Abstract
Objectives
The goal of this study was to review the feasibility of local bivalirudin injection for adjunct treatment of venous congestion of head and neck reconstructive flaps.
Methods
A retrospective chart review of patients who underwent bivalirudin treatment for venous congestion of head and neck reconstructive flaps in a single institution from September 1, 2012 to September 1, 2015 was undertaken. Individuals were treated with variable number of intradermal injections directly into the flap followed by a small skin incision to allow extended passive bleeding. The main outcome measure was improvement of flap congestion.
Results
Ten patients with free flap reconstruction (4 anterolateral thigh flaps, 2 pectoralis major flaps, 2 fibula osseocutaneous flaps, 1 supraclavicular flap, and 1 radial forearm free flap) of various head and neck defects underwent treatment with bivalirudin. Bivalirudin injections were utilized as adjunct therapy in 6 patients. Two individuals underwent alternate therapy for venous congestion immediately following injection and therefore the efficacy could not be assessed. Of the 8 remaining flaps, 4 developed partial necrosis, and 1 developed complete necrosis requiring additional reconstruction. Two individuals required blood transfusions during bivalirudin treatment.
Conclusions
Bivalirudin is a safe and feasible adjunct therapy for treatment of flap congestion. It may serve as a useful alternative to traditional leech therapy, as bivalirudin negates the need for antibiotic prophylaxis, eliminates the psychological aversion associated with leech therapy, and avoids the potential for leech migration. Further work to determine the efficacy of bivalirudin to standard leech therapy is warranted.
Keywords: Head and neck reconstruction, Free flaps, Venous Congestion, Leech, Bivalirudin
INTRODUCTION
Flap surgery is commonly used for the complex reconstruction of head and neck defects of the oral cavity, pharynx, face, and skull base.1 Free flaps, specifically, have permitted increasingly aggressive ablative surgery with single stage reconstruction, with flap survival rates reported greater than 95%.1,2 Flap compromise, however, can be devastating to the patient, family and care-providers, and there are significant costs associated with the subsequent interventions.3 One of the primary reasons for failure is vascular compromise,4 with some studies reporting a compromise rate of around 15%.1
First-line management of vascular compromise includes surgical re-exploration, which is critical for evaluation of the vascular pedicle in cases of arterial or venous clot, hematoma, or torsion of the pedicle itself.2 Often times, one can differentiate between arterial and venous insufficiency based on the examination of the flap, which helps direct subsequent intervention. Venous congestion occurs more commonly due to slower flow, more compressible vessels, and lower pressure of blood flow compared to the artery. Flaps may become venously engorged secondary to a number of processes including problems with the pedicle and anastomosis, large flap size, poor perforators, excessive fluid administration, dependency, and lymphedema.1,5 Some cases of venous congestion have been successfully managed using adjuvant leech therapy.6,7 Leeches release many active salivary products including hirudin, a tremendously potent natural anticoagulant, thereby promoting bleeding and the relief of venous congestion.6 Leech therapy, however, is associated with an increased risk of blood transfusions,7 infection,7–9 and leech migration,6 as well as psychological aversion on behalf of the patient.8,10
Recombinant hirudin derivatives, such as bivalirudin, act in a similar fashion to their parent compound and are used for anticoagulation during coronary angiogplasty11 and for prophylaxis against deep vein thrombosis.12 Bivalirudin is a direct inhibitor of thrombin (which activates both platelets and other clotting factors), thereby leading to an inhibition of the clotting cascade.11 Additionally, local injection of natural or recombinant hirudin directly into congested flaps have revealed evidence of improved flap survival in rat,13 rabbit,14 and pig models.15,16 The extent to which recombinant hirudin, such as bivalirudin, may aid in relief of venous congestion in head and neck reconstructive flaps in patients has not previously been studied.
In this study, we review the feasibility of the use of local injection of bivalirudin into flaps used for head and neck reconstruction with venous congestion. We hypothesized that bivalirudin will be safe and easy to administer to reduce venous congestion and may be an acceptable alternative adjunct therapy. To our knowledge, this represents the first evaluation of the potential use of bivalirudin for venous congestion of reconstructive flaps in humans.
