Abstract
Background
Adjuvant chemotherapy (adCx) is an integral part of multimodal treatment in resected pancreatic ductal adenocarcinoma (PDAC) and is recommended by the German S3 guideline since 2007 in all patients. We aimed to investigate the impact of this guideline at our institution.
Methods
In 151 of 403 pancreatic resections performed histopathology revealed PDAC. Follow-up data were available from 143 patients (95%) representing our study group. The rate of recommended, initiated and fully completed adCx was analyzed for period 1 (09/2003–07/2007) and period 2 (08/2007–08/2014).
Results
Our study group comprised 49 patients in period 1 and 94 patients in period 2. AdCx was recommended, initiated and completed in 42/49 (86%), 34/49 (69%) and 22/49 (45%) patients in period 1 and in 93/94 (99%), 78/94 (83%) and 49/94 (52%) patients in period 2, respectively. Only the increase in recommendations for adCx was statistically significant (p = 0.0024). Overall, only 50% (71/143) of patients fully completed the Cx protocol. Completed adCx resulted in a significantly longer (p = 0.0225) overall survival compared to patients with incomplete or without adCx. Multiple logistic regression revealed adCx (p = 0.0046) as independent factor of survival. The hazard ratio for fully completed adCx was 0.406 and for incomplete adCx 0.567.
Conclusion
Our results indicate a high acceptance of the S3-guidline recommendation for adCx in resected PDAC in a routine setting, which, however, is completed in only 50% of all patients. Fully completed adCx had the most powerful effect on improving overall survival.
Keywords: Adjuvant chemotherapy, Resected pancreatic ductal adenocarcinoma, Guide-line compliance, Long-term survival, Cohort study
Highlights
-
•
After S3 guideline implementation only the increase in recommendations for adCx was statistically significant.
-
•
Overall, only 50% (71/143) of patients fully completed their Cx protocol.
-
•
Completed adCx resulted in a significantly longer overall survival compared to patients with incomplete or without adCx.
-
•
Multiple logistic regression revealed adCx as an independent factor of survival.
-
•
Our results indicate a high acceptance of the S3-guidline recommendation for adCx in resected PDAC in the routine setting.
1. Introduction
Pancreatic cancer is one of the most common tumors in western countries with an increasing incidence of 14.0 per 100.000 in men and 10.6 in women in Germany (2012) [1]. Due to its aggressive tumor biology with extensive local and early metastatic spread it carries one of the most dismal prognosis of all cancers with overall survival rates below 5% after five years. Up to date, oncologic resection offers the only chance for cure and improves five-year survival to at least 10% [[2], [3], [4], [5], [6], [7]], however, only about 20% of all patients remain candidates for surgery.
Dissatisfaction with the disappointing long-term survival lead to the search for additional therapeutic concepts beyond surgical resection. A number of randomized controlled trials have convincingly shown that adjuvant chemotherapy significantly adds to improved survival [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11]] and is now recommended by national [[12], [13], [14], [15]] and international [16,17] treatment guidelines. In Germany, the first S3 guideline for pancreatic ductal adenocarcinoma (PDAC) was launched in 2007 [12] and updated in 2013 [13]. Hence, no data exist about the compliance to this guideline recommendation since its implementation in Germany. A very recent study from the Netherlands could show that the national guideline from 2011 lead to a significant increase of adjuvant Cx from 45% to 54% outside clinical studies [18]. In the USA, compliance with National Comprehensive Cancer Network (NCCN) guidelines was reported to be only 35% [19]. The rate of fully completed adjuvant treatment is ranging between 55% and 75% in randomized controlled trials [[4], [5], [6], [7], [8], [9]], but is fairly unknown in the clinical routine setting. Recent evidence from the ESPAC-3 trial has shown that only fully completed adjuvant chemotherapy is an independent prognostic factor for survival after oncologic resection [20].
In the present study we investigated the compliance to the German S3 guideline 2007 in terms of recommendations, initiations and completion rates of adjuvant chemotherapy after oncologic resections in PDAC at our institution.
