Abstract
Objective
To test the effectiveness of a collaborative depression care model in improving depression and hepatitis C virus (HCV) care.
Data Sources/Study Setting
Hepatitis C virus clinic patients who screened positive for depression at four Veterans Affairs Hospitals.
Study Design
We compared off‐site depression collaborative care (delivered by depression care manager, pharmacist, and psychiatrist) with usual care in a randomized trial. Primary depression outcomes were treatment response (≥50 percent decrease in 20‐item Hopkins Symptoms Checklist [SCL‐20] score), remission (mean SCL‐20 score, <0.5), and depression‐free days (DFDs). Primary HCV outcome was receipt of HCV treatment.
Data Collection
Patient data were collected by self‐report telephone surveys at baseline and 12 months, and from electronic medical records.
Principal Findings
Baseline screening identified 292 HCV‐infected patients with depression, and 242 patients completed 12‐month follow‐up (82.9 percent). Intervention participants were more likely to report depression treatment response, remission, and more DFDs than usual care participants. Intervention participants were more likely to receive antiviral treatment; however, the difference was not statistically significant.
Conclusion
Off‐site depression collaborative care improved depression outcomes in HCV patients and may serve as a model for collaboration between mental health and specialty physical health providers in other high co‐occurring conditions.
Keywords: Physical health, depression, hepatitis C
Depression is one of the most common mental health disorders affecting persons with chronic hepatitis C virus (HCV) infection (El‐Serag et al. 2002; Lehman and Cheung 2002; Bini et al. 2005; Butt et al. 2005). Despite availability of simple and effective treatments, depression is underdiagnosed and undertreated in routine HCV care (Lehman and Cheung 2002; Evon et al. 2007). Patients with both depression and HCV are at high risk for morbidity and mortality. Depression in patients with HCV may be associated with fatigue, functional disability, and poor health‐related quality of life (Dwight et al. 2000; Dan et al. 2006; Lim et al. 2006; Ozkan et al. 2006). While depression had been the main exclusionary criterion to interferon‐based antiviral treatment (Cawthorne et al. 2002; Bini et al. 2005; Butt et al. 2005; Evon et al. 2007), new treatments can now be safely used in patients with depression. Yet depression continues to play a role in predicting receipt and success of HCV treatment through its effect on retention in care and treatment adherence, in addition to the intrinsic value of improving mental health‐related quality of life.
In general primary care, structured depression collaborative care models are both effective and cost‐effective, improving both mental health process quality and clinical outcomes (Katon et al. 1996; Lave et al. 1998; Simon et al. 2000, 2001; Hedrick et al. 2003). Like many other chronic conditions that require specialized knowledge or procedures, patients are predominantly evaluated and treated for HCV in dedicated specialty clinics rather than primary care. Intensive yet focused collaborative care in HCV clinics may be effective in improving depression and HCV care. Compared to the usual referral‐based models, a collaborative care model in HCV may be more accessible and potentially less stigmatizing than receiving care in the specialty mental health clinics. Moreover, centralizing care around an HCV clinic may increase the likelihood that patients will stay in both HCV and mental health care.
This study adapted an evidence‐based primary care model of depression collaborative care to HCV clinic settings in four Veterans Administration (VA) facilities and evaluated the model's clinical effectiveness. We hypothesized that depressed HCV patients who were assigned to the Hepatitis C Translating Initiatives for Depression Into Effective Solutions (HEPTIDES) intervention would report improvement in depression outcomes and be more likely to receive antiviral treatment compared with patients receiving usual care. Six‐month intervention response was reported previously, demonstrating modest improvements in depression outcomes (Kanwal et al. 2016). We now report data from the 12‐month follow‐up (predefined study end point) of HCV patients included in the randomized controlled trial.
Methods
Design
The HEPTIDES study compared depression collaborative care with usual care in a randomized controlled effectiveness trial. The study was approved by the VA Central Institutional Review Board and registered in Clinicaltrials.gov.
Participants
We recruited a continuously enrolled, longitudinal sample of patients with confirmed HCV infection (positive HCV RNA test) who were seen in HCV clinics and who screened positive for major depression using the 9‐item Patient Health Questionnaire (PHQ‐9) depression screening instrument (score ≥10). Exclusion criteria included the following: already on HCV treatment; no telephone access; bereavement; current suicidal ideation; significant cognitive impairment (score ˃10 on the Blessed Orientation Memory and Concentration Test) (Katzman et al. 1983), court‐appointed guardian; diagnosis of schizophrenia; and bipolar disorder with admission for mental health diagnosis in the previous 12 months.
Participants were randomly assigned to intervention or usual care in a 1 : 1 ratio using a computer‐generated random assignment sequence stratified by clinic. Participants were enrolled from May 2012 through September 2013. Subjects provided written informed consent after receiving a complete description of the study prior to baseline screening.
Depression Screening and Intervention Adaptation by Clinic
The HCV clinic staff, including nurses, physician assistants, and HCV clinicians, conducted the depression screening and the research team delivered the intervention. Depression screening methods were adopted at each clinic as part of routine care using the PHQ‐9 before each HCV clinic visit. HCV clinic staff was aware of the depression screening results for all participants.
HEPTIDES Intervention
The purpose of the HEPTIDES intervention was to support HCV and mental health clinicians in delivering evidence‐based depression treatment. The depression care team consisted of a registered nurse depression care manager, a clinical pharmacist, and a psychiatrist. This team was centrally located off‐site at one VA medical center and convened once a week and as needed by telephone or in person. Clinicians were not blinded to the depression care received by the patient. The depression care team communicated with treating HCV and mental health clinicians (if patients were seen by both) via electronic medical record progress notes. The DCM communicated with patients via telephone. The HEPTIDES depression care team made treatment suggestions. Treatment decisions were made by the HCV or mental health clinicians at each site.