PATIENTS AND METHODS
A retrospective chart review was conducted for patients who underwent bivalirudin (The Medicines Company, Parsippany, NJ) treatment for venous congestion of flaps used in head and neck reconstruction from September 1, 2012 to September 1, 2015 in the Department of Otolaryngology-Head and Neck Surgery at the Johns Hopkins Hospital. These patients were the first 10 patients with venous compromise at our institution after adoption of the bivalirudin injection protocol. All cases included in this study had documented flap venous congestion in their medical record and at least 3 months of post-operative follow-up. Patients were excluded if their follow-up was less than 3 months and if they were treated with bivalirudin for any indication other than flap venous congestion. The hospital institutional review board approved this study and all research was done in accordance with the Standards of the Committee on Human Experimentation of our institution.
Bivalirudin Treatment
Bivalirudin was injected intradermally at a dose of 0.3 milligrams per kilogram directly into the reconstructive flap by a member of the surgical team, and a small 1 cm bleb was raised (Figure 1A). A #15 blade was then used to make a cruciate cut over the bleb to allow for passive bleeding from the incision (Figure 1B). Anywhere from 1 to 4 areas of the flap were injected at a time depending on the size of the flap and areas of venous congestion. Gauze dressing was usually placed over the bleeding sites to collect the blood and prevent drainage into the pharynx or tracheostoma. A data sheet was placed at bedside to record the frequency, number, and duration of injections.
Figure 1.
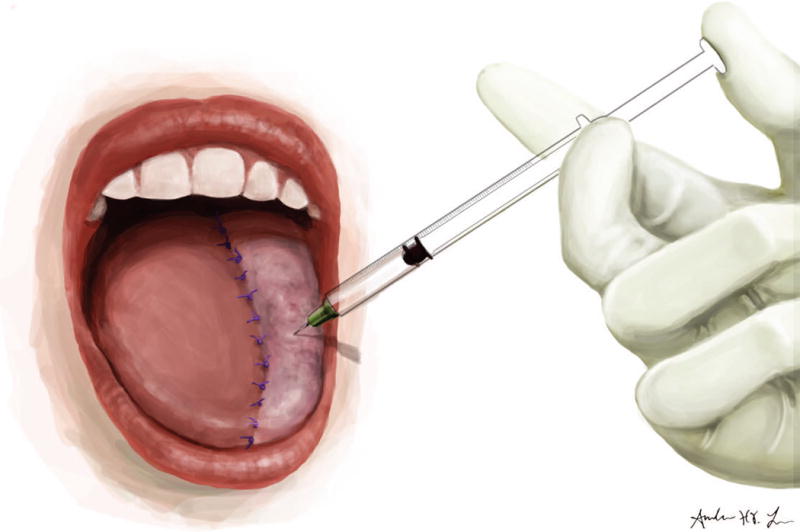
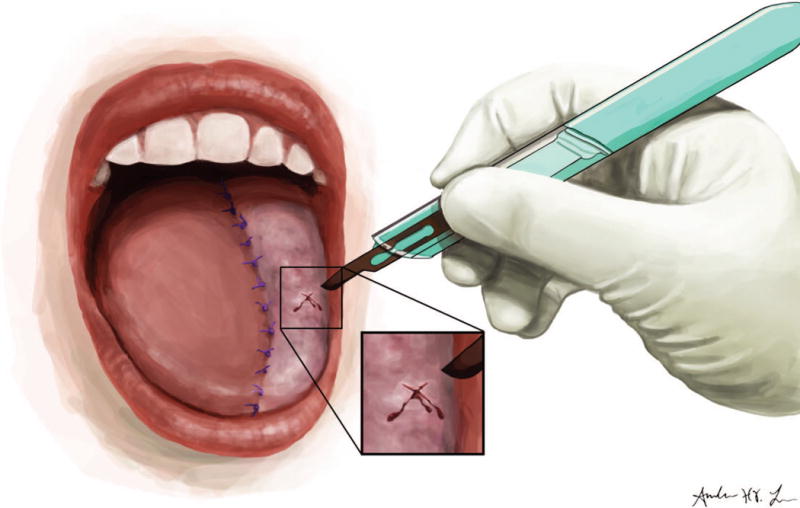
A: Intradermal injection of bivalirudin into reconstructive flap. B: Cruciate cut over the injection bleb.