2. Material and methods
Between September 2003 and August 2014 a total of 403 pancreatic resections due to PDAC, distal bile duct cancer, ampullary cancer, other tumors (benign and malignant) and chronic pancreatitis were performed at our institution. There were 179 patients with PDAC of whom 151 underwent resection in curative intent. In this group in-hospital mortality was 5.3% (8/151) and 30-day mortality 2.0% (3/151).
Postoperatively, all resected patients were discussed in our local interdisciplinary tumor board as to whether adjuvant treatment was indicated. During September 2003 until July 2007 (period 1) – prior to publication of the German S3 guideline “exocrine pancreatic cancer” [12] – TNM stage, resection status and individual postoperative patient performance were the basis for recommendation of adjuvant chemotherapy. Thereafter, starting in August 2007 (period 2), adjuvant chemotherapy was generally recommended according to the new S3 guideline irrespective of TNM and resection status. Adjuvant chemotherapy regimen was generally based on Gemcitabine and was started within 6 weeks after resection in all patients.
Data were collected retrospectively until 2009, a prospective database was established since 2010. Written informed consent for data collection and follow-up was obtained from all patients by signing a contract with our University Medical Center. Follow-up data were obtained by telephone inquiries either directly from the patients, their relatives, oncologists/house practitioners or the local University Cancer Registry. The following variables were assessed: overall and disease free survival, time and localization of tumor recurrence diagnosed by contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI) or cytology/histology, if available. We further assessed whether adjuvant chemotherapy was recommended and consecutively applied, the chemotherapeutic agent(s), number of cycles and reasons for treatment discontinuation. Follow-up data could be obtained from 143 resected patients with PDAC who represent our study group. Data were entered in an electronic database (MS Access for Windows©, version 2010, Microsoft Corporation, Redmond, W.A., USA) and analyzed.
Baseline statistical calculations were done with the MedCalc® software package, version 2004 [21]. Continuous variables are presented mean ± standard deviation or as medians and quartile ranges or 95% confidence intervals (CI). For qualitative parameters, absolute and relative frequencies are given. For all remaining calculations the SAS software (release 9.3, SAS Institute Inc., Cary, N.C., USA) was employed. Variables approximately normally distributed were compared by a 2 sample t-test. For quantitative variables with skewed distribution Mann Whitney U test has been applied instead. In order to compare qualitative factors χ2 test or Fisher's exact test were used. Ordinally scaled data has been evaluated by Cochran-Armitage trend test. Kaplan-Meier's survival analysis was employed to analyse overall and disease-free survival after surgery; survival rates have been compared with the log-rank test. For survival times assessed according to the Kaplan-Meier algorithm median and 95% confidence intervals have been calculated. Furthermore, a multiple Cox regression model has been used in order to assess Hazard ratios. In general, test results with p-values < 0.05 were considered statistically significant.
3. Results
Our study group of 143 patients comprised 68 women and 75 men with a mean age of 65.5 ± 9.4 (mean ± standard deviation; range 41–84) years. A pylorus-preserving Whipple was performed in 95 (66%), a classic Whipple in 17 (12%), a total pancreatectomy in 15 (11%), a left resection in 15 (11%) and a central resection in 1 (1%) patient. Venous resections were necessary in 25 (18%) and arterial resections in 2 (1%) patients. Median ICU stay was 4 (range 1–28) days and median in-hospital stay 18 (range 8–92) days.
The most important characteristics of the study group are shown in Table 1. Among the variables tested, surgical radicality showed a significant increase in period 2 with a higher lymph node retrieval (p < 0.0001), a higher number of vascular resections (p = 0.0005), a lower incidence of R1 resections (p = 0.0062), and a lower need of transfusions (p = 0.0127).
Table 1.
Patient, operative and pathologic characteristics by time periods before and after S3 guideline implementation in 143 patients after oncologic resection for pancreatic ductal adenocarcinoma.
