Abstract
Development of biocompatible 3D scaffolds is one of the most important challenges in tissue engineering. In this study, we developed polymer scaffolds of different design and microstructure to study cell growth in them. To obtain scaffolds of various microstructure, e.g., size of pores, we used double- and one-stage leaching methods using porogens with selected size of crystals. A composite of poly(3-hydroxybutyrate) (PHB) with poly(ethylene glycol) (PEG) (PHB/PEG) was used as polymer biomaterial for scaffolds. The morphology of scaffolds was analyzed by scanning electron microscopy; the Young modulus of scaffolds was measured by rheometry. The ability to support growth of mesenchymal stem cells (MSCs) in scaffolds was studied using the XTT assay; the phenotype of MSC was preliminarily confirmed by flow cytometry and the activity of alkaline phosphatase and expression level of CD45 marker was studied to test possible MSC osteogenic differentiation. The obtained scaffolds had different microstructure: the scaffolds with uniform pore size of about 125 µm (normal pores) and 45 µm (small pores) and scaffolds with broadly distributed pores size from about 50–100 µm. It was shown that PHB/PEG scaffolds with uniform pores of normal size did not support MSCs growth probably due to their marked spontaneous osteogenic differentiation in these scaffolds, whereas PHB/PEG scaffolds with diverse pore size promoted stem cells growth that was not accompanied by pronounced differentiation. In scaffolds with small pores (about 45 µm), the growth of MSC was the lowest and cell growth suppression was only partially related to stem cells differentiation. Thus, apparently, the broadly distributed pore size of PHB/PEG scaffolds promoted MSC growth in them, whereas uniform size of scaffold pores stimulated MSC osteogenic differentiation.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1350-8) contains supplementary material, which is available to authorized users.
Keywords: Poly(3-hydroxybutyrate), Poly(ethylene glycol), Scaffolds, Mesenchymal stem cells, Microstructure
Introduction
Poly(3-hydroxyalkanoates) (PHAs) attract particular attention among biomedical polymers for tissue engineering and regenerative medicine due to their biodegradability and high biocompatibility. Poly(3-hydroxybutyrate) (PHB) is the most studied member of PHAs family, which can be produced microbiologically by different types of microorganisms (Bonartsev et al. 2008; Lim et al. 2017). Previously, we showed that biotechnological method of PHB and its copolymers production allow to achieve high degree of purity, and to control and specify physicochemical properties of biopolymers within narrow limits during their biosynthesis (Myshkina et al. 2008, 2010). PHB is biodegradable and biocompatible polymer with high mechanical strength, thermal plasticity, and specific diffusion properties (Artsis et al. 2010; Domínguez-Díaz et al. 2015; Iordanskii et al. 2006; Monnier et al. 2016). PHB was able to form composites with synthetic polymers and inorganic materials (Artsis et al. 2010; Misra et al. 2010; Chan et al. 2013; Biazar and Heidari Keshel 2015; Domínguez-Díaz et al. 2015). The perspective area of PHB application is development of implanted medical devices for dental, cranio-maxillofacial, orthopaedic, cardiovascular, hernioplastic, and skin surgery (Bonartsev et al. 2008; Lim et al. 2017).
However, PHB homopolymer has certain disadvantages, as well: high hydrophobicity and crystallinity, long-term biodegradation, and low plasticity, which, in some cases, severely limits their use as bioengineered materials in medicine, e.g., high hydrophobicity of PHB can impair cell attachment and growth on devices from this polymer (Chan et al. 2013; Domínguez-Díaz et al. 2015). One of the most promising approaches to improve the properties of PHB is blending or copolymerization of PHB with hydrophilic polymers (Cheng et al. 2003; Iordanskii et al. 2006; Nemets et al. 2008; Chan et al. 2013; Monnier et al. 2016; Reyes et al. 2016). Poly(ethyleneglycol) (PEG) is a nontoxic hydrophilic polymer approved by the U.S. Food and Drug Administration (FDA) for internal consumption (D’souza and Shegokar 2016), and it is used often to modify different polymer properties, such as hydrophobicity, surface charge, and others (Nemets et al. 2008; Chan et al. 2013; Monnier et al. 2016). It was shown that PHB can be mixed with PEG with blend formation and the produced composites (with PEG weight content more than 10%) have improved hydrophilicity and biocompatibility (Cheng et al. 2003; Nemets et al. 2008; Chan et al. 2013).
Mesenchymal stem cells (MSC) and fibroblasts are used as a typical cell cultures to estimate biological properties of devices for tissue engineering in vitro and to develop model artificial tissues and organs, especially, such as bone, cartilage, and skin. Moreover, MSC have a potential to proliferate to different cell types and is commonly used in regenerative medicine (Boxall and Jones 2012; Fraioli et al. 2016). To produce such tissue engineering systems biodegradable 3D polymer scaffolds are used as carriers for the cultivation of cells: they should mimic the extracellular matrix in a regenerating tissue environment, easily integrate with the adjacent tissue, and favor new tissue in growth. Finally, the scaffold should be biocompatible and resorbable (Lim et al. 2017; Houben et al. 2017). PHAs and their composites as biomaterial for such scaffolds manufacture meet completely these conditions, especially for bone tissue engineering (Cheng et al. 2003; Lim et al. 2017; Reyes et al. 2016; Shishatskaya et al. 2014). However, in addition to the properties of the material, microstructure of polymer scaffolds has a great importance for cell adhesion and growth (Criscenti et al. 2017).
However in spite of high attention and numerous investigations of scaffolds from various polymers, the relationship between microstructure of scaffold on the basis of PHB and cell growth in these biopolymer scaffolds remains a relevant problem. Thus, the purpose of our research was to study MSC and fibroblasts growth in vitro in PHB/PEG porous scaffolds with different microstructure.
Materials and methods
Polymer production
PHB [molecular weight (Mw) = 1.5 × 105] were produced by original biotechnological technique using highly productive strain-producer of PHB Azotobacter chroococcum 7B, then polymer was isolated and purified for biomedical application, and PHB Mw was determined by viscosimetry as previously described (Myshkina et al. 2008).
