Abstract
The molecular substrates underlying cocaine reinforcement and addiction have been studied for decades, with a primary focus on signaling molecules involved in modulation of neuronal communication. Brain-derived neurotrophic factor (BDNF) is an important signaling molecule involved in neuronal dendrite and spine modulation. Methyl CpG binding protein 2 (MeCP2) binds to the promoter region of BDNF to negatively regulate its expression and cocaine can recruit MeCP2 to alter the expression of genes such as BDNF that are involved in synaptic plasticity. For several decades, BDNF has been implicated in mediating synaptic plasticity associated with cocaine abuse, and most studies report that neurons are the primary source for BDNF production in the brain. The current study assessed the effects of intravenous cocaine self-administration on microglial activation, and MeCP2 and BDNF expression in reward regions of the brain in vivo, as well as determined specific effects of cocaine exposure on MeCP2 and BDNF expression in human primary neurons and microglia. The results from this study highlight a distinct molecular pathway in microglia through which cocaine increases BDNF, including the phosphorylation of MeCP2 its subsequent translocation from the nucleus to the cytosol, which frees the BDNF promoter and permits its transcriptional activation. Results from these studies show for the first time that cocaine self-administration increases microglial activation, and that microglial MeCP2 is a sensitive target of cocaine resulting in increased release of BDNF from microglia, and possibly contributing to cocaine-induced synaptic plasticity.
Keywords: cocaine, microglia, neuron
1. Introduction
The molecular substrates underlying cocaine reinforcement and addiction have been studied for decades, with a primary focus on signaling molecules involved in modulation of neuronal communication. By contrast, fewer studies have investigated how alteration of resident CNS immune cell function may play a role in altered neurotransmission and neuroplasticity in the instance of cocaine taking behavior. One signaling pathway that has recently received attention is the regulation of brain derived neurotrophic factor (BDNF) by the transcription factor methyl CpG binding protein 2 (MeCP2). For example, alterations in MeCP2 expression have profound effects on cell morphology, neurotransmission, and cellular processes that support learning and memory. MeCP2 binds to methylated cytosine residues of CpG sites in DNA to silence gene transcription (Chen et al., 2003; Nan et al., 1998; Zhou et al., 2006). Several studies have now shown that psychostimulants can recruit MeCP2 to alter the expression of genes involved in synaptic plasticity (Bodetto et al., 2014; Cassel et al., 2006; Sadri-Vakili et al., 2010). Specifically, MeCP2 binds to the promoter region of BDNF to negatively regulate its expression. Following activity-dependent calcium influx into cells, MeCP2 becomes phosphorylated and dissociates from the BDNF promoter (Chen et al., 2003), thereby permitting other transcription factors to bind to the BDNF promoter and facilitate its transcriptional activation. MeCP2 loss of function leads to numerous neuronal morphological changes and alterations in synaptic plasticity in mice, and MeCP2 is the gene that causes the neurodevelopmental disorder Rett Syndrome in humans.
Im et al demonstrated that MeCP2 expression in the dorsal striatum of rodents increases with escalating cocaine self-administration and serves to regulate the effects of cocaine on striatal BDNF, an important signaling molecule involved in dendritic and spine modulation (Im et al., 2010). In the Im study, the increased protein expression of MeCP2 was localized almost exclusively with neurons as compared to astrocytes. Importantly, these effects were only seen in rats given access to cocaine for 6hr daily sessions (so called “extended” access, or escalation protocol) and not in rats given access to cocaine for 2hr daily sessions (so called “restricted” or “short” access conditions). This self-administration paradigm was developed by Ahmed and Koob, published in Science in 1998, wherein they demonstrated that rats given the opportunity to self-administer cocaine during daily 6 hr sessions would escalate intake and craving over time compared with the more stable self-administration profile seen by rats given daily 2 hr access to cocaine (Ahmed and Koob, 1998). In subsequent studies, this group and several others also described molecular changes which occurred only under the condition of 6 hr access and not the 2 hr access, suggesting that these changes were specifically associated with escalated drug taking and craving, and not just with cocaine exposure per se.
BDNF is a key mediator of neuronal survival, differentiation, and plasticity (Parkhurst et al., 2013). BDNF is synthesized as a proneurotrophin in the endoplasmic reticulum and is cleaved by proteases into its mature protein form. The cleavage of the precursor domain of BDNF aids in the proper folding and secretion of mature BDNF. BDNF and proBDNF are both biologically active and have been described to have opposing effects. Mature BDNF preferentially binds to and activates tropomycin kinase B (TrkB) receptors (Deinhardt and Chao, 2014). Activation of neuronal TrkB triggers a signaling cascade that leads to the expression of synaptic plasticity-related genes (Li and Wolf, 2015). Conversely, there is evidence that proBDNF induces axonal degeneration and its involved in synaptic pruning through binding to the p75 receptor (Hempstead, 2015). For several decades, BDNF has been implicated in mediating synaptic plasticity associated with cocaine abuse (Ghitza et al., 2010; McGinty et al., 2010; Russo et al., 2009). Although neurons appear to be the primary source of BDNF in the adult brain, BDNF is also detected in microglia (Dougherty et al., 2000; Rauskolb et al., 2010). Microglial BDNF plays an important role in the brain by regulating learning-induced synapse formation (Parkhurst et al., 2013). BDNF can be released from microglia and can act on neighboring neurons via TrkB receptors.
Other emerging evidence suggests that cocaine exposure stimulates inflammatory responses by activating innate and adaptive immune responses, including microglial activation in vitro (Guo et al., 2015), in rodent models (Periyasamy et al., 2017) and in humans (Little et al., 2009). For example, non-contingent injection of cocaine to rodents can increase microglial activation in the hippocampus and striatum (Blanco-Calvo et al., 2014). An increasing literature shows that microglia are critical in regulating neuronal circuitry and shaping their connectivity in activity- and experience-dependent ways (Li et al., 2012; Pascual et al., 2012; Sierra et al., 2010; Tremblay et al., 2010), making these cells attractive candidate players in the development of neuroplastic changes associated with cocaine addiction. Like macrophages, microglia have multiple phenotypes related to various functional states, including activated proinflammatory states and activated repair-promoting states. It remains to be determined how cocaine may alter microglial phenotype to change their functional role in neuronal communication. Lastly, microglial-specific MeCP2 regulates gene expression in response to inflammatory stimuli (Cronk et al., 2015), and can play a critical role in the pathogenesis of Rett Syndrome.
Thus, the current set of experiments sought to determine the effects of intravenous cocaine self-administration on microglial activation, and MeCP2 and BDNF expression in reward regions of the brain in vivo, as well as to assess the specific effects of cocaine exposure on MeCP2 and BDNF expression in human primary neurons and microglia.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Taconic) (approximately 10 weeks at start of study) were singly housed on a reverse 12-hour light-dark cycle (lights off 9 AM). Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of Temple University approved experimental protocols.
2.2 Jugular catheterization surgery and self-administration study
Rats habituated to the vivarium for 7 days, followed by surgical implantation of indwelling silicone catheters into the right jugular vein. For catheter implantation, rats were anesthetized with isoflurane (5% induction, 2–3% maintenance). Silicone catheters (13.5 cm long) (CamCath, Cambridgeshire, UK) were implanted into the right external jugular vein, as previously described (Ward et al., 2003). Following surgery, rats were housed individually and allotted 2–3 days of recovery prior to testing. Baytril (10 mg/kg, SC) was administered for 5 days beginning the day before surgery as a prophylactic antibiotic, and meloxicam (1 mg/kg, SC) was administered preoperatively and for 2 days following surgery for analgesia. Catheters were flushed pre- and post-daily cocaine self-administration sessions with 0.2 mL heparinized saline (100 USP/mL) and locked after sessions with gentamicin-saline solution (4 mg/mL).
Operant behavior was measured in ventilated chambers contained within sound-attenuating cubicles (Med Associates, St. Albans, VT). Each chamber was equipped with two retractable levers with a circular light above each and a house light on the opposite side of the chamber. Intravenous infusions of cocaine (Sigma Aldrich) were delivered from a 10 mL syringe placed into a computer-controlled pump and connected to a swivel and tether system fitted to the rat’s catheter port. Each session began with illumination of the house light, illumination of the stimulus light above the active lever, and a priming infusion of cocaine given via the infusion pump by the experimenter. Responses on the active lever produced a cocaine infusion (2.5–3.5 seconds depending on body weight), which coincided with extinguishing of the stimulus light above the active lever for 20 seconds to signal a timeout. Responses on the inactive lever were recorded but had no programmed consequences. Upon termination of each session, both levers were retracted and all lights were extinguished.
Nine rats were trained to self-administer 0.375 mg/kg/infusion for 7 days under a fixed-ratio 1 (FR1) schedule of reinforcement during daily 2h sessions. Extended access to cocaine self-administration was then given to a subset of the rats for an additional 12 days during daily 6h sessions (n=6), while the other rats continued to self-administer for 12 more days during daily 2h sessions (n=3). Cocaine was dissolved in sterile saline and delivered via intravenous infusion in a volume of 0.25 mL/400 g body weight over 2.5–3.5 seconds. One hour following the last self-administration session, rats were heavily anesthetized with isoflurane and decapitated immediately. An additional 3 rats received jugular catheter surgery and remained in their home cages for the length of the study to serve as sham controls.
