Abstract
Objective
To evaluate the impact of an HIV pre‐exposure prophylaxis (PrEP) education intervention on PrEP awareness and use among men who have sex with men (MSM) attending a sexually transmitted diseases (STD) clinic.
Data Sources/Study Setting
Men who have sex with men STD clinic patients.
Study Design
We estimated a difference‐in‐differences linear regression model, comparing MSM whose first visit to the clinic was before (“control”) or after (“treatment”) intervention implementation and controlling for patient.
Data Collection/Extraction
We used self‐reported data on PrEP awareness and use from STD clinic intake forms.
Principal Findings
Pre‐exposure prophylaxis awareness between first and second clinic visits increased 27.2 percentage points (pp) in the treatment group, relative to 13.7 pp in the control group. Similarly, PrEP use increased 7.1 pp in the treatment group versus 2.4 pp in the control group. Based on adjusted estimates, the PrEP intervention increased PrEP awareness by 24 pp (p < .01) and PrEP use by 5 pp (p = .01), increases of 63 percent and 159 percent relative to the 6 months prior to the intervention.
Conclusion
A brief, scalable STD clinic PrEP education intervention led to significantly increased PrEP awareness and use among MSM. Health care providers should consider implementing brief PrEP education interventions in sexual health care settings.
Keywords: Human immunodeficiency virus, PrEP, prevention, intervention, health education
The United States has more than 44,000 newly diagnosed cases of human immunodeficiency virus (HIV) each year (Centers for Disease Control and Prevention 2015). Gay, bisexual, and other men who have sex with men (MSM) bear a disproportionate burden of HIV, accounting for 66 percent of newly diagnosed cases in the United States in 2014. There are also racial and ethnic disparities in the burden of HIV; the lifetime risk of HIV is one in two for black MSM and one in four for Hispanic MSM, relative to one in eleven white MSM in the United States (Centers for Disease Control and Prevention 2015; Hess et al. 2016). Pre‐exposure prophylaxis (PrEP) has the potential to reduce the high burden of HIV among MSM and other populations at high risk of HIV (Juusola et al. 2012). Clinical trials have demonstrated that daily oral PrEP is safe and efficacious (Grant et al. 2010; Molina et al. 2015; McCormack et al. 2016) and observational studies have shown that PrEP is acceptable and effective outside of clinical trial settings (Grant et al. 2014; Liu et al. 2014; Volk et al. 2015). Cost‐effectiveness studies also indicate that PrEP is cost‐effective relative to other cost‐effective alternatives and to the status quo (Juusola et al. 2012; Drabo et al. 2016).
The Centers for Disease Control and Prevention (CDC) recommends PrEP for adult MSM who are not in a monogamous relationship, have had any condomless anal sex in the past 6 months, have had any sexually transmitted disease (STD) diagnosis in the past 6 months, or are in a relationship with a partner who is HIV positive (Centers for Disease Control and Prevention 2014). Despite interest in using PrEP among MSM (Grant et al. 2014; Liu et al. 2014; Volk et al. 2015), PrEP scale‐up has been slow since its Food and Drug Administration approval in July 2012 (Kirby and Thornber‐Dunwell 2014; Mimiaga et al. 2014; Flash et al. 2014; Eaton et al. 2015). Estimates of PrEP awareness from Internet‐based surveys of MSM range from 23 to 86 percent (Hamel et al. 2014; Mayer et al. 2015; Eaton et al. 2015; Goedel et al. 2016; Grov et al. 2015). Among MSM surveyed in 20 cities across the United States in 2014, <5 percent of MSM had taken PrEP (Hoots et al. 2016). The CDC estimates that up to 25 percent of MSM have indications for PrEP (Smith et al. 2015). Improved PrEP uptake could lead to significant reductions in new HIV infections among MSM (Juusola et al. 2012). PrEP scale‐up has also been slow among health care providers, with the CDC estimating that one‐third of primary care providers had not heard of PrEP in 2014 (CDC 2015).
State and local health departments are beginning to step up efforts to increase PrEP awareness and use through mass media campaigns and programs in clinical settings (The New York City Department of Health and Mental Hygiene 2015; Anon n.d.; Holt 2016; Mayer 2016). The impacts of these efforts have not been well characterized. The goal of the current study was to evaluate the impact of a brief, clinic‐wide education intervention to promote PrEP awareness and use among MSM presenting to an STD clinic. The brief intervention was incorporated into routine clinical care and offered to all MSM presenting to the clinic starting in 2013. We used a quasi‐experimental, difference‐in‐differences analytical approach, comparing changes in PrEP awareness, and use at a second clinic visit among MSM who presented for a first visit after versus before intervention implementation (Dimick and Ryan 2014). To our knowledge, this is the first evaluation of an education‐based, structural‐level intervention on PrEP awareness and use in a high‐risk population. Using data collected continuously for nearly 2 years prior and for more than 2 years following intervention implementation, we were able to compare PrEP awareness and use among MSM before and after the intervention began.
Methods
Sample
The Rhode Island STD Clinic is the only publicly funded STD clinic in the state. We reviewed de‐identified data on demographic and behavioral variables and PrEP awareness and use from intake forms for all HIV‐negative MSM who made two or more visits to the Rhode Island STD Clinic between January 1, 2012 and December 31, 2015. Patient intake forms allowed for partial anonymity and included information on first name, sex, birth date, phone number, race, and ethnicity. We identified repeat visitors as those who reported the same phone number or birth date and the same sex, race, and ethnicity on different dates. We identified MSM as men who reported sexual behavior with one or more men in the past 12 months during any of their clinic visits, regardless of sexual behavior with women. We excluded those who reported previously testing positive for HIV. Among MSM visiting the clinic, the HIV prevalence is 6.5 percent, and HIV incidence is 2.0 percent (Chan et al. 2015). As a negative control, we assessed changes in PrEP awareness and use among HIV‐negative men who have sex with women only (MSWO) who visited the STD clinic more than twice; MSWO did not receive the PrEP education intervention and we expected no effect of the intervention in this group.