The DCM delivered the following intervention components: participant education and activation, assessment of treatment barriers and possible resolutions, depression symptom and treatment monitoring, substance abuse monitoring, and instruction in self‐management (e.g., encouraging patients to exercise and participate in social activities). The DCM used prewritten scripts that were supported by the Web‐based decision support system (DSS). The intervention used a stepped‐care model for depression treatment. The five‐step model included the following components plus DCM monitoring: self‐management education, depression care team treatment suggestions (counseling or pharmacotherapy, considering participant preference), pharmacotherapy suggestions after review of depression treatment history by the clinical pharmacist, combination pharmacotherapy and specialty mental health counseling, and referral to specialty mental health. Specific treatment suggestions were based on the VA/Department of Defense Depression Treatment Guidelines. At any time, HCV providers were free to refer participants directly to specialty mental health care. The stepped‐care model was used to increase treatment intensity when participants did not respond to treatment.
The DCM conducted telephone‐based monitoring every 2 weeks during acute treatment (before achieving a sustained 50 percent decrease in PHQ‐9 score) and every 4 weeks during self‐management education or continuation treatment (for 2 months after maintaining remission [PHQ‐9 score <5] or 6 months after maintaining a 50 percent decrease in PHQ‐9 score). The DSS identified the potential need to change treatment when antidepressant regimen adherence was less than 80 percent during the past 14 days, counseling adherence was less than 75 percent during the past month, participant reported severe adverse effects during two consecutive DCM encounters, participant reported a 5‐point increase in depression severity from the enrollment PHQ‐9 score based on two consecutive DCM encounters, or lack of participant response (<50 percent decrease from enrollment PHQ‐9 score during two consecutive DCM encounters) following an 8‐week antidepressant or 12‐week counseling trial.
Usual Care
Usual care included depression screening with the same PHQ‐9 screener used for intervention patients. Usual care patients received “standard of care” depression treatment, typically including referral to specialty mental health clinics or depression treatment at integrated primary care mental health clinics where patients had access to on‐site evidence‐based psychotherapy and pharmacotherapy. Usual care patients did not have access to the DCM or depression care team.
Data Collection
Baseline and follow‐up data were collected by a single telephone interviewer who was blinded to treatment assignment. At baseline, the Depression Outcomes Module (DOM) was administered to assess patient demographics, depression history, and co‐occurring physical health problems (Rost, Smith, and Burnam 1992; Smith et al. 2000). Mental health comorbidity was measured using the Mini‐International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998). Acceptability of antidepressant treatment was measured using an item developed for the Quality Improvement for Depression studies (Wells et al. 2000; Rost et al. 2001).
Outcome Measures
The primary end points were improvement in depression outcomes and antiviral treatment for HCV. Secondary end points were health‐related quality of life, street drug or alcohol use, treatment satisfaction, and antidepressant medication regimen adherence.
Depression symptom severity during the past 2 weeks was measured using the 20‐item Hopkins Symptom Checklist (SCL‐20), which includes the 13‐item depression scale plus 7 depression‐related items from the Hopkins Symptom Checklist 90–Revised (Derogatis 1994). The items are scored from 0 to 4 and averaged to provide a mean depression severity score ranging from 0 to 4, with higher scores indicating greater severity. Depression outcomes based on the SCL‐20 included response, remission, and depression‐free days (DFDs). Depression treatment response at 12 months was defined as a 50 percent or greater decrease in the mean SCL‐20 score compared with baseline, and remission was defined as a mean SCL‐20 score of less than 0.5. DFDs were calculated as a summative measure of depression severity based on baseline and 12‐month SCL‐20 data using formulas originally developed by Lave and colleagues and adapted for the SCL‐20 (Katon et al. 1996; Lave et al. 1998; Simon et al. 2000, 2001). For each assessment, an SCL‐20 score of less than 0.5 was considered depression free, a score of 2.0 or higher was considered fully symptomatic, and scores in between were assigned a linear proportional value. Antiviral treatment was defined as receipt of at least one prescription of pegylated interferon within 12 months of baseline.
Health‐related quality of life was measured using the physical and mental health component summary scores (PCS and MCS) from the Medical Outcomes Study Veterans 12‐Item Short‐Form Health Survey (SF‐12) (Ware, Kosinski, and Keller 1996). The MINI alcohol abuse/dependence, at‐risk drinking, and street drug use modules were used to measure quantity and frequency and diagnostic criteria for alcohol and drug use (Sheehan et al. 1998). At‐risk drinking was defined as exceeding four standard drinks per day (>14/week) for men and more than three per day (>7/week) for women (National Institute on Alcohol Abuse and Alcoholism 2016) Illicit street drug use was measured by self‐report. Patient satisfaction with HEPTIDES was measured using a measure that was adapted from the Group Health Association of America Consumer Satisfaction Survey (1991). Pharmacy refill data were used to calculate a medication possession ratio (MPR) (Valenstein et al. 2002), by dividing the number of days' supply of antidepressant medications received by the number of days' supply needed to take the medication continuously.
Statistical Analyses
Participants were the unit of the intent‐to‐treat analysis. We based sample size calculations on data from a previous study that implemented this intervention in HIV clinics (Pyne et al. 2011). Assuming that 35 percent of patients in HEPTIDES would experience improvement in depression outcomes compared to 18 percent in usual care, we needed 170 patients to complete 12‐month follow‐up in order to test the hypothesis at 80 percent power and 5 percent significance level.