2.3 Tissue Harvest
Brains were quickly removed and placed into ice-cold 1X PBS. Brains were then separated into right and left hemispheres. Right hemispheres were dissected into hippocampus, striatum and frontal cortex and stored at −80°C. Left hemispheres were fixed in 10% buffered formalin for 24h and processed for immunolabeling by standard paraffin embedding and sectioning.
2.4 Protein Extraction from Tissue
Brain tissue was homogenized by mechanical dounce disruption on ice in TNN buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% NP40, 1: 200 protease inhibitor cocktail; Calbiochem, San Diego, CA, USA) as previously described (Jatiani et al., 2010; Kovalevich et al., 2012; Kovalevich, 2012; Ozdemir et al., 2013). Tissue homogenates were centrifuged at 13,000 × g at 4°C for 5 min. The supernatant containing proteins was collected and protein concentrations were determined by the Bradford assay.
2.5 Immunofluorescence Labeling of Rat Brain Tissue
Formalin fixed paraffin embedded tissues were cut into 5μm sagittal sections using a Leica vibratome and placed onto electromagnetically charged glass slides. Tissue sections were incubated at 65°C for 30 min, deparaffinized in xylene and rehydrated through descending grades of ethanol down to water. Following non-enzymatic antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 30 min at 95°C, slides were washed with 1X PBS and placed in blocking solution for 2h at room temperature. Sections were incubated with anti-Iba1 antibody (1:500, Wako) overnight in a dark, humidified chamber at room temperature, rinsed 3 times with 1X PBS, and incubated with Alexa fluor 488/Alexa fluor 588 (1:500, Abcam) conjugated secondary antibodies for 1h at room temperature in the dark. Sections were washed 3 times with 1X PBS, cover slipped with an aqueous based hard set mounting medium containing DAPI for nuclei labeling (Vectashield, Vector Laboratories), and visualized with an Olympus BX51 microscope, Olympus DP70 camera, and Olympus MicroSuite 5 software.
2.6 Primary Cell Culture
Human primary neurons and microglia were provided by the Temple University Comprehensive NeuroAIDS Center (CNAC) and processed as previously reported (Kovalevich, 2012; Kovalevich, 2015). Fetal brain tissue (gestational age 16–18 weeks) was obtained from the CNAC at Temple University from elective abortion procedures performed in full compliance with National Institutes of Health and Temple University ethical guidelines. Briefly, the tissue was washed with cold Hanks balanced salt solution (HBSS) and meninges and blood vessels were removed. For primary neuronal isolation, tissue in HBSS was digested with papain (Sigma-Aldrich, St. Louis, MO) for 30 min at 37°C. The tissue was further dissociated to obtain single-cell suspensions by repeated pipetting. Neurons were plated at a density of approximately 1.8 × 106 cells/60mm dish coated with poly-D lysine in neurobasal media with B27 supplement, horse serum, and gentamicin (Invitrogen). After approximately 2h, the media was changed and unattached cells were discarded. Twenty-four hours later, neuron cultures were re-fed with a complete change of neurobasal media without horse serum. Four days later, one fourth of the media was removed and replaced with neurobasal media supplemented with fluoro-deoxyuridine (FDU) and uridine. Following FDU treatment, neurons were maintained in neurobasal medium containing Glutamax and B27 supplement, with half-media changes every other day. For microglia, after tissue dissociation in trypsin, cells were plated in mixed glial growth media; DME:F12 (1:1) media supplemented with insulin, 10% FBS, L-glutamine, and gentamicin. The mixed culture was maintained under 10% CO2 for 5 days, and the medium was fully replaced to remove any cell debris. To enrich for microglia, flasks were placed on an orbital shaker for 14–18h at 200 rpm in growth media. Detached cells constituted the microglial component of the culture and were collected and plated into a new flask containing microglial media. Purity of cell types was assessed by immunolabeling for cell-type specific markers including MAP2 and neurofilament for neurons and Iba-1 for microglia. Neurons and microglia were cultured separately with and without cocaine exposure.
2.7 Cocaine Treatments
Cocaine hydrochloride (Sigma-Aldrich) was dissolved in sodium citrate buffer (pH 5.0) to increase stability (Dey, 2007). Ten millimolar stock solution and subsequent dilutions were prepared daily and added directly to cell culture medium. Sodium citrate buffer was used as the control vehicle during cocaine treatments. A physiologically relevant dose of 5 μM was utilized for all experiments based on our previous experiments and those of others (Costa et al., 2013; Dey and Snow, 2007; Kovalevich, 2012; Lee et al., 2008; Yao et al., 2009a; Yao et al., 2009b; Zheng and Zhan, 2012).
2.8 Immunocytochemical labeling
Following the treatments, cells plated on chamber slides were washed once with 1X PBS and fixed with 4 % formaldehyde for 15 min at room temperature as previously described (Kovalevich, 2012; Kovalevich, 2015). Following fixation, slides were washed with 1X PBS three times and cells were permeabilized with 0.2 % Triton X-100 for 15 min, washed again, and placed in blocking solution (5 % normal goat or horse serum; Vector Laboratories, Burlingame, CA) for 1h. Slides were then incubated in primary antibodies overnight at room temperature. Other primary antibodies include synaptophysin, (1:200, Abcam), MAP2 (1:200, Abcam), Iba-1 (1:200, Abcam), MeCP2 (1:200, Abcam). Following incubation with primary antibodies, slides were rinsed 3X with PBS, and incubated with fluorescein isothiocyanate (FITC) (1:500; Vector Laboratories) or tetramethylrhodamine (TRITC) (1:500; Vector Laboratories)-conjugated secondary antibodies for 1h at room temperature in the dark. Slides were washed 3X with PBS, cover-slipped with an aqueous based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), and visualized with the Leica Advanced Wide field imaging system (Leica Microsystems; Buffalo Grove, IL).
2.9 Cell Harvest and Protein Extraction
Following treatments, cells were washed in 1X PBS, scraped from the culture plate and collected in ice-cold 1X PBS. The cell suspension was centrifuged at 5,000 × g for 10 min at 4°C and the supernatant was discarded. The cell pellet was then re-suspended in ice-cold RIPA lysis buffer (50 mM Tris-HCL (pH 8.0), 150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS,) containing protease and phosphatase inhibitor cocktails (Calbiochem, San Diego, CA), vortexed, and incubated on ice for 25 min to complete cell lysis. Samples were then centrifuged at 14,000 × g for 10 min at 4°C to separate insoluble material. The supernatant containing proteins was collected and placed into clean, pre-chilled Eppendorf tubes and samples were stored at −80°C until protein analysis was performed. For experiments requiring nuclear/cytoplasmic fractionation, cells were collected as described but lysed and fractionated using the NE-PER nuclear and cytoplasmic extraction kit according to the manufacturer’s protocol (Thermo-Scientific, Waltham, MA). Following fractionation, nuclear and cytoplasmic lysates were stored at −80°C until analysis.
2.10 Western Blotting
Equal amounts of protein were loaded onto pre-cast midi-gels (4–12% Bis-Tris; Invitrogen), separated by electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% non-fat milk or 5% bovine serum albumin (BSA) in Tris-buffered saline, 0.1% Tween-20 (TBST) for 1h before incubation with primary antibodies. Primary antibodies included: Phospho-MeCP2 pSer421 (1:1000, Thermo Scientific), MeCP2 (1:1,000; GeneTex), BDNF (1:1000; Origene), MAP2 (1:1,000; Abcam), and the loading controls tubulin (1: 1000; Cell Signaling), lamin (1:1000, Cell Signaling) or GAPDH (1:1000; Abcam). Membranes were incubated with primary antibodies overnight at 4°C, washed in 1X TBST, incubated with appropriate secondary anti-mouse or -rabbit antibodies (1:10,000; Thermo Scientific) for 1h, and developed with ECL Prime (Amersham Pharmacia Biotech, Piscataway, NJ). Band intensities were calculated using ImageJ software and normalized to the loading control (Rasband, 1997).
2.11 BDNF ELISA
Cell culture supernatant from control and cocaine-treated human primary microglia and human primary neurons w analyzed by ELISA for levels of secreted BDNF. Cells were treated with 5 μM cocaine and the cell culture medium was collected at various time points following treatment. Any non-adherent cells were removed by centrifugation and the remaining supernatants were analyzed using a human BDNF ELISA kit according to manufacturer’s instructions (Millipore). One hundred microliters of each sample were measured in duplicate and BDNF levels were assessed by absorbance at 450 nm. Values were obtained and compared to the standard curve to extrapolate BDNF concentrations.