Intervention
The Rhode Island STD Clinic implemented a clinic‐wide, education‐based intervention for all MSM patients in November 2013. STD clinic staff members were HIV counselors without formal medical training. Prior to implementing the PrEP intervention, clinic staff provided one‐on‐one sexual risk reduction counseling in a private clinic room while waiting for 20 minutes for results from the OraQuick rapid HIV test (OraSure Technologies, Inc., Bethlehem, PA, USA). For the PrEP education intervention, all clinic staff received training to deliver a brief (≤5 minutes) education session on PrEP effectiveness, dosing, follow‐up, side effects, and discussion of any other questions or concerns about PrEP. Clinic staff integrated the PrEP education intervention into the sexual risk counseling. In a private clinic room in a one‐to‐one counseling session, clinic staff provided patients with information on what PrEP is, who should consider it, and commonly asked questions. They answered any patient questions and referred patients who were interested in PrEP care. Clinic staff also shared with patients a one‐page flyer with this information (provided in Appendix S1). Clinic staff offered PrEP education to all MSM regardless of risk behaviors, with the aim of improving PrEP awareness in the general MSM population. The PrEP education session was typically appended to the sexual risk counseling session. Each patient only received the PrEP education intervention one time, based on whether the patient indicated he or she had already had the session.
We considered men whose first clinic visit took place prior to intervention implementation in November 2013 to be in the “control group” and men whose first clinic visit took place after the intervention began to be in the “treatment group.” We considered PrEP awareness and use recorded upon intake at each man's first clinic visit to be “before” potential intervention and PrEP awareness and use recorded upon intake at each man's second clinic visit to be “after” potential intervention.
The intervention was implemented in a busy clinical setting. Although most MSM received the intervention, not all did due to limited staffing availability or time. We conducted an intention‐to‐treat analysis, considering all MSM patients who had prior clinic visits after intervention implementation began to have been exposed to the intervention. Our estimates reflect the real‐world effectiveness of intervention implementation, rather than the efficacy of the intervention implemented under strict trial conditions.
Outcomes
The main outcomes of interest were PrEP awareness and ever‐use as reported at patient intake at the beginning of the first clinic visit and at the beginning of the second clinic visit. We assessed PrEP awareness based on whether patients answered “yes” or “no” to a single question on intake forms, “Have you heard of taking HIV medications to prevent infection in people who are HIV negative? (Pre‐exposure prophylaxis, PrEP).” We also assessed PrEP use based on whether patients answered “yes” or “no” to a single question on intake forms, “Have you ever taken pre‐exposure prophylaxis?” Patients are prescribed PrEP outside of the STD clinic setting.
Analysis
We assessed differences in PrEP awareness by race/ethnicity and by age group using logistic regression analysis. We categorized patients as aged 16–19, 20–24, 25–29, 30–34, 35–44, 45–54, and 55 years or older. We also assessed the extent to which the study population was representative of the broader HIV‐negative MSM population visiting the STD clinic. We estimated logistic regression models and Wald chi‐squared tests comparing the race/ethnicity and age group of HIV‐negative MSM patients visiting the clinic two or more times, who were in the study sample, to the characteristics of all HIV‐negative MSM patients who visited the clinic.
For the main analysis, we used a difference‐in‐differences approach to estimating the impact of the intervention on PrEP awareness and use. Difference‐in‐differences is an interrupted time series analytical approach that entails comparing before–after changes in an outcome in a treatment group to before–after changes in a control group (Angrist and Pischke 2008; Wooldridge 2010; Dimick and Ryan 2014). This method is commonly used to evaluate policy and other interventions not implemented in a randomized fashion (Copeland et al. 2012). Rather than comparing before–after outcomes in two separate clinics, we use a historical control from the same clinic (Schneeweiss et al. 2001, 2002).
The two main assumptions required for the difference‐in‐differences approach are parallel trends and common shocks (Dimick and Ryan 2014); in addition to these assumptions, in our use of a historical control, we assume that the timing of the intervention implementation was exogenous and not based on patient characteristics, so patient characteristics should not differ between the treatment and control groups (Schneeweiss et al. 2002; Wooldridge 2010). Parallel trends mean that time trends in the outcome should be equivalent in the treatment group and control group prior to the intervention; the corollary is that the trends in the treatment and control group would have continued to be the same in the absence of an intervention. We assessed whether there were systematic differences between patients who first visited the clinic before and after intervention implementation by comparing the race/ethnicity and age characteristics of the control and treatment groups through logistic regression analyzes and Wald chi‐squared tests. We categorized patients as non‐Hispanic white, non‐Hispanic black, Hispanic/Latino, or other race/ethnicity. We assessed the parallel trends requirement by estimating a logistic regression model of PrEP awareness and use by year that included a variable interacting year and treatment group. A statistically significant interaction term would indicate baseline differences in treatment and control time trends in PrEP awareness and use before intervention implementation. The common shocks assumption requires that there were no events that would affect PrEP awareness and use differently for the treatment and control groups, which we anticipate is the case in this study.
For the main difference‐in‐differences analysis, we estimated the impact of the intervention on PrEP awareness and use through linear regression models with patient fixed effects, half‐year fixed effects, and with an interaction term for being in the treatment group and making a second visit. We estimated linear rather than logit models due to their unbiased estimation properties with fixed effects analyzes (Greene 2004). Including patient fixed effects means the analysis is based on within‐patient changes in PrEP awareness and use and controls for time‐invariant patient characteristics such as race, ethnicity, education, income, propensity to seek health care, age group, and other patient characteristics that would not change during the weeks or months between clinic visits. We used robust standard errors to account for an increasing number of patients visiting the STD clinic over time. We conducted a sensitivity analysis with quarter‐year, rather than half‐year, fixed effects. We also depicted changes in PrEP awareness and use over time by first and second patient visit and by treatment and control group (Figures 1 and 2).
Figure 1.
