Abstract
Bone metastasis is a complication of advanced breast and prostate cancer. Tumor-secreted Dickkopf homolog 1 (DKK1), an inhibitor of canonical Wnt signaling and osteoblast differentiation, was proposed to regulate the osteoblastic response to metastatic cancer in bone. The objectives of this study were to compare DKK1 expression with the in vivo osteoblastic response in a panel of breast and prostate cancer cell lines, and to discover mechanisms that regulate cancer DKK1 expression. DKK1 expression was highest in MDA-MB-231 and PC3 cells that produce osteolytic lesions, and hence a suppressed osteoblastic response, in animal models of bone metastasis. LnCaP, C4-2B, LuCaP23.1, T47D, ZR-75-1, MCF-7, ARCaP and ARCaPM cancer cells that generate osteoblastic, mixed or no bone lesions had the lowest DKK1 expression. The cell lines with negligible expression, LnCaP, C4-2B and T47D, exhibited methylation of the DKK1 promoter. Canonical Wnt signaling activity was then determined and found in all cell lines tested, even in the MDA-MB-231 and PC3 cell lines despite sizeable amounts of DKK1 protein expression expected to block canonical Wnt signaling. A mechanism of DKK1 resistance in the osteolytic cell lines was investigated and determined to be at least partially due to down-regulation of the DKK1 receptors Kremen1 and Kremen2 in the MDA-MB-231 and PC3 cell lines. Combined DKK1 and Kremen expression in cancer cells may serve as predictive markers of the osteoblastic response of breast and prostate cancer bone metastasis.
Introduction
Bone metastasis is a common complication of advanced prostate and breast cancer and defines a point in the disease when cure is no longer possible. The invasion of tumor cells into bone irrevocably alters the bone microenvironment and initiates a skeletal response that is dependent on the type of tumor [1]. Breast cancer bone metastasis typically results in massive osteolysis from the secretion of osteoclast-activating factors, such as parathyroid hormone-related protein and others [2]. Prostate cancer classically forms osteoblastic lesions under the direction of osteoblast-activating factors that include endothelin-1 (ET-1), Wnt signaling proteins, and bone morphogenetic proteins [3], [4]. Both osteolytic and osteoblastic bone metastases represent heightened states of bone turnover but differ in the extent to which osteoblast bone formation or osteoclast bone resorption predominates.
Dickkopf homolog 1 (DKK1) is a secreted inhibitor of canonical Wnt signaling that may predict cancer cell behavior in bone. In normal bone homeostasis, DKK1 is secreted from mature osteoblasts that then feeds-back to inhibit Wnt signaling of osteoblast precursors [5]. DKK1 operates by sequestering the LDL-related proteins 5 and 6 co-receptors from the G protein-coupled protein receptor Frizzled and thus blocks Wnt signaling activation [6]. The actions of DKK1 are reinforced by Kremen, a DKK1 co-factor receptor, that participates in the binding of the Frizzled complex and down-regulation of Wnt signaling [7], [8]. Negative feedback by DKK1 supports tight control of bone formation and thus prevents excessive osteoblast activity. This role of DKK1 in bone is illustrated by the osteopenic phenotype of DKK1 transgenic overexpression in mice [9], [10].
DKK1 regulates the osteoblastic response to invading cancer cells in bone and therefore influences the balance between bone formation and resorption [5], [11]. This idea was first proposed when DKK1 was identified as a causal factor in osteoblast suppression characteristic of multiple myeloma bone disease [12]. Since this first report, DKK1 has been implicated in other forms of cancer and bone metastasis. In animal models of prostate cancer bone metastasis, DKK1 overexpression in the prostate cancer cell line C4-2B, which normally forms mixed osteoblastic-osteolytic bone lesions, resulted in the formation of primarily osteolytic lesions [13]. Conversely, knockdown of DKK1 expression in the PC3 prostate cancer cell line resulted in increased osteoblastic potential [13].
Sclerostin, another Wnt signaling inhibitor, is a product of osteoblasts and osteocytes. It operates differently from DKK1 in that it also binds to and sequesters LRPs away from the activation complex, but is not dependent on the Kremen co-receptor. As a consequence of DKK1 itself, Sclerostin expression from osteoblasts and stromal, and possibly myeloma cells, is increased in myeloma bone disease, and represents another avenue for osteoblast suppression [14], [15].
Cancer cells not only secrete DKK1 but also are able to manipulate the secretion of DKK1 from the osteoblast. This is mediated by tumor-secreted ET-1, which activates the osteoblast endothelin A receptor (ETAR) and down-regulates osteoblast DKK1 [16]. ET-1 therefore promotes pathologic bone formation by ensuring DKK1 is quelled, permitting excessive osteoblast activity and bone formation. ETAR antagonists slow progression of osteoblastic lesions in animal models of osteoblastic bone metastasis as well in human clinical trials, which suggests an important role of DKK1 in bone metastasis [3], [17], [18]. Collectively, DKK1 secreted by both cancer cells and mature osteoblasts contribute to bone microenvironment DKK1, and influences osteoblast development and pathologic bone formation in bone metastasis.
We set out to examine the extent to which DKK1 expression in breast and cancer cell lines predicts behavior in bone. In a panel of breast and prostate cancer cell lines, DKK1 expression correlated with the osteolytic skeletal phenotype. Both epigenetic methylation of the DKK1 promoter and transcriptional mechanisms were found to regulate DKK1. In the osteolytic cell lines that secreted the most DKK1, Wnt signaling was unexpectedly found to be active. We provide evidence that active Wnt signaling reported in these aggressive osteolytic cell lines is maintained by a mechanism of DKK1 resistance.
Materials and Methods
Reagents
The prostate cancer cell lines LnCaP and PC3, the breast cancer cell lines T47D, ZR-75-1 and MCF-7, and the colon cancer cell line COLO205 were obtained from ATCC (Manassas, VA). The ARCaP and ARCaPM prostate cell lines were obtained from Novicure Biotechnology (Birmingham, AL). Dr. Leland Chung, Cedars-Sinai Medical Center, provided the prostate cancer cell line C4-2B. Dr. Robert Vessella, University of Washington, provided the LuCaP23.1 prostate cancer xenograft. The MDA-MB-231(SA) (referred to as MDA-MB-231 in the text) human breast cancer cell line was a gift from Dr. Theresa Guise (Indiana University School of Medicine). This cell line is a bone-avid variant and was maintained as previously described [2]. 5-aza-2′-deoxycytidine was obtained from Sigma-Aldrich (St. Louis, MO).