2.12 Quantification of cocaine self-administration effects on microglia
Sagittal sections from approximately 2.4 mm lateral from midline were imaged and analyzed to determine the effect of sham surgery, 2hr daily cocaine access, or 6h daily cocaine access on microglial number, microglial cell body size, and microglial morphology. Images were taken of the hippocampus, frontal cortex, and core of the nucleus accumbens at 20x and 40x magnification. Regions were identified using the Paxinos and Watson rat brain atlas (see Figure 2a) (Paxinos, 2013). Microglial number: Total microglia within the field of the 20x microscope images from three slides for each animal were averaged, and this mean number for each animal in the experimental groups was averaged to determine mean total microglial number within the hippocampus, frontal cortex, and nucleus accumbens core for sham, 2h and 6h access rats. Images of the frontal cortex did not occupy the entire image frame, and this was accounted for in the analysis by applying a standard grid to each image and counting microglia in the same number of quadrants for each image. Only microglia co-labeled with DAPI were included in the count. Microglial cell body area: Microglial cell body area from 5 microglia closest to the center of the 40x microscope images from three slides for each subject were determined and averaged using the Image J Process tool, and this mean number for each animal in the experimental groups was averaged to determine mean microglial cell body area within the hippocampus, frontal cortex, and nucleus accumbens core for sham, 2h and 6h access rats. Microglia morphology: Microglia were categorized into one of four morphological states modified from previous studies (Byrnes et al., 2012; He and Crews, 2008). Ten microglia within the field of the 20x images from one slide for each animal were placed into one of four categories based on morphology as follows: ramified with small cell bodies and long, thin, and highly branched processes; hypertrophic with larger cell bodies and thicker long branched processes; bushy with multiple short processes around an enlarged cell body; and amoeboid (Byrnes et al., 2012). Ten microglia within the field of the 20x images from one slide for each subject were placed into one of four categories based on morphology as follows: ramified, bushy, activated, amoeboid (see Figure 2d), and the number in each category for each animal in the experimental groups was averaged to determine mean percent of each morphology within the hippocampus, frontal cortex, and nucleus accumbens core for sham, 2h and 6h access rats. The rater was blinded to the experimental conditions of each slide.
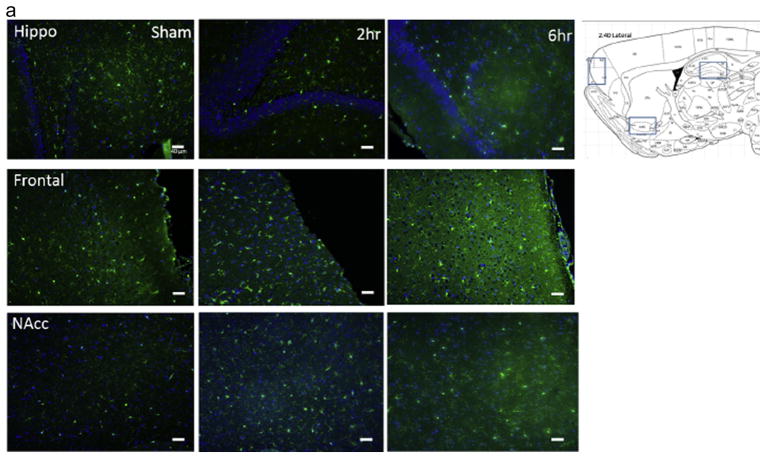
Figure 2. Cocaine activates microglia in the brains of rats that self-administered cocaine.
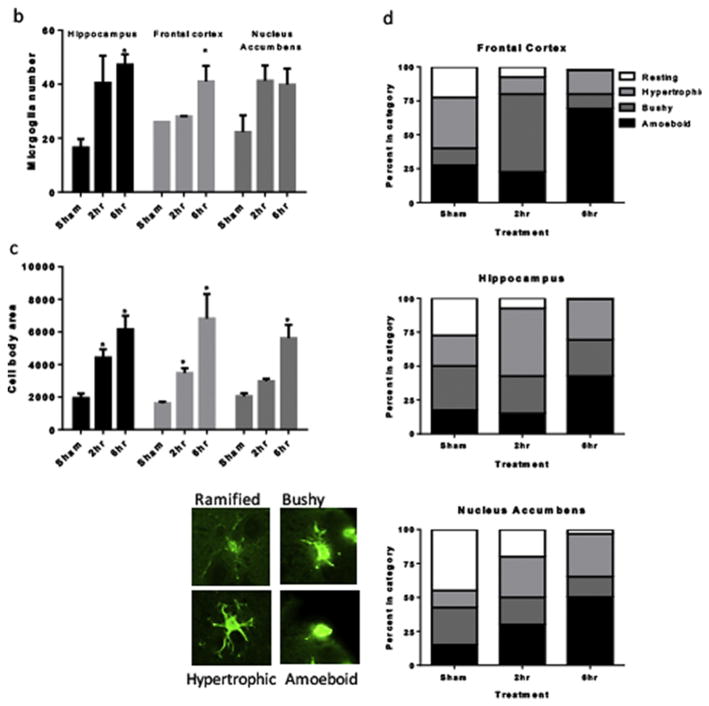
A) Representative immunohistochemical labeling of hippocampus, frontal cortex and nucleus accumbens of sham rats and rats that self-administered cocaine for 19 days for either 2 or 6h/day. Sagittal 5 um thick sections from approximately 2.4 mm lateral to midline were immunolabeled with Iba-1 (green) and DAPI (blue). Magnification 20X. B). Quantification of microglial cell number within the field of the microscope images, data are expressed as mean and SEM. C). Quantification of microglial cell body area (in arbitrary units determined using ImageJ) within the field of the microscope images, data are expressed as mean and SEM and were analyzed utilizing One-way ANOVA with Tukey’s multiple comparison test. D). Categorization of microglial morphology in sham, 2h or 6h/day access conditions. Representative microglial morphologies for each category are shown.
2.13 Statistical analysis
All data were analyzed using either the student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s post hoc testing where appropriate using GraphPad Prizm® (GraphPad Software Inc., San Diego, CA). Results were expressed as mean ± SEM. Values of p ≤ 0.05 were considered statistically significant.
3. Results
3.1 Effect of extended access cocaine self-administration on microglial activation and MeCP2 and BDNF expression in rat brain
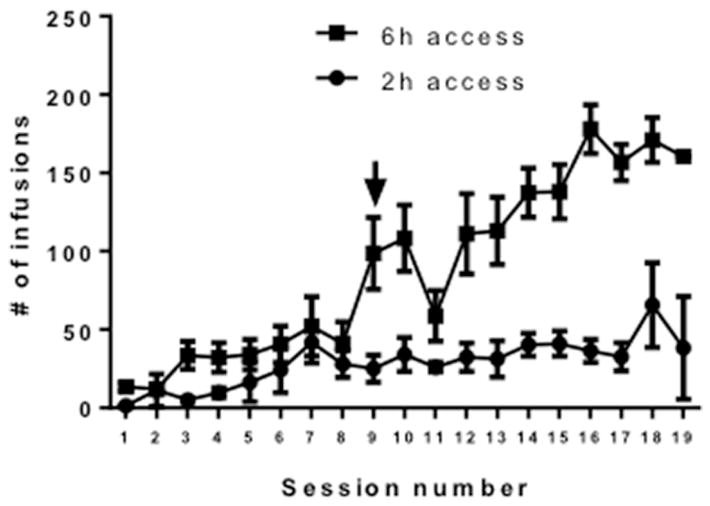
All rats acquired cocaine self-administration under the 2h access conditions. On day 9, 6 of the rats were switched to 6h access conditions. In rats switched from the 2h to 6h per day access conditions, cocaine intake increased robustly and cocaine taking escalated throughout the 10-day period (Figure 1). Increased immunolabeling for Iba-1 was observed in the hippocampus, frontal cortex and nucleus accumbens of rats with cocaine self-administration experience as compared to sham controls, and these effects were statistically significant for the hippocampus and frontal cortex (Figure 2a). Specifically, one-way ANOVAs showed significant effects of cocaine self-administration on microglial cell number in the hippocampus (F = 2.79, p< 0.05) and frontal cortex (F = 7.094, p < 0.05), with Tukey’s multiple comparisons tests showing post hoc significance of 6h daily self-administration compared with sham rats. Both 2h and 6h access conditions increased microglial number within the nucleus accumbens but this effect did not reach statistical significance (p = 1.0) (Figure 2b). Cocaine self-administration was also associated with increased microglial cell body size. Specifically, one-way ANOVAs showed significant effects of cocaine self-administration on microglia cell body area in the hippocampus (F = 9.59, p< 0.05), frontal cortex (F = 11.93, p< 0.05), and nucleus accumbens (F = 19.38, p< 0.05), with Tukey’s multiple comparisons tests showing post hoc significance of 2h and 6h daily self-administration compared with sham rats in the hippocampus and frontal cortex, and 6h daily self-administration compared with sham rats in the nucleus accumbens (Figure 2c).
Figure 1. Intravenous cocaine self-administration under 2h and 6h access conditions.
X-axis: Session number. Y-axis: # of cocaine infusions earned (0.375 mg/kg/infusion). Arrow denotes change from 2h to 6h access in the 6h access group. N=6/group.