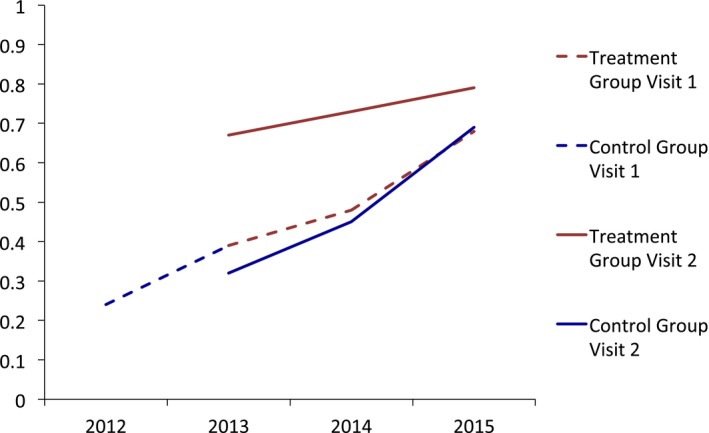
- Notes. We did not plot PrEP awareness in the Control Group at visit 2 in 2012 because only five patients in this category made a second visit in 2012.
Figure 2.
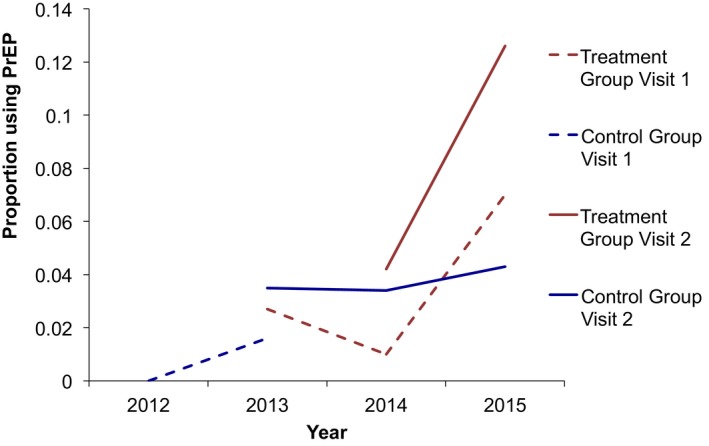
- Notes. We did not plot PrEP awareness in the Control Group at visit 2 in 2012 because only five patients in this category made a second visit in 2012.
We highlight that PrEP awareness and use outcomes data were from clinic intake forms that patients filled out prior to clinic visits. Patients in the control group made their first clinic visit prior to intervention implementation; thus, they filled out the intake form at their second visit prior to receiving any intervention, even if they went on to receive the intervention during their second visit. Patients in the treatment group filled out the intake form prior to their first visit, and then received the intervention during their first visit; when these patients filled out their intake form for their second visit, it was after receiving the intervention during their first visit.
As a validity check, we conducted the same difference‐in‐differences analysis among MSWO. Clinic staff did not deliver the PrEP intervention to MSWO because of low rates of HIV among MSWO (Centers for Disease Control and Prevention 2014).
IRB Approval
The Miriam Hospital Institutional Review Board approved review of clinical records with a waiver of consent.
Results
A total of 3,736 unique patients presented to the Rhode Island STD Clinic during the time period; of these, 1,055 (28 percent) were MSM, and 964 (26 percent) were HIV‐negative MSM. Of all HIV‐negative MSM, 316 (33 percent) visited the clinic more than once during the study period, with a median of three total visits (range: 2–11). We included only participants who had two or more clinic visits between January 2012 and December 2015 and restricted the analysis to PrEP awareness and use at first and second visits. First and second visits were an average of 8 months apart (range: 1–38 months). Among HIV‐negative MSM visiting the clinic two or more times, 82 (26 percent) first visited prior to the PrEP intervention and 234 (74 percent) first visited after the PrEP intervention began. At their first visits, 149 (47 percent) MSM were aware of PrEP, and 8 (2.5 percent) were taking PrEP. There were no significant differences between the race/ethnicity or age categories of the subset of HIV‐negative MSM who visited the clinic more than once and those of all HIV‐negative MSM who visited once or more (Table S1).
We describe patient demographics in Table 1. Just under two‐thirds of the MSM population identified as non‐Hispanic white, with 9 percent identifying as non‐Hispanic black, 16 percent identifying as Hispanic/Latino, and 11 percent identifying as another race. About half of the patients were under the age of 30 years. There were no statistically significant differences between the treatment group (whose first STD clinic visit took place after intervention implementation) and the control group (whose first STD clinic visit took place before intervention implementation).
Table 1.
Characteristics of MSM Visiting the Rhode Island STD Clinic
| Overall Sample (N = 316) | Control Group (N = 82) | Treatment Group (N = 234) | Wald Chi‐Squared Test of Differences between the Control and Treatment Groups | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | OR | 95% CI | |
| Race/ethnicity | ||||||||
| Non‐Hispanic white | 20 | 64 | 50 | 61 | 154 | 66 | 1.23 | 0.73–2.07 |
| Non‐Hispanic black | 34 | 11 | 12 | 15 | 21 | 9 | 0.58 | 0.27–1.23 |
| Hispanic/Latino | 50 | 16 | 13 | 9 | 37 | 16 | 1.11 | 0.46–2.71 |
| Other/Unknown | 29 | 9 | 7 | 16 | 22 | 9 | 1.00 | 0.50–1.99 |
| Age group | ||||||||
| 16–19 | 17 | 5 | 1 | 1 | 16 | 7 | 5.94 | 0.78–45.56 |
| 20–24 | 84 | 27 | 22 | 27 | 62 | 27 | 0.98 | 0.56–1.74 |
| 25–29 | 61 | 19 | 16 | 20 | 46 | 20 | 1.01 | 0.54–1.90 |
| 30–34 | 55 | 17 | 13 | 16 | 42 | 18 | 1.16 | 0.59–2.29 |
| 35–44 | 34 | 11 | 7 | 9 | 27 | 12 | 1.40 | 0.58–3.34 |
| 45–54 | 44 | 14 | 16 | 20 | 27 | 12 | 0.54 | 0.27–1.06 |
| 55+ | 21 | 7 | 7 | 9 | 14 | 6 | 0.68 | 0.27–1.75 |
Notes. We compared differences in demographic characteristics between the control group (MSM who made their first STD clinic visit before intervention implementation) and the treatment group (MSM who made their first STD clinic visit after intervention implementation) using logistic regression and Wald chi‐squared tests.