We compared categorical variables using a Χ2 test and continuous variables using a two‐tailed t‐test or its nonparametric analogue. We used logistic and ordinary least squares regression analyses to estimate intervention effects for dichotomous and continuous outcomes, respectively, adjusting for baseline covariates that significantly predicted dependent variables at p < .20 in bivariate analyses. The only exceptions were age and race variables, which we entered in the models based on conceptual considerations. Furthermore, several variables captured the same construct (examples: history of depression and depression treatment). We selected only one of these conceptually related variables for entry into the multivariate models based on the presence of consistent and strong associations with the end points.
We did not adjust for potential nesting of participants within VA medical centers because the intraclass coefficient (−0.0004) was close to zero with respect to our main outcomes, and there were no significant differences in outcomes across sites.
Results
Patient Characteristics
A total of 292 patients completed baseline interviews. Follow‐up data collection interviews were completed for 263 participants (90.1 percent) at 6 months (Kanwal et al. 2016) and 242 (82.8 percent) at 12 months (Figure 1).
Figure 1.
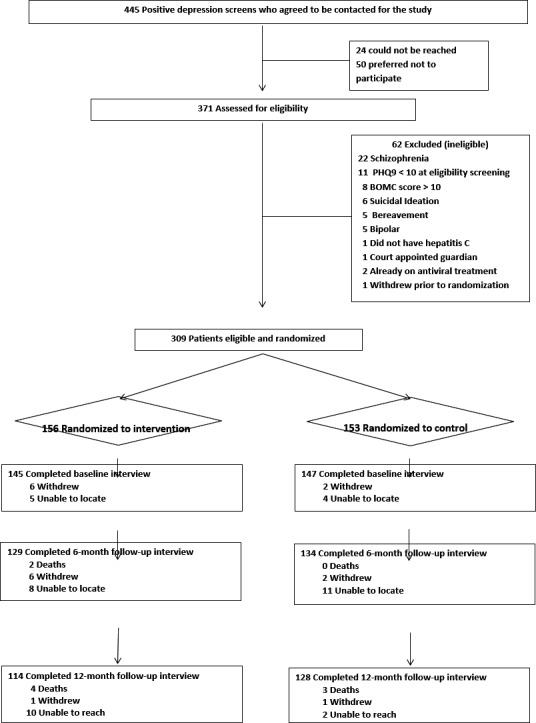
Study Flow Diagram
The majority of 242 patients who completed 12‐month follow‐up were men (mean age 59 years). Approximately half of our sample was African American and 41.3 percent was married. The annual income was greater than $20,000 in only 29.3 percent of participants. Most reported a history of mood disorder and 120 (49.5 percent) reported at least one active prescription for an antidepressant medication at baseline, yet still scored positive for depression on the SCL‐20. A total of 103 (42.5 percent) patients preferred not to take an antidepressant. Over half of the sample had comorbid generalized anxiety disorder and 26 (10.7 percent) patients met criteria for at‐risk drinking. Patients on average had five physical comorbidities and a total of 40 (16.5 percent) patients had already progressed to advanced liver fibrosis or cirrhosis.
Table 1 presents baseline characteristics for the two study groups. Intervention and usual care participants were similar at baseline with the exception of marital status and at‐risk drinking; usual care participants were more likely to be married and meet criteria for at‐risk drinking than intervention participants.
Table 1.
Baseline Characteristics of Study Participants (n = 242)
| Characteristics | Intervention (N = 114) | Usual care (N = 128) | ||
|---|---|---|---|---|
| N/mean | %/SD | N/Mean | %/SD | |
| Site | ||||
| Houston | 71 | 62.3 | 78 | 60.9 |
| Little Rock | 13 | 11.4 | 11 | 8.6 |
| Los Angeles | 10 | 8.8 | 11 | 8.6 |
| Saint Louis | 20 | 17.5 | 28 | 21.9 |
| Demographics | ||||
| Age, mean (SD) | 59.0 | 5.6 | 59.4 | 4.9 |
| Male | 110 | 96.5 | 123 | 96.1 |
| Race | ||||
| White | 48 | 42.1 | 35 | 27.3 |
| African American | 55 | 48.3 | 80 | 62.5 |
| Hispanic | 6 | 5.3 | 7 | 5.5 |
| Other race | 5 | 4.4 | 6 | 4.7 |
| Marital statusa | ||||
| Single/never married | 12 | 10.5 | 14 | 10.9 |
| Married | 35 | 30.7 | 65 | 50.8 |
| Divorced/separated/widow | 67 | 58.8 | 49 | 38.3 |
| Education | ||||
| High school graduation or lower | 58 | 50.9 | 60 | 46.9 |
| College or higher | 56 | 49.1 | 68 | 53.1 |
| Annual income ≥$20,000 | 33 | 28.9 | 38 | 29.7 |
| Clinical characteristics | ||||
| Short Form (SF)‐12 physical component score, mean (SD) | 35.1 | 12.4 | 35.2 | 11.3 |
| SF‐12 mental component score, mean (SD) | 41.8 | 13.8 | 39.4 | 11.9 |
| Satisfied with care | 86 | 75.4 | 93 | 72.7 |
| Physical health comorbidity score, mean (SD) | 5.2 | 3.0 | 4.9 | 2.6 |
| Cirrhosis | 21 | 18.4 | 19 | 14.8 |
| SCL‐20 score, mean (SD) | 1.9 | 0.8 | 1.7 | 0.8 |
| SCL‐20 < 0.5 at baseline | 4 | 3.5 | 12 | 9.4 |
| Major depression | 90 | 79.0 | 93 | 72.7 |
| Panic disorder | 9 | 7.9 | 4 | 3.1 |
| Generalized anxiety disorder | 66 | 57.9 | 65 | 50.8 |