| Period 1 (09/’03–07/’07) (n = 49) | Period 2 (08/’07–08/’14) (n = 94) | p | ||
|---|---|---|---|---|
| Age (mean ± SD) | 64.3 ± 9.7 | 66.2 ± 9.3 | 0.2503 | |
| ASA | 1 | n = 0 | n = 5 (5%) | |
| 2 | n = 23 (47%) | n = 41 (44%) | 0.5351 | |
| 3 | n = 26 (53%) | n = 48 (51%) | ||
| OR time (minutes; median, quartile ranges) | 320 (245–458) | 309 (260–376) | 0.2011 | |
| Transfusions | n = 24 (49%) | n = 25 (27%) | 0.0127 | |
| pT stage | T1/2 | n = 4 (8%) | n = 3 (3%) | |
| T3 | n = 43 (88%) | n = 89 (95%) | 0.7514 | |
| T4 | n = 2 (4%) | n = 2 (2%) | ||
| Grading | GI | n = 7 (14%) | n = 6 (6%) | |
| GII | n = 25 (51%) | n = 41 (44%) | 0.0543 | |
| GIII | n = 17 (35%) | n = 47 (50%) | ||
| pN stage | N0 | n = 16 (33%) | n = 25 (27%) | |
| N1 | n = 32 (65%) | n = 69 (73%) | 0.2633 | |
| Nx | n = 1 (2%) | – | ||
| Lymph node retrieval (median, quartile ranges) | 11 (8–13.5) | 18 (13–24) | <0.0001 | |
| Lymphatic infiltration | L0 | n = 16 (33%) | n = 54 (57%) | |
| L1 | n = 29 (59%) | n = 40 (43%) | 0.0008 | |
| Lx | n = 4 (8%) | – | ||
| Venous infiltration | V0 | n = 36 (74%) | n = 76 (81%) | |
| V1 | n = 10 (20%) | n = 18 (19%) | 0.0723 | |
| Vx | n = 3 (6%) | – | ||
| Perineural infiltration | Pn0 | n = 7 (14%) | n = 21 (22%) | |
| Pn1 | n = 40 (82%) | n = 73 (78%) | 0.0960 | |
| Pnx | n = 2 (4%) | – | ||
| Resection status | R0 | n = 20 (41%) | n = 64 (68%) | |
| R1 | n = 26 (53%) | n = 30 (32%) | 0.0062 | |
| R2 | n = 3 (6%) | – | ||
| Vascular Resection | Yes | n = 1 (2%) | n = 24 (26%) | 0.0005 |
| No | n = 48 (98%) | n = 70 (74%) | ||
SD: standard deviation.
Overall, adjuvant chemotherapy was recommended in 135 patients (94%). The rates of recommended, initiated and fully completed chemotherapy are shown in Table 2 for the two time periods investigated. A significant increase (p = 0.0024) in the number of chemotherapy recommendations and a trend toward an increase of initiated chemotherapies (p = 0.0862) was observed in period 2.
Table 2.
Adjuvant chemotherapy in 143 patients after oncologic resection for pancreatic ductal adenocarcinoma before (period 1) and after (period 2) S3 guideline implementation.
| Period 1 (09/’03–07/’07) (n = 49) | Period 2 (08/’07–08/’14) (n = 94) | p | ||
|---|---|---|---|---|
| Adjuvant chemotherapy | ||||
| Recommended | Yes | n = 42 (86%) | n = 93 (99%) | 0.0024 |
| No | n = 7 (14%) | n = 1 (1%) | ||
| Initiated | Yes | n = 34 (69%) | n = 78 (83%) | 0.0862 |
| No | n = 15 (31%) | n = 16 (17%) | ||
| Completed (6 cycles) | Yes | n = 22 (45%) | n = 49 (52%) | 0.8490 |
| No | n = 12 (24%) | n = 29 (31%) | ||
The chemotherapeutic agents used in period 1 were Gemcitabine in 32 of 34 patients, one of them underwent additional radiation therapy. Two patients received chemoradiation with Mitomycin C/5-FU and Cisplatin/5-FU. In period 2 Gemcitabine was administered in 77 patients, 1 patient received FOLFIRINOX. In 4 patients Gemcitabine was combined with Erlotinib, 1 patient underwent chemoradiation with Gemcitabine.
Long-term follow-up results for period 1 and 2 are shown in Table 3. The percentage of survivors was significantly longer in period 2 (p = 0.0005). Median overall survival, disease-free survival and tumor recurrence did not differ in the two periods.
Table 3.
