Abstract
Objective
To characterize spending patterns for Medicare patients with incident breast, prostate, lung, and colorectal cancer.
Data Sources/Study Setting/Study Design
2007–2012 data from the Surveillance, Epidemiology, and End Results Program linked with Medicare fee‐for‐service claims.
Data Collection/Extraction Methods
We calculate per‐patient monthly and yearly mean and median expenditures, by cancer type, stage at diagnosis, and spending category, over the years of diagnosis and death.
Principal Findings
Over the year of diagnosis, mean spending was $35,849, $26,295, $55,597, and $63,063 for breast, prostate, lung, and colorectal cancer, respectively. Over the year of death, spending was similar across different cancer types and stage at diagnosis.
Conclusions
Characterization of Medicare spending according to clinically meaningful categories may assist development of oncology alternative payment models and cost‐effectiveness models.
Keywords: Oncology, Medicare, alternative payment model
Aging of the U.S. population, along with an increasing number of innovative but expensive cancer therapies, have accelerated spending growth in cancer care (Yabroff et al. 2011). Even without accounting for expensive new therapeutics such as immunotherapy, U.S. cancer spending is projected to increase 26 percent from $124 billion in 2010 to $157 billion by 2020 (Mariotto et al. 2011).
The Centers for Medicare & Medicaid Services (CMS) and other payers have sought to combat rising costs by transitioning from traditional fee‐for‐service reimbursement toward payment models that place more responsibility for spending on oncology practices and care systems. CMS recently announced the Oncology Care Model; starting in the summer of 2016, participating oncology practices will receive a monthly per‐patient payment plus additional bonuses if they successfully reduce their Medicare expenses and meet quality benchmarks over 6‐month periods (Centers for Medicare & Medicaid Services 2015). The American Society of Clinical Oncology and private payers have developed their own reimbursement proposals, which would similarly provide monthly payments to providers in exchange for their taking accountability over their patients' costs over defined episodes of care (Clough and Kamal 2015).
The relative merits of these payment proposals are controversial, in part because of our still‐limited understanding of the costs of cancer care as patients transition from diagnosis to survivorship and end of life (Mariotto et al. 2011; Katel et al. 2013; Polite and Miller 2015). Academic efforts to study cancer costs have focused on calculating spending by incidence, prevalence, and phase of care, which divides cancer care into the year of diagnosis, the last year of life, and the intervening period (Riley et al. 1995; Taplin et al. 1995; Fireman et al. 1997; Mariotto et al. 2006; Yabroff, Warren, and Brown 2007; Yabroff et al. 2008, 2011; Katel et al. 2013; Yabroff, Borowski, and Lipscomb 2013). Although these analytic approaches provide an epidemiologic understanding of cancer spending, they are less helpful for researchers, policymakers, and practice managers who need to understand the magnitude of expenditures in a way that fosters their ability to influence spending. They are also of limited utility for cost‐effectiveness researchers who need more precise estimates to construct meaningful models to evaluate the relative value of cancer interventions.
We describe monthly and yearly Medicare fee‐for‐service spending for patients with the four most common cancers—breast, prostate, lung, and colorectal—during the year of diagnosis and the year of death. We stratified results by cancer site, cancer stage at diagnosis, and category of medical service. Establishing expenditure benchmarks for key cancer patient cohorts will facilitate efforts by researchers and policymakers to evaluate and improve the value of cancer care in the United States (Brooks et al. 2013, 2014; Xu et al. 2016).
Methods
We used the National Cancer Institute (NCI)'s Surveillance, Epidemiology, and End Results‐Medicare (SEER‐Medicare) dataset to estimate spending for cancer patients enrolled in Medicare fee‐for‐service. The SEER registry combines clinical data on incident cancer patients from 17 participating regional cancer registries that together cover about 30 percent of the U.S. population or about 86 million people (National Cancer Institute 2015a). For each patient, the SEER registry contains the diagnosis year and month; cancer site, stage, and histology; patient vital status; and cause of death.