| Post‐traumatic stress disorder | 40 | 35.1 | 35 | 27.3 |
| A drink of alcohol in the last year | 61 | 53.5 | 77 | 60.2 |
| At‐risk drinkinga | 6 | 5.3 | 20 | 15.6 |
| Any inpatient mental health admission | 43 | 37.7 | 49 | 38.3 |
| Any past depression treatment | 87 | 76.3 | 90 | 70.3 |
| Any antidepressant in past 6 months | 43 | 37.7 | 47 | 36.7 |
| Any depression treatment in past 6 months | 62 | 54.4 | 64 | 50.0 |
| Current antidepressant prescription | 55 | 48.3 | 65 | 50.9 |
| Skipped antidepressant in past 4 days | 8 | 7.0 | 18 | 14.1 |
| Depression treatment preference | ||||
| Watchful waiting acceptable | 80 | 70.2 | 83 | 64.8 |
| Antidepressant medication acceptable | 70 | 61.4 | 69 | 53.9 |
| Individual counseling acceptable | 92 | 80.7 | 106 | 82.8 |
| Group counseling acceptable | 64 | 56.1 | 78 | 60.9 |
| Psychoactive drugs in past 6 months | 49 | 43.0 | 62 | 48.4 |
Statistically significant differences.
Primary End Points
Among 114 patients in the intervention group, 36 (31.6 percent) met criteria for treatment response compared to 19 of the 128 patients (14.8 percent) in the usual care group at 12 months (p‐value .002) in unadjusted analyses. Similarly, intervention patients were more likely to experience remission at 12 months than those in the usual care group (22/114 or 19.3 percent vs. 9/128 or 7.0 percent (p‐value .004). Intervention patients also had more DFDs than usual care patients. However, this difference did not reach statistical significance in unadjusted analysis (mean 118.7 vs. 109.3, p‐value .53) (Table 2).
Table 2.
Comparison of Primary Outcomes between Intervention and Usual Care at Twelve Months
| Outcomes | HEPTIDES (n = 114) | Usual Care (n = 128) | p‐value | Adjusted Odds Ratio or Difference (95% Confidence Interval) |
|---|---|---|---|---|
| Depression severity | ||||
| Response—no/ total no (%) | 36/114 (31.6) | 19/128 (14.8) | .002 | 3.35 (1.71–6.53) |
| Remission—no/ total no (%) | 22/114 (19.3) | 9/128 (7.0) | .004 | 3.69 (1.56–8.74) |
| Depression‐free days, mean(SD)a | 118.7 (106.7) | 109.3 (117.9) | .53 | 30.70 (10.5–50.8) |
| HCV treatment | 11/114 (9.7) | 7/128 (5.5) | .22 | NA |
Intervention n = 109, usual care n = 123.
Only baseline covariates that predicted the outcomes in bivariate analyses (p < .20) were included in each multivariable regression model.
Response: age, race, cirrhosis status, baseline SCL‐20, depression treatment in the past 6 months, generalized anxiety disorder.
Remission: age, race, cirrhosis status, baseline SCL‐20, depression treatment in the past 6 months, generalized anxiety disorder.
Depression‐free days: age, race, cirrhosis status, baseline SCL‐20, depression treatment in the past 6 months, generalized anxiety disorder, panic disorder, post‐traumatic stress disorder.
Figure 2 displays the effect of the intervention on depression outcomes at 6 months and 12 months among patients who completed both 6‐ and 12‐month follow‐ups (n = 232). The effect of intervention was more pronounced at 12‐month follow‐up.
Figure 2.
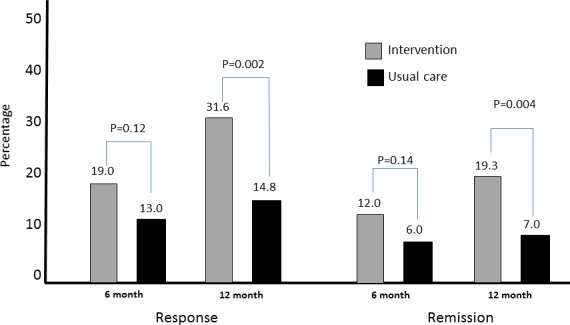
Hepatitis C Translating Initiatives for Depression into Effective Solutions Intervention Effects on Depression at Six and Twelve Months [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2 also presents the effect of the intervention after adjusting for age, race, and other clinical covariates that were significant in the bivariate analyses for each outcome. Baseline depressed HCV patients in the intervention group were more likely to meet criteria for response (adjusted odds ratio, OR = 3.35, 95 percent CI = 1.71–6.53), remission (adjusted OR = 3.69, 95 percent CI = 1.56–8.74) and had more DFDs (adjusted beta = 30.70 days, 95 percent CI = 10.5–50.8) than usual care participants.
A total of 18 patients started antiviral treatment during study follow‐up. Intervention participants were more likely to receive antiviral treatment (9.7 percent vs. 5.5 percent); however, the difference was not statistically significant. We did not perform a multivariable analysis given the small number of patients who met this end point.