The duration of passive bleeding and the presence of signs of venous congestion of the flap determined the frequency of bivalirudin doses. The maximum frequency of bivalirudin administration was every six hours. The dose was selected based upon previously published success when used for deep vein thrombosis prophylaxis.12 Given its anticoagulant properties,11 all patients had a baseline complete blood count (CBC) and coagulation profile before administration. Also, given that bivalirudin is cleared by the kidneys,17 all patients had a baseline basic metabolic panel to evaluate for renal function. Throughout the duration of the treatment, all patients underwent routine laboratory evaluation: activated partial thromboplastin time (aPTT) every 6 hours and daily CBC. All patients provided informed consent before receiving their first dose.
Medical records were reviewed to extract demographic information including age and gender. Indication for and type of head and neck reconstructive flap was documented. Signs and symptoms of venous congestion were collected, as well as number and duration of bivalirudin injections. A bivalirudin treatment was defined as a single dose administered to the patient. The pre- and post-bivalirudin injection course was documented. Improvement in flap congestion was defined as improvement in color, temperature, capillary refill, and bleeding time with pinprick. Early improvement in venous congestion was defined as improvement in the first 48 hours.
The main outcome of interest was improvement in flap congestion, leading to at least partial flap salvage. Complications during treatment of bivalirudin were also reported. Characteristics of the patient population are provided including patient age, gender, and indication for head and neck reconstruction. Flap congestion course is described including time and duration of congestion, bivalirudin therapy, and post-injection course.
RESULTS
There were 10 individuals who underwent bivalirudin injection therapy for venous congestion. The mean age of the population was 63.3 years (range 35–81 years). Fifty percent of the population was male. Four patients underwent reconstruction with an anterolateral thigh free flap for their initial defect. Two patients underwent a fibula osteocutaneous free flap and two individuals underwent a pectoralis major regional flap. A radial forearm free flap and a supraclavicular island flap were performed in one individual each. The mean number of bivalirudin treatments was 5.4 (range 1–12 treatments), and each treatment was administered over an average of 2.4 injection areas of the flap (range 1–4 injections). The mean duration of therapy was 2.1 days (range 1 to 4 days). Bivalirudin injections were utilized as adjunct therapy for venous congestion in 6 patients (5 patients received bivalirudin after operative removal of an arterial or venous clot, and 1 individual was treated with one day of standard leech therapy prior to injections).
The description of the bivalirudin cases is presented in Table 1. Two individuals underwent alternate therapy for venous congestion immediately after injection due to surgeon preference, and therefore the efficacy of bivalirudin could not be assessed (one patient underwent surgical exploration of the flap vascular pedicle and one patient underwent standard leech therapy). The remaining 8 individuals developed improvement of their venous congestion (6 cases in the first 48 hours after initiation of bivalirudin therapy). Of these 8 congested flaps, 3 (37.5%) had complete flap salvage and 4 (50%) eventually developed partial necrosis requiring local wound care. One flap developed complete necrosis requiring additional surgical intervention and reconstruction.
Table 1.
Description of head and neck flap reconstructions with venous compromise that received bivalirudin.