Messenger RNA Expression Analysis
Messenger RNA expression was determined by real-time RT PCR using an iScript SYBR Green RT-PCR kit (Bio-Rad, Hercules, CA) and a MyIQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The following primers were utilized: DKK1-F: tagcaccttggatgggtatt, DKK1-R: atcctgaggcacagtctgat, ET-1-F: ctttgagggacctgaagctg, ET-1-R: caagccacaaacagcagaga, LRP5-F: gttcggtctgacgcagtaca, LRP5-R: gtccatcacgaagtccaggt, LRP6-F: cccatgcacctggttctact, LRP6-R: ccaagccacagggatacagt, SOST-F: ggaaagtccagggactggtt, SOST-R: catctacagttgcccccagt, MESD-F: agctgggaggtgcttcagta, MESD-R: cagcagggactatgcagtga, KRM1-F: atccagatggagacgtgagc, KRM1-R: tccttgtagcagccaaggtt, KRM2-F: acacctgagatgctgtgctg, KRM2-R: ttcctgtccgacttttggtc. Relative differences in mRNA concentration were determined by subtracting the Ct (threshold cycle) of the study gene from the Ct of the housekeeping gene RPL32 (F: cagggttcgtagaagattcaaggg and R: cttggaggaaacattgtgagcgatc) (Δ = Ctgene – CtRPL32). The mean of the lowest DKK1 expressing cell line (Δlow) was subtracted from each of the cell lines (Δsample); (mean Δlow – Δsample = ε). The fold difference was calculated as 2ε.
ELISA Assays
DKK1 and ET-1 protein in cancer cell conditioned media was determined by ELISA (R&D Systems, Minneapolis, MN). After collection of media, the cells were trypsinized and counted.
Methylation-Specific Sequencing
Genomic DNA was isolated from the selected cancer cell lines and prostate cancer xenograft. The DKK1 CpG island and flanking DNA were PCR amplified to produce a 449 bp fragment (F: aggggtgaagagtgtcaaagg; R: aggttcttgatagcgttgga). The fragment was sequenced to confirm the published sequence. Isolated DNA was then subjected to bisulfite treatment using an EZ DNA Methylation-Direct kit (Zymo Research Corporation, Irvine, CA). The bisulfite-treated DNA was PCR amplified using published primers (F: ggggtgaagagtgttaaagg; R: aaaccatcatctcaaaaaaactcaa) that flank the DKK1 CpG island to produce a 326 bp fragment [19]. Amplified fragments were sequenced. The presence of a cytosine indicated that the base was protected by methylation.
Immunohistochemistry
Cancer cells were grown on collagen-coated glass cover slips and fixed for 30 minutes in 4% paraformaldehyde/1% Triton X-100. Samples were washed and incubated with 0.3% hydrogen peroxide for 30 minutes and washed in PBS. Cells were blocked with 1% BSA (Vector Laboratories), PBS washed and incubated for 30 minutes with a mouse anti-β-catenin antibody (Millipore, Billerica, MA) at a 1:500 dilution. Slides were washed with PBS and incubated with Alexa 488 goat anti-rabbit (1:400 dilution) (Invitrogen) for 30 minutes. Cells were washed with PBS/0.1% Triton X-100. Samples were nuclear counterstained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes.
Cell Number Assay
Cancer cells (3000–10,000 cells) were plated in total volume 100 μl per well in 96-well black walled, clear bottom plate. Human recombinant DKK1 (R&D Systems, Minneapolis, MN) 50 ng/ml or vehicle control was then added. Cells were incubated for 48 hours at 37 °C. The relative number of viable cells was then determined using the Cell Titer-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) according to manufacturer's directions. Luminescence was measured using a BioTek Synergy HTX plate reader with a one second integration time. Data were normalized to the control groups.
Wnt Reporter Assay
Cells were transfected with TOPFlash or FOPFlash Wnt reported vectors (Millipore, Billerica, MA) plus Renilla luciferase as a normalization standard using Lipofectamine 2000 transfection reagent (Life Technologies, Grand Island, NY). Forty-eight hours after transfection, Dual-Luciferase Reporter Assays (Promega, Madison, WI) were performed using a BioTek Synergy 2 microplate reader (BioTek, Winooski, VT). The construction of the dominant-negative TCF3 construct has been described (Wong, et al., J Cell Biol, 2003). A human Kremen1 cDNA vector clone was obtained from ATCC (Manassas, VA). The cDNA was then subcloned into the pCMV-Sport6 expression plasmid (Life Technologies, Grand Island, NY).
