Abstract
PURPOSE: To investigate breast cancers total hemoglobin concentration (THC) characteristics and its association with clinical pathologic findings. MATERIALS AND METHODS: The study was approved by the institutional review board and all patients provided written informed consent. 447 breast cancer patients, totally 455 lesions were included in our study. The size and THC of breast lesions were measured by conventional ultrasound (US) and US-guided Diffuse Optical Tomography (DOT) 1–2 days before surgery. Clinical and pathology information of patients was collected. RESULT: The average THC values of ER- or PR- lesions were significantly higher than the positive ones (P = .005 and P = .01,respectively); The average THC values of axillar LN+ or LVI+ were higher than the negative ones (P = .042 and P = .043, respectively). No significant THC difference was found in groups of infiltrating vs. non-infiltrating, HER2+ vs. HER2-, Ki67 high vs. Ki67 low, and different menstrual phases (P = .457, P = .917, P = .417, P = .213, respectively).The incidence ages and the lesion-nipple distances of T3 patients were lower than that of T1 and T2 (P < .001 and P < .001 respectively). The THC values and Ki67 indexes of T2 and T3 lesions were similar, but were higher than that of the T1 group (P < =0.001 and P = .006, respectively). CONCLUSION: Clinicopathological features of breast cancer, such as ER and PR status, axillary lymph node metastasis, lymphovascular invasion, correlate with THC values. Furthermore, the Ki67 indexes can be predicted using tumor size and THC, useful for pre-surgical evaluation of cancer biology and real-time, non-invasive monitoring of NAC efficacy.
Introduction
The prognosis of breast cancer, one of the top lethal diseases for women, is affected by many factors such as age, tumor size, lymph node metastasis, lymphovescular invasion(LVI), Ki67 index, and ER, PR, HER2 immunohistochemistry status, which requires invasive biopsy [1], [2], [3], [4]. Angiogenesis is one of the most important invasiveness markers for breast cancer, as it is critically needed for tumor growth and metastasis, i.e., the higher the blood supply, the more invasive the tumor gets, and the worse the prognosis [5], [6], [7], [8]. Ultrasound-guided Diffuse optical tomography (US-guided DOT) is an emerging functional modality, which measures the total hemoglobin concentration (THC) of breast lesions to quantitatively reflect tumor metabolic activity and angiogenesis. Zhu Q et al. have confirmed that THC has a positive correlation with microvessel density (MVD) and the mean maximum THC moderately correlated with tumor histologic grade and nuclear grade, which correlated with tumor progression [9], [10]. However, few studies addressed associations between THC and histopathologic prognostic factors of breast carcinoma [11], [12]. Our current study used so-far the largest cohort of cases, to scrutinize the correlations of breast cancer THC and clinicopathological variables, aiming to identify impacting factors of US-guided DOT THC, as well as hoping to pave the way for future use of this non-invasive technology to predict pre-surgical biological behaviors of breast cancer.
Materials and Methods
Patients
Our study was approved by the institutional review board, and all patients provided written informed consent. The study was performed from September 2014 to February 2016 in Shanghai Cancer Center US department. During this period, 550 patients with 558 breast lesions classified as US BI-RADS 4a, 4b, 4c, or 5 were prospectively recruited to undergo conventional US and US-guided DOT. 87 benign lesions in 87 patients were excluded by biopsy pathology. Sixteen breast cancer lesions in 16 patients were also excluded due to the following reasons: 7 lesions showed artifacts owing to improper contact between probe and protruding skin; 6 lesions were behind areola, as different optic absorbance of areolar and peri-areolar skin caused data inaccuracy; 3 lesions were without contralateral normal control tissue due to total mammospectomy. In all, 455 breast cancer lesions in 447 patients were included, with 8 bilateral breast cancer patients having primary lesions on both sides.
Conventional US and Evaluation
Conventional US images were obtained by a radiologist (W.X.Z., 9 years of experience in breast US inspection and diagnosis), to ascertain the locations of breast lesions and to evaluate features of the lesions using the Aixplorer system (SuperSonic Imagine, Aix enProvence, France) with a 4 -15 MHz linear transducer. Patients' age, BMI, menstrual status, i.e. pre- or post-menopause (>3 months of menopause), were recorded. Pre-menopause patients were further classified into menstrual, ovulatory and luteal groups. Conventional US measured tumor size at the maximum diameter plane, and the skin-nipple distance.
US-guided DOT Imaging System and Method
The patient in the supine position, maximum diameter section of breast lesion was found with Conventional US, the lesions was then scanned with US-guided DOT, using Optimus -01HWS breast diagnostic system (XinAo-MDT Technology, Hebei, China) which is a dual imaging modality combining Conventional US (Terason T3000 ultrasound, Teratech, USA) and near-infrared(NIR) optical tomography, which was used to measure functional tissue properties with optical spectroscopic analysis. The main functional parameter was the total hemoglobin concentration (THC) calculated from absorption coefficients measured by using two optical wave-lengths (785 nm and 830 nm). The technical details of this imaging system, including system configurations, imaging acquisition methods, and the data processing algorithms, have been previously described by us [13]. After freezing the frame at the maximal section, raw optic data acquisition was performed 3 times for each breast lesion, and the corresponding normal area in the contralateral breast. The final data was obtained by automaticdefining the region of interest, imaging and data computing, a process that takes about 2–3 minutes. The data were input into Excel files and mean values of THC (THCmean) were established. The optical imaging measured normal site in the symmetrical region of the contralateral breast was used as references in the reconstruction. All examinations were performed by the same radiologist who participated in the evaluation of conventional US images.