Secondary End Points
Significant intervention effects were observed for mental health‐related quality of life, but not for physical health quality of life, satisfaction with care, antidepressant medication regimen adherence, or alcohol and street drug use at 12 months. The adjusted intervention effect resulted in significantly better 12‐month SF‐12 mental health component summary score compared with usual care (adjusted mean difference = 3.73, 95 percent CI = 0.47–6.99) (Table 3). The unadjusted and adjusted intervention effects on SF‐12 physical component summary score, satisfaction with care, antidepressant regimen adherence, and at‐risk alcohol or drug use were not significant, although there was a trend toward higher medication adherence (adjusted OR = 1.25, 95 percent CI = 0.67–2.33) and lower alcohol or street drug use (adjusted OR = 0.59, 95 percent CI = 0.33–1.02) in the intervention group.
Table 3.
Comparison of Secondary Outcomes between Intervention and Usual Care at Twelve Months
| Outcomes | HEPTIDES (n = 114) | Usual Care (n = 128) | p‐value | Adjusted Odds Ratio or Difference (95% CI) |
|---|---|---|---|---|
| Short Form (SF)‐12 physical component score, mean (SD) | 35.1 (12.4) | 35.2 (11.3) | .96 | −0.85 (−3.52 to 1.80)a |
| SF‐12 mental component score, mean (SD) | 41.8 (13.8) | 39.4 (11.9) | .16 | 3.73 (0.47–6.99)a |
| Satisfied with care—no/total no (%) | 80 (70.2) | 91 (71.1) | .87 | 0.98 (0.55–1.75)b |
| Antidepressant adherence (medication possession ratio >90%)—no/total no (%) | 40 (35.1) | 33 (25.8) | .12 | 1.25 (0.67–2.33)b |
| At‐risk drinking or street drug use—no/total no (%) | 40 (35.1) | 55 (43.0) | .21 | 0.59 (0.33–1.02)b |
Only baseline covariates that predicted the outcomes in bivariate analyses (p < .20) were included in the multivariable regression model for each outcome.
SF‐12 physical component score (PCS): age, race, marital status, baseline PCS, cirrhosis, depression treatment in the past 6 months, preference regarding waiting to treat, individual counseling, and group counseling.
SF‐12 mental component score(MCS): age, race, baseline MCS, cirrhosis, baseline SCL‐20, depression treatment in the past 6 months, generalized anxiety disorder, panic disorder, post‐traumatic stress disorder, preference regarding antidepressants, current prescription of antidepressants, and self‐report of skipping antidepressants.
At‐risk drinking or street drug use: age, race, depression treatment in the past 6 months, generalized anxiety disorder, panic disorder, preference regarding individual and group counseling, and study site.
Satisfied with care: age, race, baseline SCL‐20, baseline satisfaction with care, depression treatment in the past 6 months, panic disorder, preference regarding individual counseling, study site.
Antidepressant adherence: age, race, major depression, depression treatment in the past 6 months, panic disorder, post‐traumatic stress disorder, preference regarding antidepressants and group counseling, and current prescription of antidepressants.
Adjusted differences were calculated for continuous variables.
Adjusted Odds Ratios were calculated for categorical variables.
Discussion
We found that structured remote collaborative care was associated with higher rates of depression response and remission outcomes as well as more DFDs at 12 months compared to usual care. The effect of intervention on depression outcomes was consistent across the four study sites and across several patient subgroups.
We also found that the effectiveness of the HEPTIDES intervention improved over time. We had found a trend toward higher rates of depression response and remission outcomes compared to usual care at 6 months (Kanwal et al. 2016). By 12 months, the odds of meeting depression outcomes were threefold higher in the intervention compared to usual care. These data suggest that depression symptoms improved gradually in the intervention group—a finding in contrast to previous studies in medical specialty populations in the VA (Pyne et al. 2011). For example, in a previous study that implemented a similar collaborative care intervention in HIV clinics, depression symptoms improved more rapidly in the intervention group compared with usual care. Usual care participants caught up with intervention participants in terms of response and remission rates at 12‐month follow‐up (Pyne et al. 2011). In contrast, intervention patients in our study continued to report better depression outcomes throughout the study with higher response and remission rates as 12 months than 6 months.
There may be several reasons underlying the observed difference in rapidity of effect. Our study included patients with characteristics that may have slowed their response to the intervention. For example, in addition to being chronically infected with HCV, participants on average had five other comorbid physical health conditions and one of five had already progressed to cirrhosis (advanced liver disease). Half of our patients screened positive for depressive symptoms while being on antidepressant medications and ~40 percent had a history of inpatient mental health admission—substantially higher than those reported in previous studies (Fortney et al. 2007; Pyne et al. 2011). Our patients were also less accepting of antidepressants, and a substantial proportion reported ongoing drug and alcohol use—all factors that could contribute to a slower response to intervention. Given these differences, it is perhaps not surprising that only 32 percent of HCV patients in the intervention group achieved depression response at 12 months compared to ~40 percent in HIV infected patients (Pyne et al. 2011). However, the magnitude of these improvements was similar to, if not larger than, those seen in other VA studies (Fortney et al. 2007; Pyne et al. 2011). The adjusted incremental 12‐month DFDs result from the HEPTIDES intervention (30.7 DFDs) was larger when compared to 19.3 incremental DFDs during 12 months in VA HIV infected patients (Pyne et al. 2011) and 14.6 incremental DFDs during 9 months in a VA primary care sample (Liu et al. 2003). Overall, our data show that depressed HCV patients benefit from collaborative depression care. However, they might need to be in the intervention program for longer than 6 months for the treatment to be effective.