| ID/age/sex | Flap | Co-morbidities | # of Rx | # of Days | Pre-injection Interventions | Post-injection Course | Bivalirudin Complications | Flap survival? | |
|---|---|---|---|---|---|---|---|---|---|
| 1/71/M | ALT free flap for oral cavity defect | HTN | 1 | 1 | OR exploration, leech therapy | Injection on POD 2 | None | Complete | |
| Early | Late | ||||||||
| Improvement in congestion after 24 hours | Resolution of congestion, wound dehiscence after discharge | ||||||||
| 2/51/M | ALT free flap for oropharynx defect | HTN | 3 | 3 | OR exploration | Injection on POD 1 to 3 | None | Complete | |
| Early | Late | ||||||||
| Improvement in congestion after 48 hours | Resolution of congestion, wound dehiscence | ||||||||
| 3/81/F | ALT free flap for parotid defect | HTN | 1 | 1 | None | Injection on POD1 | None | No | |
| Early | Late | ||||||||
| Improvement in congestion; flap immediately taken for OR exploration | Hematoma POD9, necrosis POD 14 and reconstruction with pectoralis flap | ||||||||
| 4/72/M | Pec. Flap for parotid defect | CAD | 1 | 1 | None | Injection on POD 2 | None | Partial | |
| Early | Late | ||||||||
| No improvement in congestion, leech therapy initiated after single injection | Partial necrosis of flap | ||||||||
| 5/35/F | ALT free flap for skull base defect | None | 12 | 2 | Leech therapy | Injection on POD 3 to 4 | None | Partial | |
| Early | Late | ||||||||
| Improvement in congestion within hours | Progressive necrosis | ||||||||
| 6/62/M | Fibular free flap for oral cavity defect | None | 8 | 3 | OR exploration | Injection on POD 1 to 2, 4 | None | No | |
| Early | Late | ||||||||
| Improvement in congestion by 48 hours | Wound dehiscence, complete necrosis by POD 9 | ||||||||
| 7/52/F | Pec. flap for oropharynx defect | Hypo-thyroidism | 11 | 4 | None | Injections on POD 1 to 5 | Blood transfusion for hemoglobin <8.0 g/dl | Partial | |
| Early | Late | ||||||||
| No improvement in congestion | Improvement in congestion over 4 days, partial necrosis, wound dehiscence | ||||||||
| 8/71/M | Supra-clavicular island flap for parotid defect | CVD | 8 | 2 | None | Injections on POD 2 to 4 | None | Partial | |
| Early | Late | ||||||||
| Improvement in congestion by 48 hours | Partial necrosis of skin paddle | ||||||||
| 9/64/F | Radial forearm free flap for oral cavity defect | HTN, hypo-thyroidism | 4 | 2 | OR exploration | Injections on POD 1 to 2 | None | Complete | |
| Early | Late | ||||||||
| Improvement in congestion in 24 hours | Small area of wound dehiscence | ||||||||
| 10/74/F | Fibular free flap for oral cavity defect | HTN, chronic kidney disease, CVD | 5 | 2 | OR exploration | Injections on POD 2 to 3 | Blood transfusion for hemoglobin <8.0 g/dl | Partial | |
| Early | Late | ||||||||
| Improvement in congestion within hours | Wound dehiscence, partial necrosis | ||||||||
M: Males. F: Females. Rx: Treatments. ALT: anterolateral thigh. Pec: pectoralis major regional flap. OR: Operating room. HTN: Hypertension. CVD: cardiovascular disease. POD: postoperative day (from original reconstruction).
All of the cases with complete flap salvage underwent surgical exploration prior to initiation of bivalirudin injections; whereas, only one of the flaps that were partially salvaged underwent exploration in the operating room. Two of the individuals with partial flap salvage had a history of cardiovascular disease.
Two individuals with partial flap salvage required blood transfusions during bivalirudin treatment when their hemoglobin dropped below 8.0 grams/deciliter. Additionally, two patients developed a drop in their creatinine clearance (CrCl) below 60 milliliters/minute (ml/min) after bivalirudin treatment. One individual had a history of chronic kidney disease and a CrCl<60 ml/min at baseline. The second individual was evaluated by nephrology, and it was believed that the decline in kidney function was likely secondary to the use of vancomycin. These individuals did not have a corresponding rise in their aPTT compared to those with normal kidney function.
DISCUSSION
Venous congestion remains one of the most common major complications in head and neck flap reconstruction. Despite early surgical intervention with correction of unfavorable factors (e.g. anastomotic thrombosis, kinking, excessive tension, etc.), venous congestion may persist and presents a challenging clinical problem. In this initial report of the use of intradermal injection of bivalirudin, we found that bivalirudin is feasible and safe to administer as an adjunct therapy for venous congestion of head and neck reconstruction flaps. Additional research into bivalirudin efficacy as a potential alternative to standard leech therapy for treatment of venous congestion is therefore warranted.