Statistical Analyses
Statistical analyses were performed using Prism 4.00 software. Comparisons of two groups were performed using an unpaired, two-tailed t test. Significant differences are indicated (* = P < .05; ** = P < .01; *** = P < .001).
Results
Cancer Cell DKK1 and ET-1 Expression Predicts Bone Phenotype
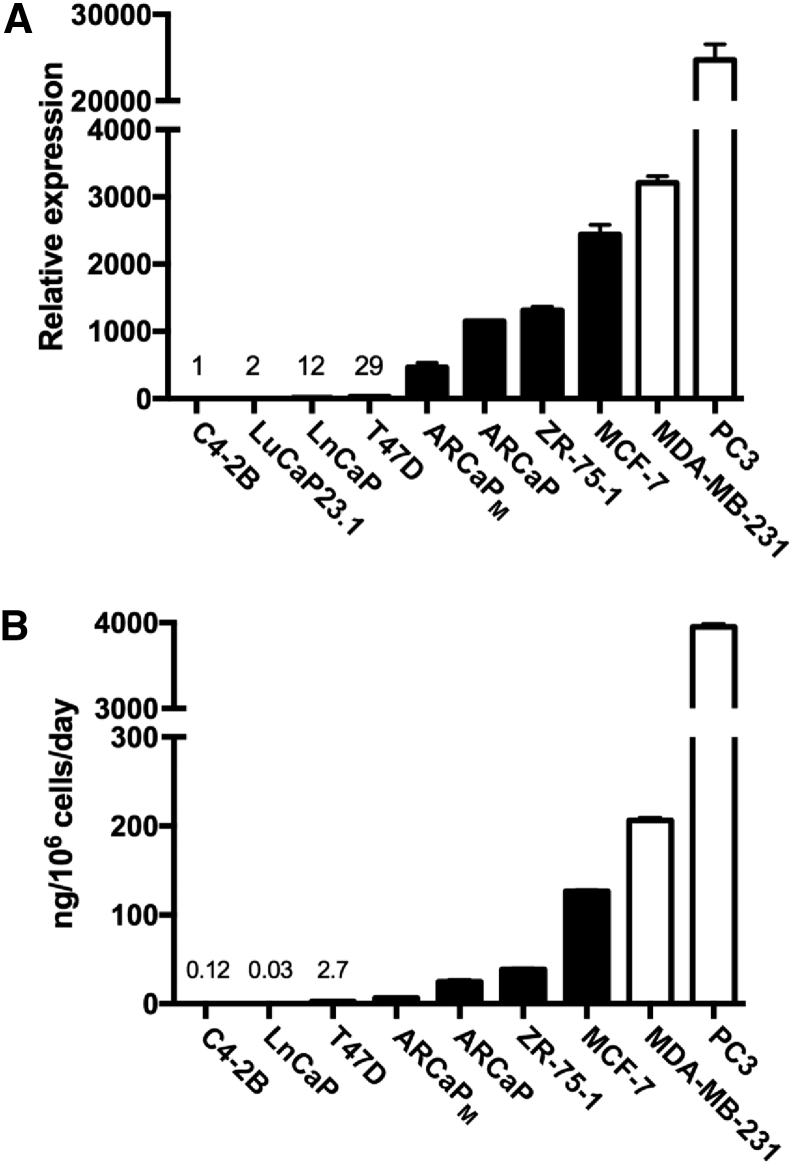
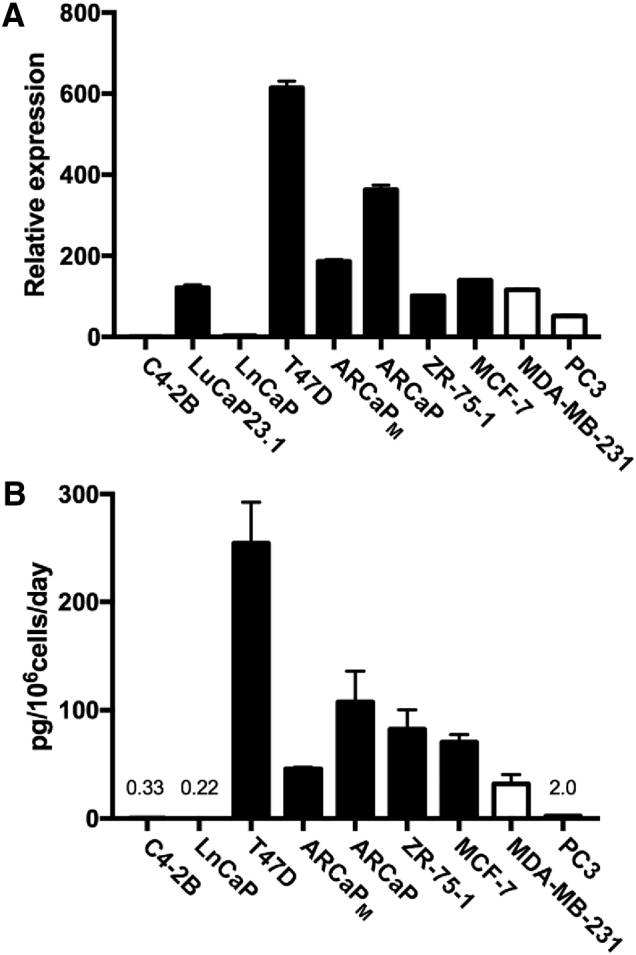
DKK1 mRNA expression and protein secretion into the surrounding medium was surveyed in selected breast and prostate cancer cell lines and compared with the phenotypic response of these cancer cells in bone (Figure 1). The androgen-dependent prostate cancer cell line LNCaP and the subline derivative C4-2B [20] expressed nearly undetectable DKK1. Of these, only C4-2B elicits a skeletal response after inoculation with mixed osteoblastic and osteoclastic characteristics. The human prostate cancer xenograft LuCaP23.1 produces osteoblastic bone lesions with intratibial inoculation [21] and little DKK1 expression was detected. The prostate cancer cell line ARCaP and the bone avid osteoblastic subline ARCaPM [20] expressed more DKK1. ARCaPM is a reliable model of osteoblastic bone metastasis. The breast cancer cell lines T47D, ZR-75-1 and MCF-7 also produce osteoblastic lesions in animal models [3] and expressed detectable amounts of DKK1. The breast cancer cell line MDA-MB-231 [2] and the prostate cancer cell line PC3 [22] elicit strong osteolytic responses in animal models of bone metastasis. These two cell lines expressed the highest amounts of DKK1. Among the cancer cell lines tested, mRNA concentration correlated well with absolute protein secreted into the surrounding medium (Figure 1).
Figure 1.
DKK1 mRNA and protein expression in cancer cells correlates with the bone response. (A) DKK1 mRNA was measured in a panel of breast (T47D, ZR-75-1, MCF-7 and MDA-MB-231) and prostate cancer (C4-2B, LnCaP, ARCaPM, ARCaP and PC3) cell lines and a prostate cancer xenograft (LuCaP23.1). (B) In the cell lines, the rate of DKK1 secreted into the surrounding medium was also measured by ELISA and normalized to cell number. Absolute DKK1 values in the cell lines with the lowest expression are reported. Closed bars represent cancer cells that produce osteoblastic, mixed or no bone lesions in animal models of bone metastasis. Open bars represent cancer cells that produce osteolytic lesion in animal models of bone metastasis.
ET-1 expression was also tested in the panel of cancer cell lines. This secreted factor activates osteoblast proliferation and new bone formation by down-regulating DKK1 [3], [16], [17], [23]. We predicted that ET-1 expression correlates with the blastic response of cancer cells in bone, opposite of DKK1 expression, consistent with previously published reports [3], [16], [17], [24], [25]. ET-1 mRNA and secreted protein expression did in fact inversely correlate with DKK1 expression in most cancer cell lines tested (Figure 2). Interestingly, C4-2B and LnCaP expressed little ET-1. The osteoblastic response of C4-2B may in part be due to the production of other osteoblast activating factors such as Wnt ligands, as has been previously reported [13].
Figure 2.
ET-1 mRNA and protein expression in cancer cells closely correlates with DKK1 expression in the cancer cells. (A) ET-1 mRNA was measured in the panel of breast and prostate cancer cell lines and the prostate cancer xenograft LuCaP23.1. (B) In the cell lines, the rate of ET-1 secreted into the surrounding medium was also measured by ELISA and normalized to cell number. Absolute ET-1 values in cell lines with the lowest expression are reported. Closed bars represent cancer cells that produce osteoblastic, mixed or no bone lesions in animal models of bone metastasis. Open bars represent cancer cells that produce osteolytic lesion in animal models of bone metastasis.
