SUMMARY
There is a growing body of research on the neural control of immunity and inflammation. However, it is not known if the nervous system can regulate the production of inflammatory myeloid cells from hematopoietic progenitor cells in disease conditions. Myeloid cell numbers in diabetic patients were strongly correlated with plasma concentrations of norepinephrine, suggesting the role of sympathetic neuronal activation in myeloid cell production. The spleens of diabetic patients and mice contained higher number of tyrosine hydroxylase (TH)-expressing leukocytes that produced catecholamines. Granulocyte macrophage progenitors (GMP) expressed the β2 adrenergic receptor, a target of catecholamines. Ablation of splenic sympathetic neuronal signaling using surgical, chemical and genetic approaches diminished GMP proliferation and myeloid cell development. Finally, mice lacking TH-producing leukocytes had reduced GMP proliferation, resulting in diminished myelopoiesis. Taken together, our study demonstrates that catecholamines produced by leukocytes and sympathetic nerve termini promote GMP proliferation and myeloid cell development.
eTOC blurb
Neural control of immunity and inflammation has been reported. Vasamsetti and colleagues demonstrate that the sympathetic nervous system controls the development of inflammatory myeloid cells from their progenitors in inflammatory conditions.
INTRODUCTION
Recent studies have shown that the immune system and the nervous system can communicate using common molecular signaling cues in various organ systems such as the central nervous system and the digestive system(Veiga-Fernandes and Pachnis, 2017). The myenteric plexus of the enteric nervous system contains macrophages and mast cells(Schemann and Camilleri, 2013), which are under neural control. Moreover, neuroimmune communication affects functions of an organ. For example, gut macrophage-derived bone morphogenesis protein 2 (BMP2) in response to enteric neuron signaling regulates gastrointestinal activity(Muller et al., 2014). Immune cells, such as macrophages and T cells, can produce catecholamines(Andersson and Tracey, 2012; Barnes et al., 2015; Brown et al., 2003; Jung et al., 2016; Laukova et al., 2013). Neural regulation of inflammation has been demonstrated by several studies(Andersson and Tracey, 2012). Vagus nerve stimulation dampens pro-inflammatory activity of macrophages(Wang et al., 2003), and vagotomy decreases group 3 innate lymphoid cell (ILC3) numbers(Dalli et al., 2017). Acetylcholine-producing T cells mediate anti-inflammatory responses after vagal nerve stimulation(Rosas-Ballina et al., 2011). Moreover, splenic sympathetic neuronal activation deploys T cells to the aorta and kidney, mediating hypertension(Carnevale et al., 2014; Tracey, 2014). Additionally, adrenergic signaling regulates hematopoietic stem cell mobilization(Scheiermann et al., 2012). Collectively, these studies suggest that the autonomic nervous system has promise as a therapeutic target to reduce inflammation in disease. As discussed above, most of the studies focused on the role of the autonomic nervous system in inflammation. However, little is known about the involvement of the autonomic nervous system in inflammatory cell development in disease.
The aim of the present study is to understand the role of the sympathetic nervous system (SNS) in hematopoietic progenitor cell proliferation and their differentiation into inflammatory myeloid cells in disease. To investigate this, we used mouse models of type 1 and 2 diabetes since SNS activation in diabetes is well known(Esler et al., 2001; Seals and Bell, 2004; Thorp and Schlaich, 2015). We investigated the effect of SNS activation in splenic myeloid cell development because the spleen plays a pivotal role in emergency hematopoiesis in different diseases like myocardial infarction (MI) (Leuschner et al., 2012) and in cancer(Cortez-Retamozo et al., 2012). The spleen acts as a reservoir of inflammatory monocytes, which are released into the bloodstream and recruited to sites of inflammation(Swirski et al., 2009).
In the current study, we observed a previously unappreciated regulation of myelopoiesis by the SNS. We found that leukocyte, monocyte and inflammatory monocyte numbers in patients were strongly correlated with plasma concentration of norepinephrine (NE), suggesting the role of sympathetic neuronal activation in inflammatory myeloid cell production. We showed, in type 1 and 2 diabetes mouse models, elevated number of myeloid cells in the spleen and blood. Moreover, the spleens of diabetic mice harbored elevated numbers of highly proliferating granulocyte macrophage progenitors (GMP). Adoptive transfer of GMP in diabetic mice revealed that the progenitors gave rise to higher number of myeloid cells in the spleen. Splenic myeloid expansion was not secondary to the increased bone marrow (BM) hematopoietic progenitors and their subsequent mobilization to the spleen. Additionally, we observed that the spleen of diabetic mice had high SNS activity. Surgical and chemical denervation of splenic sympathetic neurons in diabetic mice demonstrated reduced GMP differentiation and decreased splenic myelopoiesis, whereas sympathetic neuronal ablation did not change BM myelopoiesis. Furthermore, we found that splenic GMP expressed high amounts of the β2 adrenergic receptor. Selective β2 blocker treatment in diabetic mice reduced splenic GMP proliferation and myeloid cell production. Diabetic mice harbored increased number of TH+ leukocytes, which expressed high amounts of neuropeptide Y receptors, involved in neuroimmune signaling. Finally, diabetic mice lacking TH-producing leukocytes had reduced GMP proliferation and differentiation. This resulted in diminished splenic myelopoiesis. Altogether, these data indicate that elevated splenic sympathetic neuronal tone and paracrine activity of the catecholamines released by splenocytes in diabetes lead to GMP proliferation and differentiation into myeloid cells.
RESUTS
Inflammatory monocyte numbers correlate with plasma concentration of NE in patients
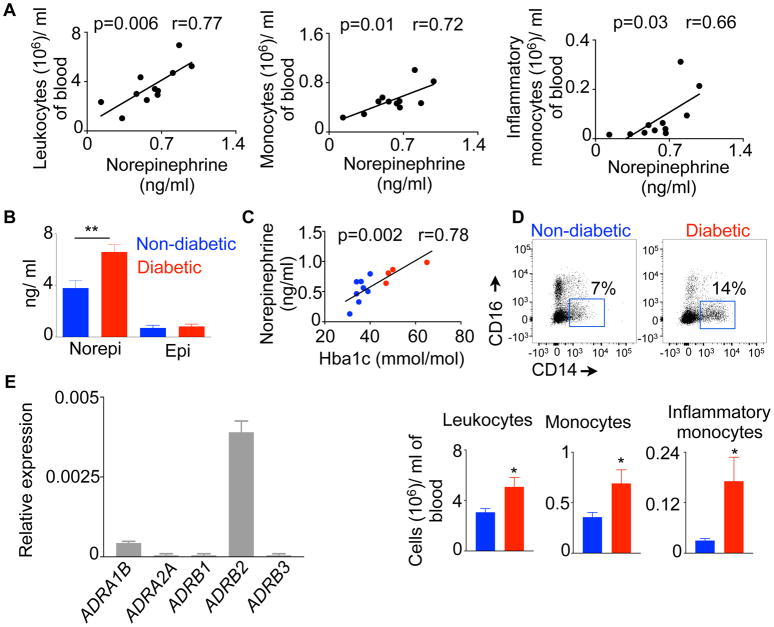
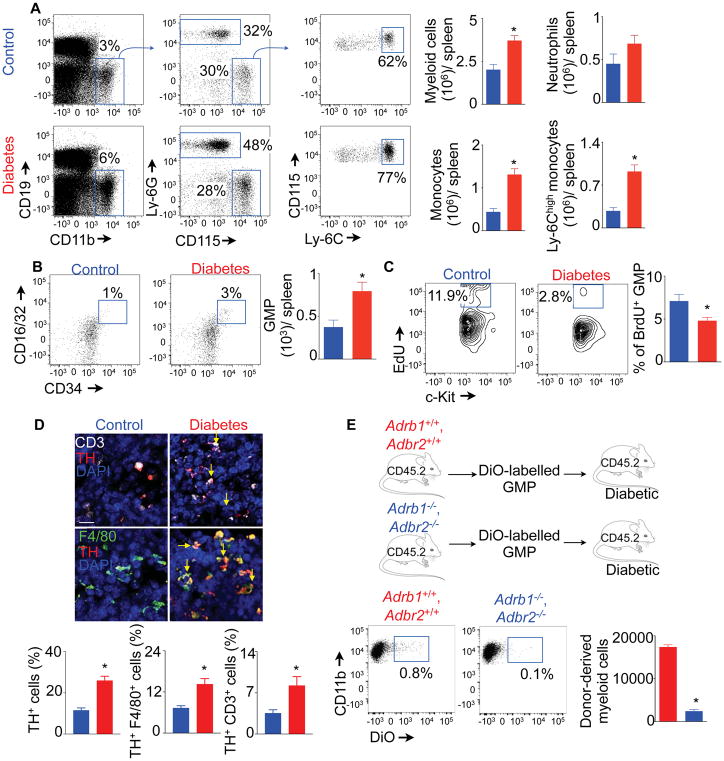
To investigate association between SNS activation and leukocytosis, we measured plasma NE and circulatory leukocyte contents in humans. We found that leukocyte, monocyte and inflammatory monocyte numbers strongly correlated with plasma NE concentrations (Figure 1A). Diabetic patients had higher plasma concentrations of NE than non-diabetic patients (Figure 1B), and plasma NE amounts correlated with glycosylated hemoglobin (Hba1c) quantities (Figure 1C). Additionally, we found that diabetic patients had elevated number of leukocytes, monocytes and inflammatory monocytes compared to non-diabetic individuals (Figure 1D). Collectively, these data reveal a strong association between SNS activation and myeloid cell frequency. Since NE binds to the adrenergic receptors and mediate a cascade of cellular responses, we measured the expression of the adrenergic receptors on GMP, the immediate progenitors of myeloid cells. We found that human GMP expressed high amount of the β2 adrenergic receptor (Figure 1E). Altogether, these data suggest a causative link between sympathetic neuronal activation and myeloid cell development.
Fig. 1. Plasma Norepinephrine (NE) concentrations strongly correlate with circulatory myeloid cell numbers in patients.
A) Plasma NE concentrations are correlated with circulatory leukocyte, monocyte and inflammatory monocytes in patients. B) Catecholamine concentrations in diabetic patients. C) Correlation between NE and Hba1c in patients. D) Circulatory leukocyte, monocyte and inflammatory monocyte numbers determined by flow cytometry. E) Relative expression of the adrenergic receptors in GMP isolated from human spleens. n=4–8/group. Mean ± s.e.m. * P < 0.05, ** P < 0.01.