First-line management of flap vascular compromise remains surgical exploration of the flap pedicle, as expeditious relief of venous congestion leads to increased flap salvage.4 Flap failure can have a profound effect on the patient and providers, as it may lead to prolonged hospital stays, delay in oncologic and other treatments, as well as significantly increased costs.3 In some cases, venous congestion may be treated with multimodal therapy. In additional to surgical management, adjunct or salvage techniques may be administered to help improve survival, including hyperbaric oxygen therapy and exsanguination techniques, such as leech therapy.18
The use of leeches to address a wide range of ailments from infectious to neurologic diseases dates back centuries.19 Treatment of venous congestion in cutaneous flaps by leeches was first described by Derganc et al. over 50 years ago,20 and they were approved by the Federal Drug Administration as a medical device in 2004.21 Leech therapy is now commonly used to help alleviate flap venous congestion.6,7 In addition to actively removing blood volume by ingestion, leeches release many salivary byproducts that lead to protein breakdown, vasodilation, and decreased platelet aggregation, all of which promote passive bleeding which may last for hours after the leech has engorged and unlatched.6 Nonetheless leech therapy may have several drawbacks, such as psychological aversion10 and risk of infection,7,9 which has led investigators to search for alternate therapies.
Several alternatives to medicinal leeching for the treatment of venous congestion have been proposed.22–26 Pharmacologic leeching with the delivery of nitrous oxide to flap tissue, for instance, has shown improvement of flap perfusion and survival in rats.25 So-called mechanical leech devices have also been developed. Hartig et al.23 described a small glass suction device that can be placed over compromised flap tissue. The device is then coupled to a heparin-impregnated subcutaneous disk and can be manually turned to allow for mechanical anticoagulation, and a continuous heparin irrigation system promotes passive bleeding. It was found to have similar efficacy as leech therapy in improving perfusion in venous compromised flaps in a porcine model.22 The use of mechanical leech devices has also been described in rats.26 The effect that such devices may help in compromised reconstruction flaps in patients, however, is not known. The use of local heparin administration has been described to treat venous congestion in re-implanted digits with some success.24. The distal tip of digits, for instance, may have a tenuous blood supply given the small size and distribution of vessels.27 Therefore, local delivery of anticoagulants, such as bivalirudin, may provide relief of venous congestion caused by decreased venous outflow.
Previous work reports the use of recombinant or natural hirudin in the treatment of venous congestion in animal models.13–16 Ying-Xin et al. reported increased survival in compromised free flaps in rats after subdermal injections of both natural or recombinant hirudin compared to controls injected with saline.13 Similarly, increased survival of surface area of compromised random pattern skin flaps after local subdermal injection of natural hirudin compared to controls have been reported in the porcine models.15,16 Furthermore, intravenous injection of recombinant hirudin in regional flaps in rabbits has been reported to increase surface area survival compared with injection of low molecular weight heparin or no injection.14 Our current report builds on this preclinical experience and, to our knowledge, is the first case series to describe the use of bivalirudin in humans in the management of flap venous congestion.
Bivalirudin treatment for an adjunct treatment of venous congestion in head and neck reconstructive flaps may have several advantages over standard leech therapy. For instance, leech therapy requires the use of prophylactic antibiotics in order to protect against infection.7,9 Nonetheless, infection may occur despite the use of antibiotics. In one systematic review of leech therapy in reconstructive flaps, a pooled infection rate of 14.4% was reported and was associated with decreased flap survival.7 The majority of these cases were found to harbor Aeromonas hydrophila, a common leech pathogen.8 Moreover, infections acquired during leech therapy may be secondary to an increase in drug resistance.9 The use of bivalirudin negates the need for prophylactic antibiotics and may decrease the risk of surgical site infection.
There are other advantages of avoiding the use of live leeches. Leech migration has been reported28 and is a very real concern for intraoral flaps. One technique to avoid this is to place a suture (“leash”) through the leech’s tail and tape it to the neck or chest. Leeches that migrate and are “lost” require an intensive and expensive work-up to confirm that they have not latched-on in the pharynx, esophagus or trachea as this could result in potential obstruction of the airway or continued blood loss.6 Psychological aversion with the use of leeches has also been reported.10 We have appreciated this in patients, family members, and nurses who handle the leeches, especially when placed intraorally. Bivalirudin, therapy, therefore, may eliminate these difficulties if utilized as an alternative.