Long-term follow-up results in 143 patients after oncologic resection for pancreatic ductal adenocarcinoma before (period 1) and after (period 2) S3 guideline implementation.
| Period 1 (09/’03–07/’07) (n = 49) | Period 2 (08/’07–08/’14) (n = 94) | p | |
|---|---|---|---|
| Follow-up results | |||
| Deaths (total) | n = 47 (96%) | n = 67 (71%) | 0.0005 |
| Survivors | n = 2 (4%) | n = 27 (29%) | 0.0005 |
| Tumor recurrence | n = 43 (88%) | n = 74 (79%) | 0.1432 |
| Survival overall a | 17.3 (12–24) | 18.6 (15–22) | 0.3048 |
| Disease free survival a | 11.3 (9–17) | 11.6 (9–13) | 0.9215 |
Median survival time, 95% CI (months).
Table 4 shows the reasons for refusing chemotherapy. In 2 patients of period 1 the initial tumor board recommendation was changed, 1 patient underwent chemoradiation and 1 adjuvant chemotherapy. Overall, the main reason for not initiating adjuvant chemotherapy was the lack of patients' consent in 32% (10/31) followed by surgical complications in 19% (6/31), and general complications in 2 patients (6%). There were no significant changes in the two observation periods.
Table 4.
Reasons for non-initiation of adjuvant chemotherapy and for discontinuation of initiated adjuvant chemotherapy after oncologic resection for pancreatic ductal adenocarcinoma before (period 1) and after (period 2) S3 guideline implementation.
| Period 1 (09/’03–07/’07) | Period 2 (08/’07–08/’14) | p | |
|---|---|---|---|
| Reasons for non-initiation | (n = 15) | (n = 16) | |
| Not recommended a | n = 7 (47%) | n = 1 ( 6%) | 0.0155 |
| Patient refused | n = 5 (33%) | n = 5 (31%) | 1.0000 |
| Surgical complications | n = 3 (20%) | n = 3 (19%) | 1.0000 |
| Non-surgical complications b | n = 0 | n = 2 (13%) | 0.4839 |
| Low performance status | n = 0 | n = 2 (13%) | 0.4839 |
| Missing information | n = 1 ( 7%) | n = 3 (19%) | 0.5996 |
| Death | n = 1 ( 7%) | n = 0 | 0.4839 |
| Reasons for discontinuation | (n = 12) | (n = 29) | |
| Toxicity | n = 3 (25%) | n = 12 (41%) | 0.4799 |
| Tumor recurrence | n = 2 (17%) | n = 9 (31%) | 0.4566 |
| Patients decision | n = 0 | n = 2 ( 7%) | 1.0000 |
| Death | n = 2 (17%) | n = 1 ( 3%) | 0.2002 |
| Concomitant diseases c | n = 2 (17%) | n = 5 (17%) | 1.0000 |
| Missing information | n = 3 (25%) | n = 0 | 0.0206 |
In two patients of period 1 the original recommendation against adjuvant chemotherapy was skipped: one patient received adjuvant chemotherapy and one patient additive chemoradiation.
Cerebral insult, pulmonary embolism, myocardial infarction.
Infections, thrombosis, pulmonary embolism, necessity for other operative interventions.
The reasons for discontinuation of adjuvant chemotherapy are displayed in Table 4. Out of 112 initiated adjuvant chemotherapies, 71 were fully completed and 41 discontinued after a median of 3 (range 1–5) cycles. The predominant reasons for discontinuing chemotherapy were drug-related side effects in 37% (15/41), cancer recurrence in 27% (11/41), and death in 7% (3/41). No significant changes were observed in the two observation periods.