**p ≤ .05, ***p ≤ .01.
MSM, men who have sex with men.
We describe PrEP awareness and use at the first patient visit in Table 2. PrEP awareness was lower among non‐Hispanic black MSM (26 percent) relative to MSM who were non‐Hispanic white (56 percent, p = .014). PrEP awareness and use were statistically significantly greater among MSM between the ages of 25–54 years (47–59 percent) than among MSM between the ages of 16–19 years (18 percent, p < .05). A total of eight (2.5 percent) patients reported using PrEP at their first visit, too few to analyze differences by demographic characteristics.
Table 2.
PrEP Awareness and Use among MSM Visiting the Rhode Island STD Clinic at First Visit (N = 316)
| PrEP Awareness | PrEP Use | |||
|---|---|---|---|---|
| % | OR | 95% CI | % | |
| Race/ethnicity | ||||
| Non‐Hispanic white (N = 203) | 51 | Ref | 3 | |
| Non‐Hispanic black (N = 34) | 26 | 0.35** | 0.16–0.79 | 0 |
| Hispanic/Latino (N = 50) | 40 | 0.65 | 0.35–1.21 | 4 |
| Other/unknown (N = 29) | 58 | 1.38 | 0.63–3.03 | 4 |
| Age group | ||||
| 16–19 (N = 17) | 18 | Ref | 0 | |
| 20–24 (N = 84) | 35 | 2.46 | 0.65–9.26 | 0 |
| 25–29 (N = 61) | 59 | 6.72*** | 1.75–25.85 | 10 |
| 30–34 (N = 55) | 58 | 6.49*** | 1.67–25.23 | 0 |
| 35–44 (N = 34) | 47 | 4.15** | 1.01–17.11 | 0 |
| 45–54 (N = 44) | 59 | 6.74*** | 1.69–26.91 | 2 |
| 55 + (N = 21) | 33 | 2.33 | 0.50–10.91 | 0 |
Notes. PrEP use was not great enough to analyze differences in PrEP use by demographic characteristics.
**p ≤ .05, ***p ≤ .01.
MSM, men who have sex with men; PrEp, pre‐exposure prophylaxis; STD, sexually transmitted diseases.
In Table 3, we describe the results of the parallel trends test. We found that there were not statistically significant differences in time trends in PrEP awareness (adjusted odds ratio (AOR): 1.0, p = .527) and PrEP use (AOR: 1.0, p = .630) in the treatment group relative to the control group. We also depict unadjusted trends in PrEP awareness and use graphically in Figures 1 and 2. The figures capture parallel time trends in PrEP awareness and use in the treatment and control groups.
Table 3.
Test of Parallel Trends Assumption (N = 316)
| PrEP Awareness | PrEP Use | |||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p‐Value | AOR | 95% CI | p‐Value | |
| Year | 2.1 | 1.41–3.13 | <.001 | 3.3 | 0.57–18.58 | .182 |
| Year*Treatment group | 1.0 | 1.00–1.00 | .527 | 1.0 | 1.00–1.00 | .630 |
| Race/ethnicity | ||||||
| Non‐Hispanic White | Ref | |||||
| Non‐Hispanic Black | 0.36 | 0.14–0.88 | .026 | |||
| Hispanic/Latino | 0.57 | 0.29–1.14 | .113 | |||
| Other/Unknown | 1.17 | 0.49–2.79 | .726 | |||
| Age group | ||||||
| 16–20 | Ref | |||||
| 21–24 | 3.74 | 0.88–15.92 | .074 | |||
| 25–29 | 9.41 | 2.15–41.1 | .003 | |||
| 30–34 | 10.34 | 2.33–45.98 | .002 | |||
| 35–44 | 5.35 | 1.16–24.78 | .032 | |||
| 45–54 | 9.59 | 2.09–43.95 | .004 | |||
| 55+ | 3.60 | 0.67–19.11 | .133 | |||
Notes. We conducted a logistic regression analysis on PrEP awareness and PrEP use at first patient visit. For the analysis of PrEP awareness, we controlled for all variables in the table and used robust standard errors. With only eight patients reported PrEP use at first visit, we did not have sufficient sample size to control for demographic characteristics in the analysis of PrEP use. Time trends in PrEP awareness and PrEP use were not statistically significantly different between the treatment group and the control group.
AOR, adjusted odds ratio; PrEp, pre‐exposure prophylaxis.
Pre‐exposure prophylaxis awareness increased from 34.5 percent at first visit to 48.2 percent at second visit (+13.7 percentage points [pp]) in the control group, relative to an increase from 49.6 to 76.8 percent (+27.2 pp) in the treatment group. PrEP use increased from 1.1 to 3.5 percent (+2.4 pp) in the control group and from 2.8 to 9.9 percent (+7.1 pp) in the treatment group.
We present the adjusted difference‐in‐differences estimate of the impact of the PrEP intervention in Table 4. Among HIV‐negative MSM patients, PrEP awareness increased over time (11.5 percentage points/year, p < .001) and PrEP use increased over time (2.8 percentage points/year, p = .033). Visiting the STD Clinic after the PrEP intervention began was associated with a 23.6 percentage point increase in PrEP awareness (p < .001) and a 5.2 percentage point increase in PrEP use (p = .010). Relative to PrEP awareness and use in the 6 months before the intervention began, the intervention effect is equivalent to a 63 percent increase in PrEP awareness and 159 percent increase in PrEP use. These effects were robust to the use of quarter‐year fixed effects for PrEP awareness (22.8 percentage points, p < .001) and PrEP use (4.8 percentage points, p = .037).
Table 4.