Diabetes exaggerates splenic myelopoiesis
To investigate the role of SNS activation in myelopoiesis, we used mouse models of streptozotocin (stz)-mediated type 1 and high fat diet-induced type 2 diabetes. Since splenic myelopoiesis is important in pathogenesis of different diseases(Murphy et al., 2011; Robbins et al., 2012; Tall and Yvan-Charvet, 2015), we investigated splenic myelopoiesis in diabetic mice. We found that, at 1 month after stz treatment, mice had elevated numbers of different splenic myeloid cell subsets compared to non-diabetic control (Figure S1A). The elevated myeloid cell numbers persisted at 2 and 4 months after diabetes induction. Confocal imaging of splenic sections confirmed the increased frequency of CD11b+ myeloid cells in diabetic mice (Figure S1B).
Myeloid cells produced in the spleen in disease conditions egress into the blood and infiltrate inflamed organs(Leuschner et al., 2012; Swirski et al., 2009). To check this phenomenon in diabetes, we enumerated different myeloid cell subsets in blood. We found increases in number and frequency of these cells in blood circulation 1, 2 and 4 months after stz injection (Figures S1C and S1D). In contrast, the frequencies of B and T lymphocytes in the blood were unaltered in diabetic mice (Figure S1E).
Splenic granulocyte macrophage progenitors (GMP) are activated in diabetic mice
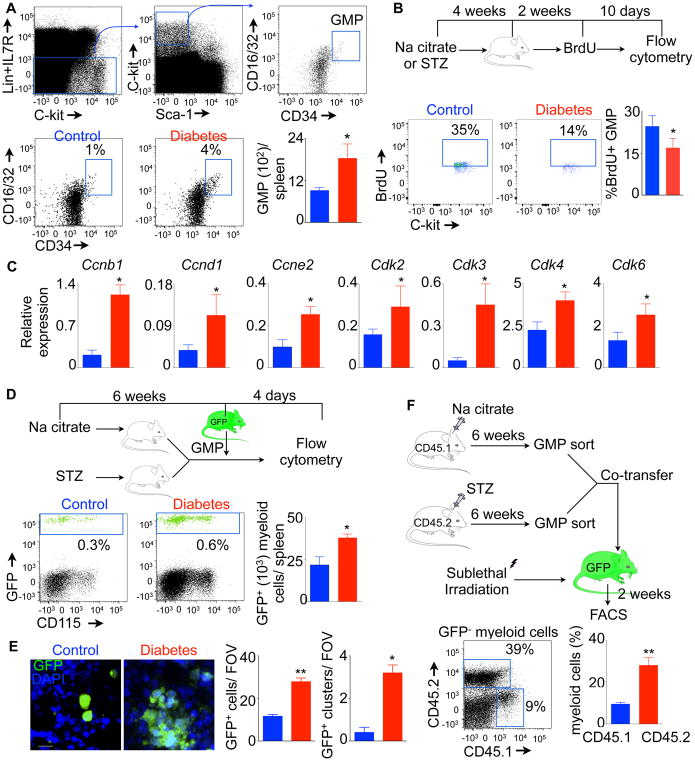
Activation and proliferation of GMP have been reported in cardiovascular disease such as atherosclerosis(Westerterp et al., 2012) and MI (Dutta et al., 2015). Activated GMP differentiate into myeloid cells, which are mobilized to sites of inflammation. To investigate the source of myeloid cells in diabetes, we enumerated GMP in the spleen of diabetic mice. We found about 2-fold increase in GMP numbers in the spleen of diabetic mice (Figure 2A), indicating a higher proliferation rate of the progenitors in diabetes. To test GMP proliferation in diabetic mice, we used BrdU, which can be incorporated into the DNA strand during the S phase of the cell cycle. After 2 weeks of BrdU pulse (Figure 2B), we found that most of the splenic progenitors were BrdU+ (Figure S1F). The dilution of BrdU in the progenitor cells of diabetic mice was higher than non-diabetic control mice (Figure 2B), suggesting that the splenic progenitor cells proliferated highly in diabetic mice. Additionally, genes involved in cell cycle progression, such as cyclins (Ccnb1, Ccnd1 and Ccne2) and cyclin-dependent kinases (Cdk2, 3, 4 and 6) were enriched in splenic GMP of diabetic mice (Figure 2C). Altogether, these data indicate that diabetes drives splenic GMP into the cell cycle.
Fig. 2. Splenic GMP proliferate and differentiate into myeloid cells in diabetic C57BL/6 mice.
A) Shows representative flow cytometric gating strategy and quantification of splenic GMP. B) Quantification of BrdU+ GMP at the end of 10 days of chase after BrdU saturation. C) Quantitation of cell cycle regulating genes in splenic GMP using quantitative RT-PCR. Enumeration of donor-derived myeloid cells in the spleen after GFP+ GMP transfer in diabetic and non-diabetic control mice using flow cytometry (D) and confocal microscopy (E). Scale bar = 10 μm. F) Quantification of donor GMP-derived myeloid cells in sublethally irradiated mice. With an exception of Fig. 2F, all data are pooled from at least two independently performed experiments. n=10/group (A) and n=5–6 mice/group (B–F). Mean ± s.e.m. * P < 0.05, ** P < 0.01. Please also see Figure S1.
Highly proliferative GMP in the spleen of diabetic mice can differentiate into myeloid cells(Herault et al., 2017). To ascertain differentiation ability of splenic GMP in diabetic mice, we isolated the progenitor cells from GFP+ mice and injected into either diabetic or non-diabetic mice (Figure 2D). We found that the donor-derived GMP generated higher number of myeloid cells in the spleen of diabetic mice. Confocal microscopy of the spleen revealed GMP transferred into diabetic mice produced higher number of progenies and clusters compared to non-diabetic control mice, indicating the active proliferation and differentiation of GMP in diabetes(Herault et al., 2017) (Figure 2E). Next, we investigated if the splenic progenitors isolated from diabetic mice retain higher proliferation ability in non-diabetic mice. Towards this end, we isolated GMP from congenically different diabetic and non-diabetic control mice and co-transferred them in sub-lethally irradiated GFP+ non-diabetic mice (Figure 2F). Two weeks after the transfer, we found that GMP-derived from diabetic donors produced higher proportion of myeloid cells in the spleen. Taken together, these data suggest that splenic GMP in diabetic mice have higher proliferation and differentiation ability.
Splenic GMP differentiation and myelopoiesis in diabetes are regulated by sympathetic neuronal activity
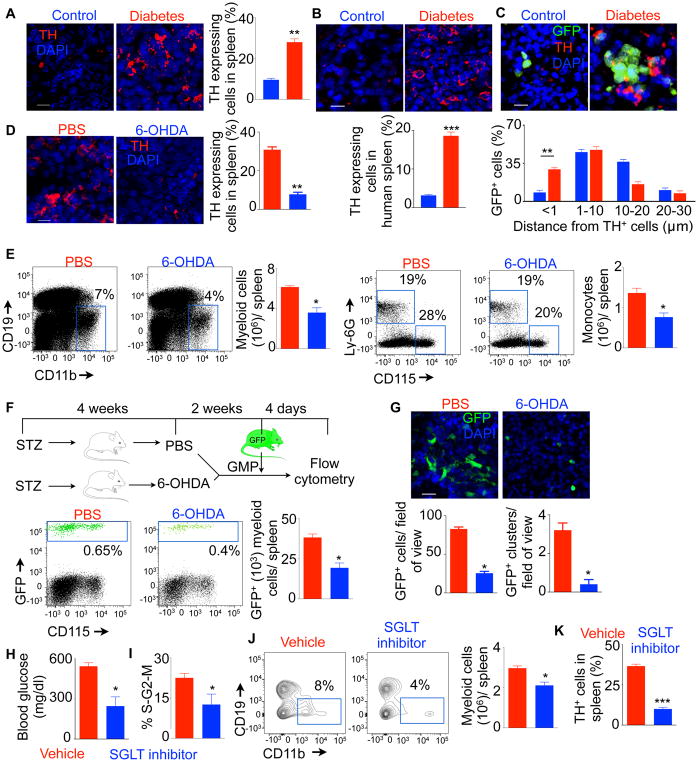
High sympathetic neuronal activity in the BM of diabetic mice(Ferraro et al., 2011) and humans(Esler et al., 2001; Seals and Bell, 2004; Thorp and Schlaich, 2015) has been reported. To investigate sympathetic neuronal activity in the spleen in diabetes, we stained spleen sections for tyrosine hydroxylase (TH), a rate limiting enzyme in catecholamine synthesis. We found increased number of TH-expressing splenocytes in diabetic mice (Figure 3A) and patients (Figure 3B). Confocal microscopy of the spleen after GFP+ GMP transfer revealed that GMP formed clusters near TH-expressing cells in diabetic mice (Figure 3C, Supplementary Movie 1 and Supplementary Movie 2). In fact, about 30% of GFP+ cells derived from the transferred GMP were within <1 μm distance from TH+ splenocytes. To investigate the effect of sympathetic neuronal activation on splenic myelopoiesis in diabetic mice, we performed sympathetic neuronal ablation with 6-hydroxydopamine (6-OHDA). 6-OHDA treatment reduced TH-producing splenocytes (Figure 3D). Concomitantly, the spleens of diabetic mice after 6-OHDA treatment contained about 2-fold reduced myeloid cells and monocytes compared to PBS-treated control (Figure 3E). Four days after adoptive transfer of GMP, the spleens of 6-OHDA-treated mice had fewer GFP+ myeloid cells than PBS-treated mice (Figure 3F). Confocal microscopy of the spleen demonstrated similar reduction of GFP+ cell numbers and clusters after 6-OHDA treatment (Figure 3G, Supplementary Movie 2 and Supplementary Movie 3). Collectively, these data indicate that higher activity of sympathetic neurons in the spleen of diabetic mice induces GMP differentiation into myeloid cells.
Fig. 3. Splenic myelopoiesis is regulated by sympathetic neuronal activity in diabetic C57BL/6 mice.
Immunofluorescent confocal images and quantification of tyrosine hydroxylase (TH)+ cells in the spleen of control and diabetic C57BL/6 mice (A) (Scale bar = 20 μm.) and humans (B) (Scale bar = 10 μm.). C) Measurement of distances between GFP+ splenocytes derived from transferred GMP and TH+ cells in the spleen. Scale bar = 10 μm. D) TH-stained cells in the spleen after 6-OHDA treatment. Scale bar = 20 μm. E) Representative flow cytometric plots and bar graphs showing quantification of myeloid cells after 6-OHDA treatment in the diabetic mice. Quantification of donor GMP-derived GFP+ myeloid cells after 6-OHDA treatment by flow cytometry (F) and confocal microscopy (G) (Scale bar = 20 μm). H) Fasting blood glucose concentrations after SGLT inhibitor treatment. Flow cytometric analysis for GMP proliferation (I) and myeloid cell enumeration (J). K) The frequency of TH-expressing splenic leukocytes was determined using confocal microscopy. All data except H–K are pooled from at least two independently performed experiments. n=8/ group (A–C), n=10/ group (D), n=6/ group (E–G) and n=4 / group (H–K). Mean ± s.e.m. * P < 0.05, ** P < 0.01, *** P < 0.001.