In the hippocampus, there was a relatively even distribution of different microglial morphologies in the sham rats (Figure 2D, graph 1). Following 2h access to cocaine self-administration, the majority of microglia were morphologically categorized as hypertrophic, with only a small proportion determined to be ramified/resting. Following 6h access to cocaine self-administration, more than 50% of the microglia in the hippocampus were categorized as amoeboid. In the frontal cortex, approximately 40% of microglia in sham rats were categorized as hypertrophic (Figure 2D, graph 2). Following 2h access to cocaine self-administration, the vast majority of microglia in the frontal cortex were categorized as bushy, with a much smaller proportion determined to be resting, hypertrophic, or amoeboid. Following 6h access to cocaine self-administration, well over 50% of the microglia in the frontal cortex were categorized as amoeboid. In the nucleus accumbens, almost half of microglia in sham rats were categorized as ramified/resting (Figure 2D, graph 3). Following 2h access to cocaine self-administration there was a decrease in the number of resting microglia and an increase in the number of hypertrophic and amoeboid morphologies. Following 6h access to cocaine self-administration, almost half of the microglia in the hippocampus were categorized as amoeboid. Taken together, these results show that the cocaine administration paradigms used in this study significantly affect the activation state of microglia in the rat brain in an access- and brain region-dependent manner.
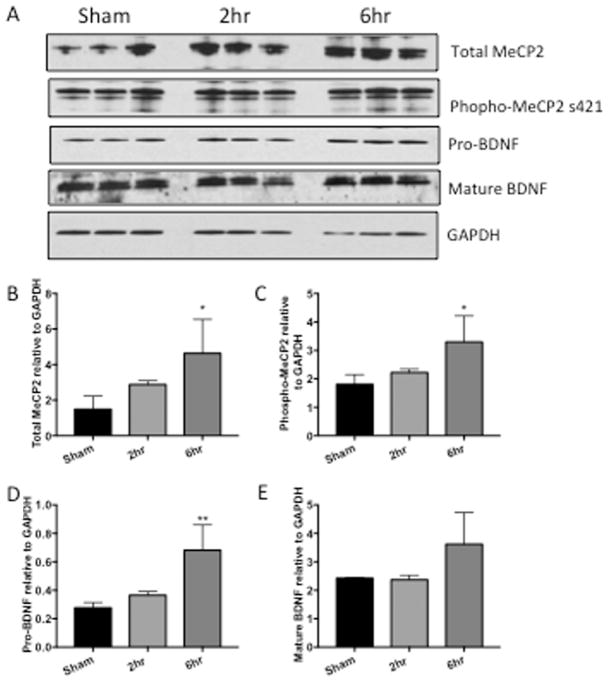
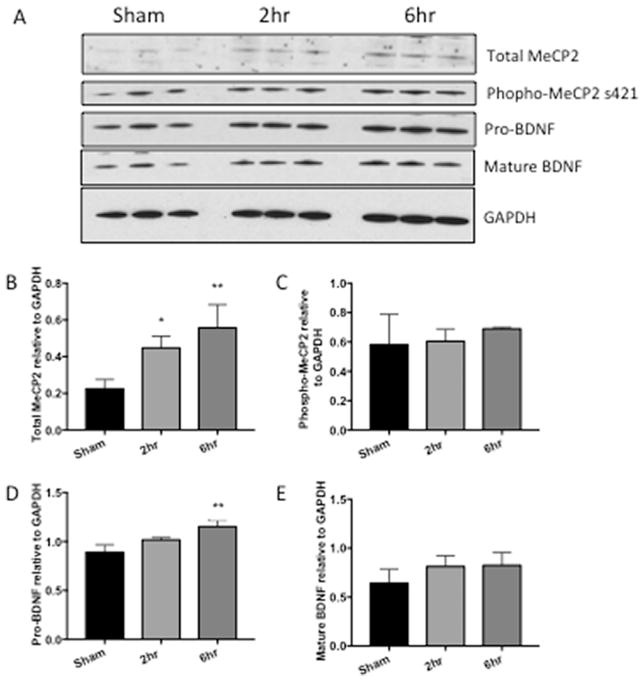
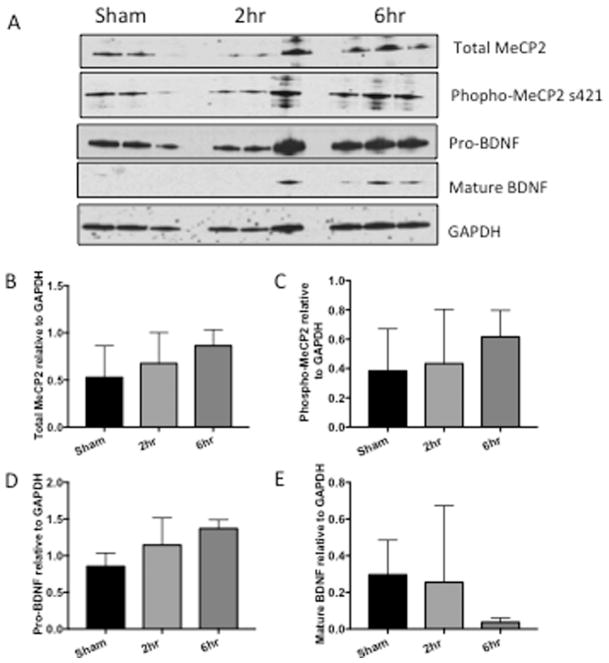
Because MeCP2 regulates BDNF, we assessed MeCP2 and BDNF expression levels in hippocampus, frontal cortex and nucleus accumbens of rats with and without cocaine administration. Western analyses showed a significant increase of total MeCP2 in the rats with 6h access to cocaine in the hippocampus (Figure 3B, *p=0.0406) and increase of total MeCP2 with 2h and 6h in the nucleus accumbens (Figure 5B, *p=0.0447, **p=0.0079). In the frontal cortex, total MeCP2 was also increased although increases did not reach statistical significance (Figure 4B, p=0.8085, 0.3847). Phosphorylation of MeCP2 at serine 421 causes its dissociation from the BDNF promoter allowing for increased expression of BDNF. Therefore, we also assessed changes in phosphorylation levels of MeCP2. Phospho-MeCP2 was increased at 6h in all brain regions from cocaine-administered rats, although these increases only reach statistical significance in the hippocampus (3C, hippocampus, *p= 0.0418). Western analyses indicated significant increases (**p < 0.01) in pro-BDNF in the hippocampus and nucleus accumbens in the 6hr access group (Figures 3D and 5D). While mature (mBDNF) increased in the hippocampus, the increase was not significantly significant (Figures 3E p=0.1391). Taken together, these data show that extended access cocaine increases levels of MeCP2 and pro-BDNF in the brains of rats that self-administered cocaine, replicating the results reported in the striatum of cocaine self-administering rats (Im et al., 2010) and extending these results to the hippocampus and frontal cortex. Cell-type specific changes in MeCP2 and BDNF were further investigated in vitro in human primary neurons and microglia exposed to cocaine.
Figure 3. MeCP2 and BDNF are increased in hippocampus of rats that self-administer cocaine.
A) Representative western blot of hippocampal tissue from rats that self-administered cocaine for 19 days for either 2 or 6h/day. B) Graphic representation of changes in total MeCP2 normalized to GAPDH, p=0.3859, *p=0.0406. C) Graphic representation of changes in phosphorylated MeCP2 normalized to total MeCP2, p=0.6624, *p=0.0418. D) Graphic representation of changes in precursor BDNF (proBDNF) normalized to GAPDH, p= 0.5903, **p= 0.008. E) Graphic representation of changes in mature BDNF (mBDNF) normalized to GAPDH, p= 0.9942, 0.1391. Results are expressed as mean ± SEM, n ≥ 3 and were analyzed utilizing One-way ANOVA with Tukey’s multiple comparison test. Sham represents saline control. NS is not statistically significant.
Figure 5. MeCP2 and BDNF are increased in striatum of rats that self-administer cocaine.
A) Representative western blot of striatal tissue from rats that self-administered cocaine for 19 days for either 2 or 6h/day. B) Graphic representation of changes in total MeCP2 normalized to GAPDH, *p=0.0447, **p=0.0079. C) Graphic representation of changes in phosphorylated MeCP2 normalized to total MeCP2, p=0.9749, 0.5916. D) Graphic representation of changes in precursor BDNF (proBDNF) normalized to GAPDH, p=0.0852, **p=0.0039. E) Graphic representation of changes in mature BDNF (mBDNF) normalized to GAPDH, p=0.3086, 0.2691. Results are expressed as mean ± SEM, n≥ 3 and were analyzed utilizing One-way ANOVA with Tukey’s multiple comparison test. Sham represents saline control. NS is not statistically significant.
Figure 4. MeCP2 and BDNF are increased in frontal cortex of rats that self-administer cocaine.
A) Representative western blot of frontal cortex tissue from rats that self-administered cocaine for 19 days for either 2 or 6h/day. B) Graphic representation of changes in total MeCP2 normalized to GAPDH, p= 0.8085, 0.3847. C) Graphic representation of changes in phosphorylated MeCP2 normalized to total MeCP2, p= 0.9760, 0.6147. D) Graphic representation of changes in precursor BDNF (proBDNF) normalized to GAPDH, p= 0.3800, 0.0949. E) Graphic representation of changes in mature BDNF (mBDNF) normalized to GAPDH, p= 0.9807, 0.4970. Results are expressed as mean ± SEM, n≥ 3 and were analyzed utilizing One-way ANOVA with Tukey’s multiple comparison test. Sham represents saline control. NS is not statistically significant.