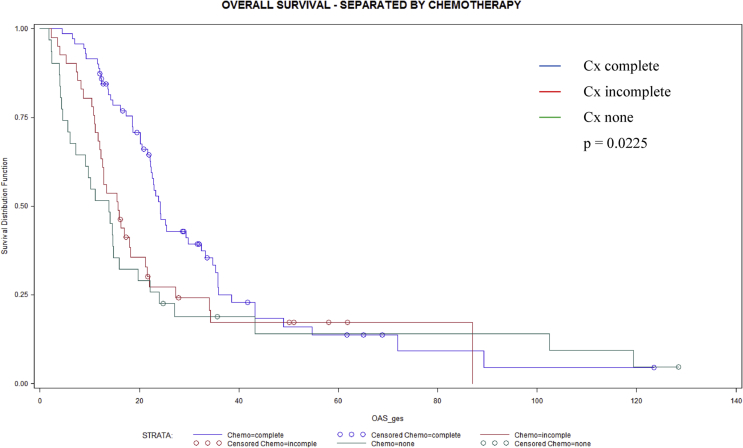
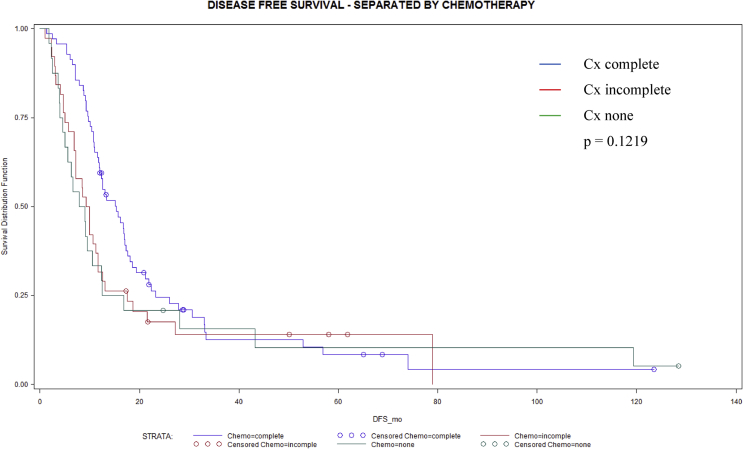
In Table 5 long-term survival data are compared between three different groups of adjuvant chemotherapy regimen. Whenever adjuvant chemotherapy was fully administered median overall survival was significantly longer (p = 0.0225) compared to patients with incomplete or without adjuvant chemotherapy. Kaplan-Meier analysis confirmed a significant benefit for overall (Fig. 1) and a trend toward longer disease free (Fig. 2) survival in patients who completed adjuvant chemotherapy with 6 cycles.
Table 5.
Long-term follow-up results in 143 patients without, with incomplete and complete adjuvant chemotherapy after oncologic resection for pancreatic ductal adenocarcinoma.
| Adjuvant Chemotherapy |
||||
|---|---|---|---|---|
| None (n = 31) | Incomplete (n = 41) | Complete (n = 71) | p | |
| Deaths (total) | n = 28 (90%) | n = 33 (80%) | n = 53 (75%) | 0.1920 |
| Survivors | n = 3 (10%) | n = 8 (20%) | n = 18 (25%) | 0.1920 |
| Tumor recurrence | n = 23 (74%) | n = 34 (83%) | n = 59 (83%) | 0.5380 |
| Overall survivala | 13.8 (6–20) | 15.7 (12–18) | 22.6 (20–26) | 0.0225 |
| Disease free survivala | 8.5 (4–13) | 9.6 (7–12) | 13.3 (12–17) | 0.1219 |
Median survival time, 95% CI (months).
Fig. 1.
Overall survival (months) in 143 patients without (n = 31), with incomplete (n = 41) and complete (n = 71) adjuvant chemotherapy after oncologic resection for pancreatic ductal adenocarcinoma (p = 0.0225).
Fig. 2.
Disease free survival (months) in 143 patients without (n = 31), with incomplete (n = 41) and complete (n = 71) adjuvant chemotherapy after oncologic resection for pancreatic ductal adenocarcinoma (p = 0.1219).
Multiple logistic regression analysis revealed tumor grading, lymph node ratio (number of positive/total lymph node count) and adjuvant chemotherapy as independent prognostic factors for overall survival. Fully completed adjuvant chemotherapy showed the most powerful effect on improving overall survival (Table 6).
Table 6.
Independent factors of overall survival after oncologic resection for pancreatic ductal adenocarcinoma by multiple logistic regression.
| p | Hazard Ratio with confidence intervals | |
|---|---|---|
| Chemotherapy | 0.0046 | |
| Cx complete versus none | 0.443 (0.270–0.727) | |
| Cx incomplete versus none | 0.567 (0.323–0.996) | |
| Lymph node (LN) ratio (unit 0.10) | 0.0003 | 1.218 (1.117–1.328) |
| Tumor grading (G) | 0.0001 | 2.003 (1.424–2.816) |
4. Discussion
Our study presents, to our knowledge, the first analysis of compliance to treatment guidelines in an academic referral center in Germany on adjuvant chemotherapy after oncologic resection of PDAC in a clinical routine setting.