Porous scaffold preparation
The scaffolds used in our study was prepared by various modifications of salt-leaching method, which is widely used for the manufacture of porous polymer scaffolds in tissue engineering (Misra et al. 2010; Rumian et al. 2013; Moisenovich et al. 2015; Houben et al. 2017): one-stage salt-leaching method using ammonium carbonate as porogen and novel double-leaching technique using ammonium carbonate and sucrose as porogens. As polymer material for scaffold production, we used blend of PHB with PEG 1500 (Sigma-Aldrich, Germany) in ratio 80:20 (PHB/PEG). The content of PEG in the composite was chosen according to the literature data as the most appropriate for cell cultivation due to its greater hydrophilicity (Cheng et al. 2003; Chan et al. 2013; Nemets et al. 2008). We used double-leaching method to produce scaffolds S1 and S2 that differ in pores size. A solution of PHB and PEG 1500 in the ratio 80:20 in trichloromethane (EKOS-1, Russia) with a concentration of 60 mg/ml was mixed with crystals of ammonium carbonate as blowing agent (Khimmed, Russia) and sucrose (Sigma-Aldrich, Germany) as leaching agent in a ratio of 1:3 (w/w) each layer-by-layer in a Petri dish. To create pores in scaffolds with different size, preliminarily, the sucrose and ammonium carbonate was sieved through laboratory pharmaceutical sieves U1-ESL (Kraft, Russia) with a mesh size of 40, 94, and 315 µm and only 94 µm, respectively. Ammonium carbonate was used as porogen due to possibility to decompose to gases at high temperature (> 60 °C) and by hydrolysis in water solutions. To produce PHB/PEG scaffolds S1, we used sucrose crystals that were obtained by sieving through sieves with mesh size of 94 and 315 µm, whereas, for manufacturing of PHB/PEG scaffolds S2, we sieved sucrose through sieves with 40 and 94 µm mesh size. The properly mixed solution of PHB/PEG with was poured in Petri dish with subsequent evaporation and then was placed in hot water (approximately 65 °C) to produce porosity both by gas releasing and sucrose leaching. The scaffold S3 was prepared by salt-leaching method using only one porogen—ammonium carbonate. The ammonium carbonate powder with a particle size 20–94 µm was mixed with PHB/PEG polymer solution in chloroform with concentration 60 mg/ml in mass ratio 5:1. The mixed solution was poured in Petri dish with subsequent chloroform evaporation and then was also placed in hot water. The obtained scaffolds was washed five times in distilled water and dried about 24 h at room temperature (Bonartsev et al. 2016).
Microscopy
The photos of scaffolds were obtained using Cyber-shot DSC-RX100 digital camera (Sony, Japan) with Macro function. Microstructure of scaffolds was studied by scanning electron microscopy (SEM). For SEM investigation, scaffolds were mounted on aluminium stumps, coated with gold in a sputtering device for 15 min at 15 mA (IB-3,Giko, Japan), and examined under a scanning electron microscope (JSM-6380LA, JEOL, Japan). To study the scaffolds with cultivated MSC and 3T3 cells, the specimens were specially treated previously by dehydration procedure using glutaraldehyde and ethanol. Scaffolds with ink testing were examined by wide-field light microscopy using stereomicroscope Nikon SMZ1500 (Nikon, Japan). Diameter of micropores was calculated by Image J software using SEM images. The data present as an average (n = 15) (Bonartsev et al. 2016).
Porosity
Scaffold porosity was calculated through Eqs. (1) and (2) of apparent densities and porosity, respectively:
| 1 |
where D is apparent density (g/cm3), m is mass of scaffold (g), h is height (or thickness) (cm), l is length (cm), and w is width (cm) of scaffold:
| 2 |
where P (%) is porosity, D is the apparent density that calculated using Eq. 1 (g/cm3), and d is a theoretical bulk density of monolithic sample of scaffold without pores (g/cm3).
The composite PHB/PEG 80:20 has a bulk density of 1.08 g/cm3 (PHB has 1.243 g/cm3; PEG 1500 has 0.450 g/cm3). Weight was measured on the laboratory scale AL-64 (Acculab, USA). Height (thickness), length, and width of scaffolds were measured with a caliper (Krino, Italy).
Ink test was used to determine interconnection of pores in scaffolds. To determine the presence of closed pores in scaffolds, they were immersed in ink solution and dried (for S3 samples) or immersed in ink solution, dried, and then was cross-sectioned (for S1 and S2 samples). Ink distribution in the scaffold volume (to ensure the absence of closed pores, which look on the cut as white spots) was examined by wide-field light microscopy using stereomicroscope Nikon SMZ1500 (Nikon, Japan) (Bonartsev et al. 2016).
Mechanical properties
Mechanical properties of scaffolds were studied by rheometry using MCR 302 rheometer (Anton Paar, Austria). The rheometer was equipped with a plate–plate measuring system. First, an amplitude test was performed to determine the linear viscoelasticity range at an angular frequency of 10 rad/s. After measuring the characteristic range of linear viscoelasticity (0.04%) and the optimal gap (relative deformation 0.1–0.3), a frequency test was carried out on the other sample at an angular frequency of 0.1–100 rad/s. The Young’s modulus was measured by compression of samples of scaffolds. The dependence of the normal pressure on the relative strain was made. Then, the Young’s modulus was calculated from the slope of the linear part of the chart (Ziv et al. 2014).
Cell viability test
To study biocompatibility of scaffolds in vitro two different types of cell lines were used: primary mesenchymal stem cells (MSC) culture (after 7th day cultivation) and 3T3 cells (second passage). To estimate cells attachment and growth on the scaffolds, a cell viability test was used.
The NIH 3T3 cells (Biolot, Russia) were maintained in DMEM, and supplemented with 10% FBS and 1% penicillin streptomycin antibiotics. The confirmation of all characteristics of this cell line as mouse fibroblasts are given in the certificate from the Biolot company (# 1.5.2). MSC were isolated from the femurs of young (3–5 days old) Wistar rats and cultured for 2 weeks in DMEM (Dubecco’s Modified Eagle Medium, PanEco, Russia) supplemented with 10% fetal calf serum (FCS, Biological Industries, Israel), 100 U/ml penicillin.. The approvals for all surgical experimental procedures and ethical guidelines were issued by the ISO 10993-1:2009. MSCs were cultivated for three passages. The cells were removed by incubation in a solution of trypsin–versene for 5 min, and then counted using hemocytometer. Then, suspension of 105 cells in 100 µl of PE buffer (2 ml EDTA-0.5% ETS in PBS) was prepared and incubated with antibodies to positive surface markers of MSC phenotype: СD90 and CD29; negative surface markers: CD45 and CD11b/c (eBioscience, USA); the dye cell viability 7-Aminoactinomycin D (7AAD) in the dark for 40 min at a temperature of 5 °C (Boxall and Jones 2012; Harting et al. 2008). Cells were washed by centrifugation once in PBS and analyzed on flow cytometer (FACS ARIA II, USA). Analysis of results and construction of graphs were performed using the program Flowing Software 2.5.1 (Supplemental materials, fig. S1).