Cancer patients in the SEER registry are identified in the Medicare master enrollment file and linked by the National Cancer Institute to their associated Medicare fee‐for‐service claims to create the SEER‐Medicare dataset. These linkages are updated approximately every 2–3 years, and at least 93 percent of SEER patients age 65 or greater have been identified each update within the Medicare claims data. The Medicare files include claims for inpatient, skilled nursing facility, outpatient, hospice, home health, durable medical equipment, and prescription services. The NCI's process for linking SEER data with Medicare fee‐for‐service records has been described previously, and SEER‐Medicare data have been used extensively for research on the economics of cancer care (Warren et al. 2002; National Cancer Institute 2015a). The SEER‐Medicare population has been found to be similar in age, sex, poverty level, and education level as the overall Medicare population (Warren et al. 2002; National Cancer Institute 2015b), and there are no consistent differences between SEER versus non‐SEER regions in terms of overall population demographics, cancer incidence and mortality, and Medicare enrollment (National Cancer Institute 2017). SEER regions do not have consistently higher or lower Medicare prices than non‐SEER regions, although there is heterogeneity within both SEER and non‐SEER regions.
We stratified our analysis into two groups: an incident cohort and a decedent cohort. The incident cohort included patients diagnosed with breast, prostate, lung, or colorectal cancer between January 1, 2007, and December 31, 2011. The decedent cohort included all members of the incident cohort who died prior to December 31, 2012, regardless of the cause of death. Patients were excluded if they (1) had a previous cancer diagnosis, (2) had non‐invasive cancer or missing stage information at diagnosis, or (3) were not continuously enrolled in fee‐for‐service Medicare from diagnosis until either death or December 31, 2012, whichever came first.
For the incident cohort, we calculated mean per‐patient Medicare spending over the year of diagnosis by summing Medicare spending from the month of diagnosis to the 11th month after diagnosis and averaging the result, regardless of when the patient died. Patients contributed $0 after death. We also calculated mean monthly spending from the month before diagnosis to the 11th month after diagnosis; patients who died sooner than 11 months after diagnosis were included in monthly spending calculations up to their month of death and censored from calculations thereafter.
For the decedent cohort, we calculated mean per‐patient Medicare spending over the year of death by summing Medicare spending in the month of death and the 11 preceding months regardless of the date of the patient's cancer diagnosis. We also calculated mean monthly spending for the 12 months leading up to death; patients were included in monthly spending calculations only if they carried a cancer diagnosis in that month. Patients who died less than a year from cancer diagnosis were thus included in both the incident and decedent cohorts over the same months.
For the decedent cohort, all deaths, including noncancer deaths, were included, because cause of death is not reliably coded on death certificates and because under proposed alternative payment models, oncologists are responsible for the spending and clinical outcomes of their patients regardless of the ultimate cause of death. We conducted sensitivity analyses comparing incident and decedent spending associated with patients with cancer‐related versus non‐cancer‐related causes of death.
We stratified cancer spending estimates for both the incident and decedent according to cancer type, stage at diagnosis, and Medicare spending category. Our spending estimates represent Medicare reimbursements, including for both cancer‐related and noncancer‐related services and exclude patient copayments and payments from supplemental insurance. Reimbursements were reported in 2013 U.S. dollars (Federal Hospital Insurance Medical Insurance Trust Funds 2012). Stage I and II prostate cancers were grouped as a single category because the SEER registry typically classifies them together as localized disease (National Cancer Institute 2016). Otherwise, stage was categorized according to the American Joint Committee on Cancer version 7 criteria as recorded in SEER based on the best available report, irrespective of neoadjuvant treatment.
Results
The incident cohort consisted of 238,795 Medicare fee‐for‐service patients who were diagnosed with breast (n = 52,279), prostate (n = 74,480), lung (n = 68,010), and colorectal (n = 44,026) cancer. Of these patients, 95,178 (40 percent) died during the study period and were included in the decedent cohort: 10,262 or 20 percent of incident breast cancer patients, 10,523 or 14 percent of incident prostate cancer patients, 54,305 or 80 percent of incident lung cancer patients, and 20,088 or 46 percent of incident colorectal cancer patients. Age, race/ethnicity, sex, and stage at diagnosis distributions for the incident and decedent cohorts appear in Table 1.
Table 1.