Few studies on either enquiries or analysis of national data bases could show that guideline compliance is overall low ranging from 35% in the USA [19] to 54% in the Netherlands [18]. In Germany, a national survey on the oncologic treatment of pancreatic cancer after implementation of the S3 guideline in 2007 revealed that adjuvant chemotherapy was considered by only 71% of physicians after R0 resection and 62% after R1 resection [22]. In our study, recommendation for adjuvant chemotherapy was with 86% already high before the S3 guideline introduction, but significantly rose to almost 100% thereafter. This is much higher than previous rates reported by national registries and may be due to the single center analysis and our status of a tertiary referral center.
We observed only a trend toward an increase of adjuvant chemotherapy after the S3 guideline implementation from 69% to 83% in our cohort. However, this rate is coming close to the rates reported by randomized controlled trials such as the CONKO-1 trial with 96% [5,7], the Japanese trials with 84%–98% [4,6,9] and the ESPAC-3 trial with 88% [8] postoperative adjuvant treatment. A guideline driven increase of adjuvant chemotherapy from 45% to 54% was also observed in the Dutch study by van Rijssen et al. [18]. Interestingly, the overall chemotherapy rates in this study are far below our results. A major reason for this difference may be again our single center analysis which may not represent the general situation outside tertiary referral centers in Germany.
Pancreatic cancer surgery is well known to carry a high morbidity even in experienced high volume centers and not all patients recover at the same rate. In our cohort, the major reason for not starting adjuvant treatment was patients‘ refusal in 30% followed by surgical complications in 20% of all resected patients, both remaining unchanged. Patients‘ refusal is still a common problem even in study settings, e.g. in the ESPAC-3 trial, in which 40% and 51% of patients not receiving intervention withdrew their consent after randomization [8]. This important and prevailing problem probably demands improved patient information and attendance to decrease the still high withdrawal rates in the future. A recent US American study analyzed the association between postoperative complications and adjuvant chemotherapy use or treatment delay (>70 days from surgery). Overall adjuvant chemotherapy receipt was 58%: 62% among patients not experiencing any complication and 44% among those who had a serious complication [23]. One attempt to improve postoperative recovery may be the use of laparoscopic techniques [24]. Another approach is providing treatment upfront in a neoadjuvant setting, which has gained considerable interest in the past years [[25], [26], [27]]. Neoadjuvant therapy carries the advantage of providing early treatment of micrometastatic disease, avoids surgery in patients with rapidly progressive disease and allows to observe patient tolerance to therapy. Currently, randomized controlled trials on this issue are under way and will show any benefits of this approach [28].
In our study, the percentage of patients completing all 6 cycles of chemotherapy reached 45% in period 1 and 52% in period 2. These figures are lower than those reported by randomized clinical trials in which 55% and 60% [8], 60% [5,7] and up to 66% [9], 69% [4] and 75% [6] completions of chemotherapy regimens were achieved. This is most likely due to the study settings, in which follow-up usually adheres to strict protocols and thresholds to discontinue treatment on both patients‘ and physicians‘ side are higher than in a routine setting. On the other hand, toxicity was the prevailing reason to discontinue adjuvant treatment in more than one third of patients in our series. With the trend toward new and more aggressive chemotherapy regimen [11,[28], [29], [30]] this problem will not subside and remains a major focus oncologists will have to cope with in the future.
Our results show that fully completed chemotherapy had the most powerful effect on improving overall survival after oncologic resection in PDAC. This observation matches well with a recent analysis of the ESPAC-3 trial [20]. The authors could show that completion of all 6 cycles of adjuvant chemotherapy rather than early initiation was an independent prognostic factor after resection for PDAC. The key message from this study is to delay the start of adjuvant chemotherapy until the patient is fully recovered and aim to give them the full 6 cycles of treatment. Unfortunately, the exact time from surgical resection to the start of chemotherapy could not be assessed in our study, since only the month and year of adjuvant treatment initiation was documented.
