperitoneal (i.p.) administration in a volume of 0.1 mL/10 g mouse weight. SS-20 (H-Phe-D-Arg-Phe-Lys-NH2) was synthesized as previously described9 and was dissolved in normal saline for continuous or bolus subcutaneous administration.
Oxaliplatin-Induced Peripheral Neuropathy Model
Oxaliplatin (10 mg/kg) was injected intraperitoneally in a volume of 0.1 mL/ 10 g mouse weight, once per week for 3 weeks. The dose of oxaliplatin administration was chosen according to our previous report.3 Behavioral testing (see below) was performed prior to the first oxaliplatin administration (baseline data) and 5 days after the third oxaliplatin administration.
Behavioral Testing
The presentation of neuropathic pain was determined by the development of hypersensitivity to mechanical and cold non-noxious stimuli. The mechanical hypersensitivity was assessed by the decrease in paw withdrawal mechanical thresholds using the von Frey test. Mice were placed in a clear plastic chamber with a wire mesh floor, which provided full access to the planter surface of the hind paws. Mice were allowed to habituate for at least 15 min before testing. The paws were touched with one of a series of 10 von Frey filaments (Semmes-Weinstein Monofilaments; Stoelting Co., Wood Dale, IL) with logarithmically incremental stiffness (0.005–3.630 g) starting with the filament of 0.407 g. The 50% mechanical withdrawal thresholds were determined using the up–down method.20 Cold hypersensitivity to non-noxious cold stimulus was assessed by counting the number of forepaw-shaking behavior of mice placed on a cold plate (LHP-1700CP; TECA, Chicago, IL) set at 15 °C (15 °C cold plate test) during a 2.5 min test period. The cold plate test was performed following the von Frey test. Behavioral testing was performed in a blinded manner with respect to all drug administration.
Effect of Continuous SS-20 Treatment during Neuropathy Development
In order to examine the effects of continuous administration of SS-20 during repeated oxaliplatin administrations, SS-20 at 5 mg/kg/day, 10 mg/kg/day or vehicle (normal saline) was continuously administered via subcutaneously implanted Alzet Micro-Osmotic Pumps (model 1004, Alzet, Cupertino, CA; pumping rate of 0.11 μL/h for 28 days). Two pumps were implanted to bilateral sides of the back in each mouse under sevoflurane anesthesia 2 days before the first oxaliplatin injection to allow the infusion rate of the pumps to stabilize. SS-20 or vehicle was administered continuously via the pumps until the end of the experiment. Behavioral testing was performed prior to the implantation of the pumps (baseline data) and 5 days after the last injection of oxaliplatin.
Quantification of IENFs
After the last behavioral testing of mice that were administered SS-20 at 10 mg/kg/day or vehicle, planter skin specimens were excised from the hind paw between the calcaneous and the digital tori under sevoflurane anesthesia. The specimens were immediately soaked in 4% paraformaldehyde for overnight fixation and then transferred to 30% sucrose and left overnight at 4 °C. The specimens were then embedded in TissueTek OCT compound (Sakura Finetek Europe B.V., Zouterwoude), frozen and sliced on a cryostat into 25 μm sections. The sections were collected in 0.01 M phosphate buffer saline (PBS) and processed by a free-floating protocol. The sections were incubated in a blocking solution that consisted of 0.5% normal goat serum and 0.3% Triton-X100 in PBS for 30 min at room temperature. They were then incubated overnight at 4 °C in primary antibodies in the blocking solution. Antibodies against protein gene-product 9.5 (rabbit, affinity purified, Enzo Life Sciences, Farmingdale, NY, USA) were used. Following this step, the sections were incubated in biotinylated goat anti-rabbit IgG (Vector Laboratories, Belmont, CA, USA) solution for 90 min at room temperature, then incubated with Alexa Fluor 488 streptavidin conjugate (Molecular Probes, Eugene, OR, USA) for 60 min at room temperature. Between each step, the sections were rinsed with PBS three times. The sections were observed under a fluorescence microscope. The number of IENFs per mm of the epidermal border was counted in 3 random sections and averaged for each mouse. Quantification of IENFs was performed blindly with respect to drug administration.