Difference‐in‐Differences Estimation of Associations between Intervention and PrEP Awareness and Use among MSM (N = 316)
| PrEP Awareness | PrEP Use | |||||
|---|---|---|---|---|---|---|
| Percentage Points | 95% CI | p‐Value | Percentage Points | 95% CI | p‐Value | |
| PrEP education intervention | 23.55 | 13.72–33.38 | <.001 | 5.20 | 1.26–9.13 | .010 |
| Half‐year | ||||||
| 2012: Jan–Jun | Ref | Ref | ||||
| 2012: Jul–Dec | 53.54 | 1.29–105.78 | .045 | −1.78 | −7.02 to 3.45 | .503 |
| 2013: Jan–Jun | 34.44 | −4.42 to 73.30 | .082 | −5.22 | −14.01 to 3.57 | .244 |
| 2013: Jul–Dec | 35.61 | 0.84–72.05 | .055 | 0.99 | −3.98 to 5.97 | .695 |
| 2014: Jan–Jun | 56.10 | 20.80–91.40 | .002 | −1.84 | −5.32 to 1.64 | .299 |
| 2014: Jul–Dec | 42.34 | 6.82–77.86 | .020 | 0.87 | −4.50 to 6.24 | .750 |
| 2015: Jan–Jun | 50.11 | 14.18–86.05 | .006 | 2.72 | −3.23 to 8.66 | .369 |
| 2015: Jul–Dec | 49.63 | 11.63–87.64 | .011 | 5.39 | −1.84 to 12.63 | .143 |
Notes. We estimated linear models with patient and half‐year fixed effects and robust standard errors. The patient fixed effects control for time‐invariant patient characteristics, including race, ethnicity, age group, and socioeconomic status. The mean duration between the first visit and the second visit was 8 months.
MSM, men who have sex with men; PrEp, pre‐exposure prophylaxis.
We present the results of the comparative analysis of PrEP awareness and use among MSWO during the study period in Table 5. The education intervention was not provided to MSWO. There was no difference in PrEP awareness (1.01 percentage points, p = .760) or use (0.45 percentage points, p = .564) at second visit among MSWO who presented for a first visit after relative to before intervention implementation.
Table 5.
Difference‐in‐Differences Estimation of Associations between Intervention and PrEP Awareness and Use among MSWO (N = 243)
| PrEP Awareness | PrEP Use | |||||
|---|---|---|---|---|---|---|
| Percentage Points | 95% CI | p‐Value | Percentage Points | 95% CI | p‐Value | |
| Treatment group | 1.01 | −5.51 to 7.54 | .760 | 0.45 | −1.09 to 1.99 | .564 |
| Half‐year | ||||||
| 2012: Jan–Jun | Ref | Ref | ||||
| 2012: Jul–Dec | 39.37 | −15.15 to 93.89 | .156 | 0.14 | −0.65 to 0.93 | .733 |
| 2013: Jan–Jun | 26.58 | −21.50 to 74.66 | .277 | −0.22 | −0.70 to 0.25 | .359 |
| 2013: Jul–Dec | 34.05 | −14.31 to 82.40 | .167 | 0.09 | −0.53 to 0.70 | .786 |
| 2014: Jan–Jun | 37.85 | −10.92 to 86.61 | .128 | −0.81 | −2.84 to 1.22 | .432 |
| 2014: Jul–Dec | 31.28 | −18.07 to 80.62 | .213 | 2.12 | −1.06 to 5.31 | .190 |
| 2015: Jan–Jun | 34.82 | −14.65 to 84.29 | .167 | −1.67 | −4.25 to 0.90 | .202 |
| 2015: Jul–Dec | 35.91 | −14.27 to 86.08 | .160 | 0.99 | −2.10 to 0.47 | .529 |
Notes. We estimated linear models with patient fixed effects and robust standard errors, controlling for patient age. The patient fixed effects control for time‐invariant patient characteristics, including race, ethnicity, and socioeconomic status.
PrEp, pre‐exposure prophylaxis.
Discussion
A brief, PrEP education intervention delivered at a busy STD clinic increased PrEP awareness by 24 percentage points and increased PrEP use by 5 percentage points, equivalent to relative increases of 63 and 159 percent, respectively. Increasing awareness is an important element of PrEP scale‐up (Cohen et al. 2013; Hamel et al. 2014). To our knowledge, this is the first published study evaluating the effectiveness of an education‐based intervention on PrEP awareness and use. Using the difference‐in‐differences method of analysis, including annual trends in PrEP awareness and use, and including patient fixed effects, we were able to distinguish between increases in PrEP awareness and use over time and further increases in PrEP awareness and use associated with the intervention. PrEP awareness among MSM remained below 50 percent and PrEP use remained below 3 percent prior to the intervention, indicating a need to increase PrEP awareness and use for MSM to benefit from the protective effects of PrEP.
The intervention is concise and straightforward, making it easy to scale up in other clinical settings. Our results are based on an intention‐to‐treat analysis and reflect PrEP education intervention effectiveness as implemented in a realistic clinical setting. Prior studies indicate that STD clinics are ideal settings for reaching populations at high risk of HIV and for delivering PrEP (Liu et al. 2014). The intervention could also work well in other high‐volume setting settings such as primary care because it is brief and straightforward. Primary care is an ideal setting for scaling up PrEP awareness and use because HIV‐negative MSM are more likely to have contact with primary care than HIV care providers (Baeten et al. 2013; Norton, Larson, and Dearing 2013). In the context of slow PrEP scale‐up (Mimiaga et al. 2009; Flash et al. 2014; Kirby and Thornber‐Dunwell 2014; Hamel et al. 2014), broad scale‐up of the intervention in primary care and STD clinic settings could help improve PrEP awareness and use. As PrEP awareness increases among MSM, it will also be important to focus greater attention on increasing PrEP use and addressing other barriers to PrEP use, including willingness of providers to prescribe (Centers for Disease Control and Prevention 2014), cost of medications and clinical care, stigma, and adherence (Grant et al. 2010; Hosek 2015).