The intervention also resulted in significant improvement in mental health quality of life outcome, providing further support to the effectiveness of HEPTIDES intervention in improving depression outcomes. Lack of detectable differences in adherence suggests that other mechanisms, beyond optimizing antidepressant adherence, led to improved depression and mental health‐related quality of life outcomes. However, it is possible that we did not detect adherence effects because of measurement error inherent in relying on pharmacy data for treatment adherence. Intervention patients were slightly more likely to receive specialty mental health care than those in the usual care group; however, this difference did not reach statistical significance (74.5 percent vs. 65.7 percent, respectively p = .12). Furthermore, among those with any specialty mental health care, on average, usual care patients had more visits to specialty mental health than intervention patients (12 [standard deviation, SD 17] vs. 9 [SD 5] visits, respectively, p = .01). During these visits, the intervention may have led to greater dose intensification or treatment switching not detected by our measurement methods. Another mechanism for depression improvements may have been DCM promotion of self‐management activities and/or brief interventions for alcohol and other drug abuse. Indeed, we saw a trend toward a reduction in alcohol and street drug use in the intervention patients (adjusted OR = 0.59, 95 percent CI, 0.33–1.02). Although this effect did not reach statistical significance, perhaps due to a delayed response similar to what we observed for depression outcomes, extending the duration of intervention might have resulted in statistically significant improvements in substance use outcomes.
Only a few of our patients received antiviral treatment during the study follow‐up. HCV treatment landscape has changed in the last few years. During the HEPTIDES study period, boceprevir or telaprevir in combination with pegylated interferon and ribavirin constituted the standard of care: a treatment with substantial toxicity and a low cure rate. For this reason and given the anticipated approval of all‐oral direct acting antiviral agents (approved shortly after HEPTIDES ended), most patients and their clinicians deferred antiviral treatment; this might explain the low rate of HCV treatment in our study. In a recent study, an integrated care model with a mid‐level mental health provider co‐located in the VA HCV clinics led to an increase in the proportion of patients who started antiviral therapy (Ho et al. 2015). The co‐located mental health provider in this study provided brief mental health interventions, case management, and also directly facilitated antiviral therapy. HEPTIDES did not directly facilitate antiviral therapy but rather got patients ready for antiviral therapy (by controlling depression symptoms). These differences, in addition to the earlier time period for the integrated care study (2009–2011), likely explain the observed differences in antiviral treatment rates between the two studies.
We believe that the off‐site nature of the HEPTIDES intervention is a key strength of our study and may have broader implications for the organization of HCV and other specialty health care settings. Collaborative care models, such as used in the current study, that employ off‐site mental health specialists to support physical health specialty clinics using telemedicine may improve health care and cost less than hiring on‐site mental health clinicians. In one of the few buy versus build depression collaborative care studies, the off‐site collaborative care team (buy) was more cost‐effective than the on‐site team (build) (Pyne et al. 2015). Given this, our data may be relevant to other specialty physical health clinical settings with a high burden of comorbid mental illness.
With the advent of new antiviral treatment, depression may not impact treatment decisions to the same extent as with the previous standard treatment (Jacobson et al. 2013; Kowdley et al. 2013). However, one of the most important considerations in prescribing the new antiviral treatment is patients’ ability to comply with clinic visits and medication. Depression may continue to play a pivotal role in predicting antiviral treatment response in HCV patients through its effect on treatment adherence. Regardless of the considerations related to antiviral treatment, HCV is a particularly important chronic condition for the depression collaborative care intervention because depression is so prevalent and because treating depression can improve depression outcomes and has the potential to improve a wide range of life‐saving self‐management and adherence behaviors.
The study has several limitations. It was a four‐site study, and thus, the results may not be generalizable to all veterans with HCV and to nonveterans seen in other health care systems. However, the geographic and clinical practice differences across the four sites allowed us to examine the impact of HEPTIDES in a variety of practice settings. There was a single DCM team, and our results may have been specific to the team members. However, the team was based on a widely implemented model in the VA that has been shown to be effective in primary care and HIV. The new HCV treatment can be as short as 3 months. However, the duration of experience in the HCV clinic is usually longer than the duration of HCV treatment, allowing sufficient time for the intervention to be effective.
In conclusion, the HEPTIDES intervention improved depression outcomes in a highly comorbid and treatment‐resistant group of patients with HCV. This improvement was consistent across all sites and was slow to occur but improved over time. HCV clinics should consider collaborative care to improve depression outcomes for their patients. If confirmed in future studies, this collaborative care program may serve as a model for other physical health specialty populations with high rates of comorbid depression.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (SDP 10‐044). Dr. Kanwal is the principal investigator for the project and is based at the Center for Innovations in Quality, Effectiveness and Safety (#CIN 13‐413), Michael E. DeBakey VA Medical Medical Center, Houston Texas. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Disclosures: None.
Disclaimer: None.