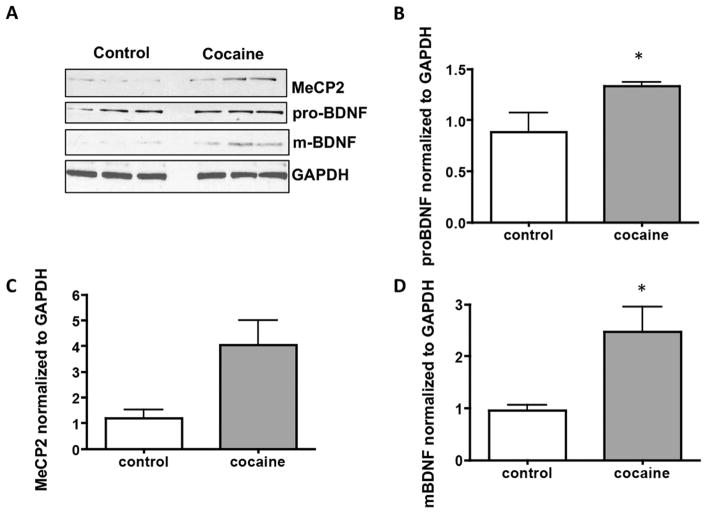
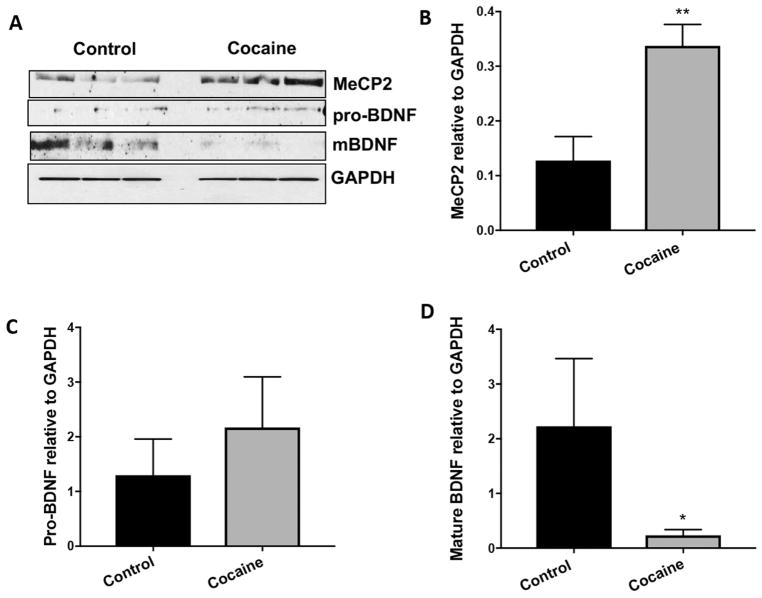
3.2 Cocaine Exposure Induces MeCP2 and BDNF expression in Neurons and Microglia
Our data show that MeCP2 was significantly increased in both neurons (Figure 6A and B, p=0.046) and microglia (Figure 7A and B, p=0.004) following cocaine exposure. Pro-BDNF levels were increased in both neurons and microglia but not to statistically significant levels (Figures 6A and C, p=0.215 and 7A and C, p=0.253). Interestingly, levels of the mature form of BDNF were significantly increased in neurons (Figure 6A and D, p=0.0417), while levels were significantly decreased in microglia (Figure 7A and D, p=0.0499).
Figure 6. MeCP2 and BDNF are increased in human primary neurons that are exposed to cocaine.
A) Representative western blot of lysate from human primary neurons exposed to cocaine for 72h. B) Graphic representation of changes in total MeCP2 normalized to GAPDH, *p=0.046. C) Graphic representation of changes in precursor BDNF (proBDNF) normalized to GAPDH, p=0.215 D) Graphic representation of changes in mature BDNF (mBDNF) normalized to GAPDH, *p=0.042. Results are expressed as mean ± SEM, n ≥ 3 using the unpaired T-test. NS is not statistically significant.
Figure 7. MeCP2 is increased and mature BDNF is decreased in human primary microglia that are exposed to cocaine.
A) Representative western blot of lysate from human primary microglia exposed to cocaine for 72h. B) Graphic representation of changes in total MeCP2 normalized to GAPDH, **p=0.004. C) Graphic representation of changes in precursor BDNF (proBDNF) normalized to GAPDH, p=0.253. D) Graphic representation of changes in mature BDNF (mBDNF) normalized to GAPDH, *p=0.05. Results are expressed as mean ± SEM, n ≥ 3 using the unpaired T-test. NS is not statistically significant.
3.3 Time Course of BDNF release following cocaine exposure
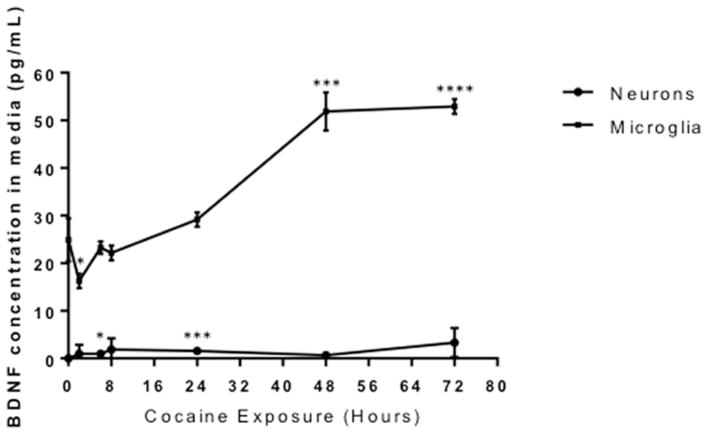
Given that data showed a significant decrease in cellular microglial mature BDNF in response to cocaine (Figure 7A and D), levels of BDNF in the media from microglia and neurons were assessed over time. A significant amount of microglial-derived BDNF was released into the media following cocaine treatment after 48 and 72 hours (51.87, 52.9 pg/mL, respectively) (Figure 8, p ≤ 0.0001). However, neuronal BDNF secretion was significantly elevated after 6h and 24h, but at much lower levels than observed in microglia (0.96, 1.57 pg/mL, respectively) (Figure 8, p=0.01 and p=0.0001, respectively). These results suggest that in response to cocaine, microglial release of BDNF is robust and markedly increases the exposure of neurons to BDNF.
Figure 8. Microglia secrete significantly more BDNF than neurons following cocaine exposure.
Neurons (circles) and microglia (squares) were exposed to cocaine from 0–72 hours and conditioned media was collected and ELISA for BDNF was performed. Neurons: 6h, *0.0168; 24h, ***p=0.0001. Microglia: 2h, *p=0.0202; 48h, ***p=0.0001; 72h, ****p<0.0001 as determined by the unpaired T-test. n=6 per time point.