Hyperglycemia induces myelopoiesis and TH expression in splenic leukocytes
Next we investigated the mechanisms of myelopoiesis in diabetic mice. Because type 1 diabetic mice have reduced insulin and high blood glucose concentrations, we assessed if hyperglycemia in these mice triggers myeloid cell development. To this end, we injected the mice with phlorizin, an inhibitor of sodium-glucose co-transporters (SGLT). This treatment reduced the fasting blood glucose concentrations (Figure 3H). Concomitantly, splenic GMP proliferation (Figure 3I) and myeloid cell number (Figure 3J) were diminished. Furthermore, the spleens of diabetic mice treated with phlorizin contained fewer TH-expressing splenocytes (Figure 3K). In aggregate, these data indicate that hyperglycemia induces myelopoiesis and TH expression in splenocytes of diabetic mice.
β2 adrenergic receptor blockade reduces splenic GMP proliferation and myelopoiesis
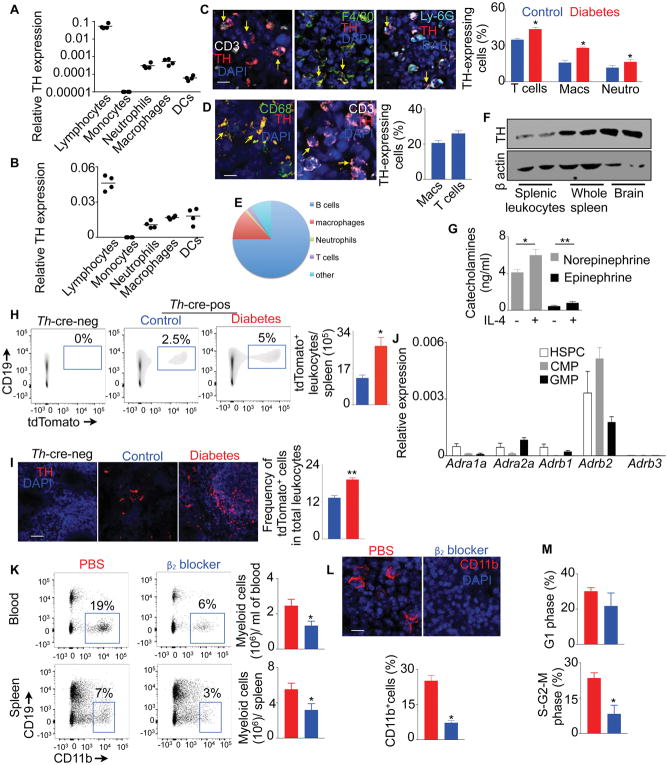
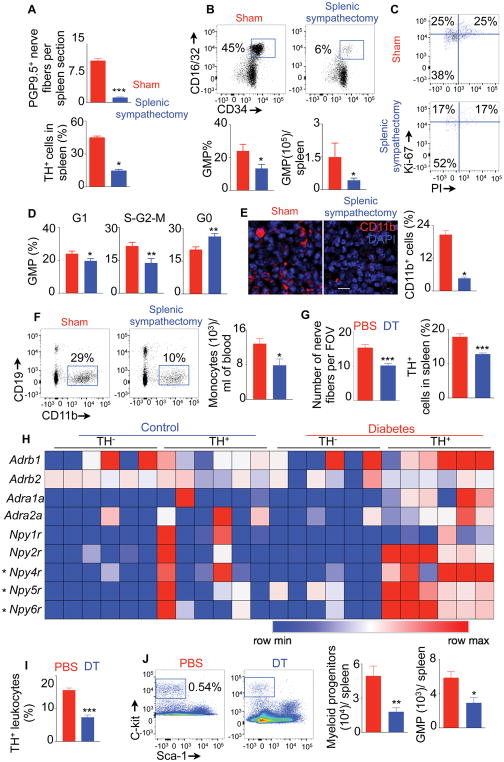
Since spleens of diabetic mice harbored increased number of TH+ splenocytes, we investigated cellular sources of TH. We observed that TH+ cells were CD45+, confirming that they are leukocytes (Figure S2A). As expected, splenic nerves (PGP9.5+) also expressed TH. Lymphocytes, neutrophils and macrophages sorted from spleens of diabetic mice (Figure 4A) and humans (Figures 4B & S2B) expressed TH mRNA. Consistently, confocal microscopy of splenic sections of diabetic mice (Figure 4C) and humans (Figure 4D) showed that these splenocytes produced TH protein. Spleens of diabetic mice harbored higher proportions of TH-expressing T cells, macrophages and neutrophils. To further confirm that leukocytes produce TH, we generated a reporter mouse (TH-tdTomato) that express tdTomato in TH-expressing cells. We checked tdTomato expression in splenic leukocytes using flow cytometry (Figures 4E & S3A) and confocal microscopy (Figure S3B). We observed that B lymphocytes and macrophages were the most abundant TH-expressing cells in the spleen. We also confirmed TH expression in isolated splenic leukocytes by immuno blot (Figure 4F). Consistent with low TH mRNA expression (Figure 4A & 4B), splenic monocytes did not express TH protein (Figure S3C). To determine if monocytes express TH after they differentiate into macrophages, we differentiated THP-1 cells, a human monocyte cell line. After differentiating into macrophages, these cells elevated Th expression (Figure S3D). Consistent to their TH expression, macrophages produced NE and epinephrine (Figure 4G). The production of the catecholamines increased in presence of IL-4, consistent with a previous finding(Nguyen et al., 2011). Flow cytometry (Figure 4H & S3E) and confocal imaging (Figure 4I, S3F, and Supplementary Movie 4 & 5) confirmed that the spleens of diabetic mice harbored elevated numbers of tdTomato+ leukocytes, B cells, macrophages, neutrophils and T cells.
Fig. 4. β2 adrenergic receptor blockade reduces splenic myelopoiesis.
Quantitation of TH expression by lymphocytes (CD11b− CD3+ or CD19+), monocytes (CD3− CD19− CD11b+ CD115+ Ly-6G−), neutrophils (CD3− CD19− CD11b+ CD115− Ly-6G+), macrophages (CD3− CD19− F4/80+ CD64+) and DCs (CD3− CD19− F4/80− CD11c+) sorted from the spleens of non-diabetic C57BL/6 mice (A) and humans (B) using quantitative RT-PCR. Please also see Figure S2. Quantification of TH-expressing splenic T cells, macrophages and neutrophils in mouse (C) (Scale bar = 10 μm) and human (D) (Scale bar = 10 μm) spleens by confocal microscopy. E) Distribution of TH-tdTomato+ cells among various leukocyte population in non-diabetic Th-cre-ROSA-tdTOmato mice. Please also see Figure S3. F) Immuno blot to detect TH in sorted splenic leukocytes, whole spleen and brain of non-diabetic wild type mice. G) Catecholamine production by splenic macrophages in culture in the presence or absence of IL-4. Quantification of tdTomato+ leukocytes in Th-cre-ROSA-tdTOmato mice by flow cytometry (H) and immunofluorescence (I) (Scale bar = 100 μm). J) Quantification of mRNA amounts of the adrenergic receptors expressed by splenic hematopoietic progenitors sorted from non-diabetic mice using quantitative RT-PCR. K) Flow cytometric enumeration of myelo cells in the blood and spleen after β2 blocker treatment in diabetic mice. L) Confocal images and quantification of myeloid cells in the spleen after β2 blocker treatment in diabetic mice (Scale bar = 10 μm). M) Flow cytometric analysis of the cell cycle of GMP after β2 blocker treatment in diabetic mice. All data except Fig. 4B are pooled from two independently performed experiments. n=4/ group (A–D), n=2/ group (F), n=7/group (E, H & I), n=5/ group (G) and n=9–10/ group (K–M). Mean ± s.e.m. * P < 0.05, ** P < 0.01.
The catecholamines bind to the adrenergic receptors and mediate a cascade of cellular responses. BM multipotent progenitors express adrenergic receptors(Muthu et al., 2007). Similarly, we found that splenic GMP and other hematopoietic progenitors in mice expressed high amounts of the β2 adrenergic receptor (Figure 4J), which is consistent with high expression of this adrenergic receptor in human GMP (Figure 1E). A specific β2 blocker treatment reduced myeloid cell numbers in the blood and spleen (Figures 4K & 4L). Consistently, the treatment lowered splenic GMP proliferation (Figure 4M). Taken together, these data indicate that β2 adrenergic receptor signaling mediates splenic GMP proliferation and their differentiation into myeloid cells.
Sympathetic neuronal regulation of myelopoiesis in type 2 diabetic mice
We found about 2-fold increase in splenic myeloid cell, monocyte and Ly-6Chi monocyte content after feeding C57BL/6 mice with a high fat diet for 16 weeks (Figure 5A), similar to type 1 diabetic mice. These diabetic mice had increased number of highly proliferative splenic GMP (Figures 5B & 5C). Additionally, spleens harbored higher number of TH+ leukocytes, macrophages and T cells (Figure 5D). Finally, to investigate the role of adrenergic signaling in myelopoiesis in type 2 diabetes, we adoptively transferred DiO-labeled GMP from β1 and β2 adrenergic receptor-deficient (Adrb1−/− and Adrb2−/−) or wild type mice into diabetic mice. Four days after the adoptive transfer, we found that GMP isolated from Adrb1−/− and Adrb2−/− diabetic mice produced lower number of myeloid cells in the spleen (Figure 5E). Collectively, these data show that myelopoiesis in type 2 diabetic mice is dependent on local splenic catecholamine exposure.
Fig. 5. Sympathetic neuronal activation triggers myelopoiesis in type 2 diabetes.
C57BL/6 mice were fed with a high fat diet for 16 weeks. A) Flow cytometric enumeration of myeloid cells in the spleens. B) Quantification of splenic GMP. C) Measurement of GMP proliferation with a BrdU dilution assay. D) Quantification of TH+ leukocytes, macrophages and T cells in the spleen. (Scale bar = 10 μm). E) Schematic representation of the experimental design and quantification of donor GMP-derived myeloid cells. All of the experiments were performed at least twice. n=8/ group (A&B) and n=8–12 mice/ group (C–E). Mean ± s.e.m. * P < 0.05, ** P < 0.01. See also Figure S6.