The implementation of the S3 guideline did not affect median overall or disease free survival in our cohort. This is remarkable, since patients in period 2 underwent a more aggressive surgical approach as shown in Table 1. This change was independent of the S3 guideline recommendations and was due to the implementation of a more radical surgical technique since 2008. It is well known that surgical radicality per se is no longer the key factor for improved survival in pancreatic cancer. Studies on extended lymph node dissection or extended vascular resection approaches have failed to show any benefit in this respect [31,32] and are well supported by our results.
Although parts of our results match well with observations from randomized controlled studies it has to be emphasized that our study still represents a single institutional experience. As a result of the long observation period of 11 years and a retrospective study design in about half of the patients there were missing informations, especially in group 1. The strong adherence to the S3 guideline observed in our study may not necessarily represent the situation all over Germany. For the assessment of more representative data as done by the Netherlands [18] or the USA [19] the implementation of national surgical registries is a necessary prerequisite. In Germany, national registries on pancreatic surgery have started only recently [33] and are still facing problems of widespread acceptance. However, this data bases will allow meaningful insights into the state of oncologic treatment of pancreatic cancer in Germany in the future.
In summary, the results of our single institutional study could show that there is a high adherence to the S3 guideline concerning adjuvant chemotherapy in resected PDAC in the routine setting. The rate of recommended adjuvant treatment reached almost 100% after guideline publication, the percentage of initiated and completed chemotherapy regimen showed no changes and is still lower than in randomized controlled trials. Predominant reasons for non initiating and discontinuing adjuvant treatment were patients’ refusal and toxicity, respectively. Fully completed adjuvant chemotherapy had the most powerful effect on improved overall survival and should therefore, whenever possible, aimed at.
Ethical approval
The present study is a registry study. Written informed consent for data collection and follow-up inquiries was obtained from all patients by signing a contract with our University Medical Center.
Sources of funding
There was no funding for the present work.
Author contribution
Malte Weinrich: analysis of data, writing of manuscript.
Johanna Bochow: data collection and documentation, maintenance of database.
Anna-Lisa Kutsch: data collection and documentation, maintenance of database.
Guido Alsfasser: analysis of data, supervision of data recruitment, writing of manuscript.
Christel Weiss: statistical analysis, writing of manuscript.
Ernst Klar: writing of manuscript.
Bettina M. Rau: study design, supervision of data collection, analysis of data, writing of manuscript.
Conflicts of interest
There are no conflict of interests.
Research registration number
The study is based on a local registry “Database of Pancreatic Resections” which was run by the Dep. of General, Thoracic-, Vascular, and Transplantation Surgery of the University of Rostock.
UIN: researchregistry3437.
Guarantor
Corresponding author: Prof. Dr. med. Bettina M. Rau.
Acknowledgements
None.
References
- 1.Baras N., Barnes B., Bertz J. Krebs in Deutschland 2011/2012-10. Ausgabe, Robert Koch-Institut, Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V.; Berlin: 2015. Bauchspeicheldrüse; pp. 50–53.http://www.gekid.de/Doc/krebs_in_deutschland_2015.pdf [Google Scholar]
- 2.Neoptolemos J.P., Dunn J.A., Stocken D.D. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos J.P., Stocken D.D., Friess H. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 4.Kosuge T., Kiuchi T., Mukai K. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn. J. Clin. Oncol. 2006;36:159–165. doi: 10.1093/jjco/hyi234. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H., Post S., Neuhaus P. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. J. Am. Med. Assoc. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Ueno H., Kosuge T., Matsuyama Y. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br. J. Canc. 2009;101:908–915. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oettle H., Neuhaus P., Hochhaus A. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. J. Am. Med. Assoc. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos J.P., Stocken D.D., Bassi C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. J. Am. Med. Assoc. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 9.Uesaka K., Boku N., Fukutomi A. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 10.Liao W.C., Chien K.L., Lin Y.L. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–1103. doi: 10.1016/S1470-2045(13)70388-7. [DOI] [PubMed] [Google Scholar]