Characteristics of the Incident and Decedent Cohorts, by Type of Cancer
| Breast Cancer | Prostate Cancer | Lung Cancer | Colorectal Cancer | |||||
|---|---|---|---|---|---|---|---|---|
| Incident Cohort (N = 52,279) | Decedent Cohort (N = 10,262) | Incident Cohort (N = 74,480) | Decedent Cohort (N = 10,523) | Incident Cohort (N = 68,010) | Decedent Cohort (N = 54,305) | Incident Cohort (N = 44,026) | Decedent Cohort (N = 20,088) | |
| Age | ||||||||
| <65 years | 8.0% | 8.0% | 6.2% | 4.7% | 9.6% | 9.6% | 6.8% | 6.5% |
| >=65 years | 92.0% | 92.0% | 93.8% | 95.3% | 90.4% | 90.4% | 93.2% | 93.5% |
| Mean (SD) | 74.2 (9.0) | 77.7 (10.1) | 72.6 (6.9) | 76.6 (8.0) | 73.8 (8.2) | 74.1 (8.4) | 76.4 (9.2) | 78.1 (9.5) |
| Racea | ||||||||
| White | 86.6% | 84.3% | 81.2% | 80.2% | 85.7% | 85.3% | 84.8% | 84.0% |
| Black | 8.7% | 12.5% | 11.7% | 14.7% | 9.6% | 10.0% | 9.4% | 10.9% |
| Asian | 3.8% | 2.6% | 3.4% | 3.2% | 4.2% | 4.2% | 5.0% | 4.4% |
| Other | 0.9% | 0.6% | 3.7% | 1.9% | 0.5% | 0.5% | 0.9% | 0.6% |
| Ethnicity | ||||||||
| Hispanic | 6.6% | 6.0% | 9.9% | 8.0% | 4.9% | 4.9% | 7.2% | 6.8% |
| Sex | ||||||||
| Female | 99.0% | 98.5% | 0% | 0% | 47.4% | 45.4% | 52.3% | 51.8% |
| Male | 1.0% | 1.5% | 100% | 100% | 52.6% | 54.6% | 47.7% | 48.2% |
| Stage at Diagnosisb | ||||||||
| Stage I | 51.1% | 25.8% | 0.2% | 0.3% | 21.2% | 12.1% | 25.4% | 15.7% |
| Stage II | 31.8% | 31.6% | 87.0% | 71.9% | 4.6% | 3.8% | 28.7% | 21.3% |
| Stage III | 10.5% | 18.9% | 6.4% | 3.2% | 26.9% | 28.1% | 26.0% | 25.7% |
| Stage IV | 6.5% | 23.7% | 6.4% | 24.6% | 47.4% | 56.0% | 19.9% | 37.3% |
Race categories were defined to be mutually exclusive.
Patients with unstaged or stage 0 cancer were excluded. Stage I and II prostate cancers were grouped as a single category because the SEER registry frequently classifies them together as localized disease (20).
Over the year of diagnosis, mean per‐patient annual Medicare spending varied substantially by cancer type: $35,849 for breast cancer, $26,295 for prostate cancer, $55,597 for lung cancer, and $63,063 for colorectal cancer (Figure 1A). More advanced stage at diagnosis was associated with higher annual spending for breast, prostate, and colorectal cancer (Figure 1B). Over the year of death, mean per‐patient annual spending was more consistent across cancer types: Breast cancer patients had an average of $61,429 in annual spending, compared to prostate ($62,351), lung ($59,912), and colorectal ($72,883) (Figure 1A).
Figure 1.
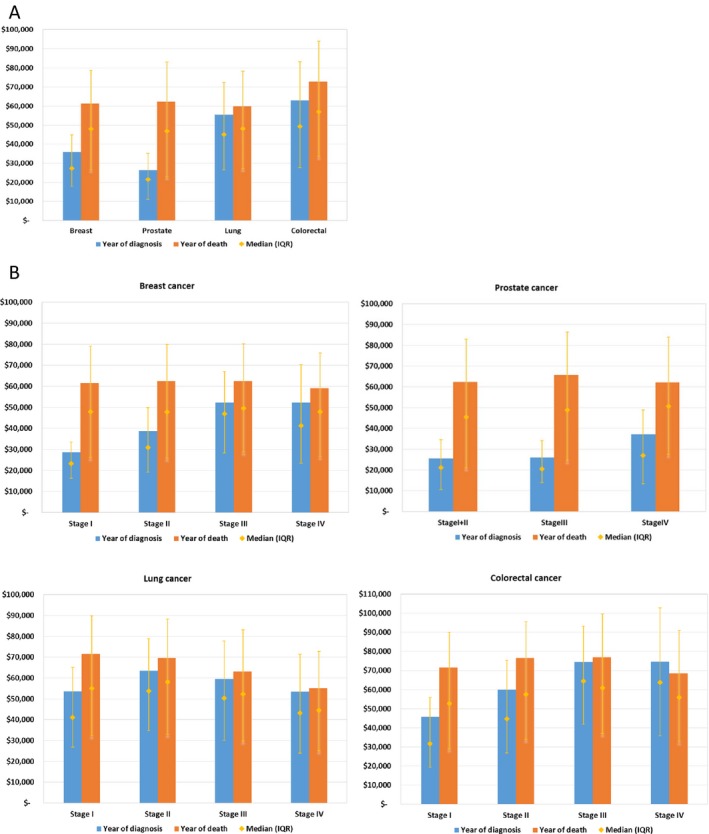
Mean and Median Annual Per‐Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer in the Year of Diagnosis and in the Year of Death [Color figure can be viewed at http://wileyonlinelibrary.com]
Note: (A) The height of the columns represents mean spending. The orange diamond represents the median spending, with the corresponding orange whiskers representing the 25th to 75th interquartile range (IQR). Mean spending in the year of diagnosis included the 12 months beginning with the month of diagnosis, regardless of the month of death (patients contribute $0 after death). Mean spending in the year of death included the 11 months prior to death and the month of death, regardless of the timing of the cancer diagnosis. (B) Mean and median annual per‐patient Medicare spending for breast, prostate, lung, and colorectal cancer in the year of diagnosis and in the year of death, stratified by stage at diagnosis.