Breast Cancer Tumor Stage Criteria
According to the 7th Edition of the American Joint Committee on Cancer (AJCC) staging [14], All tumors were divided into T1,T2,and T3 three groups according to maximum diameter on US in our study. These were T1 with lesions less than or equal to 20 mm,T2 with lesions greater than 20 mm, less than or equal to 50 mm, and T3 with lesions greater than 50 mm.
Pathology and Immunohistochemistry Parameters Evaluation
All breast lesions went through surgical dissection, lymphadenectomy, and pathology/immunohistochemistry diagnosis. The pathology evaluation parameters included estrogen receptor (ER) and progesterone receptor (PR) status, and human epidermal growth factor receptor2 (HER-2)status, Ki67 proliferation indices, LVI, lymph node (LN) status by using both modified San Antonio and American Society of Clinical Oncology–College of American Physicians scoring guidelines [15], [16]. Positive status for ER and PR was defined as nuclear staining in 1% of the tumour cells. Immunohistochemical staining for HER2 was scored according to standard criteria as 0, 1+, 2+, or 3+. Scores of 0 and 1 + were considered as negative. For scores of ≥2(moderate complete membranous staining in ≥10% of tumor cells), fluorescence in situ hybridisation (FISH) was used to determine HER2 amplification, and positive FISH amplification was considered as HER2 positive. Cells with Ki-67 nuclear staining were considered to be positive (cancerous). The Ki-67 proliferation index was determined as the percentage of Ki-67-positive cells among at least 500 cancer cells in hot spots per slide. Our study defined Ki67 index “high” as ≥14%, “low” as <14%. LVI positive is the presence of tumor cells in blood and/or lymphatic vessels [17]. For this study, 2 pathologists performed final diagnosis and discrepancies were resolved by consensus.
Statistical Analysis
Statistical analyses were performed with MedCalc software (version 15.2.2; MedCalc, Mariakerke, Belgium), Continuous data were expressed as the means ± standard deviation. The One-Way ANOVA and Independent sample T test were used to estimate intergroup differences for clinicopathology parameters, a P value <.05 was regarded as significantly significant. A stepwise multiple regression analysis was used to isolate variables significantly and independently associated with THC. The enter and remove limits for the regression models were 0.05 and 0.1, respectively.
Result
The average age of 447 breast cancer patients was 51.1 years ±10.0 (mean ± standard deviation) (range19–77 years). The average size of 455 breast lesions was 29.8 mm ±15.8 (range 6–78 millimeters). The average THC value was 209.7 μmol/L ± 63.9 (range 84-395 μmol/L). The correlations of clinical pathologic parameters with breast cancer THC are listed in Table 1. In details, THC values of ER- or PR- lesions were significantly higher than the positive ones: 227.8 μmol/L ± 55.5 vs. 196.1 μmol/L ± 66.4 for ER, or 225.1 μmol/L ± 57.3 vs. 193.0 μmol/L ± 66.5 for PR (P = .005 and P = .010, respectively). Similarly, axillar lymph node positive or LVI positive lesions had higher THC than the negative ones: 216.9 μmol/L ± 60.0 vs. 204.2 μmol/L ± 66.2 for the former, or 217.4 μmol/L ± 60.1 vs. 204.6 μmol/L ± 65.8 for the latter (P = .042 and P = .043, respectively).
Table 1.
Relationships between THC and clinicopathological parameters of 455 breast cancers
| Clinicopathology Characteristics | Number | THC (μmol/L) | P Value |
|---|---|---|---|
| Histopathologic classification | |||
| Non-invasive breast carcinoma | 30 | 196.9 ± 65.8 | 0.457 |
| Invasive breast carcinoma | 425 | 210.6 ± 63.7 | |
| Tumor stage | |||
| T1 | 148 | 181.6 ± 70.4 | 0.000 |
| T2 | 251 | 221.0 ± 56.0 | |
| T3 | 56 | 233.1 ± 54.0 | |
| ER | |||
| Negative | 195 | 227.8 ± 55.5 | 0.005 |
| Positive | 260 | 196.1 ± 66.4 | |
| PR | |||
| Negative | 236 | 225.1 ± 57.3 | 0.01 |
| Positive | 219 | 193.0 ± 66.5 | |
| HER2 | |||
| Negative | 346 | 208.4 ± 64.0 | 0.917 |
| Positive | 109 | 213.5 ± 63.6 | |
| Axillary lymph node | |||
| Negative | 260 | 204.2 ± 66.2 | 0.042 |
| Positive | 195 | 216.9 ± 60.0 | |
| Lymphovascular invasion status | |||
| Negative | 276 | 204.6 ± 65.8 | 0.043 |
| Positive | 179 | 217.4 ± 60.1 | |
| Menopausal status | |||
| Postmenopausal | 251 | 211.2 ± 62.2 | 0.213 |
| Menstrual phase* | 45 | 192.7 ± 70.7 | |
| Follicular phase | 81 | 207.2 ± 68.6 | |
| luteal phase | 78 | 217.1 ± 59.1 |
Note.—THC = total hemoglobin concentration;* There were significant differences between Menstrual phase and luteal phase (P = .042).
Non-invasive breast cancer includes ductal carcinoma in situ;
Invasive breast cancer includes invasive ductal carcinoma, invasive lobular carcinoma and mucinous adenocarcinoma and papillary carcinoma and Malignant lobulated tumor and neuroendocrine cancer.