References
- Bini, E. J. , Brau N., Currie S., Shen H., Anand B. S., Hu K. Q., Jeffers L., Ho S. B., Johnson D., Schmidt W. N., King P., Cheung R., Morgan T. R., Awad J., Pedrosa M., Chang K. M., Aytaman A., Simon F., Hagedorn C., Moseley R., Ahmad J., Mendenhall C., Waters B., Strader D., Sasaki A. W., Rossi S., and Wright T. L.. 2005. “Prospective Multicenter Study of Eligibility for Antiviral Therapy Among 4084 U.S. Veterans with Chronic Hepatitis C Virus Infection.” American Journal of Gastroenterology 100: 1772–9. [DOI] [PubMed] [Google Scholar]
- Butt, A. A. , Wagener M., Shakil A. O., and Ahmad J.. 2005. “Reasons for Nontreatment of Hepatitis C in Veterans in Care.” Journal of Viral Hepatitis 12: 81–5. [DOI] [PubMed] [Google Scholar]
- Cawthorne, C. H. , Rudat K. R., Burton M. S., Brown K. E., Luxon B. A., Janney C. G., and Fimmel C. J.. 2002. “Limited Success of HCV Antiviral Therapy in United States Veterans.” American Journal of Gastroenterology 97: 149–55. [DOI] [PubMed] [Google Scholar]
- Dan, A. A. , Martin L. M., Crone C., Ong J. P., Farmer D. W., Wise T., Robbins S. C., and Younossi Z. M.. 2006. “Depression, Anemia and Health‐Related Quality of Life in Chronic Hepatitis C.” Journal of Hepatology 44 (3): 491–8. [DOI] [PubMed] [Google Scholar]
- Derogatis, L. 1994. 2014. SCL‐90‐R: Administration, Scoring, and Procedures Manual, 3d Edition Minneapolis, MN: National Computer Systems Inc. [Google Scholar]
- Dwight, M. M. , Kowdley K. V., Russo J. E., Ciechanowski P. S., Larson A. M., and Katon W. J.. 2000. “Depression, Fatigue, and Functional Disability in Patients with Chronic Hepatitis C.” Journal of Psychosomatic Research 49 (5): 311–7. [DOI] [PubMed] [Google Scholar]
- El‐Serag, H. B. , Kunik M., Richardson P., and Rabeneck L.. 2002. “Psychiatric Disorders among veterans with Hepatitis C Infection.” Gastroenterology 123 (2): 476–82. [DOI] [PubMed] [Google Scholar]
- Evon, D. M. , Verma A., Dougherty K. A., Batey B., Russo M., Zacks S., Shrestha R., and Fried M. W.. 2007. “High Deferral Rates and Poorer Treatment Outcomes for HCV Patients with Psychiatric and Substance Use Comorbidities.” Digestive Diseases and Sciences 52 (11): 3251–8. [DOI] [PubMed] [Google Scholar]
- Fortney, J. C. , Pyne J. M., Edlund M. J., Williams D. K., Robinson D. E., Mittal D., and Henderson K. L.. 2007. “A Randomized Trial of Telemedicine‐Based Collaborative Care for Depression.” Journal of General Internal Medicine 22 (8): 1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group Health Association of America (GHAA) . 1991. GHAA Consumer Satisfaction Survey and User's Manual, 2d Edition. Washington, DC: Group Health Association of America (GHAA). [Google Scholar]
- Hedrick, S. C. , Chaney E. F., Felker B., Liu C. F., Hasenberg N., Heagerty P., Buchanan J., Bagala R., Greenberg D., Paden G., Fihn S. D., and Katon W.. 2003. “Effectiveness of Collaborative Care Depression Treatment in Veterans’ Affairs Primary Care.” Journal of General Internal Medicine 18 (1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S. B. , Brau N., Cheung R., Liu L., Sanchez C., Sklar M., Phelps T. E., Marcus S. G., Wasil M. M., Tisi A., Huynh L., Robinson S. K., Gifford A. L., Asch S. M., and Groessl E. J.. 2015. “Integrated Care Increases Treatment and Improves Outcomes of Patients with Chronic Hepatitis C Virus Infection and Psychiatric Illness or Substance Abuse.” Clinical Gastroenterology and Hepatology 13 (11): 2005–14. [DOI] [PubMed] [Google Scholar]
- Jacobson, I. M. , Gordon S. C., Kowdley K. V., Yoshida E. M., Rodriguez‐Torres M., Sulkowski M. S., Shiffman M. L., Lawitz E., Everson G., Bennett M., Schiff E., Al‐Assi M. T., Subramanian G. M., An D., Lin M., McNally J., Brainard D., Symonds W. T., McHutchison J. G., Patel K., Feld J., Pianko S., Nelson D. R., Study P., and Study F.. 2013. “Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options.” New England Journal of Medicine 368 (20): 1867–77. [DOI] [PubMed] [Google Scholar]
- Kanwal, F. , Pyne J. M., Tavakoli‐Tabasi S., Nicholson S., Dieckgraefe B., Storay E., Goetz M. B., Smith D. L., Sansgiry S., Gifford A., and Asch S. M.. 2016. “Collaborative Care for Depression in Chronic Hepatitis C Clinics.” Psychiatric Services 67 (10): 1076–82. [DOI] [PubMed] [Google Scholar]
- Katon, W. , Robinson P., Von Korff M., Lin, Bush T., Ludman E., Simon G., and Walker E.. 1996. “A Multifaceted Intervention to Improve Treatment of Depression in Primary Care.” Archives of General Psychiatry 53 (10): 924–32. [DOI] [PubMed] [Google Scholar]
- Katzman, R. , Brown T., Fuld P., Peck A., Schechter R., and Schimmel H.. 1983. “Validation of a Short Orientation‐Memory‐Concentration Test of Cognitive Impairment.” American Journal of Psychiatry 140 (6): 734–9. [DOI] [PubMed] [Google Scholar]