Effect of Acute SS-20 Treatment on Established Neuropathic Pain
In order to examine the effects of acute administration of SS-20 on established oxaliplatin-induced neuropathic pain symptoms, other mice that had received 3 weekly administrations of oxaliplatin were tested 5 days after the last oxaliplatin administration. A single dose of SS-20 (10 mg/kg) or saline was given subcutaneously to each mouse in a volume of 0.1 mL/10 g mouse weight. Behavioral testing was performed prior to the first oxaliplatin administration (baseline data), and before and 1 h after the administration of SS-20 or saline on the day of the drug test.
Statistical Analysis
Statistical analyses were carried out with SigmaPlot statistical software package for Windows (version 13.0; Systat, San Jose, CA). Data were analyzed using one-way analysis of variance (ANOVA) with Bonferroni correction or two-way repeated ANOVA with Bonferroni correction. For all tests, p < 0.05 was considered statistically significant.
Acknowledgments
Funding
The work was supported by a grant-in-aid for Scientific Research to M.S. (Grant No. 24592358) from the Japan Society for the Promotion of Science (Tokyo, Japan), and grants to P.W.S. from the U.S. National Institutes of Health (DA004443) and the Canadian Institutes of Health Research (MOP-89716).
ABBREVIATIONS
- CIPN
chemotherapy-induced peripheral neuropathy
- ROS
reactive oxygen species
- IENF
intraepidermal nerve fiber
- ATP
adenosine triphosphate
Footnotes
Notes
The authors declare the following competing financial interest(s): H.H.S. is the inventor of SS-20. SS-20 has been licensed by the Cornell Research Foundation to Stealth Biotherapeutics for commercial research and development. H.H.S. is the Scientific Founder and has equity ownership in Stealth Biotherapeutics.
Author Contributions
S.T. and N.S. were involved in experiments and data analysis of the study. H.H.S. was involved in planning of the study. P.W.S. was involved in the synthesis of SS-20 and planning of the study. M.S. conceived the study and was involved in experiments and data analysis.
References
- 1.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL. American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practical guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 3.Toyama S, Shimoyama N, Ishida Y, Koyasu T, Szeto HH, Shimoyama M. Characterization of acute and chronic neuropathies induced oxaliplatin in mice and differential effect of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology. 2014;120:459–473. doi: 10.1097/01.anes.0000435634.34709.65. [DOI] [PubMed] [Google Scholar]
- 4.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–626. doi: 10.1007/s00280-012-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153:704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins NM, Santos NA, Curti C, Bianchi ML, Santos AC. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008;28:337–344. doi: 10.1002/jat.1284. [DOI] [PubMed] [Google Scholar]
- 8.Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane—from discovery to clinical development. Pharm Res. 2011;28:2669–2679. doi: 10.1007/s11095-011-0476-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhao K, Zhao G, Wu D, Soong Y, Birk A, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell deaths, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci. 2011;31:11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph EK, Levine JD. Multiple PKCε-dependent mechanisms mediating mechanical hyperalgesia. Pain. 2010;150:17–21. doi: 10.1016/j.pain.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10:326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birk AV, Chao WM, Liu S, Soong Y, Szeto HH. Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim Biophys Acta, Bioenerg. 2015;1847:1075–1084. doi: 10.1016/j.bbabio.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szeto HH, Liu S, Soong Y, Birk AV. Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol. 2015;308:F11–F21. doi: 10.1152/ajprenal.00366.2014. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity. Antioxid Redox Signaling. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013;14:1585–1600. doi: 10.1016/j.jpain.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Di Cesare Mannelli L, Pacini A, Corti F, Boccella S, Luongo L, Esposito E, Cuzzocrea S, Maione S, Calignano A, Ghelardini C. Antineuropathic profile of N-palmitoylethanol-amine in a rat model of oxaliplatin-induced neurotoxicity. PLoS One. 2015;10(6):e0128080. doi: 10.1371/journal.pone.0128080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Cesare Mannelli L, Zanardelli M, Landini I, Pacini A, Ghelardini C, Mini E, Bencini A, Valtancoli B, Failli P. Effect of the SOD mimetic MnL4 on in vitro and in vivo oxaliplatin toxicity: Possible aid in chemotherapy induced neuropathy. Free Radical Biol Med. 2016;93:67–76. doi: 10.1016/j.freeradbiomed.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]