For the incident cohort, mean per‐patient monthly spending peaked 1 month after diagnosis for all stages of breast, lung, and colorectal cancer and subsequently declined over the next 2–4 months before reaching a plateau for the rest of the year (Figure 2). Among prostate cancer patients with stage I–III disease, mean per‐patient monthly spending rose until month 4 and then slowly declined. Monthly spending was more consistent in the decedent cohort: Across all cancer types and stages, mean per‐patient monthly spending climbed steadily in the 6 months leading up to death, with the month of death universally associated with the highest spending levels (Figure 3).
Figure 2.
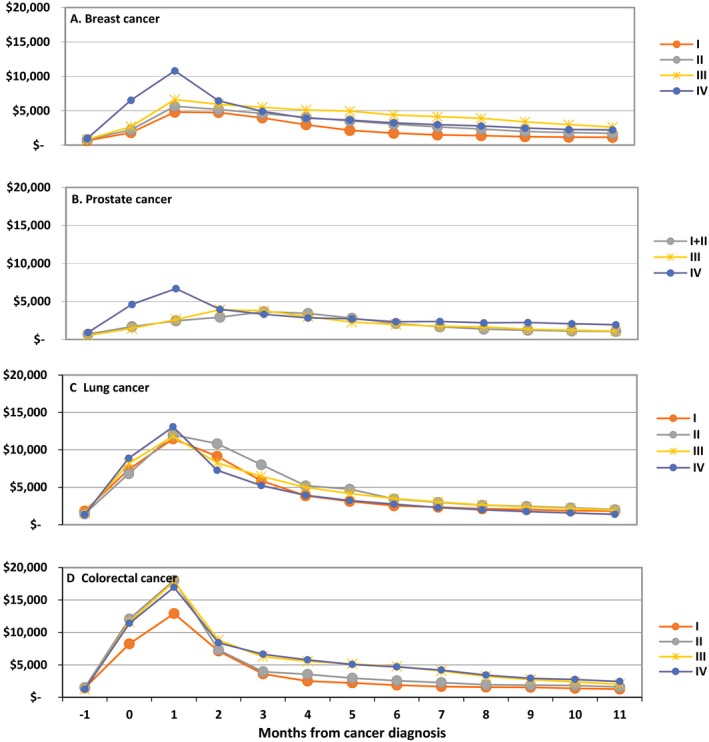
Mean Monthly Per‐Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer, Extending from the Month Prior to Diagnosis (month −1) Through the 11th Month After Diagnosis (month 11), by Stage at Diagnosis (I, II, III, or IV) [Color figure can be viewed at http://wileyonlinelibrary.com]
Note: Month 0 represents the month of diagnosis. Patients were included in monthly spending calculations up to the month of death and then subsequently censored from calculations. These monthly figures include a 13‐month period (rather than the 12‐month period for annual spending) to better reflect spending trends.
Figure 3.