Splenic sympathectomy reduces GMP proliferation and diminishes myelopoiesis
Our data indicated that SNS activation in diabetes increased splenic myelopoiesis. To investigate if this effect is specific to splenic sympathetic neuronal activation, we performed surgical splenic sympathectomy in diabetic mice. Splenic sympathectomy reduced PGP9.5+ nerve fibers in the spleen (Figure 6A), indicating successful denervation. Moreover, the surgery reduced TH+ splenic leukocyte numbers and also diminished GMP proportions and numbers (Figure 6A & 6B). Furthermore, the mice with splenic sympathectomy had lower percentage of GMP in the S-G2-M phases (proliferating cells) and higher percentage of GMP in the G0 phase (quiescent cells) of the cell cycle (Figure 6C & 6D). In contrast, GMP number (Figure S4A) and proliferation (Figure S4B) in the BM did not alter after splenic sympathectomy. Moreover, splenic sympathectomy reduced splenic monocyte numbers (Figure S4C); however, the surgery did not change myeloid cell content in the BM (Figure S4D), indicating the organ-specific effect after splenic sympathectomy. Consistent with diminished splenic GMP number and proliferation, the spleen (Figure 6E) and blood (Figure 6F) contained declined number of CD11b+ myeloid cells and monocytes, respectively, after splenic sympathectomy.
Fig. 6. Splenic sympathectomy and TH+ leukocyte depletion diminish diabetes-induced splenic myelopoiesis.
Quantification of splenic PGP9.5+ nerve fibers and TH-expressing cells (A), enumeration of splenic GMP (B) and cell cycle analysis of splenic GMP (C&D) after surgical splenic sympathectomy. E) Confocal images and quantification of CD11b+ myeloid cells in the spleen after surgical splenic sympathectomy. (Scale bar = 10 μm). F) Quantification of monocytes in the blood using flow cytometry. Scale bar = 10 μm. G) Quantification of splenic nerve termini and TH-expressing leukocytes in the spleen after DT injection in Th-cre-ROSA-iDTR mice transplanted with wild type BM cells. H) Adrenergic receptors and neuropeptide Y (NPY) receptors were quantified by PCR array in TH+ and TH− cells isolated from the spleens of non-diabetic and diabetic Th-cre-ROSA-tdTomato mice. I) Quantification of TH+ splenic leukocytes after DT injection in wild type mice reconstituted with BM from Th-cre-ROSA-iDTR. J) Myeloid progenitor and GMP numbers in the spleen determined by flow cytometry after TH+ leukocyte depletion. All data are pooled from at least two independently performed experiments. n=8–9/group (A–D), n=8/group (E–G) and n=6–7 mice/ group (H–J). Mean ± s.e.m. *P < 0.05, ** P < 0.01, *** P < 0.001. Please also see Figure S4.
TH+ leukocytes are equipped with receptors involved in neuroimmune signaling
After splenic sympathectomy, we observed reduction in TH expression in leukocytes (Figure 6A). This suggests a feed forward loop of adrenergic signaling from nerves to TH-expressing hematopoietic cells. To investigate this more carefully, we determined the distance of TH+ and TH− splenic leukocytes from splenic nerves. About 40% of TH+ and only 15% of TH− leukocytes were within 1 μm distance from splenic nerves (Figure S4E & S4F), indicating their direct contact. We generated mice that express diphtheria toxin receptor (DTR) under Th promoter in neurons by transplanting wild type BM cells in lethally irradiated Th-cre-ROSA-iDTR mice. We induced diabetes in these mice and administered DT locally in the spleen using micro-osmotic pumps. DT administration reduced nerve fibers and TH-expressing leukocytes in the spleen (Figure 6G). Collectively, these data indicate a feed forward loop of adrenergic signaling from nerves to TH-expressing hematopoietic cells. To determine if TH+ leukocytes are more effective in neuroimmune communication than the TH− leukocytes, we sorted these cells from the spleens of non-diabetic and diabetic Th-tdTomato mice. We observed that TH+ leukocytes isolated from diabetic mice had higher expression of the receptors for neuropeptide (NP) Y4, Y5 and Y6, involved in neuroimmune communication(Straub et al., 2000), compared to TH− leukocytes (Figure 6H). Furthermore, TH+ cells had elevated expression of Npy4r, Npy5r and Npy6r in diabetic mice compared to non-diabetic mice. These data suggest that NPY produced by splenic sympathetic nerve termini mediate nerve to TH+ leukocyte communication in diabetes.
TH+ leukocytes mediate myelopoiesis
We found that TH+ leukocytes are in close contact with GMP clusters, suggesting the role of TH+ leukocytes in myeloid cell production. To test this, we generated mice that express the DTR under Th promoter in hematopoietic cells (Figure S4G). We found that TH+ leukocyte depletion with DT (Figure 6I) resulted in reduced myeloid progenitor and GMP numbers (Figure 6J), and diminished myeloid cell and monocyte contents in the spleen and blood (Figure S4H and S4I). Next, we investigated if splenic nerves are important for myelopoiesis in diabetes. To accomplish this, we used mice expressing DTR under Th promoter in neurons. Depletion of splenic nerves by local administration of DT using micro-osmotic pumps in these mice resulted in diminished myeloid cell and monocyte numbers in the spleen (Figure S4J) and GMP proliferation (Figure S4K).
Ablation of sympathetic neuronal activity ameliorates diabetes complication
The major cause of death of diabetic patients is cardiovascular disease, such as atherosclerosis (Howard and Wylie-Rosett, 2002; Wendt et al., 2002), which is a complication of diabetes. To investigate this, we induced diabetes in atherosclerotic Apoe−/− mice with stz injection. Consistent to reported insidious role of myeloid cells in plaque progression and rupture(Libby, 2012, 2013; Libby et al., 2011), we found highly inflamed (Figures S5A & S5B) and vulnerable (Figure S5C) plaques in diabetic Apoe−/− mice. These findings complemented previously published data on plaque macrophages in diabetic mice(Johansson et al., 2008; Kanter et al., 2012; Parathath et al., 2011).
Additionally, we found that the diabetic Apoe−/− mice had increased numbers of myeloid cells, monocytes, Ly-6Chi monocytes and neutrophils in the spleen and blood (Figure S6A & S6B). The mice also had increased GMP numbers in the spleen (Figure S6C), suggesting the role of splenic hematopoiesis in diabetes-associated atherosclerosis. In line with this, splenectomy in diabetic Apoe−/− mice reduced myeloid cell and inflammatory monocyte numbers in the aorta (Figure S6D), resulting in reduced atherosclerotic plaque areas and thicker fibrous caps (Figure S6E).
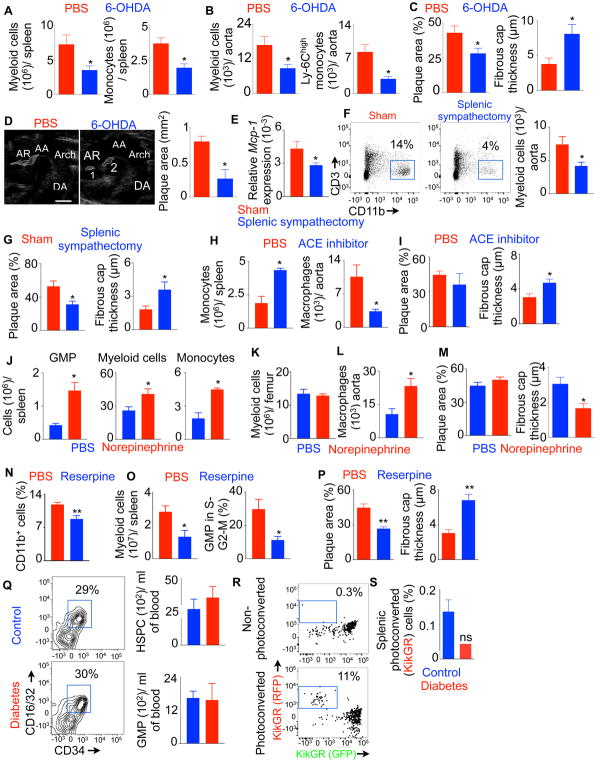
Splenic myelopoiesis has been shown to increase inflammation and vulnerability of atherosclerotic plaques(Swirski et al., 2007; Tall and Yvan-Charvet, 2015). Since we found sympathetic neuronal ablation with 6-OHDA decreased splenic myelopoiesis in diabetic mice (Figure 3), we investigated if 6-OHDA treatment could diminish inflammation in atherosclerotic lesions of diabetic mice. We found reduced splenic myeloid cell and monocyte numbers in 6-OHDA-treated mice (Figures 7A, S7A & S7B), confirming that sympathetic neuronal ablation curbs diabetes-induced splenic myelopoiesis. Next, we enumerated myeloid cells in the aortas of Apoe−/− mice (Figures 7B, S7C & S7D). We observed declined number of myeloid cells and inflammatory monocytes in the aortas of Apoe−/− mice after sympathetic neuronal ablation. Masson’s trichrome staining of aortic root sections demonstrated smaller plaque size and thicker fibrous caps in diabetic mice after the chemical sympathectic blockade (Figure 7C & S7E). To measure plaque area in vivo, we performed ultrasound imaging and found reduced plaque area in 6-OHDA-injected diabetic Apoe−/− mice (Figure 7D). Altogether, these results indicate that sympathetic neuronal blockade in diabetic mice improves the features of stable atherosclerotic lesions.
Fig. 7. Sympathetic neuronal activation in diabetes accelerates atherosclerosis.
A) Flow cytometric quantification of splenic myeloid cells in diabetic Apoe/− mice after 6-OHDA treatment. B) The bar graphs depict quantification of myeloid cells in the aorta. C) Quantification of atherosclerotic plaque area and fibrous cap thickness by Masson’s trichrome staining. D) Representative ultrasound images showing plaques in the aortic root. The bar graph shows quantification of atherosclerotic plaque area. (Scale bar = 1 mm). E) Relative Mcp-1 expression in the aorta of diabetic Apoe−/− mice after sham surgery or splenic sympathectomy. Enumeration of myeloid cells in the aortas (F) and histological analysis of atherosclerotic plaques (G) of Apoe−/− mice at 4 weeks after splenic sympathectomy. ACE inhibitor, NE, reserpine or PBS was administered into the spleens of diabetic Apoe−/− mice using micro-osmotic pumps for three weeks. H–P) Myeloid cells in the spleen, BM and aorta were enumerated by flow cytometry. Atherosclerotic plaque area and fibrous cap thickness were quantified using Masson’s trichrome staining. n=5 per group. Q) Hematopoietic progenitors in the blood were quantified in non-diabetic (control) and diabetic mice. n=4 per group. R) Representative flow cytometric plots showing photoconverted cells in the calvarium of KikGR mice. S) Enumeration of the photoconverted cells in the spleen. n=3–11 per group. n=6–8/ group (A,B,C), n=8–9/ group (D–G), n=4–5/ group (H–Q) and n=3–11 mice/ group (R&S). Mean ± s.e.m. * P < 0.05, ** P < 0.01. Please also see Figures S5–7.