3.4 Cocaine induces the translocation of MeCP2 from the nucleus to the cytoplasm in microglia
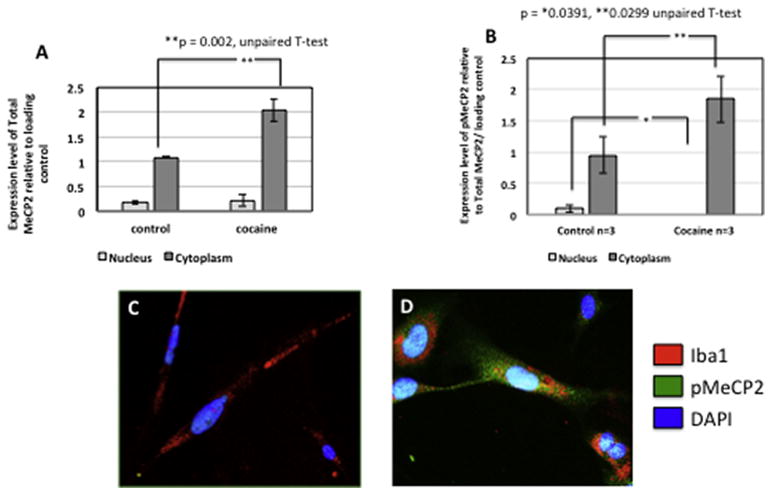
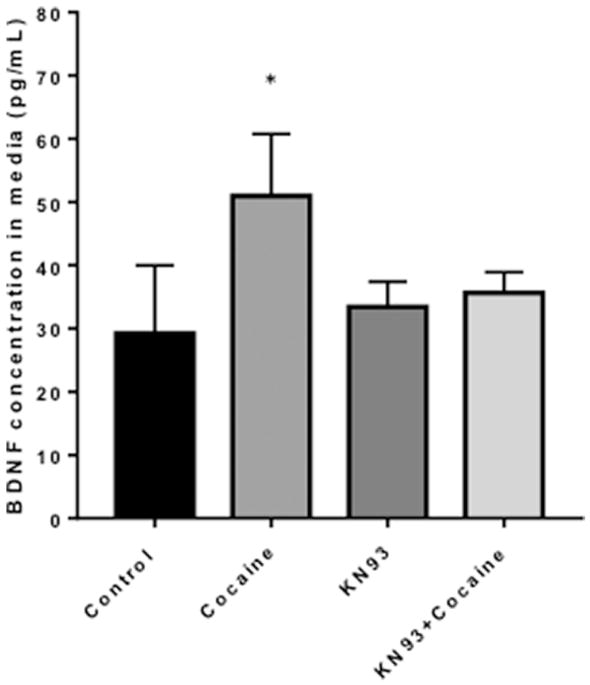
Zhou et al, found that in rat hippocampal neuronal cultures calcium influx induced the phosphorylation of MeCP2 at serine 421. This contributed to the up regulation of Bdnf transcription. Additionally, MeCP2 serine 421 phosphorylation was required for MeCP2-dependent regulation of spine morphogenesis and dendritic growth (Zhou et al., 2006). Cocaine has been shown to increase the phosphorylation of MeCP2 at serine 421 in the rat striatum, including the caudate putamen and nucleus accumbens following a single acute systemic injection (Mao et al., 2011). Having demonstrated that microglial MeCP2 levels and secreted BDNF are increased by cocaine (Figures 7, 8), we next assessed the changes in phosphorylation status and subcellular localization of MeCP2 in microglia following cocaine exposure. Interestingly, MeCP2 underwent a translocation from the nucleus to the cytoplasm in microglia exposed to cocaine (Figure 9), supporting the ability of cocaine to induce dissociation of MeCP2 from the DNA. Once phosphorylated and dissociated from the DNA, MeCP2 is readily transported out of the nucleus to the cytoplasm. Western blot analysis confirmed our finding that exposure to cocaine results in significant increases in total MeCP2 (Figure 10A, p=0.002) and p-MeCP2 (Figure 10B, p=0.029) from the nucleus into the cytoplasm. Total nuclear MeCP2 levels were unchanged, but phosphorylated nuclear MeCP2 was significantly decreased (p=0.039) (Figure 10A, B). Thus, cocaine-induced increased phosphorylation and cytoplasmic shuttling may account for increased BDNF production and release by microglia. Previous reports have demonstrated that CaMKII is responsible for the phosphorylation of MeCP2 at serine 421. The use of a specific CaMKII inhibitor, KN93, blocks MeCP2 421 serine phosphorylation, however overexpression of constitutively active CaMKII triggers this event (Buchthal et al., 2012; Zhou et al., 2006). Importantly, we found that blocking the phosphorylation of MeCP2 with the KN93 prevented the cocaine-induced release of BDNF from microglia (Figure 11).
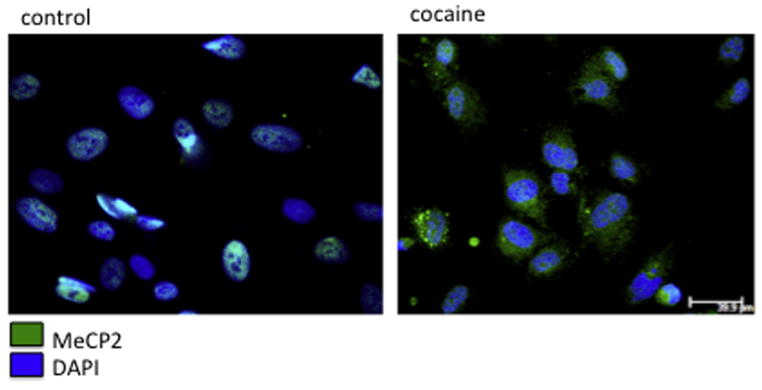
Figure 9. Cocaine induces MeCP2 translocation from nucleus to the cytoplasm in microglia.
Human primary microglia were exposed to cocaine for 72h and were labeled with anti-MeCP2 antibody (green) and DAPI (blue). Scale bar ~ 40 μm.
Figure 10. Cocaine induces increased cytoplasmic pMeCP2 and total MeCP2 and decreased nuclear pMECP2.
A) Graphic results from western analyses showing increased total MeCP2 in the cytoplasm of microglia after cocaine exposure. **p=002 via unpaired T-test. B) Graphic results from western analyses showing increased pMeCP2 in the cytoplasm (*p=0.0391 via unpaired T-test) and decreased nuclear pMeCP2 (**p=0.0299 via the unpaired T-test) of microglia after cocaine exposure. Results are expressed as mean ± SEM, n ≥ 3. C) Representative immunocytochemical labeling of control (untreated) and D) cocaine treated microglia with anti-MeCP2 (green) and Iba-1 (red) and DAPI (blue) showing cytoplasmic MeCP2. Magnification 40x, scale bar ~ 40um.
Figure 11. Inhibiting the phosphorylation of MeCP2 prevents the release of BDNF following cocaine treatment in microglia.
ELISA was used to assess levels of BNDF in media from microglia exposed to cocaine, the CamKII inhibitor KN93 or both. *p=0.01 as determined by unpaired T-test. N=6 per condition.
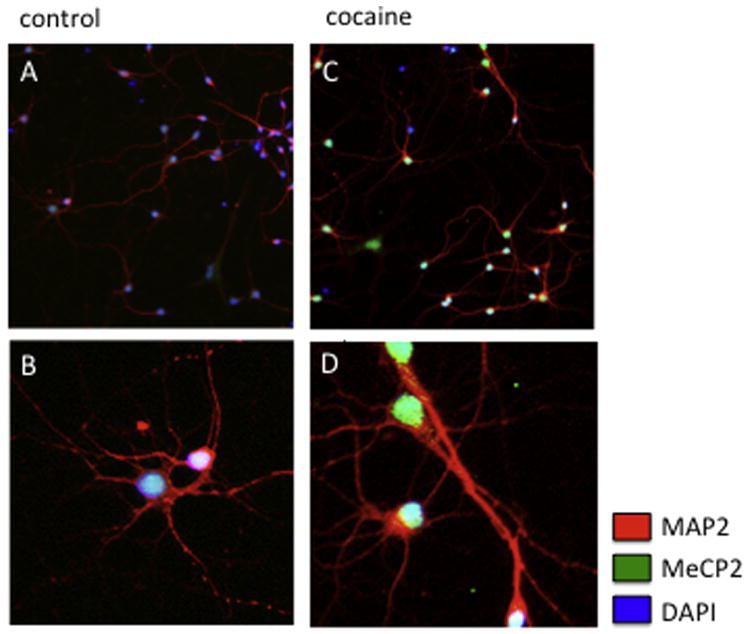
Lastly, although in neurons nuclear MeCP2 levels increase following cocaine treatment we did not observe the translocation of MeCP2 from the nucleus to the cytoplasm as seen in microglia (Figure 12), further supporting a distinct molecular pathway in microglia exposed to cocaine.
Figure 12. Neuronal MeCP2 increases following cocaine exposure however it remains localized to the nucleus.
A–B) Representative immunocytochemical labeling of control (untreated) and C–D) cocaine treated neurons with anti-MeCP2 (green) and MAP2 (red) and DAPI (blue) showing nuclear MeCP2. A, C) magnification 20X; B, D) magnification 40X.
4. Discussion
This study investigated the impact of cocaine on microglial activation and MeCP2-induced BDNF release that may influence synaptic remodeling. Our results show that cocaine self-administration under extended access significantly increased microglial number in the hippocampus and frontal cortex and produced a non-significant increase in microglial number in the nucleus accumbens. Cocaine self-administration under 2h access conditions also increased microglial number in the hippocampus and nucleus accumbens but these effects were not significant. In addition, cocaine self-administration was associated with increased microglial cell body area and changed microglial morphology in these key brain regions in the cocaine addiction circuit in rats, as supported by previous studies (Castilla-Ortega et al., 2016). As in the results for microglial cell number, results for cell body area and microglial morphology were cocaine access-dependent, in that the most robust changes were seen in the 6hr access group. These changes in number and cell body area, and especially in microglial morphology, suggest that extended access cocaine self-administration leads to microglial polarization associated with distinct molecular phenotypes and effector functions (Colton, 2009). We categorized the microglia into four cellular phenotypes which correspond with increasing activation status, and results showed that cocaine self-administration was associated with decreased numbers of ramified microglia and increased numbers of more reactive phenotypes. An increase in reactive phenotypes was observed in all three brain regions studied, and again the extended access group showed the largest shift toward highly activated versus resting state, strongly suggesting that not only the number of microglia were altered with increasing cocaine intake, but the functionality of these CNS immune cells as well. Future studies are warranted to determine how these changes in microglial morphology following cocaine self-administration relate to their expression of various cytokines etc. that can modify neuronal transmission and synaptic plasticity.
Furthermore,, extended access, but not short access, cocaine self-administration elevated MeCP2 and BDNF expression in the brains of these rats, confirming and extending upon the findings of previous studies (Im et al., 2010). As stated in the introduction, the selectivity of these effects of microglial activation and alterations in MeCP2 and BDNF expression to the 6hr, extended access group suggest that these changes are associated with escalated drug taking behavior and not simply with cocaine exposure per se.