Methylation of the DKK1 CpG Island Down-Regulates DKK1 Expression
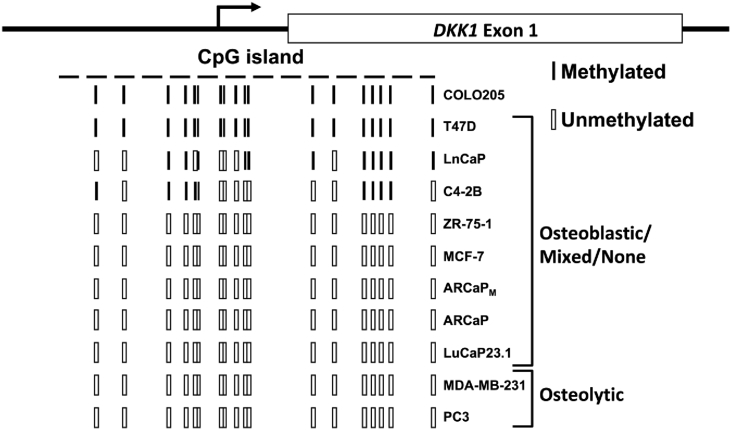
The cancer cell lines selected for study demonstrated wide variation in DKK1 expression. DKK1 methylation was a potential mechanism of DKK1 down-regulation in the cancer cells with low DKK1 expression. The DKK1 gene contains a 233 bp CpG island surrounding the transcriptional start site and a portion of the first exon [19] (Figure 3). This area has a GC content of 68% containing 18 potential cytosine methylation points where a cytosine precedes a guanine. This CpG island is a focus of methylation previously reported to regulate DKK1 transcription in colon cancer [19], [26], acute myeloid leukemia [27], malignant glioma [28], and multiple myeloma [29]. The extent to which a similar epigenetic mechanism of gene regulation regulates DKK1 expression in prostate and breast cancer cells was tested. Using a methylation-specific sequencing approach, the cell lines with negligible expression (LNCaP, C4-2B and T47D) had some degree of methylation of the CpG island indicating a putative mechanism of transcriptional repression (Figure 3). Methylation of the DKK1 CpG island was not detected in the remaining cell lines.
Figure 3.
DKK1 methylation patterns in cancer cells. A 233 bp CpG island encompasses the transcriptional start site and a portion of the first exon of DKK1. Eighteen potential methylation sites were identified within this CpG island. Methylation-specific sequencing was performed to identify the methyl-deoxycytidines within this CpG island. The COLO205 colon cancer cell line served as a control for methylation as this region of the DKK1 gene was reported methylated [19].
The impact of DNA methylation on DKK1 transcriptional repression was examined in C4-2B and T47D cells, the two cell lines with detectable DKK1 CpG island methylation that reliably produce osteoblastic lesions in animal models. The unmethylated PC3 and MDA-MB-231 cell lines served as controls. Treatment with the DNA demethylating agent 5-aza-2′-deoxycytidine resulted in a nearly 500-fold increase in DKK1 mRNA in the C4-2B cell line, strongly indicating that methylation is responsible for transcriptional repression (Figure 4). Demethylation did not significantly alter DKK1 mRNA concentration in the T47D breast cancer cell line suggesting that other transcriptional mechanisms, such as regulation by c-Jun [30], cooperate in the down-regulation of DKK1 expression. Interestingly, both MDA-MB-231 and PC3 cancer cell lines exhibited a marginal increase in DKK1 mRNA despite being unmethylated at the DKK1 promoter. This result was likely due to indirect phenomena, such as the presence of epigenetically controlled transcriptional activators that regulate DKK1 transcription.
Figure 4.
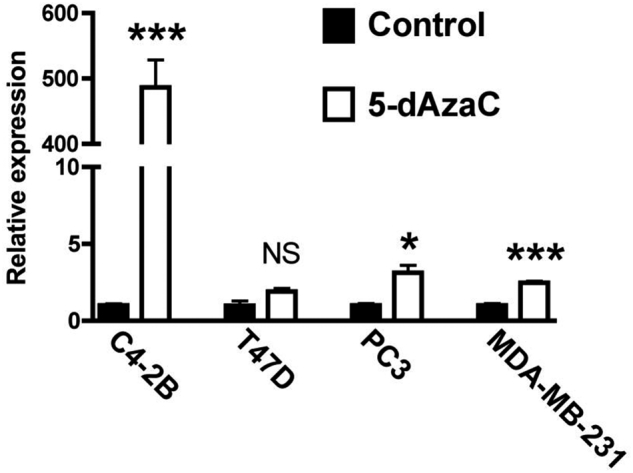
Demethylation restored DKK1 expression in C4-2B prostate cancer cells. Cancer cell lines were treated with and without 10 μM of 5-aza-2′-deoxycytidine (5-dAza-C) for 4 days. Messenger RNA was isolated from the cells and subjected to DKK1 real-time RT PCR. (NS = not significant; * = P < .05; *** = P < .001).