We found lower baseline levels of PrEP awareness among non‐Hispanic black relative to non‐Hispanic white MSM. The lifetime risk of HIV for black MSM in the United States is one in two, relative to one in eleven for white MSM, and low PrEP awareness may exacerbate existing racial and ethnic HIV disparities (Centers for Disease Control and Prevention 2015; Millett et al. 2007). Differences in health literacy may play a role in different levels of PrEP awareness by race (Osborn et al. 2007). Prior studies indicate that PrEP education led to larger increases in PrEP interest among individuals with lower educational attainment (Mimiaga et al. 2009; Liu et al. 2008). Scaling up PrEP education efforts should focus on different racial and ethnic groups and could help address disparities in HIV prevention.
Our study has a number of potential limitations. While the study population had demographic characteristics consistent with those of other HIV‐negative MSM visiting the STD clinic, the study population may not be generalizable to other MSM populations across the United States. The main outcomes of PrEP awareness and use were based on patient self‐report. A main limitation of the study is that self‐reported ever‐use of PrEP could be overreported due to social desirability bias among patients who know that the clinic is promoting PrEP. The anonymous nature of STD clinic visits and separation of PrEP prescribing from the STD clinic location may reduce social desirability bias, but the possibility of this bias remains a main limitation of the study. We also lacked detailed information on the extent of PrEP awareness and PrEP use. It would be ideal to have more comprehensive information on patient understanding of PrEP, beyond whether patients had heard of PrEP. We were also unable to determine whether patients were prescribed PrEP or currently using PrEP. In addition to lacking these details of the outcome measures, we lacked information on patient characteristics such as educational attainment and socioeconomic status. While our fixed effects analysis controls for confounding by these variables if they are time‐invariant, information on educational attainment, and socioeconomic status could provide further information on whether PrEP education efforts are reaching those with the lowest baseline levels of PrEP knowledge and the greatest burdens of HIV. The intervention was implemented in a busy clinical setting, and we do not have measures of intervention fidelity. It is possible that not all MSM patients received the intervention, in which case our intention‐to‐treat analyzes reflect underestimates of the overall efficacy of the intervention.
Conclusions
A simple, 5‐minute PrEP education intervention at a STD clinic was associated with a statistically significant increase in PrEP awareness of 23 percentage points and an increase in PrEP use of 5 percentage points among MSM patients. There is a need for interventions to increase PrEP awareness and use, especially among MSM who are racial and ethnic minorities in STD clinics, primary care practices, and other settings providing sexual health care.
Supporting information
Appendix SA1: Author Matrix.
Table S1. Characteristics of HIV‐Negative MSM Visiting the Rhode Island STD Clinic at First Visits: Study Sample Relative to Full HIV‐Negative MSM Population.
Appendix S1. PrEP Information Sheet.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This work was supported by National Institute of Allergy and Infectious Diseases [grant number T32AI102623 to JR, 1K23AI096923 to PAC, and P30AI042853], the National Institute of Mental Health [grant number R25MH083620 to JR and CO], and the National Institute on Drug Abuse [grant number T32DA013911‐12 to CO].
Disclosures: None.
Disclaimer: None.
References
- Angrist, J. D. , and Pischke J.‐S.. 2008. Mostly Harmless Econometrics: An Empiricist's Companion. Princeton, NJ: Princeton University Press. [Google Scholar]
- Anon . n.d. “Citywide Campaign Transmits Love and Shows the Sexier Side of HIV Prevention” [accessed on May 4, 2016]. Available at http://www.aidschicago.org/page/news/all-news/citywide-campaign-transmits-love-and-shows-the-sexier-side-of-hiv-prevention
- Baeten, J. M. , Haberer J. E., Liu A. Y., and Sista N.. 2013. “Preexposure Prophylaxis for HIV Prevention: Where Have We Been and Where Are We Going?” Journal of Acquired Immune Deficiency Syndromes 63: S122–9. 10.1097/qai.0b013e3182986f69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2015. “Daily Drug Can Reduce HIV Risk,” Centers for Disease Control and Prevention” [accessed on February 4, 2016]. Available at http://www.cdc.gov/vitalsigns/hivprep/index.html
- Centers for Disease Control and Prevention . 2014. “Preexposure Prophylaxis for the Prevention of HIV Infection in the United States ‐ 2014” [accessed on October 4, 2016]. Available at http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf
- Centers for Disease Control and Prevention . 2015. “HIV Surveillance Report 2014” [accessed on October 4, 2016]. Available at http://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-us.pdf