To test biocompatibility of scaffolds, their samples 5 × 5 mm were placed in the center of the 96-well plate wells. Then cultivating cells were detached using trypsin/ EDTA (0.25% w/v trypsin/0.02% EDTA, PanEco, Russia) and 100 µl cell suspension including about 2000 cells was placed on the top of the samples of scaffolds that were positioned at the bottom of wells. Plates were incubated for 1, 3, 6, and 14 days. The control samples were examined for wells of 96-well plate without scaffolds, tissue culture polystyrene (TCPS). Cells viability was measured by the cell proliferation reagent XTT according to the manual (XTT Cell Proliferation Kit, Biological Industries, Israel). For biocompatibility testing, samples of scaffolds with attached cells were gently and quickly transferred from wells of incubated tissue culture plate to respective wells of a new plate with preliminarily added 100 µl of fresh medium; then 50 µl of XTT reagent solution was added in each well. Multi-well plates were incubated at 37 °C for a further 4 h. Polymer samples were removed and absorbance measurements were conducted using a microplate spectrophotometer Zenyth 3100 Microplate Multimode Detector (Anthos Labtec Instruments GmbH, Austria) at 450 nm with reference wavelength at 640 nm (Bonartsev et al. 2016).
The change of expression level of CD45 marker on 6th and 14th days was studied to monitor the stem cells phenotype during their cultivation in PHB/PEG scaffolds. CD45, transmembrane protein-tyrosine phosphatase (leukocyte common antigen, L-CA, B220, T200, Ly-5, EC 3.1.3.48), is a glycoprotein that presents on the surface of all the representatives of the blood-forming series, except for mature erythrocytes. CD45 is widely used for phenotyping MSC as a negative marker, to identify MSC of any origin (human, rat, mouse), the absence of a surface marker CD 45 is mandatory (Boxall and Jones 2012; Harting et al. 2008).
Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity is one of the main markers of differentiation of MSC in osteogenic direction (Raucci et al. 2012; Rumian et al. 2013; Hadjicharalambous et al. 2015; Lim et al. 2017; Criscenti et al. 2017). At first, the specimens of scaffolds 10 × 10 mm were seeded at a rate of 7000 cells per specimen and cells were cultivated for 6 days. To study ALP activity of MSC, the specimens of scaffolds with MSC grown on them were washed two times with PBS, placed in lysis buffer (250 mM NaCl, 0.1% Triton X-100, 50 mM Hepes, pH 7.5), and subjected to three freeze–thaw cycles. Then, the obtained samples of cells lysate were centrifuged for 10 min at 10,000 rpm. One hundred microliters of lysate sample and 50 µl of reagent were added to the 96-well plate wells, and incubated in a thermostat for 2 h. Then, the absorbance of the samples was measured at 405 nm and ALP activity was calculated as activity units per ml of sample.
Statistical analysis
Statistical evaluation of data was performed using the software package SPSS/PC+ Statistics™ 12.1 (SPSS). Non-parametric Kruskal–Wallis test was employed for all statistical analyses. Data were averaged with the standard error to the mean (± SD) and considered significant for p < 0.05.
Results and discussion
Microstructure and mechanical properties of scaffolds
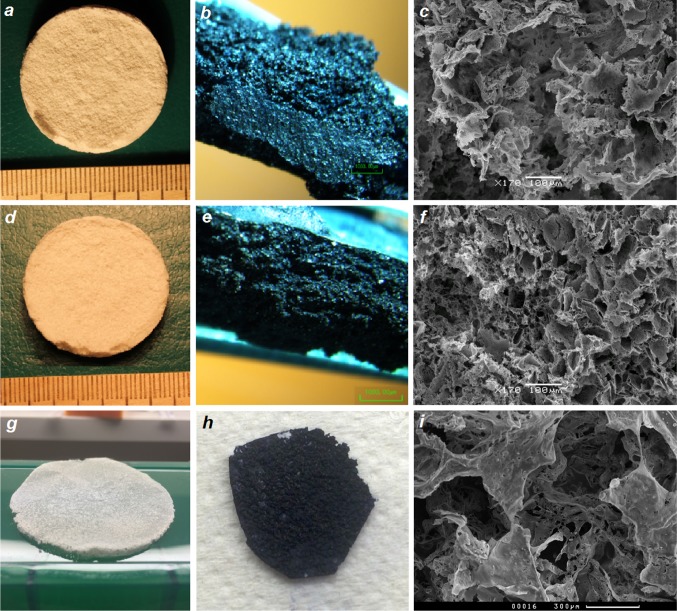
The most widespread methods to manufacture the PHB 3D scaffolds with desired microstructure for tissue engineering by means of improvement of cell adhesion and growth are salt-leaching methods (Misra et al. 2010; Stevanovic et al. 2011; Rumian et al. 2013; Houben et al. 2017; Moisenovich et al. 2015). Using one-stage and double-leaching methods, we obtained three-dimensional scaffolds from composite PHB/PEG of different shape and microstructure: S1, S2, and S3. The images of produced scaffolds are shown in Fig. 1. The design of devices differs greatly: scaffold S3 is a flat device (of 0.5 mm thick) with two sides, each of that has its own microstructure (Fig. 1g); scaffolds S1 and S2 are cylinder-shape devices with diameter of 30 mm and thickness of 3 mm of uniform pore microstructure (Fig. 1a, d). Investigation of scaffolds samples by SEM showed that all types of scaffolds have a 3D porous structure and pores of irregular shape with microroughness of pore walls. However, the porosity and pore size of PHB/PEG 3D-scaffolds obtained by one-step and double-leaching techniques using of porogen crystals of various sizes differed greatly (Fig. 1c, f, i; Table 1). It should be recalled that all three types of scaffolds were prepared using ammonium carbonate as a porogen salt, which produces gases at elevated temperature and by hydrolysis. For scaffold S3, which was produced by one-stage leaching method using only ammonium carbonate as porogen, the pores emerged by breaking polymer surface; at the same time, the porosity is also formed by folds of the polymer. The process of gas formation is very active, so the porosity is achieved not only by the removal of a blowing agent, but also due to deformation of the polymer matrix. Flat scaffolds were formed in a glass Petri dish, so they have two sides: the side bordering with glass which we call “internal” and another one “outer”. If we compare two sides of the porous structures of S3, we can see that outer side is more porous and irregular (Fig. 1i), while internal is more smooth. These differences in microstructure are seen from the pore size values given in Table 1: the pores of outer side of S3 are approximately two times larger than the pores of internal side of this scaffold. However, the pores of both sides of S3 had very irregular structure and a great diversity in size. The porosity of S3 was high: 88% (Table 1). Thus, one-stage leaching method allows to produce flat scaffolds of irregular pore structure with gradient in pore size.