Because splenic sympathectomy reduced myeloipoiesis in diabetic mice (Figure 6) and myeloid cells generated in the spleen aggravates atherosclerosis pathogenesis(Swirski et al., 2007; Tall and Yvan-Charvet, 2015), we investigated the effect of splenic sympathectomy in atherosclerosis. We found that aortas of diabetic Apoe−/− mice, after splenic sympathectomy, had reduced amounts of mRNA of monocyte chemoattractant protein-1 (Figure 7E), responsible for myeloid cell infiltration into atherosclerotic lesions. In line with this finding, we observed lower number of myeloid cells in the aortas of splenic sympathectomized mice (Figure 7F). Since splenic myelopoiesis augments inflammation and vulnerability of atherosclerotic plaques(Swirski et al., 2007; Tall and Yvan-Charvet, 2015), we checked the features of plaque vulnerability, such as plaque area and fibrous cap thickness and found that surgical denervation of splenic sympathetic neurons reduced plaque area and increased fibrous cap thickness (Figures 7G & S7F).
Next, we determined the contribution of spleen in supplying inflammatory myeloid cells to atherosclerotic plaques. Three weeks after spleen transplantation, myeloid cell enumeration revealed an increase in number of donor-derived myeloid cells, monocytes and neutrophils in the aorta and blood of diabetic recipient mice (Figure S7G). Because the egress of monocytes from the spleen depends on angiotensin 2(Swirski et al., 2009), we delivered an angiotensin converting enzyme (ACE) inhibitor into the spleen of diabetic Apoe−/− mice using micro-osmotic pumps for 3 weeks. ACE inhibitor treatment increased monocyte numbers in the spleen, whereas macrophage numbers in the aorta decreased (Figures 7H & S7H), indicating their reduced egress from the spleen. These mice also had thicker fibrous caps of atherosclerotic lesions compared to the control group (Figure 7I). To ascertain the effect of NE on splenic myelopoiesis, we exogenously administered NE in diabetic Apoe−/− mice using micro-osmotic pumps. The treatment resulted in elevated GMP and myeloid cell numbers in the spleen (Figure 7J) but not in the BM (Figure 7K). We further observed increased macrophages in the aorta (Figure 7L) and thinner fibrous cap in atherosclerotic plaques (Figures 7M & S7I) compared to the vehicle-treated group.
To determine the role of catecholamines secreted by splenic nerves in myelopoiesis and atherosclerosis, we injected diabetic Apoe−/− mice with reserpine in the spleen using micro-osmotic pumps. Reserpine blocks the uptake of catecholamines into synaptic vesicles by inhibiting the vesicular monoamine transporter 2(Rosas-Ballina et al., 2008). However, it did not reduce catecholamine secretion by leukocytes in culture (Figure S7J). In contrast to this finding, reserpine not only reduced catecholamine production by splenic nerve fibers but also by splenic leukocytes in vivo (Figure S7K), indicating a crosstalk between splenic nerves and leukocytes. Reserpine treatment reduced splenic myelopoiesis (Figures 7N & 7O). Concomitantly, atherosclerotic plaque burden decreased, and fibrous cap thickness increased in these mice compared to PBS-injected group (Figure 7P). These data indicate that catecholamines secreted by splenic nerve termini and leukocytes are important for myelopoiesis and atherosclerosis.
Collectively, these data indicate that splenic sympathetic neuronal activation in diabetes-associated atherosclerosis amplifies inflammatory myeloid cell production by increasing GMP proliferation and differentiation. These newly produced inflammatory myeloid cells in the spleen infiltrate into atherosclerotic plaques and increase the features of plaque vulnerability.
BM does not contribute to splenic myeloid expansion in diabetes
Consistent with a published report(Nagareddy et al., 2013), we found that the BM of diabetic mice harbored higher numbers of myeloid cell subsets compared to non-diabetic mice (Figure S7L). However, we did not observe any difference in the number of TH+ cells in BM between these two groups (Figure S7M). Consistently, systemic DT administration in mice expressing iDTR in TH+ hematopoietic cells (Figure S7N) and β2 blocker treatment (Figure S7O) did not alter myeloid progenitor and GMP numbers in the femur. In aggregate, these data indicate that increased BM myelopoiesis in diabetic mice is not due to augmented sympathetic neuronal activity.
To assess if splenic myeloid expansion is secondary to BM myelopoiesis, we enumerated HSPC and GMP in the blood of diabetic mice. We did not observe an increase in the number of these progenitor populations in the blood of diabetic mice compared to non-diabetic mice (Figure 7Q), with is consistent to a recent finding (Ferraro et al., 2011). To address definitively if BM hematopoietic progenitors mobilize to the spleen in diabetic mice, we generated KikGR transgenic mice that express a Kikume Green-Red photoconvertible fluorescent protein under the control of a chicken beta actin promoter. Lin− C-kit+ progenitor cells isolated from these mice were adoptively transferred in non-diabetic and diabetic wild type mice. Four days after the transfer, the GFP+ donor cells in the calvarium were photoconverted using an intravital microcope (Figure 7R). After photoconversion, GFP in these cells changed to RFP. Seven days after the photoconversion, we assessed the number of photoconverted (RFP+) cells in the spleen to determine mobilization of these cells from the BM to the spleen. We did not find an increase in photoconverted cell numbers in the spleen of diabetic mice (Figure 7S). The spleens of diabetic mice contained less number of photoconverted cells although this decrease in cell number did not reach statistical significance, supporting the finding that diabetic patients have impaired mobilization of hematopoietic progenitors from the BM (Ferraro et al., 2011). Altogether, these data indicate that diabetes does not induce mobilization of BM hematopoietic progenitor cells to the spleen; rather splenic hematopoietic progenitors proliferate and differentiate into myeloid cells.
DISCUSSION
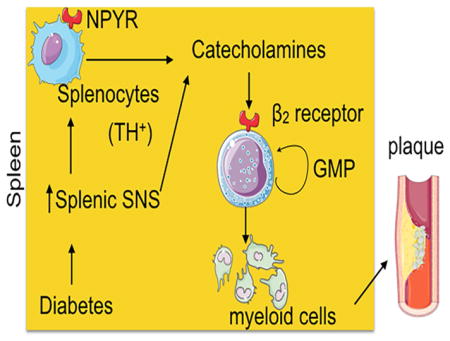
Sympathetic neuronal activation in diabetes is well known(Esler et al., 2001; Seals and Bell, 2004; Thorp and Schlaich, 2015). We found a significant correlation between plasma concentration of catecholamines and circulatory leukocyte counts in patients, suggesting the role of SNS activation in leukocyte production. The effect of the SNS and catecholamine signaling on GMP proliferation and their differentiation into myeloid cells had not been studied. We serendipitously found higher number of TH+ cells in the spleens of diabetic patients and mice. Reduced GMP proliferation and differentiation in diabetic mice after splenic sympathectomy indicates that higher sympathetic neuronal tone induces GMP proliferation. Additionally, sympathectomized spleen harbored reduced number of TH+ cells. Mice lacking TH+ cells exhibited reduced GMP proliferation and myelopoiesis. These observations indicate that diabetic mice have elevated sympathetic neuronal tone, which results in additional catecholamine production by splenic leukocytes. The data presented in the study indicate that both splenic nerve and TH+ leukocytes are crucial to splenic myelopoiesis. Furthermore, the experiment using reserpine to block postsynaptic release of catecholamines also reduced catecholamine production by leukocytes, indicating that catecholamines produced by splenic nerve termini trigger catecholamine production by leukocytes. In line with this, we found sympathetic neuronal denervation and DT-mediated depletion of splenic nerves resulted in declined frequency of TH+ leukocytes in the spleen. Because catecholamine production by leukocytes depends on splenic neurons, determining the specific roles of catecholamines produced by these two cell types is beyond the scope of this study. Additionally, we observed that TH+ leukocytes were located close to splenic nerves and had higher expression of neuropeptide Y receptors, which are involved in neuroimmune communication. Future mechanistic studies are required to determine if the expression of these receptors in TH+ cells is crucial to splenic myelopoiesis in diabetes.
Splenic emergency myelopoiesis has been studied in different diseases such as cancer(Cortez-Retamozo et al., 2012), hyperlipidemia(Robbins et al., 2012) and MI (Leuschner et al., 2012; Swirski et al., 2009). Myelopoiesis in the spleen maintains a steady flow of myeloid cells to sites of inflammation, such as atherosclerotic plaques and infarcted myocardium. Given that these cells play a critical role in tissue repair after a sterile injury and splenic monocytes exhibit a more vigorous inflammatory gene signature than BM monocytes(Dutta et al., 2012), it is imperative to know the mechanisms of splenic myelopoiesis.
The major cause of death of diabetic patients is cardiovascular complications(Howard and Wylie-Rosett, 2002; Wendt et al., 2002), primarily MI due to atherosclerotic plaque rupture. Diabetes can exacerbate pre-existing atherosclerosis by increasing plaque size and intralesional hemorrhage(Renard et al., 2004). Moreover, induction of diabetes in mice with established atherosclerosis causes plaque disruption in the brachiocephalic artery(Johansson et al., 2008). Consistently, we found that atherosclerotic plaques of diabetic mice had thinner fibrous caps, a feature of plaque vulnerability. Vulnerable atherosclerotic plaques in diabetic mice harbor highly proliferative and inflammatory macrophages(Kanter et al., 2012), which is in line with our data showing high number of plaque-dwelling myeloid cells. Although the contribution of plaque macrophages in diabetes-induced exacerbation of atherosclerosis is well accepted, the source of myeloid cells is not clear. Our data suggest that the splenic myelopoiesis is one of the sources of plaque macrophages in diabetic mice. Of note, plaque macrophages can also be derived from aortic smooth muscle cells(Shankman et al., 2015). The role of smooth muscle cell-derived macrophages in diabetic atherosclerosis is currently unknown. We found that splenic myelopoiesis is regulated by sympathetic neuronal activation in diabetes, whereas augmented sympathetic neuronal tone in diabetes does not induce BM myelopoiesis. Future studies are warranted to investigate the mechanisms of BM myelooiesis in diabetes.
The present study has identified multiple therapeutic targets, such as splenic sympathetic nerve and the β2 adrenergic receptor on GMP, to curtail the production of inflammatory cells in diabetes. β1 blockers, such as metoprolol and carvedilol, are used in diabetic patients with hypertension. The effect of β1 blockers in hyperglycemia is controversial. Most studies showed that the treatment does not alter insulin sensitivity in diabetic patients(Bokhari et al., 2014; Ostman, 1983). In contrast, carvedilol treatment was shown to reduce new onset of diabetes(DiNicolantonio et al., 2015). In the present study, we have reduced splenic GMP proliferation and inflammatory myeloid cell generation with a selective β2 blocker and genetic deficiency of the adrenergic receptor β2. We recognize the possibility of off-target effects of these methods. Generation of mice lacking the β2 adrenergic receptor on GMP will help overcome this shortcoming.