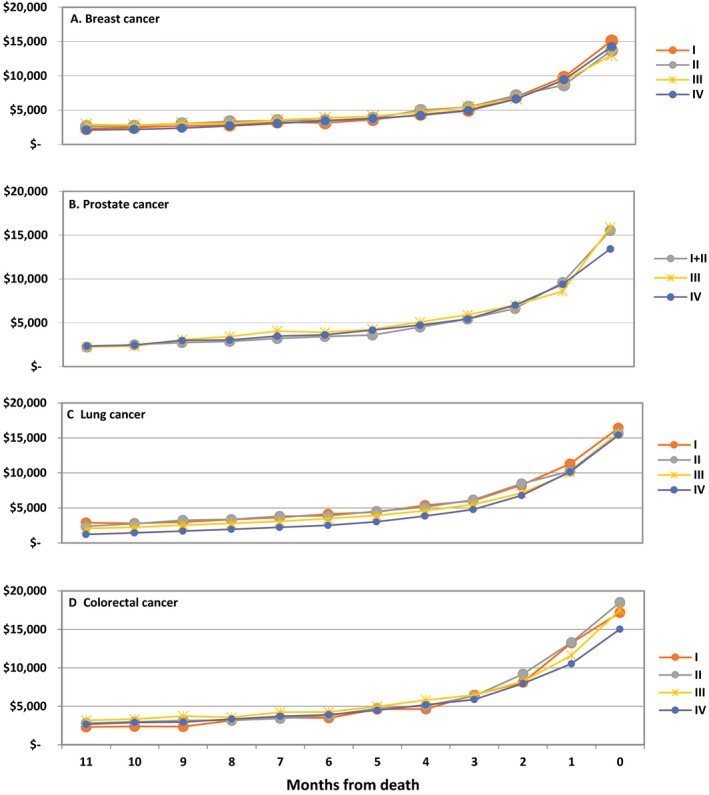
Mean Monthly Per‐Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer Extending from the 11th Month Prior to Death (month 11) Through the Month of Death (month 0), by Stage at Diagnosis (I, II, III, or IV) [Color figure can be viewed at http://wileyonlinelibrary.com]
Note: Patients were included in the monthly spending calculations only if they had been diagnosed with cancer.
Over the year of diagnosis, inpatient services accounted for 50 percent of lung and 58 percent of colorectal cancer spending but only 22 percent of breast and 25 percent of prostate cancer spending (Figure 4). Outpatient costs were responsible for the majority of initial spending among patients newly diagnosed with breast and prostate cancer (66 percent for both). Over the year of death, the distribution of spending categories was much more similar across cancer types: For the four cancers, inpatient services accounted for 52–60 percent of spending, outpatient services consisted of 27–33 percent, and hospice was responsible for 5–7 percent. Part D prescription drugs accounted for <5 percent of spending. Categorization of monthly costs shows that the steep rise in spending as patients approach death is attributable primarily to increases in inpatient spending (Figure S1a, b). It is important to note that inpatient medications billed under Medicare Part A are included in the inpatient spending category, and cancer prescription medications, including oral chemotherapy, billed under Medicare Part B are included in outpatient spending estimates.
Figure 4.
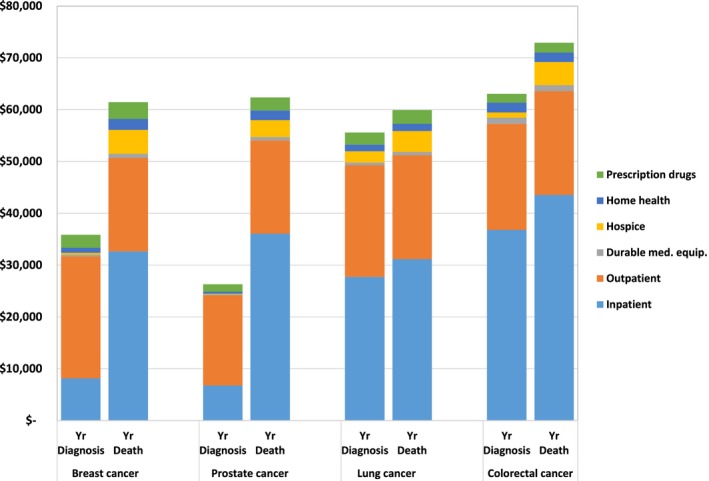
Mean Annual Per‐Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer in the Year of Diagnosis and Year of Death, by Category of Spending [Color figure can be viewed at http://wileyonlinelibrary.com]
Note: Mean spending in the year of diagnosis included the 12 months beginning with the month of diagnosis, regardless of the month of death (patients contribute $0 after death). Mean spending in the year of death included the 11 months prior to death and the month of death, regardless of when the patient was diagnosed. Categories of spending were defined by Medicare claims. Inpatient and outpatient spending included drug spending billed under Part A and Part B, respectively. Prescription drug spending refers to spending under Medicare Part D.