Fig. 1.
Appearance and microstructure of PHB/PEG porous scaffolds S1 (a, b, c), S2 (d, e, f), and S3 (g, h, i): photography of scaffolds (a, d, g); microphotography of scaffolds with ink testing, wide-field microscopy, ×75 (b, e, f); microphotography of pore structure of scaffolds, SEM, ×170 [c, g, external side of S3 (k)]
Table 1.
Porosity, pore size, and Young modulus of PHB/PEG porous scaffolds
| PHB/PEG scaffold | Porosity (%) | Pore size (µm) | Young’s modulus (kPa) | |
|---|---|---|---|---|
| Internal side | Outer side | |||
| S1 | 88 ± 9 | 126 ± 25 | 35 ± 1 | |
| S2 | 60 ± 5 | 44 ± 9 | 110 ± 2 | |
| S3 | 75 ± 16 | 53 ± 26 | 100 ± 53 | – |
Using of sucrose as second leaching agent for scaffolds S1 and S2, preparation leaded also to the formation of complex porous microstructure of these scaffolds. However, the porous structure of S1 and S2 was uniform without significant difference in pore size and shape between internal and outer sides. Therefore, this two-step technique can be applied not only for flat devices manufacture but also for 3D devices with given shape and size. Moreover, the use of sucrose crystals of different size allows to regulate porosity and pore size of scaffolds: scaffolds S1 had a high porosity (75%) that did not differ significantly from porosity of flat scaffolds S3, whereas the porosity of S2 was 15% lower compared to S1; similarly, mean pore size of S1 was 126 µm that did not differ significantly from pore size of outer side of S3, whereas mean pore size of S2 was nearly three times lower than pore size of S1 and did not differ significantly from pore size of internal side of S3.
Ink test showed that all types of scaffolds had an interconnected pore structure: closed pores that looked like black spots in obtained scaffold were absent (Fig. 1b, e, h). The high porosity and interconnected pore structure are key properties for scaffolds used for tissue engineering and essential condition for cell growth in the scaffolds (Misra et al. 2010; Murphy et al. 2010; Rumian et al. 2013; Zhang et al. 2010; Ribeiro-Samy et al. 2013; Moisenovich et al. 2015; Houben et al. 2017).
The mechanical properties, namely, Young modulus of obtained PHB/PEG scaffolds measured by rheometry differed also greatly: Young modulus of scaffolds S2 that have lowered porosity and pore size was more than three times higher than Young modulus of scaffolds S1 with normal porosity and pore size (Table 1). In general, strength of produced PHB/PEG scaffolds was lower than PHAs-based scaffolds produced by other methods but was sufficient and appropriate for long-term cells cultivation (Ribeiro-Samy et al. 2013). The relatively low Young modulus of scaffolds can be connected both with using PEG in polymer composite and double-leaching technique used for scaffold manufacture. Unfortunately, we could not measure Young modulus of scaffolds S3 due to their small thickness, whereas using of other methods of mechanical properties study can lead to obtaining of data that incorrectly will be compared with the data obtained by the method of rheometry.
Cell growth in scaffolds
Before the study of MSC growth on PHB/PEG scaffolds, we performed phenotyping of the obtained cells to prove that these cells are really mesenchymal stem cells. We studied cell culture isolated from the bone marrow of rats on the third passage. To analyze phenotype of obtained MSC, we used the most relevant markers: CD 90 and CD29 as positive markers of MSC phenotype, CD 45 and CD11b/c as negative markers (Boxall and Jones 2012), and the vital marker (7AAD) to estimate viable cells in the population studied. The flow cytometry analysis showed that the amount of living cells was 98.8 ± 0.8% of the total number. The percentage of positive cells to different MSC positive and negative phenotype markers was the following: CD 90–95%; CD 29–50%, CD 45–2%; CD11b/c – 26%, which is similar to the published data on the MSC and along with very low ALP activity (45 U/ml) in cells and their ability to adhere to culture plastic allow to characterize them as MSC (Harting et al. 2008) (Supplemental data, fig. S1).
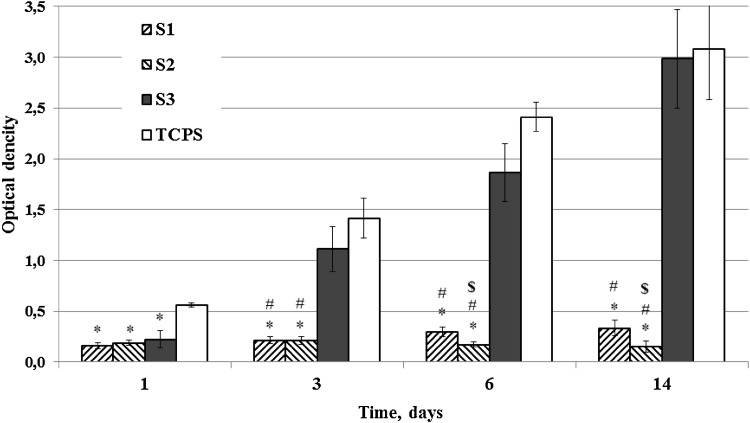
Cell viability testing was used to estimate the attachment and cell growth of MSC in the scaffolds. As indicated in Fig. 2 there was a great difference between the cell growth on the scaffolds S1 and S2 produced by double-leaching method and scaffolds S3 produced by one-stage leaching method on 3rd and 6th days of cell cultivation. The lowest MSC growth showed scaffolds S2 with small pores and low porosity on 6th and 14th days of cell cultivation. Thus, the scaffolds S3 are more suitable for MSC proliferation (Fig. 2a). The more active growth of MSC in scaffolds S3 was confirmed by SEM: If, on the surface of the pores of S1 and S2 scaffolds, we can see only single cells, on the surface of the pores of S3 scaffolds, several cells are attached (Fig. 3). To confirm the effect of pore size and porosity on cell growth, we studied also the growth of 3T3 fibroblasts in PHB/EG scaffolds. Unlike MSC, the growth of 3T3 fibroblasts in various PHB/PEG scaffolds did not differ so greatly (Supplemental data, fig. S2). On third day, the growth of 3T3 in scaffolds S3 was lower than in scaffolds S1 and S2, but, on 6th day, the 3T3 cells growth in S3 scaffolds was the same than in S1 and significantly increased compared to S2. Scaffolds S2 showed also the lowest growth of 3T3 fibroblasts on 6th day of cell cultivation. The values of cell viability estimated by XTT were similar to the values obtained in other experiments for study of cell growth in polymer scaffolds by other colorimetric assays for assessing cell metabolic activity (Zhang et al. 2010; Cruz et al. 2010; Ribeiro-Samy et al. 2013).