- 11.Jones O.P., Melling J.D., Ghaneh P. Adjuvant therapy in pancreatic cancer. World J. Gastroenterol. 2014;20:14733–14746. doi: 10.3748/wjg.v20.i40.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler G., Seufferlein T., Bischoff S.C. S3-Guidelines “Exocrine pancreatic cancer”. Z. Gastroenterol. 2007;45:487–523. doi: 10.1055/s-2007-963224. [DOI] [PubMed] [Google Scholar]
- 13.Seufferlein T., Porzner M., Becker T. S3-guideline exocrine pancreatic cancer. Z. Gastroenterol. 2013;51:1395–1440. doi: 10.1055/s-0033-1356220. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K., Okusaka T., Shimizu K. EBM-based clinical guidelines for pancreatic cancer (2013) issued by the Japan pancreas society: a synopsis. Jpn. J. Clin. Oncol. 2014;44:883–888. doi: 10.1093/jjco/hyu127. [DOI] [PubMed] [Google Scholar]
- 15.Khorana A.A., Mangu P.B., Berlin J. Potentially curable pancreatic cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016;34:2541–2556. doi: 10.1200/JCO.2016.67.5553. [DOI] [PubMed] [Google Scholar]
- 16.Ducreux M., Cuhna A.S., Caramella C. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26(Suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 17.Takaori K., Bassi C., Biankin A. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology. 2016;16:14–27. doi: 10.1016/j.pan.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Van Rijssen L.B., van der Geest L.G., Bollen T.L. National compliance to an evidence-based multidisciplinary guideline on pancreatic and periampullary carcinoma. Pancreatology. 2016;16:133–137. doi: 10.1016/j.pan.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Visser B.C., Ma Y., Zak Y. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB. 2012;14:539–547. doi: 10.1111/j.1477-2574.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valle J.W., Palmer D., Jackson R. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 2014;32:504–512. doi: 10.1200/JCO.2013.50.7657. [DOI] [PubMed] [Google Scholar]
- 21.Schoonjans F., Zalata A., Depuydt C.E. A new computer program for medical statistics. Comput. Meth. Progr. Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 22.Boeck S., Bruns C.J., Sargent M. Current oncological treatment of patients with pancreatic cancer in Germany: results from a national survey on behalf of the Arbeitsgemeinschaft Internistische Onkologie and the Chirurgische Arbeitsgemeinschaft Onkologie of the Germany Cancer Society. Oncology. 2009;77:40–48. doi: 10.1159/000226110. [DOI] [PubMed] [Google Scholar]
- 23.Merkow R.P., Bilimoria K.Y., Tomlinson J.S. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann. Surg. 2014;260:372–377. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 24.Croome K.P., Farnell M.B., Que F.G. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann. Surg. 2014;260:633–638. doi: 10.1097/SLA.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich S., Schäfer M., Weber A. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann. Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 26.Sahora K., Kuehrer I., Eisenhut A. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery (St Louis) 2011;149:311–320. doi: 10.1016/j.surg.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 27.Xia B.T., Habib D.A., Dhar V.K. Early recurrence and omission of adjuvant therapy after pancreaticoduodenectomy argue against a surgery-first approach. Ann. Surg. Oncol. 2016;23:4156–4164. doi: 10.1245/s10434-016-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo S., Saif M.W. Neoadjuvant therapy for pancreatic cancer: an ongoing debate. Therap. Adv. Gastroenterol. 2016;9:429–436. doi: 10.1177/1756283X16646524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 31.Dasari B.V., Pasquali S., Vohra R.S. Extended versus standard lymphadenectomy for pancreatic head cancer: meta-analysis of randomized controlled trials. J. Gastrointest. Surg. 2015;19:1725–1732. doi: 10.1007/s11605-015-2859-3. [DOI] [PubMed] [Google Scholar]
- 32.Giovinazzo F., Turri G., Katz M.H. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br. J. Surg. 2016;103:179–191. doi: 10.1002/bjs.9969. [DOI] [PubMed] [Google Scholar]
- 33.Wellner U.F., Lapshyn H., Bartsch D.K. Laparoscopic versus open distal pancreatectomy-a propensity score-matched analysis from the German StuDoQ|Pancreas registry. StuDoQ Pancreas study group and members of StuDoQ|Pancreas registry of the German Society for General and Visceral Surgery (DGAV) Int. J. Colorectal Dis. 2017;32:273–280. doi: 10.1007/s00384-016-2693-4. [DOI] [PubMed] [Google Scholar]