Obesity and type 2 diabetes affect the function of hematopoietic stem(Nagareddy et al., 2014; Nagareddy et al., 2013; Orlandi et al., 2010; Trottier et al., 2012) and niche cells(Ferraro et al., 2011; Spinetti et al., 2013) in the BM. Our study focused on splenic myelopoiesis in the context of diabetes. The effect of the SNS on BM myelopoiesis remains to be investigated. Furthermore, the molecular mechanisms leading to sympathetic neuronal activation in diabetes remain to be explored. We found that GMP isolated from diabetic mice, when transferred into non-diabetic mice, can still proliferate and differentiate at a higher rate, indicating epigenetic changes of the progenitors in hyperglycemic environment. Future studies are required to identify the epigenetic marking responsible for continued proliferation and differentiation of GMP in normoglycemic environment.
STAR Methods
Key Resources Table
| Reagent or resource | Source | Identifier |
|---|---|---|
| Chemicals | ||
| 42% Kcal% high fat diet for Apoe−/− | Research Diets Inc | TD88137 |
| 6-Hydroxydopamine hydrobromide (6-OHDA) | Sigma-Aldrich | H116 |
| 60% Kcal% high fat diet | Research Diets Inc | D12492 |
| AEC substrate | ScyTek | ACJ500 |
| Bovine growth serum | Fisher Scientifics | SH3054103 |
| Bovine serum albumin | Fisher Scientifics | BP1600-1 |
| BrdU APC flow kit | BD Biosciences | 552598 |
| Captopril | Alfa Aesar | J63593 |
| cDNA synthesis kit | Applied Biosystems | 4387406 |
| Cell Strainer | Fisher Scientifics | 22363548 |
| Collagenase-1 | Sigma-Aldrich | C00130 |
| Collagenase-IX | Sigma-Aldrich | C7657 |
| Diphtheria Toxin | Sigma-Aldrich | D0654 |
| Dio | Invitrogen | V22886 |
| Dream Taq PCR Master mix (2X) | Thermo Scientific | K1071 |
| EasySep mouse biotin positive selection kit | Stem Cell Technologies | 18556 |
| Epinephrine/norepinephrine ELISA kit | Abnova | KA1877 |
| Fixation buffer for nuclear staining | BD biosciences | 554655 |
| Glucose test strips | Fisher Scientific | 23111276 |
| Glycoxylic acid monohydrate | Acros Organics | 120170250 |
| Hyalouronidase | Sigma-Aldrich | H35006 |
| ICI 118,551 | Sigma-Aldrich | I127 |
| Immuno-blot HRP substrate | Millipore | WBLUR0500 |
| L-Glutamine | Gibco | 25030-081 |
| Laemmli SDS sample buffer, reducing (6X) | Alfa Aesar | J61337 |
| Micro-osmotic pumps | Alzet | Model 1002 |
| MEM non-essential amino acids | Gibco | 11140-050 |
| Monobasic potassium phosphate | Acros Organics | 212595000 |
| Norepinephrine | Sigma-Aldrich | A7256 |
| Penicillin-streptomycin mixture | Fisher Scientifics | ICN1670249 |
| Permeabilization buffer for nuclear staining | BD Biosciences | 558050 |
| Phlorizin dihydrate | Sigma-Aldrich | P3449 |
| Picopure TM RNA isolation kit | Applied Biosystems | 1220401 |
| RBC lysis buffer | Biolegend | 420301 |
| Recombinant IL-4 | R&D | 204-IL |
| Reserpine | Alfa Aesar | L03506 |
| RIPA buffer | Alfa Aesar | J63324 |
| RPMI 1640 medium | HyClone | SH30027LS |
| Sodium citrate | Fisher Scientifics | BP327 |
| Streptozotocin | Sigma-Aldrich | S0130 |
| SYBR green PCR master mix | Applied Biosystems | A25776 |
| TRIzol | Fisher Scientifics | NC9574779 |
| Vectastain ABC-HRP kit | Vector Laboratories | PK6100 |
| Vector shield mounting medium with DAPI | Vector Laboratories | H1200 |
| Primers | ||
| Human ADRA1B-F CTTTCACGAGGACACCCTTAGC ADRA1B-R GCCCAACGTCTTAGCTGCTT |
IDT | |
| Human ADRA2A-F TCGTCATCATCGCCGTGTTC ADRA2A-R AAGCCTTGCCGAAGTACCAG |
IDT | |
| Human ADRB1-F ATCGAGACCCTGTGTGTCATT ADRB1-R GTAGAAGGAGACTACGGACGAG |
IDT | |
| Human ADRB2-FTTGCTGGCACCCAATAGAAGC ADRB2-R CAGACGCTCGAACTTGGCA |
IDT | |
| Human ADRB3-F GACCAACGTGTTCGTGACTTC ADRB3-R GCACAGGGTTTCGATGCTG |
IDT | |
| Human β actin-F CATGTACGTTGCTATCCAGGC β actin-R CTCCTTAATGTCACGCACGAT |
IDT | |
| Human TH-F GCTGGACAAGTGTCATCACCTG TH-R CCTGTACTGGAAGGCGATCTCA |
IDT | |
| Mouse Adra1b- F CGGACGCCAACCAACTACTT Adra1b- R AACACAGGACATCAACCGCTG |
IDT | |
| Mouse Adra2a- F GTGACACTGACGCTGGTTTG Adra2a- R CCAGTAACCCATAACCTCGTTG |
IDT | |
| Mouse Adrb1- F CTCATCGTGGTGGGTAACGTG Adrb1- R ACACACAGCACATCTACCGAA |
IDT | |
| Mouse Adrb2- F GGGAACGACAGCGACTTCTT Adrb2- R GCCAGGACGATAACCGACAT |
IDT | |
| Mouse Adrb3- F GGCCCTCTCTAGTTCCCAG Adrb3- R TAGCCATCAAACCTGTTGAGC |
IDT | |
| Mouse Ccnb1 | Taqman | Mm03055893_gH |
| Mouse Ccnd1 | Taqman | Mm00432359_m1 |
| Mouse Ccne2 | Taqman | Mm00438077_m1 |
| Mouse Cdk2 | Taqman | Mm00443947_m1 |
| Mouse Cdk3 | Taqman | Mm00518387_m1 |
| Mouse Cdk4 | Taqman | Mm00726334_s1 |
| Mouse Cdk6 | Taqman | Mm01311342_m1 |
| Mouse Gapdh-F AGGTCGGTGTGAACGGATTTG Gapdh-R TGTAGACCATGTAGTTGAGGTCA |
IDT | |
| Mouse Mcp-1 F GTCCCTGTCATGCTTCTGG Mcp-1R GCTCTCCAGCCTACTCATTG |
IDT | |
| Mouse Th-F GGCTTCTCTGACCAGGCGTAT Th-R TGCTTGTATTGGAAGGCAATCTC |
IDT | |
| Flow cytometry Antibodies | ||
| Human CD11C, BV650, APC | BD Biosciences | 563403, 559877 |
| Human CD123, BUV395, BV605 | BD Biosciences | 564195, 564197 |
| Human CD14-biotin | Biolegend | 325624 |
| Human CD14, APC, APC-Cy7 | BD Biosciences | 555399, 557831 |
| Human CD16, APC H7, BV711 | BD Biosciences | 560715, 563127 |
| Human CD169 (siglec-1), PE | BD Biosciences | 565248 |
| Human CD2-biotin | BD Biosciences | 555325 |
| Human CD206, PE-CF594, FITC | BD Biosciences | 564063,551135 |
| Human CD24, PerCP-Cy5.5 | BD Biosciences | 561647 |
| Human CD3-biotin | BD Biosciences | 555338 |
| Human CD3, BV510 | BD Biosciences | 563109 |
| Human CD4-biotin | BD Biosciences | 555345 |
| Human CD45, AF700 | BD Biosciences | 560566 |
| Human CD56, BV421 | BD Biosciences | 562751 |
| Human CD8-biotin | BD Biosciences | 555365 |
| Human HLA-DR, APC-R700 | BD Biosciences | 565127 |
| Mouse CD 115, PerCP-Cy5.5 | eBioscience | 46-1152-82 |
| Mouse CD 34, FITC | BD Biosciences | 553733 |
| Mouse CD 48, Pac Blue | Biolegend | 103418 |
| Mouse/Human CD11b-Biotin | Biolegend | 101204 |
| Mouse CD11b, APC-Cy7 | BD Biosciences | 557657 |
| Mouse CD11c-Biotin | Biolegend | 117304 |
| Mouse CD11c, BV510 | BD Biosciences | 562949 |
| Mouse CD150, Alexa Fluor 647 | Biolegend | 115918 |
| Mouse CD16/32, PE/Cy7, APC-Cy7 | Biolegend, BD Biosciences | 101318, 560541 |
| Mouse CD19, BV605 | BD Biosciences | 563148 |
| Mouse CD206, BV605 | Biolegend | 141721 |
| Mouse CD3 molecular complex, BV421 | BD Biosciences | 564008 |
| Mouse CD4-Biotin | Biolegend | 100404 |
| Mouse CD45.1, PE-Cy7 | Biolegend | 110730 |
| Mouse CD45.2, Pac Blue, Alexa Flour 700 | Biolegend | 109820, 109822 |
| Mouse CD48, Pac Blue | Biolegend | 103418 |
| Mouse CD49b (pan NK cells), PE | Biolegend | 108908 |
| Mouse CD8a-Biotin | Biolegend | 100704 |
| Mouse F4/80, PE-Cy7 | Biolegend | 123114 |
| Mouse Ly-6G/Ly-6C (Gr1)-Biotin | Biolegend | 108404 |
| Mouse IL-7R alpha (CD127) -Biotin | Biolegend | 135006 |
| Mouse IL7R alpha (CD127), BV711 | BD Biosciences | 565490 |
| Mouse Ki-67, BV605 | Biolegend | 652413 |
| Mouse Ly-6C, PerCP Cy5.5 | eBioscience | 45-5932-32 |
| Mouse Ly-6G, APC | Biolegend | 127614 |
| Mouse MHC-II, Pac Blue, PE-Cy7 | Biolegend, eBioscience | 107620, 25-5321-82 |
| Mouse NK1.1-Biotin | Biolegend | 108704 |
| Mouse NK1.1, PE | Biolegend | 108708 |
| Mouse Sca-1, Pac Blue, APC-Cy7 | Biolegend | 108120, 108126 |
| Mouse Ter119-Biotin | Biolegend | 116204 |
| Mouse- c-kit, PerCP | Biolegend | 105822 |
| Streptavidin BV605, BV510 | BD Biosciences | 563260, 563261 |
| Mice | ||
| Adrb1−/− and Adrb2−/− | JAX | 003810 |
| Apoe−/− | JAX | 002052 |
| B6GFP | JAX | 004353 |
| C57BL/6 | JAX | 000664 |
| CD45.1 | JAX | 002014 |
| KikGR | JAX | 013753 |
| ROSA 26iDTR | JAX | 007900 |
| ROSA tdTOmato | JAX | 007914 |
| Th cre | JAX | 025614 |
| Immunofluorescence and Immunoblot antibodies | ||
| Beta-actin | Cell Signaling Technology | 4970 |
| CD 3 | BD Biosciences | 557306 |
| CD 11b | Abcam | Ab133357 |
| CD 45 | Sigma Aldrich | SAB4502541 |
| CD 68 | eBioscience, Abcam | 14-0681-82, ab955 |
| F4/80 | Invitrogen | MA1-91124 |
| Ly6G | Biolegend | 127602 |
| PGP 9.5 | Invitrogen | 38-1000 |
| Tyrosine Hydroxylase | Millipore | AB152 |
| Software and algorithms | ||
| Prism 6 and 7 | GraphPad | NA |
| Fiji (Image J) | https://fiji.sc | https://fiji.sc |
| Imaris | Bitplane | NA |
| FlowJo | NA | NA |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Partha Dutta (duttapa@pitt.edu).