Fig. 2.
Growth of MSC in PHB/PEG scaffolds for 14 days, *p < 0.05 vs. TCPS; #p < 0.05 for S1 and S2 vs. S3; $p < 0.05 for S2 vs. S1
Fig. 3.
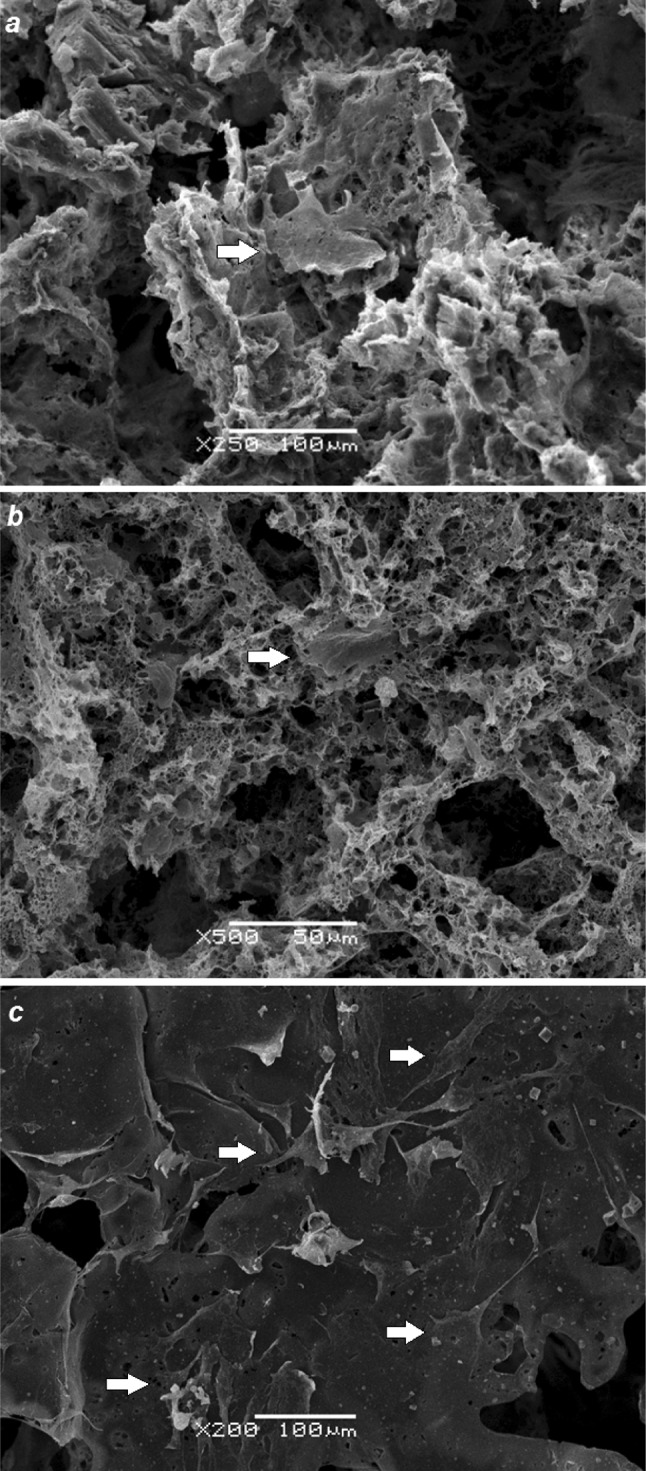
Adhesion of MSCs in PHB/PEG scaffolds on 6th day of cell cultivation: S1, SEM, ×250 (a); S2, SEM, ×500 (b); S3, SEM, ×200 (c)
The observed data on the growth of cells on polymer scaffolds can be connected both with the properties of PHB/PEG composite and the morphology of these scaffolds. The physicochemical (e.g., mechanical) properties of polymer materials have a great influence on growth and differentiation of stem cells grown on them (Cruz et al. 2010; Raucci et al. 2012; Rumian et al. 2013; Humpolicek et al. 2015; Fraioli et al. 2016; Zhang et al. 2016; Kuznetsova et al. 2016). Moreover, apparently PHB is an example of such biomaterial possessing osteoinductive properties: scaffolds based on PHB can induce spontaneous differentiation of stem cells and stimulate bone tissue regeneration in vivo (Wang et al. 2004; Bonartsev et al. 2008; Shishatskaya et al. 2014; Lim et al. 2017). However, the material, from which the studied scaffolds are made, is the same: PHB/PEG composite; nevertheless, we observed the great difference in MSC growth on them. Therefore, the obtained data are probably associated mainly with scaffold morphology. The observed low growth of both MSCs and 3T3 fibroblasts in scaffolds S2 can be obviously connected with small pores and low porosity of these scaffolds. Porosity and pore size are critical values for bone tissue engineering, where the scaffolds on the basis of PHB are mainly used (Lim et al. 2017). Scaffolds with mean pore sizes ranging from 20 to 1500 µm were used in bone tissue engineering applications (Richardson et al. 2006; Oh et al. 2007; Zhang et al. 2010; Moisenovich et al. 2015; Rumian et al. 2013; Kuznetsova et al. 2016). The minimum pore size for a significant bone growth and MSC cultivation is 40 µm with an optimal range of 100–200 µm (Klawitter et al. 1976; Richardson et al. 2006; Cruz et al. 2010; Misra et al. 2010; Zhang et al. 2010; Murphy et al. 2010; Ribeiro-Samy et al. 2013; Shishatskaya et al. 2014; Hadjicharalambous et al. 2015). However, as shown in the study of growth of different kinds of cells (chondrocytes, osteoblasts, and fibroblasts) in cylindrical polycaprolactone scaffolds with gradually increasing pore size (from 88 to 405 µm) and porosity (from 80 to 94%), the difference in scaffold microstructure is relevant only for long-term cultivation of cells, while the growth of cells with a duration of their cultivation of less than 14 days was the same and did not depended on pore size and porosity (Oh et al. 2007). The low correlation between size of the topographical units of the micropatterned electrospun scaffolds and growth/differentiation of MSC grown on them was also confirmed (Criscenti et al. 2017). Moreover, the scaffolds made from PHB, its copolymers, and composites with pores size in the range from 100 to 200 µm and porosity more than 80% have proven to be effective for both MSC cultivation and bone tissue regeneration (Nemets et al. 2008; Misra et al. 2010; Ribeiro-Samy et al. 2013; Shishatskaya et al. 2014). Therefore, the pores size of S2 about 40 µm, probably, cannot provide the normal growth of cells in spite of the known cases of using scaffolds with such a small pore size (Richardson et al. 2006; Biazar and Heidari Keshel 2015; Reyes et al. 2016). However, the inhibition of cell growth can be caused also by increased stiffness of scaffolds S2 that have three times greater Young’s modulus compared to S1 (Table 1) (Ribeiro-Samy et al. 2013; Ziv et al. 2014; Hadjicharalambous et al. 2015).