In contrast, no significant THC difference was found between lesions of HER2- vs. HER2+ (P = .917), invasive vs. non-invasive (P = .457), or Ki67 high vs. low (P = .417). No statistically significant THC variation was observed among the lesions of patients in different menstrual cycles(P = .213), except for the luteal lesions being higher than the menstrual ones: 217.1 μmol/L ± 59.1 vs. 192.7 μmol/L ± 70.7 (P = .042).
The correlations of lesion size/T stages, THC values and clinical pathologic parameters are shown in Table 2 and Figure 1, Figure 2, Figure 3. T stages were closely associated with THC value, lesion edge-nipple distance and Ki67 index (all P = .000), i.e. the higher the T stages (bigger lesion size) got, the lower the patients' age, the smaller the lesion edge-nipple distance, and the higher the THC. The average T2 lesion THC (221.0 μmol/L ± 56.0) and T3 THC (233.1 μmol/L1 ± 54.0) were markedly higher than that of T1 (181.6 μmol/L ± 70.4, P = .000), whilst no significant difference between T2 and T3 THC values. Concordantly, the Ki67 indexes of T2 and T3 were higher than that of T1: T2 39.7 ± 23.8, T3 37.0 ± 22.6 vs. T1 27.1 ± 20.9 (P = .000, P = .006, respectively), hence no difference between T2 and T3. No difference was found for BMI and lesion edge-skin distance among the T stages. Based on the aforementioned continuity variables, a stepwise multiple regression analysis generated a formula as: THC = 167.2 + 1.062size+ 0.298ki67, i.e. with 1 mm change of size, THC value varies 1.062; with 1 unit change of Ki67 index, THC value 0.298.
Table 2.
THC distribution differences and clinical characteristics of T1 - T3 breast cancer
| Clinical Stage | No. of Lesions | Age (Year) | Size (mm) | THC (μmol/L) | BMI | D1 (mm) | D2 (mm) | Ki67 |
|---|---|---|---|---|---|---|---|---|
| T1 | 148 | 53.5 ± 10.0⁎, # | 14.7 ± 3.4⁎, # | 181.6 ± 70.4⁎, # | 23.3 ± 2.8 | 7.4 ± 5.5 | 32.8 ± 17.1⁎, # | 27.1 ± 20.9⁎, # |
| T2 | 251 | 50.5 ± 10.0⁎ | 31.8 ± 8.2⁎ | 221.0 ± 56.0 | 23.5 ± 3.4 | 7.8 ± 11.1 | 24.8 ± 17.8⁎ | 39.7 ± 23.8 |
| T3 | 56 | 47.6 ± 11.1 | 61.1 ± 9.7 | 233.1 ± 54.0 | 23.7 ± 3.5 | 6.4 ± 3.4 | 14.0 ± 15.7 | 37.0 ± 22.6 |
| P value | 0.000 | 0.000 | 0.000 | 0.674 | 0.575 | 0.000 | 0.000 |
Note.—BMI:body mass index; THC:total hemoglobin concentration; D1:The distance from the lesion edge to the skin; D2; The distance from the lesion edge to the nipple;
:There were significant differences among T1 and T2 and T3;
:There were significant differences between T1 and T2.
Figure 1.
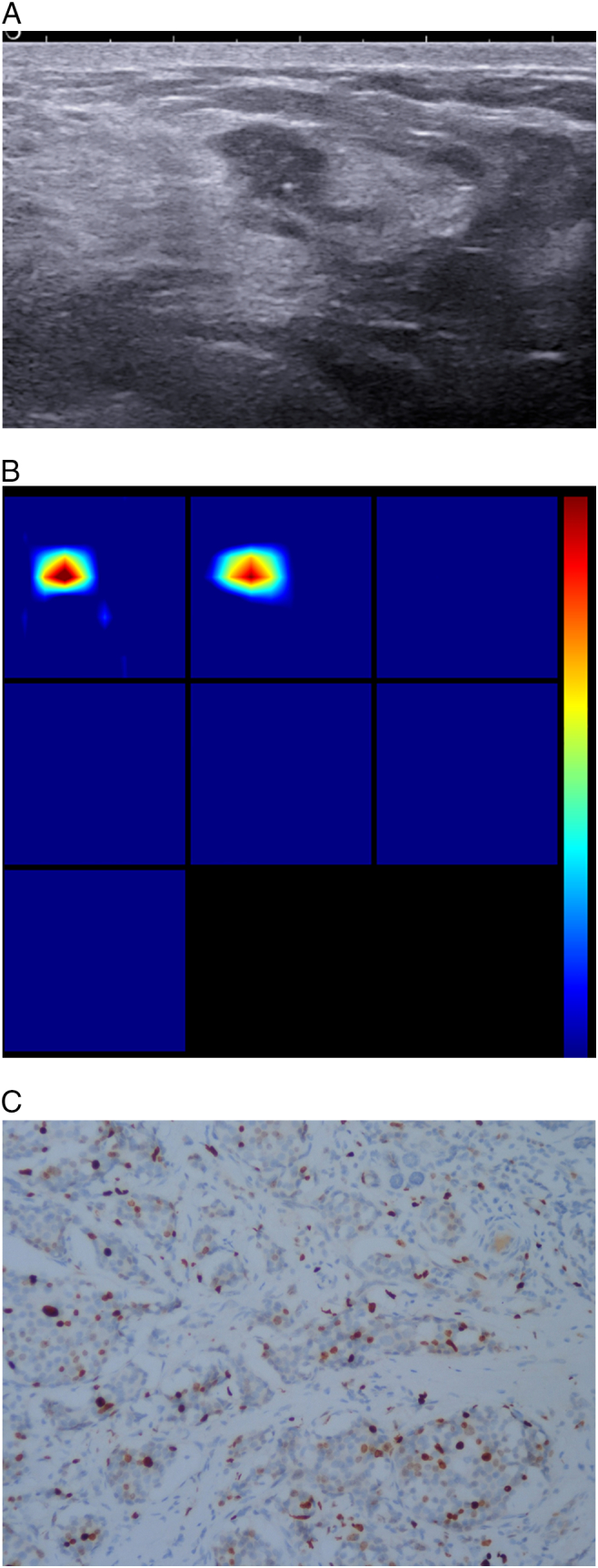
Patient age 50y, currently in the Luteal Phase, BMI20.7, pathology diagnosis of invasive ductal breast cancer, immunohistochemistry: ER+, PR+, HER -, Ki67 30%, LN +, LVI+.