Regulation of DKK1 Expression by Wnt Signaling
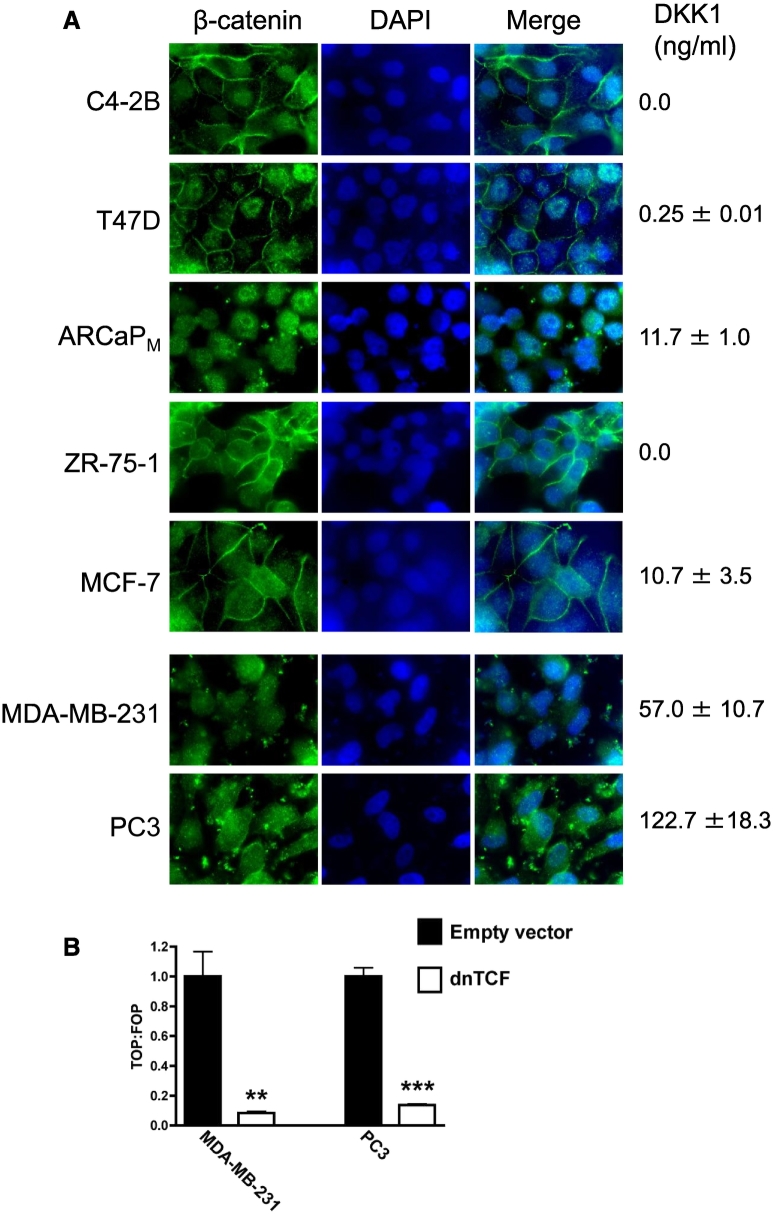
The absence of DKK1 promoter methylation within the LuCaP23.1, ARCaP, ARCaPM, ZR-75-1, MCF-7, MDA-MB-231 and PC3 cells indicated that other mechanisms regulated DKK1 expression. Wnt signaling itself is one candidate. DKK1 expression is regulated, at least partly, by TCF/LEF Wnt signaling responsive elements located within the DKK1 promoter, and thus fits with DKK1 operating in a negative feedback loop regulating Wnt signaling [31]. A unifying mechanism of DKK1 regulation by Wnt signaling in the studied cancer cell lines was investigated by assessing the degree of nuclear localization of β-catenin, a marker for active Wnt signaling. The cell lines that reproducibly form bone lesions in animal models (C4-2B, T47D, ZR-75-1, MCF-7, MDA-MB-231 and PC3) were selected for examination and all demonstrated nuclear β-catenin staining (Figure 5A). Controls using secondary antibody without primary antibody showed no staining (data not shown). C4-2B, T47D, ZR-75-1 and MCF-7 cell lines showed additional staining of the cell membrane. This staining pattern may indicate β-catenin reserve and lower level of Wnt signaling, and/or the presence of mature adherens junction complexes associated with β-catenin.
Figure 5.
Canonical Wnt signaling in cancer cell lines. (A) Seven cancer cell lines utilized in animal models of bone metastasis were analyzed for cellular location of β-catenin using fluorescence immunostaining. The cells were counterstained with DAPI to identify the nucleus. All cells had some degree of nuclear β-catenin accumulation. Conditioned media collected from this experiment was analyzed for DKK1 by ELISA. (B) MDA-MB-231 and PC3 cells were transfected with a dominant-negative TCF3 (dnTCF) or empty vector plasmids, along with Wnt reporter vectors. The ratio of TOPFlash to FOPFlash luciferase activity (TOP:FOP) indicated relative Wnt signaling activity. (** = P < .01; *** = P < .001).
A puzzling aspect of the data is that DKK1 itself is a potent inhibitor of Wnt signaling. In most cells, 10–50 ng/ml of DKK1 is sufficient to block Wnt signaling [12], [16], [32]. DKK1 was once again assayed from conditioned media of the cells that underwent immunofluorescent analysis (Figure 5A). MDA-MB-231 and PC3 cells secreted more than sufficient quantities of DKK1 (>50 ng/ml) to block Wnt signaling.
To confirm active Wnt signaling in these cell line, a dominant-negative expression construct for the mutant form of TCF3 lacking the β-catenin binding site was co-transfected along with Wnt signaling reporter vectors into the MDA-MD-231 and PC3 cell lines. The strategy efficiently down-regulated Wnt signaling, again suggesting that Wnt signaling is active in these cells (Figure 5B). These data support that MDA-MB-231 and PC3 cell lines are insensitive to the Wnt-suppressive actions of DKK1.
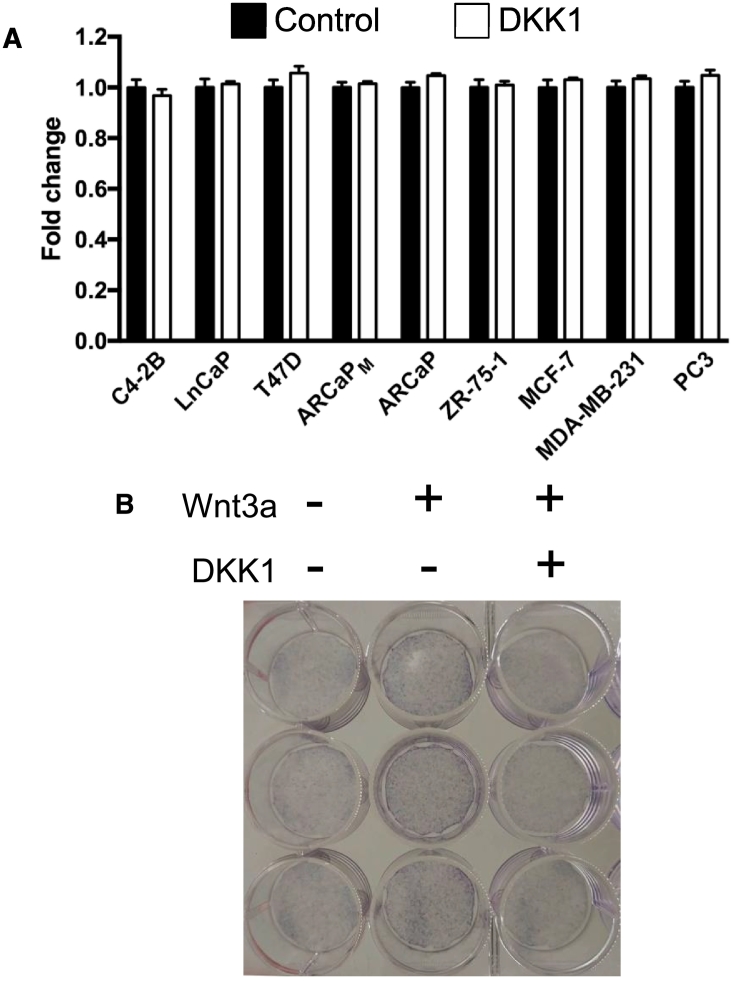
As a secondary test to confirm DKK1 resistance, MDA-BM-231 and PC3 cells were treated with DKK1 50 ng/ml and changes in total cell number after 48 hours were measured. DKK1 did not change cell number over time (Figure 6A). Similarly, DKK1 treatment did not alter cancer cell number in the remaining cancer cell line panel, likely as a consequence of lower Wnt signaling activity. As a control, DKK1 did successfully block Wnt3a-induced increase in alkaline phosphatase staining in murine calvarial osteoblasts (Figure 6B).