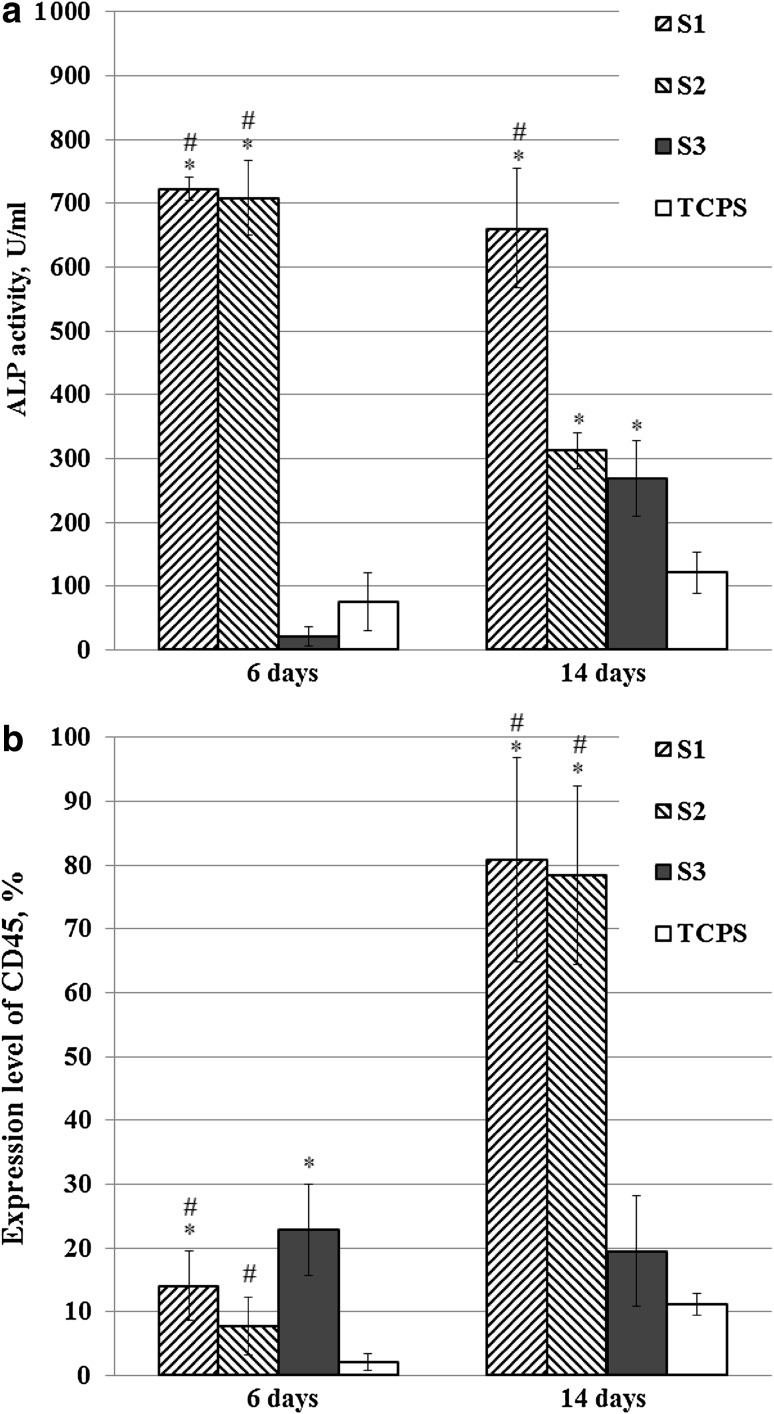
However, the greatest difference in cell growth was observed when comparing the growth of MSC in scaffolds S1 and S2 produced by double-leaching method and scaffolds S3 produced by one-stage leaching method. The 3D growth of MSC in PHB scaffolds produced by one-stage leaching (like S3) was shown earlier by confocal microscopy. The microstructure of scaffolds S1 and S2 implied also 3D cell growth (Bonartsev et al. 2016). However, the scaffolds S3 provided the normal growth of MSC, as well as 3T3 fibroblasts, whereas the growth of MSC in scaffolds S1 was suppressed in some way. It should be noted that such great difference in MSC growth in scaffolds with various microstructure is in contradiction with the data on MSC growth in 3D scaffolds from other polymer biomaterials. The difference in cell growth in scaffolds of different microstructure from collagen–glycosaminoglycan, polycaprolactone, and polylactic-co-glycolic was not so significant, especially in the early stages of cell cultivation (Oh et al. 2007; Murphy et al. 2010; Criscenti et al. 2017). We proposed that the suppressed growth of MSC in scaffolds S1, as well as S2, can be associated with spontaneous osteogenic differentiation of stem cells. To verify whether the reduced growth of MSC can be caused by their differentiation, we studied the activity of ALP and expression level of CD45 marker in MSCs on 6th and 14th days of cultivation in various scaffolds (Fig. 4). The dramatic increase (up to 30-fold and even more) in ALP activity in MSC grown in scaffolds S1 and S2 in comparison with S3 indicates spontaneous differentiation of MSC in S1 and S2 on 6th and 14th days, which is accompanied by a reduced growth of cells (Cruz et al. 2010; Raucci et al. 2012; Rumian et al. 2013). The loss of phenotype of MSC through their differentiation was also confirmed by rise in expression level of CD45 marker on 6th day in MSC grown in S1 and S3 scaffolds, and on 14th day in MSC grown in S1 and S2 scaffolds. However, equally, the same ALP activity in MSC grown in S2 and S3 and the lower level of CD45 expression in MSC grown in S2 in comparison with S3 cannot explain the greatly inhibited MSC growth in S3 scaffolds through stem cells differentiation. However, the obtained data are also in contradiction with the data on MSC differentiation in scaffolds from other polymer biomaterials, e.g., polylactic and polylactic-co-glycolic acids, with different surface topography or with small pores (62 µm), where the change in ALP activity was not practically observed (Richardson et al. 2006; Criscenti et al. 2017). These data are consistent with the data which we obtained earlier on the long-term cultivation of MSCs with passages every 4–6 days in scaffolds produced by one-stage leaching (like S3) (Andreeva et al. 2015). The observed ability of scaffolds S3 to support MSC growth can be associated also with the irregular pore structure and the great diversity of pore size with gradient between two sides of scaffold. Therefore, it was shown that polymer scaffolds with gradient in pore size or anisotropic porous morphology contribute to improving MSC growth in them (Ribeiro-Samy et al. 2013; Houben et al. 2017). The spontaneous differentiation of MSC that inhibited cell growth can be caused by microroughness of pores surface: the double-leaching method can produce pores with greater roughness in scaffolds S1 and S2 in comparison with S3 produced by one-stage leaching method. It was shown that pore microstructure of 3D scaffolds and topography of polymer surface influence greatly on both MSC growth and their differentiation in various directions: osteogenic, chondrogenic, lipogenic, etc. (Raucci et al. 2012; Rumian et al. 2013; Lim et al. 2017; Criscenti et al. 2017).