We created Lorenz curves to describe the population variance in spending by type of cancer (Figure S2). Over both the year of diagnosis and the year of death, patients in the top spending decile accounted for 28–32 percent of all spending and the top 50 percent accounted for 75–82 percent of spending. A sensitivity analysis stratified per‐year spending associated with incident cohort patients by those who died of cancer within the year of diagnosis, those who died of noncancer causes within the year of diagnosis, and those who survived the year (Figure S3). We also calculated spending by decedent cohort patients who died of a cancer‐related versus a non‐cancer‐related cause (Figure S4).
Discussion
We characterize monthly and yearly spending on Medicare fee‐for‐service patients with the four most common cancers during the year of diagnosis and the year of death. Our analysis reveals that in the months after diagnosis, lung and colorectal cancer patients incurred higher spending than breast and prostate cancer patients and that more advanced stage at diagnosis was generally associated with higher spending. Although the magnitude of spending varied between cancer types and stages, the majority of patient cohorts followed the same monthly spending pattern: They experienced an initial increase in spending in the first several months after diagnosis, after which costs started to decline and then plateau. This spending bump in the first several months likely represents the costs associated with initial diagnostic testing and staging studies as well as the cost of initial hospital care, including surgical interventions. Over the year of death, both the monthly spending magnitudes and patterns were strikingly similar across cancer types and stages: In the 6 months before death, costs rise steeply, with the month of death uniformly associated with the highest costs.
Inpatient care accounted for the significant majority of the initial spending increase in the months after diagnosis for lung and colorectal cancer patients, whereas outpatient spending was the biggest source of spending for breast and prostate cancer patients. However, over the months leading up to death, inpatient care was the largest source of spending for all four cancers; in particular, the exponential rise in spending across all four cancers in the months approaching death was almost entirely driven by an acceleration in inpatient spending (Figure S4b), without a substantial change in the trend of other spending categories. This is consistent with previous research demonstrating that hospital services are the largest category of spending and most important source of geographic variation in spending among Medicare fee‐for‐service patients with advanced cancer (Brooks et al. 2014).
Previous characterization of cancer costs using the SEER‐Medicare data relies primarily on the phase‐of‐care method that calculates per‐patient cost in three distinct categories: the initial phase of treatment, the continuing phase, and the final phase of life (Riley et al. 1995; Taplin et al. 1995; Fireman et al. 1997; Yabroff et al. 2008, 2011). The phases of care approach are valuable for researchers constructing cost‐effectiveness models but complicated by the fact that the same phases for different patients can vary significantly in length, depending on patient survival after diagnosis. Cost estimates stratified by phases of care thus have limited utility for policy makers, researchers, and health systems preparing for new bundled payment initiatives in oncology that typically require oncology providers to be accountable for the costs of their patients over fixed, predefined episodes of time in exchange for monthly fees and bonuses (Newcomer et al. 2014; American Society for Clinical Oncology 2015; Centers for Medicare & Medicaid Services 2015). For example, the American Society for Clinical Oncology's Patient‐Centered Oncology Payment proposal would create new billing codes that provide a monthly payment for care management and treatment planning services. CMS has chosen to base its Oncology Care Model on 6‐month episodes of care triggered by chemotherapy administration (Centers for Medicare & Medicaid Services 2015). The monthly spending patterns described here provide estimates that inform the design of such initiatives. Our data may also be useful as updated cost‐of‐illness inputs for cost‐effectiveness researchers seeking to create models to evaluate the relative value of cancer interventions.
Our study has a number of limitations. First, we studied Medicare beneficiaries with cancer enrolled in traditional fee‐for‐service plans, so our results may not generalize to the approximately 27 percent of Medicare beneficiaries enrolled in Medicare Advantage plans in 2012 (Jacobson et al. 2015). Second, Medicare payments to hospitals are adjusted for geographic variations in labor prices and hospital characteristics; if hospitals in SEER regions receive different geographic adjustments than non‐SEER hospitals, our data may be not representative of non‐SEER regions. Third, we do not capture decedent spending by patients who were diagnosed with cancer during our study period but survived beyond the study period, who may represent a different spending profile.