(A). US 2-D imaging showing hypoechoic lesion, maximal diameter of 10 × 6 × 7 mm, irregular shape, with no circumscribed margin, containing hyperechoic spots, lesion edge-nipple distance 33 mm, lesion edge-skin surface distance 7 mm.
(B). A reconstructed optical absorption maps show that the lesion is resolved in slices from 1 to 2 (top row, left to right). THCmean is 230.5 μmol/L. The first section (slice 1, top left) is a 6 × 6 cm spatial x-y image (coronal plane of the body) obtained at a depth of 0.5 cm, as measured from the skin surface. The last section (slice 7, bottom left) is a 6 × 6 cm spatial x-y image (coronal plane of the body) obtained at a depth of 3.5 cm towards the chest wall. Spacing between sections is 0.5 cm in the direction of propagation. The vertical color scale from blue to red is the THC in micromoles per liter from low to high.
(C). Ki-67 nuclear staining (×200). The Ki-67 proliferation index is determined as 30%.
Figure 2.
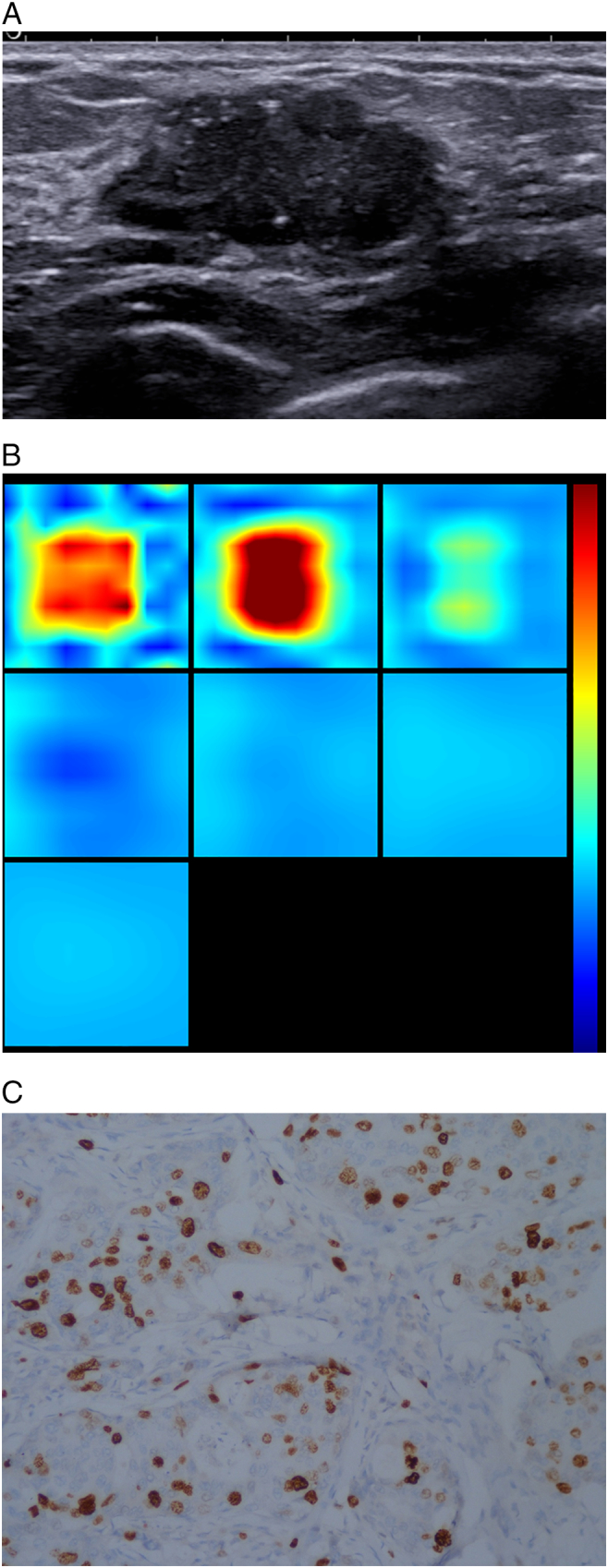
Patient age 44y, currently in the Menstrual Phase, BMI21.7, pathology diagnosis of invasive ductal breast cancer, immunohistochemistry: ER+, PR+, HER +, KI67 20%, LN-, LVI-.