Figure 6.
DKK1 treatment did not affect cancer cell number. (A) The panel of breast and prostate cancer cell lines were grown to approximately 25% confluence and then treated with recombinant human DKK1 50 ng/ml or vehicle control for 48 hours. The number of viable cells was determined. There was no significant difference in cell number in any cancer cell line tested. (B) As a control for recombinant human DKK1 activity, murine calvarial osteoblasts were treated with and without Wnt3a 50 ng/ml and DKK1 50 ng/ml for 7 days in triplicate. The cells were then stained for alkaline phosphatase activity as a marker of osteoblast differentiation. Wnt3a expectedly increased alkaline phosphatase staining but was blocked with the addition of DKK1.
Kremen Down-Regulation Causes DKK1 Resistance
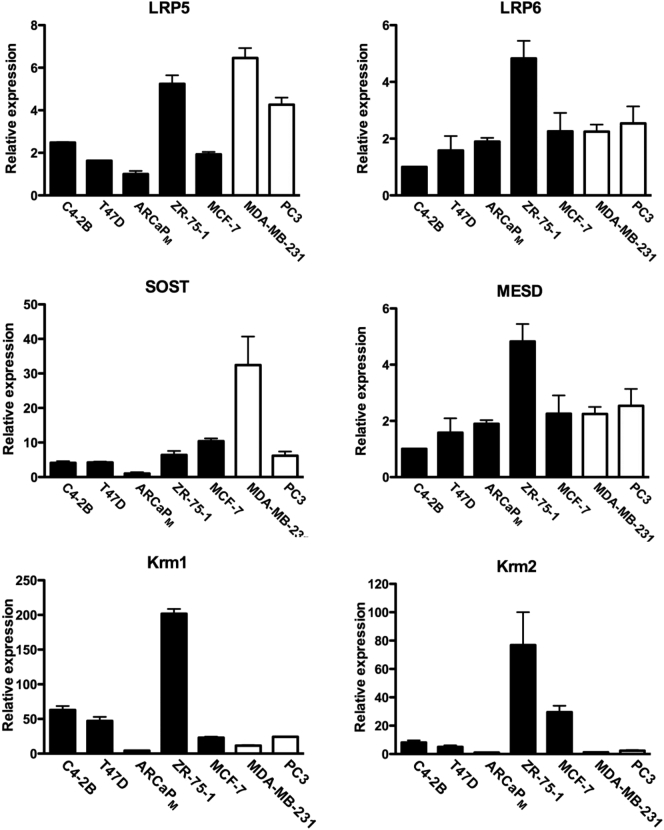
DKK1 action is dependent on other Wnt signaling components and dysregulation of these members could result in DKK1 resistance. DKK1 binds to the high-affinity transmembrane Kremen1 and Kremen2 receptors. The DKK1-Kremen receptor complex sequesters LRP5 and LRP6 away from the Wnt ligand and Frizzled receptor, leading to LRP removal from the cell membrane and down-regulation of Wnt signaling [7], [8]. Down-regulation of LRPs and/or Kremen receptors could render DKK1 inactive. Two other Wnt inhibitors, Sclerostin (encoded by the gene SOST) and mesoderm development candidate 2 protein (MESD), compete with DKK1 for LRP binding [33], [34]. Excessive expression of these Wnt antagonists could mask DKK1-mediated Wnt inhibition. Expression of these genes was assessed in the seven cell lines that produce bone lesions in animal models of bone metastasis (Figure 7). A consistent pattern of low Kremen1 and Kremen2 expression in MDA-MB-231 and PC3 cells suggested a mechanism of DKK1 resistance.
Figure 7.
Expression of DKK1 binding partners and Wnt signaling inhibitors. Real-time RT PCR was performed in seven cancer cell lines that produce bone lesions in animal models of bone metastasis. Messenger RNA was analyzed for the genes that encode for LDL-related receptor proteins 5 and 6 (LRP5, LRP6), Sclerostin (SOST), mesoderm development candidate 2 protein (MESD), Kremen1 (Krm1) and Kremen2 (Krm2).
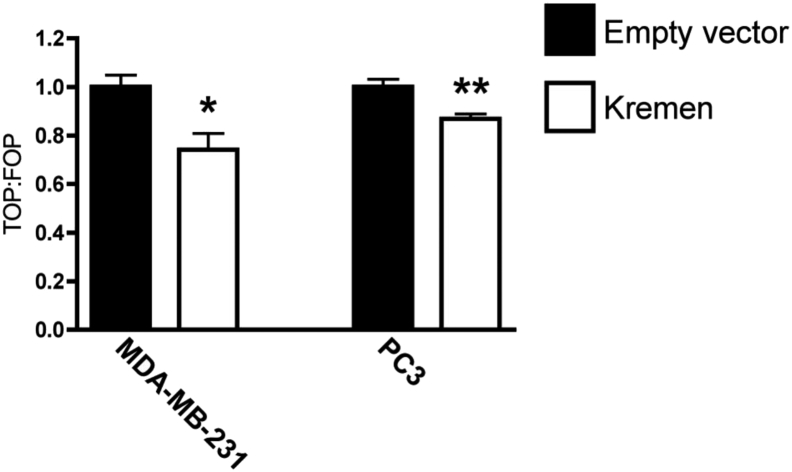
To test the extent to which expression of Kremen could restore DKK1-mediated Wnt signaling inhibition, Kremen1 was overexpressed in MDA-MB-231 and PC3 cells and Wnt signaling was measured. Kremen1 reduced Wnt signaling in these cancer cell lines suggesting that down-regulation of Kremen membrane receptors is in part responsible for DKK1 resistance (Figure 8).
Figure 8.
Overexpression of Kremen1 reduces Wnt signaling. MDA-MB-231 and PC3 cells were transfected with a Kremen1 expression construct or empty vector control, along with Wnt reporter vectors. The ratio of TOPFlash to FOPFlash luciferase activity (TOP:FOP) indicated relative Wnt signaling activity. (* = P < .05; ** = P < .01).