- Kowdley, K. V. , Lawitz E., Crespo I., Hassanein T., Davis M. N., DeMicco M., Bernstein D. E., Afdhal N., Vierling J. M., Gordon S. C., Anderson J. K., Hyland R. H., Dvory‐Sobol H., An D., Hindes R., Albanis E., Symonds W. T., Berrey M. M., Nelson D. R., and Jacobson I. M.. 2013. “Sofosbuvir With Pegylated Interferon Alfa‐2a and Ribavirin for Treatment‐Naive Patients with Hepatitis C Genotype‐1 Infection (ATOMIC): An Open‐Label, Randomised, Multicentre Phase 2 Trial.” Lancet 381 (9883): 2100–7. [DOI] [PubMed] [Google Scholar]
- Lave, J. R. , Frank R. G., Schulberg H. C., and Kamlet M. S.. 1998. “Cost‐Effectiveness of Treatments for Major Depression in Primary Care Practice.” Archives of General Psychiatry 55 (7): 645–51. [DOI] [PubMed] [Google Scholar]
- Lehman, C. L. , and Cheung R. C.. 2002. “Depression, Anxiety, Post‐Traumatic Stress, and Alcohol‐Related Problems among Veterans with Chronic Hepatitis C.” American Journal of Gastroenterology 97 (10): 2640–6. [DOI] [PubMed] [Google Scholar]
- Lim, J. K. , Cronkite R., Goldstein M. K., and Cheung R. C.. 2006. “The Impact of Chronic Hepatitis C and Comorbid Psychiatric Illnesses on Health‐Related Quality of Life.” Journal of Clinical Gastroenterology 40 (6): 528–34. [DOI] [PubMed] [Google Scholar]
- Liu, C. F. , Hedrick S. C., Chaney E. F., Heagerty P., Felker B., Hasenberg N., Fihn S., and Katon W.. 2003. “Cost‐Effectiveness of Collaborative Care for Depression in a Primary Care Veteran Population.” Psychiatric Services 54 (5): 698–704. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism . “Drinking Levels Defined” [accessed on April 17, 2017]. Available at http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- Ozkan, M. , Corapcioglu A., Balcioglu I., Ertekin E., Khan S., Ozdemir S., Karayun D., Unsalver B. O., Kocaman N., Kaymakglu S., and Koroglu G.. 2006. “Psychiatric Morbidity and Its Effect on the Quality of Life of Patients with Chronic Hepatitis B and Hepatitis C.” International Journal of Psychiatry in Medicine 36 (3): 283–97. [DOI] [PubMed] [Google Scholar]
- Pyne, J. M. , Fortney J. C., Curran G. M., Tripathi S., Atkinson J. H., Kilbourne A. M., Hagedorn H. J., Rimland D., Rodriguez‐Barradas M. C., Monson T., Bottonari K. A., Asch S. M., and Gifford A. L.. 2011. “Effectiveness of Collaborative Care for Depression in Human Immunodeficiency Virus Clinics.” Archives of Internal Medicine 171 (1): 23–31. [DOI] [PubMed] [Google Scholar]
- Pyne, J. M. , Fortney J. C., Mouden S., Lu L., Hudson T. J., and Mittal D.. 2015. “Cost‐Effectiveness of on‐Site Versus off‐Site Collaborative Care for Depression in Rural FQHCs.” Psychiatric Services 66 (5): 491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost, K. , Nutting P., Smith J., Werner J., and Duan N.. 2001. “Improving Depression Outcomes in Community Primary Care Practice: A Randomized Trial of the quEST Intervention. Quality Enhancement by Strategic Teaming.” Journal of General Internal Medicine 16 (3): 143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost, K. , Smith G., and Burnam M.. 1992. “Measuring the Outcomes of Care for Mental Health Problems.” Medical Care 30: MS266–73. [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., and Dunbar G. C.. 1998. “The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM‐IV and ICD‐10.” Journal of Clinical Psychiatry 59 (Suppl 20): 22–33. [PubMed] [Google Scholar]
- Simon, G. E. , Von Korff M., Rutter C., and Wagner E.. 2000. “Randomised Trial of Monitoring, Feedback, and Management of Care by Telephone to Improve Treatment of Depression in Primary Care.” British Medical Journal 320 (7234): 550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, G. E. , Katon W. J., VonKorff M., Unutzer J., Lin E. H., Walker E. A., Bush T., Rutter C., and Ludman E.. 2001. “Cost‐Effectiveness of a Collaborative Care Program for Primary Care Patients with Persistent Depression.” American Journal of Psychiatry 158 (10): 1638–44. [DOI] [PubMed] [Google Scholar]
- Smith, G. R. , Burnam A., Burns B. J., Cleary P. D., and Rost K.. 2000. “Depression Outcomes Module (DOM)” In Handbook of Psychiatric Measures, edited by First M. B., and Ross R., pp. 213–15. Washington, DC: American Psychiatric Association. [Google Scholar]
- Valenstein, M. , Copeland L. A., Blow F. C., McCarthy J. F., Zeber J. E., Gillon L., Bingham C. R., and Stavenger T.. 2002. “Pharmacy Data Identify Poorly Adherent Patients with Schizophrenia at Increased Risk for Admission.” Medical Care 40 (8): 630–9. [DOI] [PubMed] [Google Scholar]
- Ware Jr, J. , Kosinski M., and Keller S. D.. 1996. “A 12‐Item Short‐Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity.” Medical Care 34 (3): 220–33. [DOI] [PubMed] [Google Scholar]
- Wells, K. B. , Sherbourne C., Schoenbaum M., and Keller S. D.. 2000. “Impact of Disseminatig Quality Improvement Programs for Depression in Managed Primary Care: A Randomized Controlled Trial.” Journal of the American Medical Association 283 (2): 212–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