- Chan, P. A. , Rose J., Maher J., Benben S., Pfeiffer K., Almonte A., Poceta J., Oldenburg C. E., Parker S., Marshall B. D., Lally M., Mayer K., Mena L., Patel R., and Nunn A. S.. 2015. “A Latent Class Analysis of Risk Factors for Acquiring HIV among Men Who Have Sex with Men: Implications for Implementing Pre‐Exposure Prophylaxis Programs.” AIDS Patient Care and STDs 29 (11): 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. E. , Liu A. Y., Bernstein K. T., and Philip S.. 2013. “Preparing for HIV Pre‐Exposure Prophylaxis: Lessons Learned from Post‐Exposure Prophylaxis.” American Journal of Preventive Medicine 44 (1 0 2): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, D. L. , Basurto‐Davila R., Chung W., Kurian A., Fishbein D. B., Szymanowski P., Zipprich J., Lipman H., Cetron M. S., Meltzer M. I., and Averhoff F.. 2012. “Effectiveness of a School District Closure for Pandemic Influenza A (H1N1) on Acute Respiratory Illnesses in the Community: A Natural Experiment.” Clinical Infectious Diseases 56 (4): 509–16. [DOI] [PubMed] [Google Scholar]
- Dimick, J. B. , and Ryan A. M.. 2014. “Methods for Evaluating Changes in Health Care Policy: The Difference‐in‐Differences Approach.” Journal of the American Medical Association 312 (22): 2401–2. [DOI] [PubMed] [Google Scholar]
- Drabo, E. F. , Hay J. W., Vardavas R., Wagner Z. R., and Sood N.. 2016. “A Cost‐Effectiveness Analysis of Preexposure Prophylaxis for the Prevention of HIV among Los Angeles County Men Who Have Sex with Men.” Clinical Infectious Diseases 63 (11): 1495–504. [DOI] [PubMed] [Google Scholar]
- Eaton, L. A. , Driffin D. D., Bauermeister J., Smith H., and Conway‐Washington C.. 2015. “Minimal Awareness and Stalled Uptake of Pre‐Exposure Prophylaxis (PrEP) among at Risk, HIV‐Negative, Black Men Who Have Sex with Men.” AIDS Patient Care and STDs 29 (8): 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flash, C. , Landovitz R., Mera Giler R., Ng L., Magnuson D., Bush Wooley S., and Rawlings K.. 2014. “Two Years of Truvada for Pre‐Exposure Prophylaxis Utilization in the US.” Journal of the International AIDS Society 17 (4 suppl 3): 19730 [accessed on October 4, 2016] Available at http://www.jiasociety.org/index.php/jias/article/view/19730, 10.7448/ias.17.4.19730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedel, W. C. , Halkitis P. N., Greene R. E., and Duncan D. T.. 2016. “Correlates of Awareness of and Willingness to Use Pre‐Exposure Prophylaxis (PrEP) in Gay, Bisexual, and Other Men Who Have Sex with Men Who Use Geosocial‐Networking Smartphone Applications in New York City.” AIDS and Behavior 20 (70): 1435–42. 10.1007/s10461-016-1353-6 [DOI] [PubMed] [Google Scholar]
- Grant, R. M. , Lama J. R., Anderson P. L., McMahan V., Liu A. Y., Vargas L., Goicochea P., Casapía M., Guanira‐Carranza J. V., Ramirez‐Cardich M. E., O. Montoya‐Herrera , Fernández T., Veloso V. G., Buchbinder S. P., Chariyalertsak S., Mauro Schechter, Bekker L.‐G., Mayer K. H., Kallás E. G., Amico K. R., K. Mulligan , Bushman L. R., Hance R. J., Ganoza C., Defechereux P., Postle B., Wang F., McConnell J. J., Zheng J.‐H., Lee J., Rooney J. F., Jaffe H. S., Martinez A. I., Burns D. N., and Glidden D. V., for the iPrEx Study Team. 2010. “Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men.” New England Journal of Medicine 363 (27): 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, R. M. , Anderson P. L., McMahan V., Liu A., Amico K. R., Mehrotra M., Hosek S., Mosquera C., Casapia M., Montoya O., Buchbinder S., Veloso V. G., Mayer K., Chariyalertsak S., Bekker L.‐G., Kallas E. G., Schechter M., Guanira J., Bushman L., Burns D. N., Rooney J. F., and Glidden D. V.. 2014. “Uptake of Pre‐Exposure Prophylaxis, Sexual Practices, and HIV Incidence in Men and Transgender Women Who Have Sex with Men: A Cohort Study.” Lancet Infectious Diseases 14 (9): 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, W. 2004. “The Behaviour of the Maximum Likelihood Estimator of Limited Dependent Variable Models in the Presence of Fixed Effects.” Econometrics Journal 7 (1): 98–119. [Google Scholar]
- Grov, C. , Whitfield T. H. F., Rendina H. J., Ventuneac A., and Parsons J. T.. 2015. “Willingness to Take PrEP and Potential for Risk Compensation among Highly Sexually Active Gay and Bisexual Men.” AIDS and Behavior 19 (12): 2234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel, L. , Firth J., Hoff T., Levine S., and Dawson L.. 2014. “HIV/AIDS in the Lives of Gay and Bisexual Men in the United States” [accessed on October 4, 2016]. Available at http://kff.org/hivaids/report/hivaids-in-the-lives-of-gay-and-bisexual-men-in-the-united-states/
- Hess, K. , Hu X., Lansky A., Mermin J., and Hall H. I.. 2016. “Estimating the Lifetime Risk of a Diagnosis of HIV Infection in the United States.” Conference on Retroviruses and Opportunistic Infections (CROI). February 23, 2016.
- Holt, S. 2016. “The Important Role of the PrEP Specialist in a Multidisciplinary Team Approach to PrEP at a Community Health Center” [accessed on October 4, 2016] Available at http://www.iapac.org/AdherenceConference/presentations/ADH11_OA130.pdf
- Hoots, B. E. , Finlayson T., Nerlander L., Paz‐Bailey G., and NHBS Study Group . 2016. “Willingness to Take, Use of, and Indications for Pre‐Exposure Prophylaxis among Men Who Have Sex with Men – 20 U.S. Cities, 2014.” Clinical Infectious Diseases 65 (5): 672–7. (Online First), 10.1093/cid/ciw367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek, S. 2015. “An HIV Pre‐Exposure Prophylaxis (PrEP) Demonstration Project and Safety Study for Young Men Who Have Sex with Men in the United States (ATN 110)” [accessed on October 4, 2016]. Available at http://pag.ias2015.org/Search/Index
- Juusola, J. L. , Brandeau M. L., Owens D. K., and Bendavid E.. 2012. “The Cost‐Effectiveness of Preexposure Prophylaxis for HIV Prevention in the United States in Men Who Have Sex with Men.” Annals of Internal Medicine 156 (8): 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, T. , and Thornber‐Dunwell M.. 2014. “Uptake of PrEP for HIV Slow among MSM.” Lancet 383 (9915): 399–400. [DOI] [PubMed] [Google Scholar]