Fig. 4.
ALP activity (a) and expression level of CD45 phenotype marker (b) in MSC on 6th and 14th days of cell cultivation in PHB/PEG scaffolds in normal cultural medium, *p < 0.05 vs. TCPS, #p < 0.05 for S1 and S2 vs. S3
Conclusions
To summarize, it is suggested that microstructure of polymer scaffolds affects greatly on growth of MSCs: cylinder-shape scaffolds from PHB/PEG composite with uniform pore size of about 125 µm do not support MSCs growth probably due to their spontaneous differentiation in these scaffolds, whereas flat PHB/PEG scaffolds with diverse pore size promote stem cells growth with significantly lower level of their differentiation. In scaffolds with small pores (about 40 µm), the growth of MSC was the lowest and cell growth suppression was only partially related to stem cell differentiation. Thus, the MSC growth in PHB/PEG scaffolds significantly depended on different microstructure of these polymer carriers that can be used for specific purposes in tissue engineering.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by Russian Science Foundation, project no. 17-74-20104 with the exception of the part of poly(3-hydroxybutyrate) microbiological production by Azotobacter chroococcum 7B that was carried out within the framework of government assignment registration Federal Agency for Scientific Organizations (RU) (# 01201351358). The equipment of User Facilities Centers of M. V. Lomonosov Moscow State University (incl. the FACSAria SORP complex, Scanning electron microscope, Complex for production of micro- and nanoparticles of polymers, etc.) and Research Center of Biotechnology RAS was used in the work.
Compliance with ethical standards
The authors declare that they have no conflicts of interest regarding the publication of this article.
References
- Andreeva NV, Bonartsev AP, Zharkova II, et al. Culturing of mouse mesenchymal stem cells on poly-3-hydroxybutyrate scaffolds. Bull Exp Biol Med. 2015;159(4):567–571. doi: 10.1007/s10517-015-3015-5. [DOI] [PubMed] [Google Scholar]
- Artsis MI, Bonartsev AP, Iordanskii AL, et al. Biodegradation and medical application of microbial poly(3-hydroxybutyrate) Mol Cryst Liq Cryst. 2010;523(1):21–49. [Google Scholar]
- Biazar E, Heidari Keshel S. Electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/hydroxyapatite scaffold with unrestricted somatic stem cells for bone regeneration. ASAIO J. 2015;61(3):357–365. doi: 10.1097/MAT.0000000000000205. [DOI] [PubMed] [Google Scholar]
- Bonartsev AP, Iordanskii AL, Bonartseva GA, Zaikov GE. Biodegradation and medical application of microbial poly(3-hydroxybutyrate) J Balkan Tribol Assoc. 2008;14(3):359–395. [Google Scholar]
- Bonartsev AP, Zharkova II, Yakovlev SG, et al. Adhesion and growth of bone marrow mesenchymal stem cells on 3D scaffolds from poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer. J Biomater Tissue Eng. 2016;6(1):42–52. doi: 10.1166/jbt.2016.1414. [DOI] [Google Scholar]
- Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RTH, Marcal H, Ahmed T, et al. Poly(ethylene glycol)-modulated cellular biocompatibility of polyhydroxyalkanoate films. Polym Int. 2013;62(6):884–892. doi: 10.1002/pi.4451. [DOI] [Google Scholar]
- Cheng G, Cai Z, Wang L. Biocompatibility and biodegradation of poly(hydroxybutyrate)/poly(ethylene glycol) blend films. J Mater Sci Mater Med. 2003;14(12):1073–1078. doi: 10.1023/B:JMSM.0000004004.37103.f4. [DOI] [PubMed] [Google Scholar]
- Criscenti G, Vasilevich A, Longoni A, et al. 3D screening device for the evaluation of cell response to different electrospun microtopographies. Acta Biomater. 2017;55:310–322. doi: 10.1016/j.actbio.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Cruz DM, Gomes M, Reis RL, et al. Differentiation of mesenchymal stem cells in chitosan scaffolds with double micro and macroporosity. J Biomed Mater Res A. 2010;95(4):1182–1193. doi: 10.1002/jbm.a.32906. [DOI] [PubMed] [Google Scholar]
- D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13(9):1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Domínguez-Díaz M, Meneses-Acosta A, Romo-Uribe A, et al. Thermo-mechanical properties, microstructure and biocompatibility in poly-β-hydroxybutyrates (PHB) produced by OP and OPN strains of Azotobacter vinelandii. Eur Polymer J. 2015;63:101–112. doi: 10.1016/j.eurpolymj.2014.12.002. [DOI] [Google Scholar]
- Fraioli R, Dashnyam K, Kim JH, Perez RA, et al. Surface guidance of stem cell behavior: chemically tailored co-presentation of integrin-binding peptides stimulates osteogenic differentiation in vitro and bone formation in vivo. Acta Biomater. 2016;43:269–281. doi: 10.1016/j.actbio.2016.07.049. [DOI] [PubMed] [Google Scholar]
- Hadjicharalambous C, Mygdali E, Prymak O, et al. Proliferation and osteogenic response of MC3T3-E1 pre-osteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria. J Biomed Mater Res Part A. 2015;103A:3612–3624. doi: 10.1002/jbm.a.35475. [DOI] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Pati S, et al. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10(3):243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- Houben A, Van Hoorick J, Van Erps J, et al. Indirect rapid prototyping: opening up unprecedented opportunities in scaffold design and applications. Ann Biomed Eng. 2017;45(1):58–83. doi: 10.1007/s10439-016-1610-x. [DOI] [PubMed] [Google Scholar]