Discussion
Risk prediction tools have greatly assisted clinicians in selecting the most effective therapies for patients at the highest risk for cancer progression and metastasis, and in avoiding unnecessary treatments for low risk patients. The Gleason scoring system combined with clinical stage and serum prostate-specific antigen have improved prognostic accuracy in prostate cancer patients. The expression of HER2 and the receptors for estrogen and progesterone in conjunction with clinical stage provides prognostic information in selecting appropriate treatments for women with breast cancer. Despite these important advances in risk prediction, strategies that identify which patients will develop metastasis to specific organs are currently lacking.
DKK1 may be an ideal marker to predict bone metastasis in patients with early malignancies. DKK1 expression was reported higher in women with hormone-resistant breast cancers, which are more likely to be aggressive and metastasize [35]. Similarly, serum DKK1 was higher in women with breast cancer compared to normal subjects, and in women with breast cancer bone metastasis compared to women with breast cancer metastasis to non-bone sites [26], [36]. Although potentially a valuable bone metastasis marker, DKK1 may be an even better predictor of how the skeleton responds to the invading cancer cells. We now report that DKK1 secretion was the highest in the cell lines that produce osteolytic lesions in animal models of bone metastasis. DKK1 excess would therefore suppress Wnt-mediated osteoblast differentiation and allow uncoupled and unrestricted osteolytic bone resorption. In the case of osteoblastic bone metastasis, less DKK1 in the bone microenvironment would permit maximal canonical Wnt signaling in the osteoblast.
The range of DKK1 expression in the cancer cell lines tested was extreme, from high-expressing PC3 cells to nearly undetectable DKK1 in C4-2B cells. Epigenetic control of DKK1 had been reported in other malignancies and we examined whether a similar mode of regulation occurred, especially in the breast and prostate cancer cell lines with the lowest expression. Using a methylation-specific sequencing approach, DNA methylation of the DKK1 CpG island was detected in LnCaP and the cell line C4-2B derived from parental LnCaP cells. This result is consistent with a previous report in which DKK1 was one of 813 genes methylated in LnCaP cells [37]. This same report also showed that DKK1 was selectively methylated in metastatic prostate cancer, but not in primary prostate cancer, benign tissue adjacent to prostate cancer, or normal prostate. Whether restoration of DKK1 expression using systemic DNA demethylases would reduce prostate cancer burden or possibly convert bone metastases to a more osteolytic phenotype is unclear and merits future study.
Progressive decline in DKK1 expression from the primary tumor to bone metastasis has been reported and may in fact involve dynamic changes in DKK1 CpG island methylation [38]. The only breast cancer cell line methylated at the DKK1 promoter was T47D that translated into negligible expression. In breast cancers, DKK1 epigenetic inactivation appears to be a less common event and was reported to occur in 19% of primary tumors analyzed [39]. In the cancer cell lines not methylated at the DKK1 CpG and not subject to epigenetic control, other unidentified mechanisms regulating DKK1 exist. The heat shock family member DNAJB6 is certainly a candidate, and was reported to regulate DKK1 expression in breast cancer cells [40]. The homeobox protein MSX2 regulated DKK1 expression in mesenchymal cells but it is unclear whether this occurs in tumors [41].
Cancer cells with ample DKK1 expression alter the bone microenvironment and regulate osteoblast canonical Wnt signaling. In the cancer cell lines with low to negligible DKK1 expression, it is predicted that canonical Wnt signaling would be determined by the balance between Wnt ligand activators and repressors. Canonical Wnt signaling as measured by β-catenin nuclear staining was in fact active in these cells, likely due to secretion of Wnt ligands themselves. Paradoxically, canonical Wnt signaling was detected in MDA-MB-231 and PC3 cells that produce large amounts of DKK1. One explanation is that MDA-MB-231 and PC3 cells may secrete a biologically inactive protein. However, other groups have reported that these cell lines in fact secrete an active DKK1 protein [13], [42], [43], [44].
Resistance to the actions of DKK1 in MDA-MB-231 and PC3 is now proposed. Of the components required for DKK1 action, Kremen1 and Kremen2 were found to be consistently down-regulated in these cell lines. Kremen proteins are high-affinity DKK1 receptors that cooperate with DKK1 to increase the clearance of LRP co-receptors [7], [8], resulting in Wnt signaling down-regulation. Kremen participates in regulating bone homeostasis and osteoblast biology [45], [46]. Overexpression of Kremen1 in MDA-MB-231 and PC3 cancer cells partially rescued the ability of DKK1 to reduce Wnt signaling suggesting a mechanism of DKK1 resistance. The model of DKK1 resistance, and possible resistance to sclerostin as well, may in fact represent a mechanism by which cancer cells down-regulate Wnt signaling within the bone microenvironment but at same time require active Wnt signaling for growth in bone. Such a model is consistent with previous reports that DKK1 or Sclerostin neutralizing antibodies alter the bone response to myeloma but did change growth of myeloma cells themselves [47], [48], [49].
Conclusions
We propose a central mechanism that revolves around DKK1 and Kremen, in which canonical Wnt signaling is independently regulated in cancer cells and osteoblasts in bone metastasis. In breast cancer, Wnt signaling is clearly required for breast cancer cell proliferation and migration [50], [51]. In breast cancer bone metastasis, TGF-β released from the bone matrix during osteolysis is a critical event that supports breast cancer growth in bone [52]. Massive osteolysis characterized by uncoupled bone turnover is made possible through the osteoblast suppressive effects of tumor-secreted DKK1. It is therefore essential for breast cancer cells to possess DKK1 resistance. An analogous mechanism can be applied to PC3 animal models of prostate cancer osteolysis. In the situation of osteoblastic bone metastasis, especially in the case of prostate cancer, active canonical Wnt signaling promotes the malignant potential [53], [54]. Low or even absent DKK1 in the bone microenvironment, regardless of tumor Kremen expression, permits active canonical Wnt signaling of the tumor cells and the osteoblasts. Kremen therefore may act as a molecular switch that cooperates with DKK1 to determine how cancer cells behave in bone. Defining expression of these two Wnt regulators may have predictive value when assessing risk for progression in primary tumors and in determining the risk for bone metastasis. DKK1 and Kremen receptors also represent novel therapeutic targets for bone metastasis.
With advances in cancer therapeutics, it is likely that bone metastases will be treated as a chronic complication of an incurable disease and that control rather than cure will be viewed as success in the future. Eradication of cancer cells in bone may not feasible but at least halting the progression of disease by targeting bone-specific pathways such as the DKK1-Kremen system may serve as a potential treatment.
Acknowledgments
Acknowledgments
Dr. Barry Gumbiner, University of Virginia, kindly provided the dominant-negative TCF3 construct.