- Liu, A. Y. , Kittredge P. V., Vittinghoff E., Raymond H. F., Ahrens K., Matheson T., Hecht J., Klausner J. D., and Buchbinder S. P.. 2008. “Limited Knowledge and Use of HIV Post‐and Pre‐Exposure Prophylaxis among Gay and Bisexual Men.” Journal of Acquired Immune Deficiency Syndromes 47 (2): 241–7. [PubMed] [Google Scholar]
- Liu, A. , Cohen S., Follansbee S., Cohan D., Weber S., Sachdev D., and Buchbinder S.. 2014. “Early Experiences Implementing Pre‐Exposure Prophylaxis (PrEP) for HIV Prevention in San Francisco.” PLoS Medicine 11 (3): e1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, K. 2016. “PrEP 2016: What Will it Take to Generate Demand, Increase Access, and Accelerate Uptake?” [accessed on October 4, 2016]. Available at http://www.iapac.org/AdherenceConference/presentations/ADH11_Plenary5-Mayer.pdf
- Mayer, K. H. , Oldenburg C. E., Novak D. S., Elsesser S. A., Krakower D. S., and Mimiaga M. J.. 2015. “Early Adopters: Correlates of HIV Chemoprophylaxis Use in Recent Online Samples of US Men Who Have Sex with Men.” AIDS and Behavior 20 (7): 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack, S. , Dunn D. T., Desai M., Dolling D. I., Gafos M., Gilson R., Sullivan A. K., Clarke A., Reeves I., Schembri G., Mackie N., Bowman C., Lacey C. J., Apea V., Brady M., Fox J., Taylor S., Antonucci S., Khoo S. H., Rooney J., Nardone A., Fisher M., McOwan A., Phillips A. N., Johnson A. M., Gazzard B., and Gill O. N.. 2016. “Pre‐Exposure Prophylaxis to Prevent the Acquisition of HIV‐1 Infection (PROUD): Effectiveness Results from the Pilot Phase of a Pragmatic Open‐Label Randomised Trial.” Lancet 387 (10013): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett, G. A. , Flores S. A., Peterson J. L., and Bakeman R.. 2007. “Explaining Disparities in HIV Infection among Black and White Men Who Have Sex with Men: A Meta‐Analysis of HIV Risk Behaviors.” AIDS 21 (15): 2083–91. [DOI] [PubMed] [Google Scholar]
- Mimiaga, M. J. , Case P., Johnson C. V., Safren S. A., and Mayer K. H.. 2009. “Preexposure Antiretroviral Prophylaxis Attitudes in High‐Risk Boston Area Men Who Report Having Sex With Men: Limited Knowledge and Experience but Potential for Increased Utilization after Education.” Journal of Acquired Immune Deficiency Syndromes 50 (1): 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga, M. J. , White J. M., Krakower D. S., Biello K. B., and Mayer K. H.. 2014. “Suboptimal Awareness and Comprehension of Published Preexposure Prophylaxis Efficacy Results among Physicians in Massachusetts.” AIDS Care 26 (6): 684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, J.‐M. , Capitant C., Spire B., Pialoux G., Cotte L., Charreau I., Tremblay C., Le Gall J.‐M., Cua E., Pasquet A., Raffi F., Pintado C., Chidiac C., Chas J., Charbonneau P., Delaugerre C., Suzan‐Monti M., Loze B., Fonsart J., Peytavin G., Cheret A., Timsit J., Girard G., Lorente N., Préau M., Rooney J. F., Wainberg M. A., Thompson D., Rozenbaum W., Doré V., Marchand L., Simon M.‐C., Etien N., Aboulker J.‐P., Meyer L., Delfraissy J.‐F., and ANRS IPERGAY Study Group . 2015. “On‐Demand Preexposure Prophylaxis in Men at High Risk for HIV‐1 Infection.” New England Journal of Medicine, 373 (23): 2237–46. [DOI] [PubMed] [Google Scholar]
- The New York City Department of Health and Mental Hygiene . 2015. “Health Department Launches New PrEP & PEP Campaign: New Ways to Prevent HIV.” Press Release # 003‐15 Monday, January 12, 2015 [accessed July 7, 2017]. Available at https://www1.nyc.gov/site/doh/about/press/pr2015/pr003-15
- Norton, W. E. , Larson R. S., and Dearing J. W.. 2013. “Primary Care and Public Health Partnerships for Implementing Pre‐Exposure Prophylaxis.” American Journal of Preventive Medicine 44 (1): S77–9. [DOI] [PubMed] [Google Scholar]
- Osborn, C. Y. , Paasche‐Orlow M. K., Davis T. C., and Wolf M. S.. 2007. “Health Literacy: An Overlooked Factor in Understanding HIV Health Disparities.” American Journal of Preventive Medicine 33 (5): 374–8. [DOI] [PubMed] [Google Scholar]
- Schneeweiss, S. , Maclure M., Walker A. M., Grootendorst P., and Soumerai S. B.. 2001. “On the Evaluation of Drug Benefits Policy Changes with Longitudinal Claims Data: The Policy Maker's versus the Clinician's Perspective.” Health Policy 55 (2): 97–109. [DOI] [PubMed] [Google Scholar]
- Schneeweiss, S. , Maclure M., Soumerai S. B., Walker A. M., and Glynn R. J.. 2002. “Quasi‐Experimental Longitudinal Designs to Evaluate Drug Benefit Policy Changes with Low Policy Compliance.” Journal of Clinical Epidemiology 55 (8): 833–41. [DOI] [PubMed] [Google Scholar]
- Smith, D. K. , Van Handel M., Wolitski R. J., Stryker J. E., Hall H. I., Prejean J., Koenig L. J., and Valleroy L. A.. 2015. “Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition–United States, 2015.” Morbidity and Mortality Weekly Report 64 (46): 1291–5. [DOI] [PubMed] [Google Scholar]
- Volk, J. E. , Marcus J. L., Phengrasamy T., Blechinger D., Nguyen D. P., Follansbee S., and Hare C. B.. 2015. “No New HIV Infections with Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting.” Clinical Infectious Diseases 61 (10): 1601–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge, J. 2010. Econometric Analysis of Cross Section and Panel Data. Cambridge, MA: MIT Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Table S1. Characteristics of HIV‐Negative MSM Visiting the Rhode Island STD Clinic at First Visits: Study Sample Relative to Full HIV‐Negative MSM Population.
Appendix S1. PrEP Information Sheet.