(A). US 2-D imaging showing hypoechoic lesion, maximal diameter of 27 × 14 × 25 mm, irregular shape, with no circumscribed margin, a few hyperechoic spots close to lesion edge, lesion edge-nipple distance 27 mm, lesion edge-skin surface distance 4 mm.
(B). A reconstructed optical absorption maps show that the lesion is resolved in slices from 1 to 3 (top row, left to right). THCmean is 244.8 μmol/L. The first section (slice 1, top left) is a 6 × 6 cm spatial x–y image (coronal plane of the body) obtained at a depth of 0.5 cm, as measured from the skin surface. The last section (slice 7, bottom left) is a 6 × 6 cm spatial x–y image (coronal plane of the body) obtained at a depth of 3.5 cm towards the chest wall. Spacing between sections is 0.5 cm in the direction of propagation. The vertical color scale from blue to red is the THC in micromoles per liter from low to high.
(C). Ki-67 nuclear staining (×200). The Ki-67 proliferation index is determined as 20%.
Figure 3.
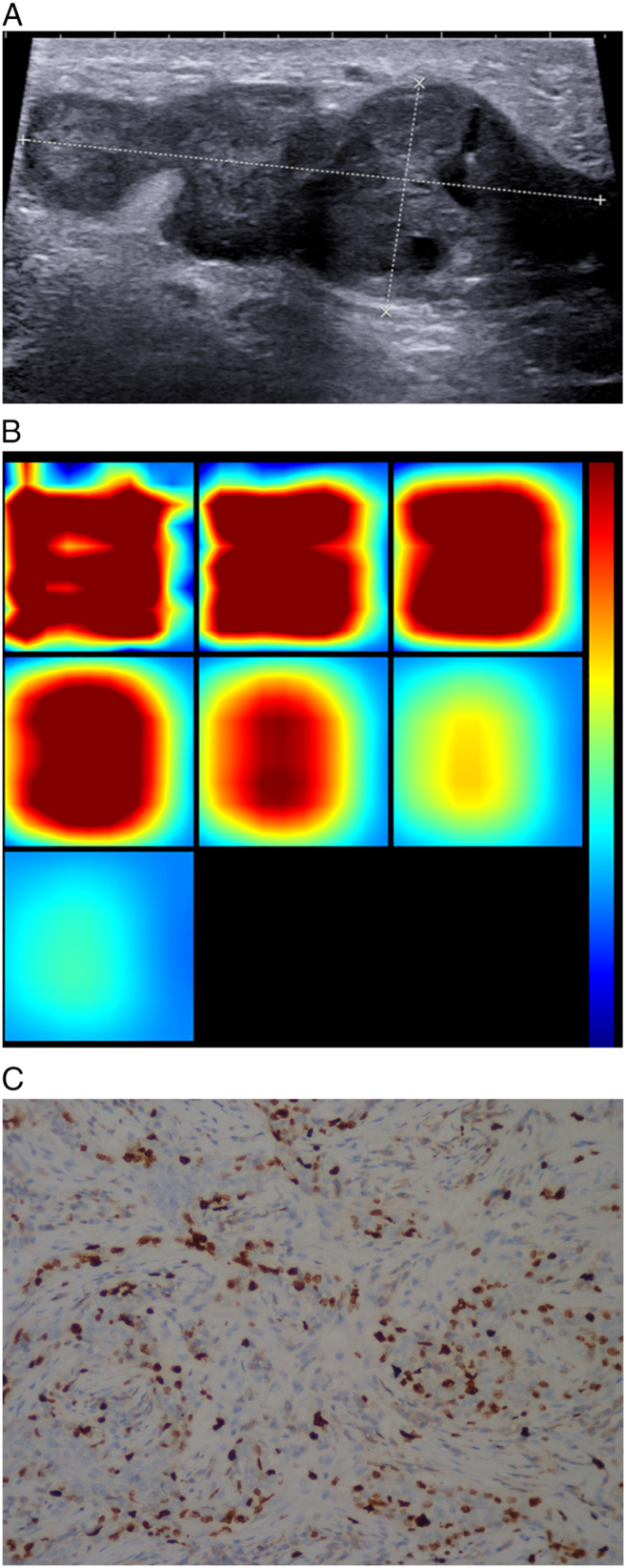
Patient age 44y, currently in the Luteal Phase, BMI22.4,pathology diagnosis of invasive ductal breast cancer, immunohistochemistry: ER-, PR-, HER -, Ki67 35%, LN -, LVI-.
(A). US 2-D imaging showing hypoechoic lesion, maximal diameter of 53 × 21 × 35 mm, irregular shape, with no circumscribed margin, heteroechoic, lesion edge-nipple distance 12 mm, lesion edge-skin surface distance 4 mm.
(B). A reconstructed optical absorption maps show that the lesion is resolved in slices from 1 to 3 (top row, left to right). THCmean was 296.1 μmol/L. The first section (slice 1, top left) is a 6 × 6 cm spatial x–y image (coronal plane of the body) obtained at a depth of 0.5 cm, as measured from the skin surface. The last section (slice 7, bottom left) is a 6 × 6 cm spatial x–y image (coronal plane of the body) obtained at a depth of 3.5 cm towards the chest wall. Spacing between sections is 0.5 cm in the direction of propagation. The vertical color scale from blue to red is the THC in micromoles per liter from low to high.