Experimental model and subject details
Patient samples
Peripheral blood from diabetic patients was collected at Academic Medical Center, Amsterdam, the Netherlands. Diabetes was defined as Hba1c concentrations equal or more than 47 mmol/mol. Blood samples from age-matched patients with Hba1c less than 40 mmol/mol were used as control. After ficol gradient-mediated separation of leukocytes, the cells were stored in 10% DMSO-containing media. Plasma samples and frozen leukocytes were shipped on dry ice to the University of Pittsburgh Medical Center for flow cytometric and catecholamine analysis. Human spleens were collected through a warm autopsy program at the University of Pittsburgh Medical Center under CORID 724.
Mice, diabetes induction and sympathetic ablation
All the strains of mice were obtained from Jackson Laboratories and housed in UPMC Animal Facility in individually ventilated cages and taken veterinary care under the supervision of Division of laboratory animal resources (DLAR). All facilities are USDA registered, covered under an Assurance with the Office of Lab Animal Welfare (OLAW) of the PHS, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). We made all attempts to minimize the number of mice required to complete all the experiments outlined, and all surgeries under anesthesia and administering post-operative analgesics. We used both male and female mice of aged more than 12 weeks old for all of the experiments. To induce type-1 diabetes, mice were fasted for 6 hours and injected with streptozotocin dissolved in sodium citrate buffer (0.1 mmol/L) pH 4.5 i.p., 22.4 mg/kg/day, for 5 consecutive days. Chemical sympathetic ablation was performed by injecting 6-hydroxydopamine hydrobromide (6-OHDA) through i.p., 250 mg/kg/week, for three weeks.
Method details
Organ harvesting, flow cytometry and cell sorting
Mice were euthenized and perfused thoroughly with 30 ml of ice cold PBS through the left ventricle. The entire aortae were harvested (from the root to the iliac bifurcation), minced into small pieces with a fine scissors and digested in enzymatic mixture containing 450 U/ml collagenase I, 125 U/ml collagenase XI, 60 U/ml DNase I, and 60 U/ml hyaluronidase at 37°C at 750 rpm for 1 h. Cells were passed through 40-μm cell strainer, washed in 10 ml FACS buffer and centrifuged (4°C, 370 g, 7 minutes). Spleens were removed, homogenized in FACS buffer and passed through 70-μm filters, followed by red blood cell lysis in the filtrate. Splenocytes were washed and resuspended in FACS buffer. Total viable cell numbers were obtained from above aliquots using Trypan Blue (Cellgro, Mediatech, Inc, VA). Following harvesting of single cell suspensions, cells were stained in FACS buffer. Cell staining and analysis were performed as described previously (Dutta et al., 2012). All antibodies used in this study were purchased from eBioscience, BioLegend or BD Biosciences. For mature myeloid cells analysis, monoclonal antibodies including anti-CD11b (M1/70), Ly6G (1A8), CD115 (AFS98), Ly6C (AL-21) were used. Neutrophils were identified as CD11b+ Ly6G+, Ly6Clow monocytes were identified as CD11b+ CD115+ Ly6Clow and Ly6Chigh monocytes were defined as CD11b+ CD115+ Ly6Chigh. For hematopoietic stem and progenitor cells analyses, cells were stained with biotin conjugated antibodies against lineage markers including B220 (RA3-6B2), CD4 (GK1.5), CD8a (53-6.7), NK1.1 (PK136), CD11b (M1/70), CD11c (N418), Gr-1 (RB6-8C5), Ter119 (TER-119) followed by streptavidin Pacific OrangeTM or APC/Cy7 conjugates, and antibodies against c- Kit (2B8), Sca-1 (D7), IL7Ra (SB/199), CD93 (AA4.1), CD16/32 (2.4G2), CD34 (RAM34), CD135 (A2F10), CD48 (HM48-1) and CD150 (TC15-12F12.2). Hematopoietic stem cells (HSC) were identified as Lin- c-Kit+ Sca-1+ CD48- CD150+, LKS were identified as Lin- c-Kit+ Sca-1+. Granulocyte-macrophage progenitors (GMP) were identified as Lin- c-Kit+ Sca-1- CD16/32+ CD34+ and macrophage and dendritic cell progenitors (MDP) were defined as Lin- c-Kit+ Sca-1- CD16/32+ CD34+ CD115+. Common lymphoid progenitors (CLP) were identified as Lin- IL7Ra + c-Kit int Sca-1int and early immature B cells were defined as Lin- B220int CD93+. Cell numbers per femur were calculated as total cells per femur sample multiplied by percentage of cells obtained from the appropriate FACS gates. Data acquisition was performed using LSRII Flow Cytometer (BD). Data were analyzed using with FlowJo software (Tree Star).
Treatment with adrenergic β2 receptor antagonist
Following induction of diabetes by stz injection, ICI 118,551 hydrochloride, a selective antagonist of β2 adrenergic receptor (12 μg/kg/hr) was delivered via an osmotic mini pump for 2 weeks.
Treatment with SGLT inhibitor
Following diabetes induction in C57BL/6 wild type mice using stz injection, mice were treated twice daily i.p for two weeks with phlorizin, a competitive inhibitor of SGLT, at a concentration of 200 mg/kg body weight dissolved in 30% DMSO to reduce the blood glucose concentrations.
Catecholamine analysis
To check catecholamine concentrations in sorted macrophages, we cultured the cells at a density of 10,000/well in DMEM medium containing 10 % FBS, non-essential amino acids, penicillin (100 U/mL), streptomycin (100 μg/mL) in the presence or absence of recombinant IL-4 (10 ng/ml). Supernatants were harvested at day 3, and the catecholamines were quantified using epinephrine/norepinephrine ELISA kit.
Cell sorting, gene expression and heat map analysis
Cells were sorted using a FACS Aria II directly in RNA extraction buffer, total RNA was extracted using ArcturusTM PicoPureTM RNA isolation kit, and cDNA was prepared from 100 ng of mRNA using the high-capacity RNA to cDNA synthesis kit. Quantitative PCR was performed using SYBBR green PCR master mix in Quantstudio 6 Flex Real-time PCR system (Thermo Fisher Scientific), and the results of the indicated genes were expressed as Ct values normalized to the house-keeping gene GAPDH. Heat maps were generated using Microsoft Excel.
Tyrosine hydroxylase (TH) expression
a) Flow cytometry
To check TH expression in splenic leukocytes, we bred the Rosa tdTomato female mice with Th cre male mice to obtain offspring that express tdTomato in TH+ cells. We analyzed the expression of tdTomato in sorted leukocyte subsets by flow cytometry.
b) Immuno blotting
To check the expression of TH in splenocytes, we sorted the splenocytes from spleen of wild type mice using magnetic activated cell sorting and lysed the cells in 1X RIPA buffer containing proteinase and phosphatase inhibitor cocktail. We also extracted the proteins from whole spleen and brain using 1X RIPA buffer and all the protein lysates were resolved by SDS–PAGE and then blotted onto nitrocellulose membrane using a semidry apparatus (Bio-Rad). Subsequently blots were blocked with 5% BSA in TBST, and incubated with either anti-tyrosine hydroxylase or anti-β-Actin (1:1000 dilution) at 4°C for 18 h. Subsequently blots were incubated with secondary IgG HRP antibody in 0.5% BSA in TBST for 60 minutes and immunoreactive proteins were developed using Luminata Crescendo Western HRP substrate and images were captured by molecular imaging system (ChemiDocTM XRS + System, Bio-Rad).
c) q-PCR
We checked the gene expression of TH in THP1cells that were cultured in 60 mm dishes for 48h in the presence or absence of 50 ng/ml PMA to differentiate monocytes into macrophages. Results were expressed as Ct values normalized to the house-keeping gene GAPDH.
TH+ leukocyte and neuron depletion
We generated mice expressing iDTR in leukocytes by transplanting bone marrow (BM) from Th-cre-ROSA iDTR mice into wild type mice. Diabetes was induced in these mice by stz injection. Diptheria toxin (DT) was injected ip. at 10 μg/kg body weight in these mice every other day for a week to deplete the TH+ leukocytes. Additionally, we generated mice expressing iDTR in neurons by transplanting BM from wild type mice into Th-cre-ROSA iDTR mice. Splenic nerves were depleted in these mice by delivering DT locally into the spleen using micro-osmotic pumps for 7 days.
Catecholamines analysis in cultured macrophages
We enriched macrophages by negative selection using B220, Ter119, Gr1, NK1.1, CD4 and CD8 antibodies. Enriched macrophages were seeded at a density of 20,000 / well in 96 well plates and cultured in the presence or absence of recombinant IL-4 (20 ng/ml). Macrophages were incubated in the presence or absence of various concentrations of reserpine for 3 days and analyzed the concentrations of epinephrine and NE in the conditioned media using ELISA.