On a cellular level, our results show that following cocaine exposure, both neurons and microglia increase expression and phosphorylation of MeCP2. Cocaine exposure concomitantly increased mBDNF levels in neurons, while it had the opposite effect of attenuating expression of mBDNF in microglia. While this effect was surprising, we discovered that BDNF release is significantly enhanced in microglia exposed to cocaine compared to neurons (Figure 8). We detected the translocation of MeCP2 from the nucleus to the cytoplasm upon cocaine exposure in microglia but not in neurons, suggesting differential roles for MeCP2 in neurons and microglia. In line with previous literature, which has illustrated that in neuronal cells cocaine induces the dissociation of MeCP2 from the BDNF promoter followed by increased expression of BDNF, our results demonstrate that microglial MeCP2 is also a sensitive target of cocaine and can contribute to the release of BDNF, possibility contributing to cocaine-induced synaptic plasticity.
There is mounting evidence that implicates microglia in cocaine effects (Guo et al., 2015; Periyasamy et al., 2017). Non-contingent cocaine exposure induces a pro-inflammatory immune response in the CNS (Lee et al., 2009), and agents known to suppress microglial activation have been shown to inhibit drug-induced cytokine production and behavior responses (Chen et al., 2009; Hutchinson et al., 2008), suggesting the significance of microglial activation in the development and maintenance of drug addiction. Here, we report for the first time that in cocaine self-administrating rats, microglial number and morphology are altered in the hippocampus, frontal cortex and nucleus accumbens, and that these effects are dependent upon cocaine access conditions (Figure 2). Activated microglia may contribute to cocaine-induced synaptic plasticity through multiple mechanisms, including altered release of pro-inflammatory mediators, changes in trophic factor supply, and synaptic remodeling. More research is necessary however to determine how changes in microglial morphology relate to microglial function and microglia-neuronal interactions.
The close proximity of microglia to neurons allows them to prune synapses in response to synaptic transmission as well as provide neurotrophic support to mediate plasticity (Parkhurst et al., 2013; Tremblay et al., 2010). Thus, the role of microglia in shaping neuronal connectivity may be important in cocaine-induced synaptic plasticity. Exposure of various drugs of abuse produce lasting morphological and physiological changes in dendritic arborization, dendritic spines, and synaptic density in reward regions of the brain (Kovacs, 2012). Cocaine in particular results in increased spine density on medium spiny neurons of the nucleus accumbens and apical dendrites of the pyramidal neurons in the prefrontal cortex (Robinson and Kolb, 2004). Microglial processes have been associated with dendritic spine density in an experience-dependent manner (Tremblay et al., 2010), supporting the involvement of microglia in synaptic remodeling. We found that in response to cocaine, microglia increase their secretion of BDNF into the extracellular environment. BDNF has been implicated to play a major role in cocaine addiction and this aspect of cocaine signaling has been extensively reviewed (Li and Wolf, 2015). Overall, previous data suggest that cocaine exposure generally increases BDNF levels in reward-related regions of the CNS including the ventral tegmental area, nucleus accumbens, prefrontal cortex, and amygdala (Li and Wolf, 2015). Although the major source of BDNF in the adult brain was believed to be neurons, new evidence supports that microglial derived-BDNF plays an important role in the healthy brain (Rauskolb et al., 2010; Trang et al., 2011). The specific depletion of microglia using CX3CR1CreER mice showed defects in multiple learning tasks and a significant reduction in motor-learning-dependent synapse formation (Parkhurst et al., 2013). Furthermore, Cre-dependent removal of BDNF from microglia recapitulated the effects of microglia depletion (Parkhurst et al., 2013). These findings highlight the importance of microglial-derived BDNF in regulating learning-induced synapse formation and reveal a possible connection between elevated microglial-derived BDNF in response to cocaine and cocaine-induced synaptic plasticity through synapse formation. In addition, it is possible that increased microglial-derived BDNF can feedback to influence microglial activity. Emerging literature has established that BDNF can trigger of the activation of microglia, and microglial activation itself can induce the release of BDNF (Gomes et al., 2013; Mizoguchi et al., 2014; Zhang et al., 2014) thereby, creating a positive feedback loop in microglia that can prolong their activation state (Zhang et al., 2014). In summary, there are two potential functions that elevated microglial-derived BDNF may have: 1) enhancing neuronal synaptic remodeling and 2) propagation of microglial activation and pro-inflammatory cytokine release. In fact, both mechanisms have been implicated in contributing to the development and maintenance of cocaine addiction. Interestingly, our findings show that in microglia the mature form of BDNF is increased in response to cocaine exposure, whereas proBDNF levels are not increased, suggesting increased processing of existing por-BDNF. This is of importance because mature BDNF has been well established to promote outgrowth, mediate synaptic plasticity, and facilitate long-term potentiation where as proBDNF, has the opposing effect. The change in the ratio of proBDNF to mature BDNF following cocaine treatment found in this study supports the role of microglia’s contribution to cocaine-induced synaptic plasticity by favoring the supply of key signaling molecules involved in the enhancement of neuronal connectivity and not synaptic retraction or pruning.
Studies have shown that chromatin remodeling is an important mechanism underlying cocaine-induced changes in gene expression (Sadri-Vakili et al., 2010). Cocaine has been demonstrated to decrease histone deacetylase (HDAC) function and to hyper-acetylate histones H3 and H4, both of which are associated with gene promoters (Kumar et al., 2005; Renthal et al., 2007). This results in an active chromatin confirmation, which allows for transcription factor binding to promoter regions. BDNF in the brain has been closely correlated to MeCP2 (Chang et al., 2006). MeCP2 is a transcription factor that binds to methylated DNA, and recruits HDACs to silence target genes (Nan et al., 1998). Loss-of-function mutations and duplication of MeCP2 gene is associated with Rett syndrome, a neurodevelopmental disorder marked by severe cognitive impairment, autism-like symptoms, and compulsive behaviors (Amir et al., 1999; Chen et al., 2003; Van Esch et al., 2005). In response to neuronal activity-dependent calcium influx, MeCP2 becomes phosphorylated on serine 421 through a CaMKII-dependent mechanism and is released from BDNF promoter permitting its transcription (Chen et al., 2003; Zhou et al., 2006). By mutating MeCP2 at serine 421, one group demonstrated the importance of MeCP2 serine 421 phosphorylation in controlling BDNF transcription, dendritic patterning, and spine morphogenesis (Zhou et al., 2006). They found that preventing the phosphorylation of MeCP2 at serine 241 blocked the ability of MeCP2 to facilitate dendritic growth, spine maturation, and activate BDNF transcription (Zhou et al., 2006). Through both pharmacological and molecular approaches, this group also identified the phosphorylation of MeCP2 was dependent specifically on CaMKII. Recently, MeCP2 has received much attention in the field of addiction. One group reported that self-administration of cocaine elevates MeCP2 in the rat dorsal striatum (Im et al., 2010). Similar findings have been reported after repeated injections of cocaine (Cassel et al., 2006). Another group expanded on these findings to show that cocaine increases the phosphorylation of MeCP2 at serine 421 in the rat striatum (Mao et al., 2011). Furthermore, other studies found a decreased association of MeCP2 with the BDNF promoter in the medial prefrontal cortex of rats that self-administered cocaine. This was correlated with a significant increase in BDNF transcription (Sadri-Vakili et al., 2010). In neuronal cells, it was reported that during differentiation, phosphorylation of MeCP2 regulates its intracellular location (Miyake and Nagai, 2007). They report that at different time points of differentiation, MeCP2 can be detected in either the nucleus or the cytosol. Cytosolic MeCP2 was phosphorylated, whereas nuclear MeCP2 was not. This suggests that phosphorylation of MeCP2 may control its transcriptional function by regulating its intracellular location. We can speculate that phosphorylation of MeCP2 removes it from the promoter region of BDNF and exports it to the cytosol allowing BDNF transcription to occur. This is in line with our findings that in microglia, MeCP2 translocates from the nucleus to the cytosol following cocaine exposure, therefore allowing BDNF to be expressed and secreted at elevated levels. It is important to note that all previous work has only focused on the phosphorylation of MeCP2 in neurons. However, a number of groups have shown that MeCP2 is present in glial cells including microglia, and can regulate their function (Cronk et al., 2015; Rousseaud et al., 2015). Furthermore, it has been suggested that the expression and regulatory function of MeCP2 is cell-type-specific and has different regulation pathways of BDNF (Rousseaud et al., 2015; Sugino et al., 2014). This agrees with our findings that microglial and neuronal MeCP2 regulation differs in response to cocaine where in microglia, but not neurons, MeCP2 is readily exported out of the nucleus following cocaine exposure. Furthermore, cocaine triggers a significantly greater release of BDNF from microglia than from neurons. This could be a result of differential MeCP2 regulation of BDNF in these two cell types. However, it is also possible that less BDNF is detected in neuronal cultures because neurons may utilize BDNF more readily.