(C). Ki-67 nuclear staining (×200). The Ki-67 proliferation index is determined as 35%.
Discussion
Clinical staging, molecular subtyping, patient's age, and mutational profile all affect the prognosis of breast cancer [18], [19], [20]. Unfortunately, most of these factors have to be determined by post-surgical analysis. As a molecular imaging technology, US guided DOT can measure THC, a parameter that associates with the clinical pathologic features of different size breast cancers.
The presence of ER and PR proteins, both hormone receptors, is predictive biomarkers for efficacious endocrinological treatment of breast cancer [21]. Studies have addressed patients who are ER, PR positive and HER2 negative have decreased incidence of both local and distant recurrence as compared to those who are ER, PR negative and HER2 positive [22], [23]. Makkat et al. reported that ER-/PR-/HER2+ breast cancer lesions had more blood supply than the ER+/PR+/HER2- ones [24]. Our current study reached a similar conclusion, showing the ER-/PR- lesions maintained higher THC than the ER+/PR+ ones. This result suggests that THC value can reflect the ER/PR status of breast cancer: the higher the THC, the lower the ER/PR levels. HER2 overexpression is a benchmark for cancer invasiveness [25], however, our result didn't support a conclusion of higher THC in HER2+ lesions.
The Ki-67 index (proliferation index) is a biological marker that is associated with cell proliferation including S-phase fraction, mitotic index, which was confirmed to strongly correlate with FLT uptake in a PET study for early prediction of positive NAC response [26], [27]. Our data showed a higher THC value in Ki67 index-high lesions, alas without statistical significance. With lesion size increasing from Stage T1 to Stages T2 and T3, THC increased along with Ki67 index; but no THC difference was found between T2 and T3 lesions, probably due to saturated blood supply when tumors grow to a certain large size, or to tumor heterogeneity [28], we speculate. Using multiple regression analysis tool, we derived a formulation of THC = 167.2 + 1.062size+ 0.298ki67, to demonstrate a linear correlation of THC and its 2 most impacting factors lesion size and Ki67 index. This is to say: when tumor THC value changes 1 μmol/L,tumor size changes 1.062 mm, and Ki67 value changes 0.298, accordingly. Using our formulation, we can calculate Ki67 index from tumor lesion size and THC value, to pre-surgically evaluate tumor biology [29]. As we know, when patients are sensitive to NAC, their breast tumors shrink, cancer blood supply decreased, consequently, THC and Ki67 index values lowered. Conventionally, Ki67 indexes can only be measured from invasive needle biopsy samples [30]. We here, however, propose to use US-guided DOT to non-invasively, in real-time, determine Ki67 index in the NAC treated breast cancer patients.
LVI is the presence of tumor cells in blood and/or lymphatic vessels, the presence of LVI are unfavorable prognostic factor in primary breast cancer [31], [32]. Nathanson et al. [33], [34] described that LVI was a predictor of both regional lymph node and systemic metastasis, whilst Zhang et al. [35] reported patients with positive LNM showed higher LVI detection rate than that of LNM negative ones, demonstrating LVI as a valuable predictor of the LNM occurrence in breast cancer. Our results presented higher THC values in LVI+ lesions, the same also found in LNM+ group, suggesting THC can measure and predict the LVI and LNM status, i.e. patient prognosis.
Invasive breast cancers, which derive from non-invasive precursors, have worse prognosis [36]. To our surprise, our data didn't show significant THC difference between invasive and non-invasive breast cancers. We reason this might result from the much fewer non-invasive cases in our cohort (30/455 cases, only 6.6%). Previous research by Coradini [37] et al. showed that estrogen up-regulated VEGF expression in serum, in ovulation phase, breast tumor had an expression peak of VEGF. All these observations suggest estrogen binds to ER to induce the expression of its target gene. Accordingly, in the late luteal phase, when the estrogen antagonizing progesterone fades, VEGF levels surge again. As known, THC is associated with VEGF, MVD [38], [39]. Our results revealed, when patients were going through the menstrual, ovulatory and luteal phases of the menstrual cycle, the THC values of breast cancer lesions were progressively increasing, peaking in the luteal phase. Therefore, our finding strongly suggest, extra care must be taken when THC values are used for differential diagnosis of benign vs. malignant breast tumors, especially for the pre-menopause patients.
Of course, there are limitations for our study. Firstly, some patients decided to receive NAC treatment right after US-guided DOT and needle biopsy, resulting in lack of histologic grading. Therefore, we were not able to analyze the impact of histology grades on THC. Secondly, multi-centered, bigger sample pooled studies are needed to validate our formulation of lesion size, THC and Ki67 values, as well as its use in evaluating NAC efficacy. Thirdly, we have to exclude all the T4 lesions that involve skin, due to inherent imaging mechanism of US-guided DOT (if the lesion is within 2 mm-distance of the skin, light emission calculation is not accurate).
In summary, we demonstrate that clinicopathological features of breast cancer, such as ER and PR status, axillary lymph node metastasis, lymphovascular invasion, correlate with THC values. Furthermore, the Ki67 indexes can be predicted using tumor size and THC, useful for pre-surgical evaluation of cancer biology and real-time, non-invasive monitoring of NAC efficacy.
Conflicts of Interest
None.
Acknowledgments
We appreciate the physicians of Breast Surgery and Ultrasound Departments of FUSCC for their helpful assistance with case acquisition.