We found that bivalirudin was safe to administer to our patient population. As is customary for any patient intentionally treated with blood-letting, meticulous attention to fluid shifts, red blood cell counts, and electrolytes is mandatory. Reports have shown the need for blood transfusion with live leech therapy may occur in up to 100% of the patients.10 Only two of the participants in our current series required blood transfusions during therapy. Although we were not able to measure objective blood loss in this study, this may indicate that our group experienced less blood loss than occurs with traditional leech therapy. Whether this is a reflection of the lack of the active component of leech therapy with bivalirudin injection or reflects a less aggressive treatment protocol in our institution in the nascent phase of this novel treatment is unknown at this point.
Our overall flap salvage rate (80% with at least partial salvage) is similar to what has been previously reported in the literature for standard leech therapy. A systematic review for the efficacy of leeches in improving venous congestion for plastic reconstructive flaps or re-implantation surgery reported the overall success rate for at least partial survival of the reconstructive site at 79%.7 It is unclear, however, to what extent our flap salvage was based solely on bivalirudin treatment, as the majority of our injections were used as adjunct therapy with other routine flap salvage techniques and our patient population was not treated in a standard manner. As we continue to investigate bivalirudin’s potential role, its efficacy on improving venous congestion in heterogenous patient populations will need to be determined. The ease of administration and tolerability of the therapy in comparison to leeching make it an attractive alternative.
One primary deterrent from using bivalirudin injection is its expense. Based upon 2016 average wholesale price (AWP), one day of bivalirudin therapy is approximately 610 USD (cost estimation based upon the AWP of one vial from which four doses could be provided).29 The cost of one day of leech therapy is estimated at approximately 190 USD (cost estimation includes application of one leech every two hours—12 leeches—and standard antimicrobial prophylaxis with trimethoprim/sulfamethoxazole at a dose of 160 milligrams enterally twice daily).29,30 Cost-effectiveness studies comparing leech therapy versus bivalirudin for the treatment of venous congestion are warranted.
The current report does have several limitations. First, a retrospective review is limited to what is available in the patients’ medical records, which may be inaccurate and poorly documented although we did attempt to use a standardized data form for all patients. Also, our small sample size, and lack of control group does not allow us to compare bivalirudin treatment to standard leech therapy or to no therapy at all. We could not control for confounding variables (such as age, co-morbidities, etc.) and patients were not treated in a standard fashion. We present a heterogeneous patient population, reconstructed with various head and neck flaps, which limits generalizability of our results. Therefore, the incremental gain of bivalirudin injection to flap salvage remains unknown. A randomized controlled trial comparing bivalirudin to other treatment methods of venous congestion is needed to further determine its potential role but admittedly would be very difficult to perform given the rarity of the situation and heterogeneous patient group. Additionally, bivalirudin for the adjunct treatment of venous congestion is an off-label use of the medication, and therefore all patients should be informed about its risks and benefits and provide consent before its use. Alternative administrative routes and doses for bivalirudin (e.g. intravenous delivery) methods should be studied as these may improve flap salvage rates. Furthermore, we emphasize that first-line management of vascular compromise still remains surgical exploration of the flap pedicle. Bivalirudin, conversely, may be considered in addition to operative and other treatments to address persistent venous congestion, particularly in a subset of patients with psychological aversion and with intraoral flaps in which leech therapy may be difficult to administer. However, more investigation beyond this initial report is needed to understand its efficacy.
CONCLUSIONS
In summary, we describe the feasibility of the use of bivalirudin injection as an alternative adjunct therapy for the treatment of venous congestion in head and neck reconstructive flaps. It may have several benefits over standard leech therapy and therefore its use warrants further investigation.
Acknowledgments
Aisha Harun was funded by a T32 Award (5T32DC000027-25).
Funding for work: Aisha Harun was funded by an NIH T32 Award (Grant number: 5T32DC000027-25)
Footnotes
Conflicts: The authors have no conflicts of interest to report
The following manuscript was presented as a poster at The Triological Society 119th Annual Meeting at COSM, May 20–21, 2016, Chicago, IL.