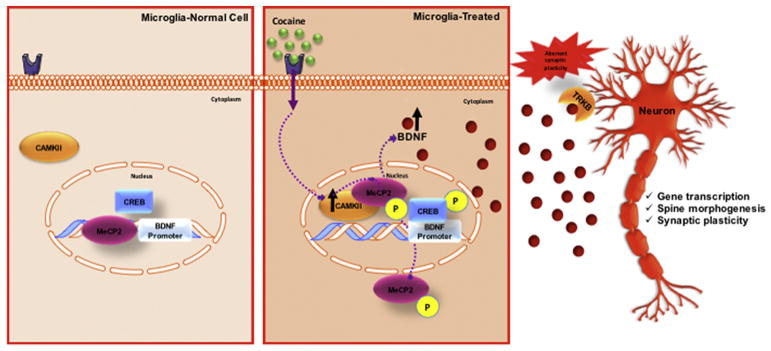
Cocaine-induced BDNF expression is mediated through various factors other than MeCP2. The promoter region of BDNF exon IV also contains specific bindings sites for cAMP response element-binding (CREB). CREB is known to play a critical role in establishing and maintaining addictive behaviors (Briand and Blendy, 2010; Carlezon et al., 2005). Cocaine can increase the activity of CREB (Barrot et al., 2002; Carlezon et al., 2005; Edwards et al., 2007). CREB is activated through its phosphorylation at serine 133. Interestingly, CaMKII has been shown to phosphorylate both CREB and MeCP2, resulting in the dissociation of MeCp2 and derepression of the BDNF promoter followed by the association of pCREB with the BDNF promoter IV is thus triggering BDNF transcription (Figure 13) (Zhou et al., 2006). This establishes a relevant connection for the importance of CaMKII for both MeCP2 and CREB for BDNF expression in response to cocaine.
Figure 13.
5. Conclusions
In conclusion, the current findings replicate and expand observations that cocaine self-administration increases MeCP2 and BDNF expression in the rat hippocampus, frontal cortex, and nucleus accumbens. In these same brain regions, cocaine self-administration also increases microglial activation. The current results also highlight a distinct molecular pathway in microglia through which cocaine increases BDNF, involving the phosphorylation of MeCP2 and subsequent translocation from the nucleus to the cytosol, which frees the BDNF promoter and permits its transcriptional activation. This leads to a significant release of BDNF by microglia (Figure 13). Future studies investigating the impact of microglial-derived BDNF in neuronal response to cocaine and the function of cytosolic MeCP2 are necessary to better understand the role of MeCP2 in cocaine-induced synaptic plasticity.
Highlights.
Cocaine self-administration increases microglial activation in the rat.
Cocaine increases BDNF in microglia, including the phosphorylation of MeCP2 its subsequent translocation from the nucleus to the cytosol, which frees the BDNF promoter and permits its transcriptional activation.
Microglial MeCP2 is a sensitive target of cocaine and possibly contributes to cocaine-induced synaptic plasticity
Acknowledgments
This work was supported by NIH P01 DA037830, Temple University’s Comprehensive NeuroAIDS Center (NIH P30 MH09217), NIH P30 DA013429 and the MacManus Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Barrot M, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–40. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Calvo E, et al. Pharmacological blockade of either cannabinoid CB1 or CB2 receptors prevents both cocaine-induced conditioned locomotion and cocaine-induced reduction of cell proliferation in the hippocampus of adult male rat. Front Integr Neurosci. 2014;7:106. doi: 10.3389/fnint.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodetto SP, et al. Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food. Int J Neuropsychopharmacol. 2014;17:2031–44. doi: 10.1017/S1461145714000972. [DOI] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–34. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal B, et al. Nuclear calcium signaling controls methyl-CpG-binding protein 2 (MeCP2) phosphorylation on serine 421 following synaptic activity. J Biol Chem. 2012;287:30967–74. doi: 10.1074/jbc.M112.382507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, et al. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J Neuroinflammation. 2012;9:43. doi: 10.1186/1742-2094-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, et al. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cassel S, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–92. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Castilla-Ortega E, et al. A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev. 2016;66:15–32. doi: 10.1016/j.neubiorev.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Chang Q, et al. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–8. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Minocycline affects cocaine sensitization in mice. Neurosci Lett. 2009;452:258–61. doi: 10.1016/j.neulet.2009.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–9. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, et al. Role of endoplasmic reticulum (ER) stress in cocaine-induced microglial cell death. J Neuroimmune Pharmacol. 2013;8:705–14. doi: 10.1007/s11481-013-9438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk JC, et al. Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity. 2015;42:679–91. doi: 10.1016/j.immuni.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Chao M. Handbook of Experimental Pharmacology. Springer; Berlin: 2014. Trk Receptors in Neurotrophic Factors. [DOI] [PubMed] [Google Scholar]
- Dey S, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNF-alpha-mediated induction of Bax and phosphorylated c-Jun NH(2)-terminal kinase. J Neurochem. 2007;103:542–56. doi: 10.1111/j.1471-4159.2007.04750.x. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, et al. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–85. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Edwards S, et al. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–13. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, et al. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–71. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes C, et al. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ML, et al. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy. 2015;11:995–1009. doi: 10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. Brain-Derived Neurotrophic Factor: Three Ligands, Many Actions. Trans Am Clin Climatol Assoc. 2015;126:9–19. [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, et al. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008;22:1248–56. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, et al. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatiani A, et al. Neuronal PINCH is regulated by TNF-alpha and is required for neurite extension. J Neuroimmune Pharmacol. 2010;6:330–40. doi: 10.1007/s11481-010-9236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. Microglia and drug-induced plasticity in reward-related neuronal circuits. Front Mol Neurosci. 2012;5:74. doi: 10.3389/fnmol.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, et al. Cocaine-Induced Loss of White Matter Proteins in the Adult Mouse Nucleus Accumbens Is Attenuated by Administration of a beta-Lactam Antibiotic during Cocaine Withdrawal. Am J Pathol. 2012;181:1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Corley G, Yen W, Kim J, Rawls S, Langford D. Cocaine decreases expression of neurogranin via alterations in thyroid receptor/retinoid X receptor signaling. Journal of Neurochemistry. 2012;121:302–313. doi: 10.1111/j.1471-4159.2012.07678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Yen William, Ozdemir Ahmet, Langford Dianne. Cocaine Induces Nuclear Export and Degradation of Neuronal Retinoid X Receptor-γ via a TNF-α/JNK-Mediated Mechanism. Journal of Neuroimmune Pharmacology. 2015 doi: 10.1007/s11481-014-9573-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lee CT, et al. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5:e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, et al. Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. J Addict Med. 2009;3:218–26. doi: 10.1097/ADM.0b013e318199d863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–54. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Little KY, et al. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009;168:173–80. doi: 10.1016/j.psychres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Mao LM, et al. Cocaine increases phosphorylation of MeCP2 in the rat striatum in vivo: a differential role of NMDA receptors. Neurochem Int. 2011;59:610–7. doi: 10.1016/j.neuint.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, et al. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–93. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Nagai K. Phosphorylation of methyl-CpG binding protein 2 (MeCP2) regulates the intracellular localization during neuronal cell differentiation. Neurochem Int. 2007;50:264–70. doi: 10.1016/j.neuint.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, et al. Brain-derived neurotrophic factor (BDNF) induces sustained intracellular Ca2+ elevation through the up-regulation of surface transient receptor potential 3 (TRPC3) channels in rodent microglia. J Biol Chem. 2014;289:18549–55. doi: 10.1074/jbc.M114.555334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–9. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Ozdemir AY, et al. PINCH in the cellular stress response to tau-hyperphosphorylation. PLoS One. 2013;8:e58232. doi: 10.1371/journal.pone.0058232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, et al. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GaW, Charles . The Rat Brain in Stereotaxic Coordinates. Academic Press; 2013. [Google Scholar]
- Periyasamy P, et al. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. NIH; Bethesda: 1997. [Google Scholar]
- Rauskolb S, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–49. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rousseaud A, et al. Differential Expression and Regulation of Brain-Derived Neurotrophic Factor (BDNF) mRNA Isoforms in Brain Cells from Mecp2(308/y) Mouse Model. J Mol Neurosci. 2015;56:758–67. doi: 10.1007/s12031-014-0487-0. [DOI] [PubMed] [Google Scholar]
- Russo SJ, et al. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–44. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, et al. Cell-type-specific repression by methyl-CpG-binding protein 2 is biased toward long genes. J Neurosci. 2014;34:12877–83. doi: 10.1523/JNEUROSCI.2674-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, et al. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 2011;7:99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, et al. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442–53. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, et al. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2003;75:301–7. doi: 10.1016/s0091-3057(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Yao H, et al. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009a;15:164–75. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, et al. Cocaine Exposure Results in Formation of Dendritic Varicosity in Rat Primary Hippocampal Neurons. Am J Infect Dis. 2009b;5:26–30. doi: 10.3844/ajidsp.2009.26.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34:715–23. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- Zheng F, Zhan CG. Modeling of pharmacokinetics of cocaine in human reveals the feasibility for development of enzyme therapies for drugs of abuse. PLoS Comput Biol. 2012;8:e1002610. doi: 10.1371/journal.pcbi.1002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–69. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]