Histo-pathological analysis and Immunofluorescence
Aortic root sections were stained with Masson’s trichrome to assess plaque size and fibrous cap thickness. For immunohistochemistry, sections were stained with anti-CD11b (clone EPR1344). Following application of appropriate biotinylated secondary antibodies, sections were developed using a Vectastain ABC kit and AEC substrate kit. All sections were counterstained with Harris Hematoxylin and images were taken using a Nikon 90-I microscope. For immunofluorescence staining, tissue sections were permeabilized with 0.1% Triton X-100 for an hour. Mouse tissue sections were stained with anti-CD11b (clone EPR1344), anti-CD3 (clone 145-2C11), anti-F4/80 (clone A3-1), anti-Ly6G (clone 1A8), anti-PGP9.5, anti-CD45 or sheep anti-mouse tyrosine hydroxylase. For tyrosine hydroxylase staining, we used Cy3-labelled donkey anti-sheep secondary antibody. For F4/80 and Ly6G staining, we used Cy5-labelled goat anti-rat secondary antibody. For CD11b, CD3 and PGP9.5 staining, we used Cy5-labelled goat anti-rabbit, Alexa fluor 488-labelled goat anti-hamster, Alexa fluor 488-labelled goat anti-rabbit secondary antibodies respectively. For CD3 and CD68 staining, we used Alexa fluor 488-labelled donkey anti-sheep secondary antibody. The sections were counterstained and fixed with vector shield DAPI, and images were taken using confocal laser scanning immunofluorescence microscopy (CLSM). Image analysis was done using Image J software (Fiji). 3D movies were made with Imaris software (bitplane).
Catecholamine Staining
The glyoxylic acid method described by de la Torre (de la Torre and Surgeon, 1976) was performed. Briefly, 10 μm spleen sections were dipped three times in a sucrose, monobasic potassium phosphate, and glyoxylic acid solution immediately after cutting and dried for 30 min using air from a hair dryer. The excess of dried solution was removed with Kimtech wipers (Kimberly-Clark) to avoid any possible background surrounding the stained tissue. Then, the slides were covered with mineral oil and heated in an oven at 95°C for 3 min, after which excess oil was removed and coverslips were added. Catecholamines were visualized with a Nikon A1 confocal microscope. Auto-fluorescent cells were considered as leukocytes. Image analysis was done using Image J software (Fiji).
BrdU experiments
For BrdU pulse experiments, 1 mg of BrdU was injected i.p twice a day for two weeks. Ten days after the BrdU pulse, the frequency of BrdU-retaining GMP was determined by flow cytometry. After staining for cell surface antigen, intracellular BrdU staining was performed according to the manufacturer’s (BD Bioscience) protocol.
Ultrasound imaging
Transcutaneous ultrasound imaging was performed on the chest using linear array transducer MS 550 (40 MHz) attached to Vevo 2100 (Visualsonics, Toronto, Canada) ultrasound system. Right longitudinal axis parasternal cine loop image of aortic root, ascending aorta, arch of aorta and descending aorta were obtained and stored for offline analysis. Cine loop images were later analyzed using Vevo 2100 software. Intima-media thickness was calculated using previously validated approach(Zhang et al., 2015) i.e. from edge of echo toward the blood stream (intima) to other echo edge (adventitia) using ImageJ (National Institutes of Health).
Splenectomy
Mice were anesthetized using isoflurane (1.5% mixed with oxygen at a flow rate of 2L/minute) and placed on a heating pad to maintain body temperature during the procedure. A sub cutaneous injection of buprenorphine (0.1mg/kg in a volume of 100 μl saline) was administered before the procedure. A laparotomy incision was made, and the spleen, renal vein and renal artery were identified and ligated using a 7-0 silk suture. The vessels were dissected, the spleen was removed and the abdomen was closed in layers using 7-0 suture.
Splenic sympathectomy
After laparotomy incision in the anaesthetized mice splenic nerve was isolated from the splenic blood vessels and connective tissue near the bifurcation of the celiac artery. The entire bundle of the nerve was cut.
Spleen transplantation
After laparotomy incision, renal vein and renal artery were identified and ligated using a 7-0 silk suture. The vessels were dissected, and the spleen was removed. A recipient mouse was also prepared as the donor mice. Laparotomy incision was performed and a part of the donor spleen was transplanted in the renal adipose tissue without vascular anastomosis. Spleens from non-diabetic or diabetic CD45.1+ mice were transplanted in non-diabetic or diabetic CD45.2+ Apoe−/− mice, respectively. Three weeks, after the transplantation, flow cytometry on the blood and aorta was performed to determine donor spleen-derived monocytes. Donor and native spleens were embedded in OCT.
Micro-osmotic pump experiments
Apoe−/− mice (10–12 weeks old) were fed with a high fat diet for 30 days. Afterwards, we induced type-1 diabetes in these mice with stz (i.p., 22.4 mg/kg/day for 5 consecutive days). Micro-osmotic pumps were installed in the spleen of the mice to deliver reserpine (5mg/kg body weight per day), captopril (ACE inhibitor) (6mg/kg body weight per day) or NE (5mg/kg body weight per day) at 0.21 μl/h for 21 days into the spleens. All drugs were dissolved in PBS, and micro-osmotic pumps containing PBS were installed in the control group.
Irradiation and bone marrow transplantation
Mice were placed in the irradiator, non-anesthetized, and performed the irradiation by exposing to 10Gy gamma irradiation for a period of 15 minutes. Then mice were placed in a cage and returned to the housing rooms and injected approximately 1 million bone marrow cells/mouse through i.v under anesthesia.
Statistics
Data are represented as mean±SEM. Statistical significance between groups was performed using Prism t test or ANOVA according to the data set. Results were considered as statistically significant when P<0.05.
Study approval
All animal experiments were conducted following NIH guidelines under protocols approved by the Institutional Animal care and Use Committee of the University of Pittsburgh. Written informed consent was received from family members of deceased patients before tissue collection for the study.
Supplementary Material
C57BL/6 mice were injected with Na citrate buffer as control. The video shows transferred GFP+ GMP (green) and TH+ cells (red) in the spleen.
C57BL/6 mice were injected with stz to induce diabetes. The video shows that transferred GFP+ GMP (green) form cell clusters near TH+ cells (red) in the spleen.
Diabetic C57BL/6 mice were treated with 6-OHDA. The video shows that 6-OHDA treatment reduces cell clusters formed by transferred GFP+ GMP (green) in the spleen. TH+ splenocytes are displayed in red.
These mice were injected with Na citrate buffer as control. The video shows tdTomato+ (red) cells in the spleen.
These mice were injected with stz to induce diabetes. The video shows tdTomato+ (red) cells in the spleen.
HIGHLIGHTS.
Sympathetic nervous system (SNS) mediates differentiation of myeloid progenitors.
TH+ leukocytes express high amounts of neuropeptide Y receptors (NPYR).
TH+ cells are required for myeloid cell generation during “emergency” hematopoiesis.
Regulation of myelopoiesis by the β2 adrenergic receptor expressed by GMP.
Acknowledgments
This work was supported by National Institute of Health grants 4R00HL121076-03 and 1R01HL143967 (to PD). The small animal imaging system was supported by NIH 1S10RR027383-01 (to KK). We would like to acknowledge the NIH supported microscopy resources in the Center for Biologic Imaging. Specifically the confocal microscope supported by grant number 1S10OD019973-01. The graphic abstract was adapted from the Servier Medical Art (www.servier.com). We would like to thank Drs. Prabir Ray and Anuradha Ray for their critical review of the manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
SBV and JF were involved in conducting experiments, data analysis, and writing the manuscript. EC was involved in conducting experiments. MUN performed the ultrasound imaging experiment, and KK provided critical insight in ultrasound image analysis. LS, KHZ and ESS provided us with serum and blood leukocytes from patients. MR and JS provided us with spleen samples from deceased donors. DL provided us with insights in experiments involving splenic SNS. PD was involved in designing research studies, conducting experiments, acquiring and analyzing data, and writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MA, Carson MJ, Nair MG. Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine. 2015;72:210–219. doi: 10.1016/j.cyto.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari S, Plummer E, Emmerson P, Gupta A, Meyer C. Glucose counterregulation in advanced type 2 diabetes: effect of beta-adrenergic blockade. Diabetes Care. 2014;37:3040–3046. doi: 10.2337/dc14-0782. [DOI] [PubMed] [Google Scholar]
- Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41:737–752. doi: 10.1016/j.immuni.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity. 2017;46:92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC, Surgeon JW. Histochemical fluorescence of tissue and brain monoamines: results in 18 minutes using the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience. 1976;1:451–453. doi: 10.1016/0306-4522(76)90095-6. [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Lavie CJ, Bell DS, O’Keefe JH. beta-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. 2015;2:e000230. doi: 10.1136/openhrt-2014-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212:497–512. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herault A, Binnewies M, Leong S, Calero-Nieto FJ, Zhang SY, Kang YA, Wang X, Pietras EM, Chu SH, Barry-Holson K, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017;544:53–58. doi: 10.1038/nature21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: A statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2002;106:523–527. doi: 10.1161/01.cir.0000019552.77778.04. [DOI] [PubMed] [Google Scholar]
- Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, et al. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WC, Levesque JP, Ruitenberg MJ. It takes nerve to fight back: The significance of neural innervation of the bone marrow and spleen for immune function. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–724. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova M, Vargovic P, Vlcek M, Lejavova K, Hudecova S, Krizanova O, Kvetnansky R. Catecholamine production is differently regulated in splenic T- and B-cells following stress exposure. Immunobiology. 2013;218:780–789. doi: 10.1016/j.imbio.2012.08.279. [DOI] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–712. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- Ostman J. beta-adrenergic blockade and diabetes mellitus. A review. Acta Med Scand Suppl. 1983;672:69–77. [PubMed] [Google Scholar]
- Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013;144:698–704. e694. doi: 10.1053/j.gastro.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetti G, Cordella D, Fortunato O, Sangalli E, Losa S, Gotti A, Carnelli F, Rosa F, Riboldi S, Sessa F, et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA-155/FOXO3a signaling pathway. Circ Res. 2013;112:510–522. doi: 10.1161/CIRCRESAHA.112.300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res. 2015;2015:341583. doi: 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Hypertension: an immune disorder? Immunity. 2014;41:673–674. doi: 10.1016/j.immuni.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Pachnis V. Neuroimmune regulation during intestinal development and homeostasis. Nat Immunol. 2017;18:116–122. doi: 10.1038/ni.3634. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4:228–237. doi: 10.1007/s11883-002-0024-4. [DOI] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ha S, Wei W, Duan S, Shi Y, Yang Y. Noninvasive imaging of aortic atherosclerosis by ultrasound biomicroscopy in a mouse model. J Ultrasound Med. 2015;34:111–116. doi: 10.7863/ultra.34.1.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C57BL/6 mice were injected with Na citrate buffer as control. The video shows transferred GFP+ GMP (green) and TH+ cells (red) in the spleen.
C57BL/6 mice were injected with stz to induce diabetes. The video shows that transferred GFP+ GMP (green) form cell clusters near TH+ cells (red) in the spleen.
Diabetic C57BL/6 mice were treated with 6-OHDA. The video shows that 6-OHDA treatment reduces cell clusters formed by transferred GFP+ GMP (green) in the spleen. TH+ splenocytes are displayed in red.
These mice were injected with Na citrate buffer as control. The video shows tdTomato+ (red) cells in the spleen.
These mice were injected with stz to induce diabetes. The video shows tdTomato+ (red) cells in the spleen.