- Humpolicek P, Radaszkiewicz KA, Kasparkova V, et al. Stem cell differentiation on conducting polyaniline. RSC Adv. 2015;5:68796–68805. doi: 10.1039/C5RA12218J. [DOI] [Google Scholar]
- Iordanskii AL, Ol’khov AA, Pankova YN, et al. Hydrophilicity impact upon physical properties of the environmentally friendly poly(3-hydroxybutyrate) blends: modification via blending. Macromol Symp. 2006;233:108–116. doi: 10.1002/masy.200690005. [DOI] [Google Scholar]
- Klawitter JJ, Bagwell JG, Weinstein AM, et al. An evaluation of bone growth into porous high density polyethylene. J Biomed Mater Res. 1976;10(2):311–323. doi: 10.1002/jbm.820100212. [DOI] [PubMed] [Google Scholar]
- Kuznetsova DS, Timashev PS, Dudenkova VV, et al. Comparative analysis of proliferation and viability of multipotent mesenchymal stromal cells in 3D scaffolds with different architectonics. Bull Exp Biol Med. 2016;160(4):535–541. doi: 10.1007/s10517-016-3214-8. [DOI] [PubMed] [Google Scholar]
- Lim J, You M, Li J, Li Z. Emerging bone tissue engineering via polyhydroxyalkanoate (PHA)-based scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;79:917–929. doi: 10.1016/j.msec.2017.05.132. [DOI] [PubMed] [Google Scholar]
- Misra SK, Ansari TI, Valappil SP, et al. Poly(3-hydroxybutyrate) multifunctional composite scaffolds for tissue engineering applications. Biomaterials. 2010;31(10):2806–2815. doi: 10.1016/j.biomaterials.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Moisenovich MM, Malyuchenko NV, Arkhipova AY, et al. Novel 3d-microcarriers from recombinant spidroin for regenerative medicine. Dokl Biochem Biophys. 2015;463:232–235. doi: 10.1134/S1607672915040109. [DOI] [PubMed] [Google Scholar]
- Monnier A, Rombouts C, Kouider D, et al. Preparation and characterization of biodegradable polyhydroxybutyrate-co-hydroxyvalerate/polyethylene glycol-based microspheres. Int J Pharm. 2016;513(1–2):49–61. doi: 10.1016/j.ijpharm.2016.08.066. [DOI] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- Myshkina VL, Nikolaeva DA, Makhina TK, et al. Effect of growth conditions on the molecular weight of poly-3-hydroxybutyrate produced by Azotobacter chroococcum 7B. Appl Biochem Microbiol. 2008;44(5):482–486. doi: 10.1134/S0003683808050050. [DOI] [PubMed] [Google Scholar]
- Myshkina VL, Ivanov EA, Nikolaeva DA, et al. Biosynthesis of poly-3-hydroxybutyrate-3-hydroxyvalerate copolymer by Azotobacter chroococcum strain 7B. Appl Biochem Microbiol. 2010;46(3):289–296. doi: 10.1134/S0003683810030075. [DOI] [PubMed] [Google Scholar]
- Nemets EA, Efimov AE, Egorova VA, et al. Micro- and nanostructural characteristics of 3D porous carriers ElastoPHB-3D. Bull Exp Biol Med. 2008;145(3):371–373. doi: 10.1007/s10517-008-0094-6. [DOI] [PubMed] [Google Scholar]
- Oh SH, Park IK, Kim JM, Lee JH. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials. 2007;28(9):1664–1671. doi: 10.1016/j.biomaterials.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Raucci MG, Alvarez-Perez MA, Demitri C, Sannino A, Ambrosio L. Proliferation and osteoblastic differentiation of hMSCs on cellulose-based hydrogels. J Appl Biomater Funct Mater. 2012;10(3):302–307. doi: 10.5301/JABFM.2012.10366. [DOI] [PubMed] [Google Scholar]
- Reyes AP, Martinez Torres A, Carreon Castro Mdel P, et al. Novel poly(3-hydroxybutyrate-g-vinyl alcohol) polyurethane scaffold for tissue engineering. Sci Rep. 2016;6:31140. doi: 10.1038/srep31140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Samy S, Silva NA, Correlo VM, et al. Development and characterization of a PHB-HV-based 3D scaffold for a tissue engineering and cell-therapy combinatorial approach for spinal cord injury regeneration. Macromol Biosci. 2013;13(11):1576–1592. doi: 10.1002/mabi.201300178. [DOI] [PubMed] [Google Scholar]
- Richardson SM, Curran JM, Chen R, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-l-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27(22):4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Rumian L, Wojak I, Scharnweber D, Pamuła E. Resorbable scaffolds modified with collagen type I or hydroxyapatite: in vitro studies on human mesenchymal stem cells. Acta Bioeng Biomech. 2013;15(1):61–67. [PubMed] [Google Scholar]
- Shishatskaya EI, Kamendov IV, Starosvetsky SI, et al. An in vivo study of osteoplastic properties of resorbable poly-3-hydroxybutyrate in models of segmental osteotomy and chronic osteomyelitis. Artif Cells Nanomed Biotechnol. 2014;42(5):344–355. doi: 10.3109/21691401.2013.816312. [DOI] [PubMed] [Google Scholar]
- Stevanovic M, Pavlovic V, Petkovic J. ROS-inducing potential, influence of different porogens and in vitro degradation of poly (d,l-lactide-co-glycolide)-based material. Express Polym Lett. 2011;5(11):996–1008. doi: 10.3144/expresspolymlett.2011.97. [DOI] [Google Scholar]
- Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25(4):669–675. doi: 10.1016/S0142-9612(03)00561-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Ma Z, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010;6(8):3021–3028. doi: 10.1016/j.actbio.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu J, Ruan YC, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv K, Nuhn H, Ben-Haim Y, et al. A tunable silk-alginate hydrogel scaffold for stem cell culture and transplantation. Biomaterials. 2014;35(12):3736–37343. doi: 10.1016/j.biomaterials.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.