Footnotes
Grant Support: This work was supported by National Major Scientific Research Equipment Development Project (Grant No. 81627804) and Shanghai Municipal Health and Family Planning Commission (Grant NO.201440424).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159:395–406. doi: 10.1007/s10549-016-3947-0. [DOI] [PubMed] [Google Scholar]
- 3.Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O'Malley FP, Page DL. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009;133:1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood H, Faheem M, Mahmood S, Sadiq M, Irfan J. Impact of age, tumor size, lymph node metastasis, stage, receptor status and menopausal status on overall survival of breast cancer patients in Pakistan. Asian Pac J Cancer Prev. 2015;16:1019–1024. doi: 10.7314/apjcp.2015.16.3.1019. [PMID:25735323] [DOI] [PubMed] [Google Scholar]
- 5.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PMID:9504686] [PubMed] [Google Scholar]
- 6.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [PMID:1281237] [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Guinea O, Álvarez-Cofiño A, Eiró N, González LO, del Casar JM, Fernandez-Garcia B, Lamelas ML, Andicoechea A, Vizoso FJ. Low microvascular density at the tumor center is related to the expression of metalloproteases and their inhibitors and with the occurrence of distant metastasis in breast carcinomas. Int J Clin Oncol. 2013;18:629–640. doi: 10.1007/s10147-012-0428-2. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Zhang Y, Zhang WH, Jia S, Kang Y, Tian R. Effects of differential distribution of microvessel density, possibly regulated by miR-374a, on breast cancer prognosis. Asian Pac J Cancer Prev. 2013;14:1715–1720. doi: 10.7314/apjcp.2013.14.3.1715. [PMID:23679262] [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, Ricci A, Jr., Hegde P, Kane M, Cronin E, Merkulov A, Xu Y, Tavakoli B, Tannenbaum S. Assessment of Functional Differences in Malignant and Benign Breast Lesions and Improvement of Diagnostic Accuracy by Using US-guided Diffuse Optical Tomography in Conjunction with Conventional US. Radiology. 2016;280:387–397. doi: 10.1148/radiol.2016151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, Kurtzma SH, Hegde P, Tannenbaum S, Kane M, Huang M, Chen NG, Jagjivan B, Zarfos K. Utilizing optical tomography with ultrasound localization to image heterogeneous hemoglobin distribution in large breast cancers. Neoplasia. 2005;7:263–270. doi: 10.1593/neo.04526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JS, Kim MJ, Youk JH, Moon HJ, Suh HJ, Kim EK. US-guided optical tomography: correlation with clinicopathologic variables in breast cancer. Ultrasound Med Biol. 2013;39:233–240. doi: 10.1016/j.ultrasmedbio.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Xiao M, Jiang Y, Zhu Q, You S, Li J, Wang H, Lai X, Zhang J, Liu H, Zhang J. Diffuse optical tomography of breast carcinoma: can tumor total hemoglobin concentration be considered as a new promising prognostic parameter of breast carcinoma? Acad Radiol. 2015;22:439–446. doi: 10.1016/j.acra.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhi W, Gu X, Qin J, Yin P, Sheng X, Gao SP, Li Q. Solid breast lesions: clinical experience with US-guided diffuse optical tomography combined with conventional US. Radiology. 2012;265:371–378. doi: 10.1148/radiol.12120086. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol. 2011;29:e458. doi: 10.1200/JCO.2011.35.2245. [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hicks DG. American Society of Clinical Oncology/College of American Pathologists Human Epidermal Growth Factor Receptor 2 Testing Clinical Practice Guideline Upcoming Modifications: Proof That Clinical Practice Guidelines Are Living Documents. Arch Pathol Lab Med. 2015;139:970–971. doi: 10.5858/arpa.2015-0074-ED. [DOI] [PubMed] [Google Scholar]
- 17.Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA. Different types of tumor vessels in breast cancer: morphology and clinical value. Springerplus. 2015;4:512. doi: 10.1186/s40064-015-1293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goorts B, van Nijnatten TJ, de Munck L, Moossdorff M, Heuts EM, de Boer M, Lobbes MB, Smidt ML. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2017;163:83–91. doi: 10.1007/s10549-017-4155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Chediak A, Alameddine RS, Hakim A, Hilal L, Abdel Massih S, Hamieh L, Mukherji D, Temraz S, Charafeddine M, Shamseddine A. Younger age is an independent predictor of worse prognosis among Lebanese nonmetastatic breast cancer patients: analysis of a prospective cohort. Breast Cancer (Dove Med Press) 2017;9:407–414. doi: 10.2147/BCTT.S130273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronin-Fenton DP, Kjærsgaard A, Nørgaard M, Pedersen IS, Thomassen M, Kaye JA, Gutierrez L, Telford C, Lewis J, Tyczynski JE. Clinical outcomes of female breast cancer according to BRCA mutation status. Cancer Epidemiol. 2017;49:128–137. doi: 10.1016/j.canep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Collins D, Jacob W, Cejalvo JM, Ceppi M, James I, Hasmann M, Crown J, Cervantes A, Weisser M, Bossenmaier B. Direct estrogen receptor (ER) / HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS One. 2017;12:e177331. doi: 10.1371/journal.pone.0177331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim HJ, Kim SH, Kang BJ, Choi BG, Kim HS, Cha ES, Song BJ. Breast cancer recurrence according to molecular subtype. Asian Pac J Cancer Prev. 2014;15:5539–5544. doi: 10.7314/apjcp.2014.15.14.5539. [PMID:25081661] [DOI] [PubMed] [Google Scholar]