Funding Sources
This publication was made possible by grants PC073756 from the Department of Defense Prostate Cancer Research Program (GAC) and CA118428 from NCI/NIH (GAC).
Footnotes
Conflicts of Interest: All authors have no conflicts of interest.
References
- 1.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Investig. 1996;98(7):1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100(19):10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68(14):5785–5794. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 7.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 8.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302(1-2):179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 12.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JDJ. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 13.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 14.Eda H, Santo L, Wein MN, Hu DZ, Cirstea DD, Nemani N, Tai YT, Raines SE, Kuhstoss SA, Munshi NC. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J Bone Miner Res. 2016;31(6):1225–1234. doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P, Zallone A, Rizzi R, Grano M, Colucci S. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- 16.Clines GA, Mohammad KS, Bao Y, Stephens O, Suva LJ, Shaughnessy JD, Fox JW, Chirgwin JM, Guise TA. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol. 2007;22:486–498. doi: 10.1210/me.2006-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake JM, Danke JR, Henry MD. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol Ther. 2010;9(8):607–614. doi: 10.4161/cbt.9.8.11112. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JB. Endothelin receptor antagonists. World J Urol. 2005;23(1):19–27. doi: 10.1007/s00345-004-0478-9. [DOI] [PubMed] [Google Scholar]
- 19.Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, Garcia JM, Munoz A, Esteller M, Gonzalez-Sancho JM. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25(29):4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 20.Zhau HE, Li CL, Chung LW. Establishment of human prostate carcinoma skeletal metastasis models. Cancer. 2000;88(12 Suppl):2995–3001. doi: 10.1002/1097-0142(20000615)88:12+<2995::aid-cncr15>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin Cancer Res. 1996;2(6):1039–1048. [PubMed] [Google Scholar]
- 22.Soos G, Jones RF, Haas GP, Wang CY. Comparative intraosseal growth of human prostate cancer cell lines LNCaP and PC-3 in the nude mouse. Anticancer Res. 1997;17(6D):4253–4258. [PubMed] [Google Scholar]
- 23.Clines GA, Mohaddad KS, Wessner LL, Chirgwin JM, Guise TA. Endothelin-1 stimulates bone formation by regulating osteoblast secretion of the paracrine regulators IL-6, Cyr61, CTGF and Dkk1. J Bone Miner Res. 2005;20(Suppl. 1):S249. [Google Scholar]
- 24.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, Simons JW. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 25.Grunda JM, Wang D, Clines GA. Development and characterization of murine models of medulloblastoma extraneural growth in bone. Clin Exp Metastasis. 2013;30(6):769–779. doi: 10.1007/s10585-013-9577-6. [DOI] [PubMed] [Google Scholar]
- 26.Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28(12):2459–2466. doi: 10.1093/carcin/bgm178. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki R, Onizuka M, Kojima M, Shimada M, Fukagawa S, Tsuboi K, Kobayashi H, Shintani A, Ogawa Y, Kawada H. Preferential hypermethylation of the Dickkopf-1 promoter in core-binding factor leukaemia. Br J Haematol. 2007;138(5):624–631. doi: 10.1111/j.1365-2141.2007.06702.x. [DOI] [PubMed] [Google Scholar]
- 28.Gotze S, Wolter M, Reifenberger G, Muller O, Sievers S. Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer. 2010;126(11):2584–2593. doi: 10.1002/ijc.24981. [DOI] [PubMed] [Google Scholar]
- 29.Kocemba KA, Groen RWJ, van Andel H, Kersten MJ, Mahtouk K, Spaargaren M, Pals ST. Transcriptional Silencing of the Wnt-Antagonist DKK1 by Promoter Methylation Is Associated with Enhanced Wnt Signaling in Advanced Multiple Myeloma. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Yu C, Dai J, Keller JM, Hua A, Sottnik JL, Shelley G, Hall CL, Park SI, Yao Z. Parathyroid hormone-related protein inhibits DKK1 expression through c-Jun-mediated inhibition of beta-catenin activation of the DKK1 promoter in prostate cancer. Oncogene. 2014;33(19):2464–2477. doi: 10.1038/onc.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23(52):8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 32.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 34.Lu W, Liu CC, Thottassery JV, Bu G, Li Y. Mesd is a universal inhibitor of Wnt coreceptors LRP5 and LRP6 and blocks Wnt/beta-catenin signaling in cancer cells. Biochemistry. 2010;49(22):4635–4643. doi: 10.1021/bi1001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96(4):646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P, Garnero P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97(7):964–970. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Dhanasekaran SM, Prensner JR, Cao X, Robinson D, Kalyana-Sundaram S, Huang C, Shankar S, Jing X, Iyer M. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 2011;21(7):1028–1041. doi: 10.1101/gr.119347.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68(13):1396–1404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98(6):1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra A, Menezes ME, Shevde LA, Samant RS. DNAJB6 induces degradation of beta-catenin and causes partial reversal of mesenchymal phenotype. J Biol Chem. 2010;285(32):24686–24694. doi: 10.1074/jbc.M109.094847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115(5):1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, Lopez A, Logothetis CJ, Lin SH, Navone NM. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2008;27(5):596–603. doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- 43.Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, Keller ET, Li Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer. 2008;123(5):1034–1042. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fillmore RA, Mitra A, Xi Y, Ju J, Scammell J, Shevde LA, Samant RS. Nmi (N-Myc interactor) inhibits Wnt/beta-catenin signaling and retards tumor growth. Int J Cancer. 2009;125(3):556–564. doi: 10.1002/ijc.24276. [DOI] [PubMed] [Google Scholar]
- 45.Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28(15):4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulze J, Seitz S, Saito H, Schneebauer M, Marshall RP, Baranowsky A, Busse B, Schilling AF, Friedrich FW, Albers J. Negative regulation of bone formation by the transmembrane Wnt antagonist Kremen-2. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R, Bilic S, Sen S, Munshi NC. A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br J Haematol. 2014;167(3):366–375. doi: 10.1111/bjh.13056. [DOI] [PubMed] [Google Scholar]
- 48.Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K, Santo L, Cirstea D, Hideshima T, Schirtzinge L. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53(2):487–496. doi: 10.1016/j.bone.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, Pettitt JA, Simic MK, Cheng TL, Morse A. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129(26):3452–3464. doi: 10.1182/blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107(11):5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Many AM, Brown AM. Mammary stem cells and cancer: roles of Wnt signaling in plain view. Breast Cancer Res. 2010;12(5):313. doi: 10.1186/bcr2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Investig. 1999;103(2):197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets. 2008;9(7):571–580. doi: 10.2174/138945008784911831. [DOI] [PubMed] [Google Scholar]
- 54.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19(6):683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]