References
- 1.Urken ML, Weinberg H, Buchbinder D, Moscoso JF, Lawson W, Catalano PJ, Biller HF. Microvascular free flaps in head and neck reconstruction. Report of 200 cases and review of complications. Archives of otolaryngology–head & neck surgery. 1994;120(6):633–640. doi: 10.1001/archotol.1994.01880300047007. [DOI] [PubMed] [Google Scholar]
- 2.Wolff KD, Holzle F, Wysluch A, Mucke T, Kesting M. Incidence and time of intraoperative vascular complications in head and neck microsurgery. Microsurgery. 2008;28(3):143–146. doi: 10.1002/micr.20468. [DOI] [PubMed] [Google Scholar]
- 3.Momeni A, Kattan A, Lee GK. Is microsurgical head and neck reconstruction profitable?: analysis at an academic medical center. Annals of plastic surgery. 2012;68(4):401–403. doi: 10.1097/SAP.0b013e31823d2dec. [DOI] [PubMed] [Google Scholar]
- 4.Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head & neck oncology. 2009;1:33. doi: 10.1186/1758-3284-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hand WR, McSwain JR, McEvoy MD, Wolf B, Algendy AA, Parks MD, Murray JL, Reeves ST. Characteristics and intraoperative treatments associated with head and neck free tissue transfer complications and failures. Otolaryngology—head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152(3):480–487. doi: 10.1177/0194599814564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitaker IS, Cheung CK, Chahal CA, Karoo RO, Gulati A, Foo IT. By what mechanism do leeches help to salvage ischaemic tissues? A review. The British journal of oral & maxillofacial surgery. 2005;43(2):155–160. doi: 10.1016/j.bjoms.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker IS, Oboumarzouk O, Rozen WM, Naderi N, Balasubramanian SP, Azzopardi EA, Kon M. The efficacy of medicinal leeches in plastic and reconstructive surgery: a systematic review of 277 reported clinical cases. Microsurgery. 2012;32(3):240–250. doi: 10.1002/micr.20971. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker IS, Josty IC, Hawkins S, Azzopardi E, Naderi N, Graf J, Damaris L, Lineaweaver WC, Kon M. Medicinal leeches and the microsurgeon: a four-year study, clinical series and risk benefit review. Microsurgery. 2011;31(4):281–287. doi: 10.1002/micr.20860. [DOI] [PubMed] [Google Scholar]
- 9.Kruer RM, Barton CA, Roberti G, Gilbert B, McMillian WD. Antimicrobial prophylaxis during Hirudo medicinalis therapy: a multicenter study. Journal of reconstructive microsurgery. 2015;31(3):205–209. doi: 10.1055/s-0034-1395395. [DOI] [PubMed] [Google Scholar]
- 10.Chepeha DB, Nussenbaum B, Bradford CR, Teknos TN. Leech therapy for patients with surgically unsalvageable venous obstruction after revascularized free tissue transfer. Archives of otolaryngology—head & neck surgery. 2002;128(8):960–965. doi: 10.1001/archotol.128.8.960. [DOI] [PubMed] [Google Scholar]
- 11.Di Nisio M, Middeldorp S, Buller HR. Direct thrombin inhibitors. The New England journal of medicine. 2005;353(10):1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg JS, Nurmohamed MT, Gent M, MacKinnon B, Sicurella J, Brill-Edwards P, Levine MN, Panju AA, Powers P, Stevens P, et al. Use of Hirulog in the prevention of venous thrombosis after major hip or knee surgery. Circulation. 1994;90(5):2385–2389. doi: 10.1161/01.cir.90.5.2385. [DOI] [PubMed] [Google Scholar]
- 13.Ying-Xin G, Guo-Qian Y, Jia-Quan L, Han X. Effects of natural and recombinant hirudin on superoxide dismutase, malondialdehyde and endothelin levels in a random pattern skin flap model. The Journal of hand surgery. 2012;37(1):42–49. doi: 10.1177/1753193411414628. European volume. [DOI] [PubMed] [Google Scholar]