- 23.Hurvitz SA, Gelmon KA, Tolaney SM. Optimal Management of Early and Advanced HER2 Breast Cancer. Am Soc Clin Oncol Educ Book. 2017;37:76–92. doi: 10.1200/EDBK_175630. [DOI] [PubMed] [Google Scholar]
- 24.Makkat S, Luypaert R, Stadnik T, Bourgain C, Sourbron S, Dujardin M, De Greve J, De Mey J. Deconvolution-based dynamic contrast-enhanced MR imaging of breast tumors: correlation of tumor blood flow with human epidermal growth factor receptor 2 status and clinicopathologic findings--preliminary results. Radiology. 2008;249:471–482. doi: 10.1148/radiol.2492071147. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, Yang Y, Jiang L, Wu W, Shao Z. Predictive factors of pathologic complete response in HER2- positive and axillary lymph node positive breast cancer after neoadjuvant paclitaxel, carboplatin plus with trastuzumab. Oncotarget. 2017;8(34):56626–56634. doi: 10.18632/oncotarget.17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crippa F, Agresti R, Sandri M, Mariani G, Padovano B, Alessi A, Bianchi G, Bombardieri E, Maugeri I, Rampa M. (1)(8)F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging. 2015;42:818–830. doi: 10.1007/s00259-015-2995-8. [DOI] [PubMed] [Google Scholar]
- 27.Ermiah E, Buhmeida A, Abdalla F, Khaled BR, Salem N, Pyrhönen S, Collan Y. Prognostic value of proliferation markers: immunohistochemical ki-67 expression and cytometric s-phase fraction of women with breast cancer in libya. J Cancer. 2012;3:421–431. doi: 10.7150/jca.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasir A, Holzer TR, Chen M, Man MZ, Schade AE. Differential expression of VEGFR2 protein in HER2 positive primary human breast cancer: potential relevance to anti-angiogenic therapies. Cancer Cell Int. 2017;17:56. doi: 10.1186/s12935-017-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Minckwitz G, Schmitt WD, Loibl S, Müller BM, Blohmer JU, Sinn BV, Eidtmann H, Eiermann W, Gerber B, Tesch H. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19:4521–4531. doi: 10.1158/1078-0432.CCR-12-3628. [DOI] [PubMed] [Google Scholar]
- 30.Alba E, Lluch A, Ribelles N, Anton-Torres A, Sanchez-Rovira P, Albanell J, Calvo L, García-Asenjo JA, Palacios J, Chacon JI. High Proliferation Predicts Pathological Complete Response to Neoadjuvant Chemotherapy in Early Breast Cancer. Oncologist. 2016;21:150–155. doi: 10.1634/theoncologist.2015-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed RA, Menon S, Martin SG, Green AR, Paish EC, Ellis IO. Prognostic significance of lymphatic invasion in lymph node-positive breast carcinoma: findings from a large case series with long-term follow-up using immunohistochemical endothelial marker. Mod Pathol. 2014;27:1568–1577. doi: 10.1038/modpathol.2014.60. [DOI] [PubMed] [Google Scholar]
- 32.Niemiec JA, Adamczyk A, Ambicka A, Mucha-Małecka A, Wysocki WM, Biesaga B, Ziobro M, Cedrych I, Grela-Wojewoda A, Domagała-Haduch M. Prognostic role of lymphatic vessel density and lymphovascular invasion in chemotherapy-naive and chemotherapy-treated patients with invasive breast cancer. Am J Transl Res. 2017;9:1435–1447. [PMID:28386369 PMCID:PMC5376034] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathanson SD, Kwon D, Kapke A, Alford SH, Chitale D. The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol. 2009;16:3396–3405. doi: 10.1245/s10434-009-0659-2. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizawa M, Shingaki S, Nakajima T, Saku T. Histopathological study of lymphatic invasion in squamous cell carcinoma (O-1N) with high potential of lymph node metastasis. Clin Exp Metastasis. 1994;12:347–356. doi: 10.1007/BF01755878. [PMID:7923987] [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Zhang D, Yi S, Gong M, Lu C, Cai Y, Tang X, Zou L. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:2863–2873. doi: 10.18632/oncotarget.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiotaki R, Polioudaki H, Theodoropoulos PA. Differential nuclear shape dynamics of invasive andnon-invasive breast cancer cells are associated with actin cytoskeleton organization and stability. Biochem Cell Biol. 2014;92:287–295. doi: 10.1139/bcb-2013-0120. [DOI] [PubMed] [Google Scholar]
- 37.Coradini D, Veneroni S, Pellizzaro C, Daidone MG. Fluctuation of intratumor biological variables as a function of menstrual timing of surgery for breast cancer in premenopausal patients. Ann Oncol. 2003;14:962–964. doi: 10.1093/annonc/mdg258. 12796038. [DOI] [PubMed] [Google Scholar]
- 38.Morabito A, Magnani E, Gion M, Sarmiento R, Capaccetti B, Longo R, Gattuso D, Gasparini G. Prognostic and predictive indicators in operable breast cancer. Clin Breast Cancer. 2003;3:381–390. doi: 10.3816/CBC.2003.n.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Q, Cronin EB, Currier AA, Vine HS, Huang M, Chen N, Xu C. Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology. 2005;237:57–66. doi: 10.1148/radiol.2371041236. [DOI] [PMC free article] [PubMed] [Google Scholar]