- 14.Duzgun S, Nisanci M, Unlu E. The effect of recombinant hirudin on rabbit ear flaps with venous insufficiency. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2014;47(1):102–108. doi: 10.4103/0970-0358.129633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo-Qian Y, Gang W, Zhi-Yong S. Investigation on the microcirculation effect of local application of natural hirudin on porcine random skin flap venous congestion. Cell biochemistry and biophysics. 2012;62(1):141–146. doi: 10.1007/s12013-011-9274-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Shi Q, Sun ZY, Yin GQ, Yang HL. Effect of natural hirudin on random pattern skin flap survival in a porcine model. The Journal of international medical research. 2012;40(6):2267–2273. doi: 10.1177/030006051204000624. [DOI] [PubMed] [Google Scholar]
- 17.Tsu LV, Dager WE. Bivalirudin dosing adjustments for reduced renal function with or without hemodialysis in the management of heparin-induced thrombocytopenia. The Annals of pharmacotherapy. 2011;45(10):1185–1192. doi: 10.1345/aph.1Q177. [DOI] [PubMed] [Google Scholar]
- 18.Kubo T, Yano K, Hosokawa K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery. 2002;22(8):391–395. doi: 10.1002/micr.10059. [DOI] [PubMed] [Google Scholar]
- 19.Whitaker IS, Rao J, Izadi D, Butler PE. Historical Article: Hirudo medicinalis: ancient origins of, and trends in the use of medicinal leeches throughout history. The British journal of oral & maxillofacial surgery. 2004;42(2):133–137. doi: 10.1016/S0266-4356(03)00242-0. [DOI] [PubMed] [Google Scholar]
- 20.Derganc M, Zdravic F. Venous congestion of flaps treated by application of leeches. British journal of plastic surgery. 1960;13:187–192. doi: 10.1016/s0007-1226(60)80036-7. [DOI] [PubMed] [Google Scholar]
- 21.Rados C. Beyond bloodletting: FDA gives leeches a medical makeover. FDA consumer. 2004;38(5):9. [PubMed] [Google Scholar]
- 22.Hartig GK, Connor NP, Heisey DM, Conforti ML. Comparing a mechanical device with medicinal leeches for treating venous congestion. Otolaryngology—head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003;129(5):556–564. doi: 10.1016/S0194-59980301587-0. [DOI] [PubMed] [Google Scholar]
- 23.Hartig GK, Connor NP, Warner TF, Heisey DM, Sarmadi M, Conforti ML. Testing a device to replace the leech for treating venous congestion. Archives of facial plastic surgery. 2003;5(1):70–77. doi: 10.1001/archfaci.5.1.70. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias M, Butron P. Local subcutaneous heparin as treatment for venous insufficiency in replanted digits. Plastic and reconstructive surgery. 1999;103(6):1719–1724. doi: 10.1097/00006534-199905060-00026. [DOI] [PubMed] [Google Scholar]
- 25.Russell JA, Connor NP, Hartig GK. Iontophoretic delivery of nitric oxide donor improves local skin flap viability. Journal of rehabilitation research and development. 2010;47(1):61–66. doi: 10.1682/jrrd.2008.10.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottler PS, Skalak TC. Development of a clinically useful mechanical leech device that promotes flap survival in an animal model of venous-congested skin flaps. Annals of plastic surgery. 2001;47(2):138–147. doi: 10.1097/00000637-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Yang JW, Lee DC, Ki SH, Roh SY. Challenges in fingertip replantation. Seminars in plastic surgery. 2013;27(4):165–173. doi: 10.1055/s-0033-1360583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin G, 2nd, Linnell JD, Yang SS, Lin J, Camarata PJ, Andrews BT. Ultrasound localization of a tunneled leech beneath a microvascular scalp reconstruction: A case report. Microsurgery. 2013;33(7):572–574. doi: 10.1002/micr.22163. [DOI] [PubMed] [Google Scholar]
- 29.Lexi-Comp Online. Lexi-Drugs Online. Hudson, Ohio: Wolters Kluwer Clinical Drug Information, Inc; Accessed May 11, 2016. [Google Scholar]
- 30.Leeches U.S.A. LTD. Medicinal Leech Price List. Available at: http://www.leechesusa.com/index.php/pricing-information/pricing. Accessed May 11, 2016.