A description of monthly and yearly Medicare spending per patient stratified by cancer type, stage at diagnosis, and spending category provides researchers and policy makers with a clear picture of oncology spending trajectories. These estimates will help policy makers design alternative payment and cost‐effectiveness models for cancer care.
Supporting information
Appendix SA1. Author Matrix.
Figure S1. (a) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer, Extending from the Month Prior to Diagnosis (Month −1) through the 11th Month after Diagnosis (Month 11), by Category of Spending. (b) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer Extending from the 11th Month Prior to Death (Month 11) through the Month of Death (Month 0), by Category of Spending.
Figure S2. Lorenz Curves for Mean Annual Medicare Spending in the Year of Diagnosis and Year of Death, by Type of Cancer.
Figure S3. Mean Annual per Patient Medicare Spending in the Year of Diagnosis, among Patients Who Died of Cancer within 12 Months, Who Died of Another Cause within 12 Months, or Who Survived 12 Months.
Figure S4. Mean Annual per Patient Medicare Spending in the Year of Death, Stratified by Cause of Death (Cancer Versus Non‐Cancer).
Table S1. (a) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer, Extending from the Month Prior to Diagnosis (Month −1) Through the 11th Month after Diagnosis (Month 11), by Stage at Diagnosis. (b) Mean Monthly Per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer Extending from the 11th Month Prior to Death (Month 11) through the Month of Death (Month 0), by Stage at Diagnosis.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Funding for this project was received through an award from the California HealthCare Foundation to DS. The funder had no role in the drafting, editing, or final approval for this manuscript.
Disclosure: None.
Disclaimer: None.
References
- American Society for Clinical Oncology . 2015. “Patient‐Centered Oncology Payment: Payment Reform to Support Higher Quality, More Affordable Cancer Care” [accessed on January 12, 2016]. Available at http://www.asco.org/sites/www.asco.org/files/asco_patient-centered_oncology_payment_final_2.pdf
- The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. “2012 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds” April 23, 2012 [accessed on January 12, 2016]. Available at https://www.treasury.gov/resource-center/economic-policy/ss-medicare/Documents/TR_2012_Medicare.pdf
- Brooks, G. A. , Li L., Sharma D. B., Weeks J., Hassett M., Yabroff K., and Schrag D.. 2013. “Regional Variation in Spending and Survival for Older Adults with Advanced Cancer.” Journal of the National Cancer Institute 105: 634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, G. A. , Li L., Uno H., Hassett M., Landon B., and Schrag D.. 2014. “Acute Hospital Care Is the Chief Driver of Regional Spending Variation in Medicare Patients with Advanced Cancer.” Health Affairs 33: 1793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services . “Oncology Care Model [CMS web site]” November 13, 2015 [accessed on December 6, 2015]. Available at http://innovation.cms.gov/initiatives/oncology-care/
- Clough, J. D. , and Kamal A. H.. 2015. “Oncology Care Model: Short‐ and Long‐Term Considerations in the Context of Broader Payment Reform.” Journal of Oncology Practice 11: 319–21. [DOI] [PubMed] [Google Scholar]
- Fireman, B. H. , Quesenberry C. P., Somkin C. P., Jacobson A., Baer D., West D., Potosky A., and Brown M.. 1997. “Cost of Care for Cancer in a Health Maintenance Organization.” Health Care Financing Review 18: 51–76. [PMC free article] [PubMed] [Google Scholar]
- Jacobson, G. , Damico A., Neuman T., and Gold M.. “Medicare Advantage 2015 Spotlight: Enrollment Market Update. Kaiser Family Foundation 2015” [published on June 30, 2015]. Available at http://kff.org/medicare/issue-brief/medicare-advantage-2015-spotlight-enrollment-market-update/
- Katel, K. K. , Morin A. J., Nadel J. L., and McClellan M. B.. 2013. Meaningful Physician Payment Reform in Oncology. Journal of Oncology Practice 9: 49s–53s. [DOI] [PubMed] [Google Scholar]
- Mariotto, A. B. , Yabroff J. R., Feuer E. J., De Angelis R., and Brown M.. 2006. “Projecting the Number of Patients with Colorectal Carcinoma by Phases of Care in the US: 2000–2020.” Cancer Causes & Control 17: 1215–26. [DOI] [PubMed] [Google Scholar]
- Mariotto, A. B. , Yabroff K. R., Shao Y., Feuer E., and Brown M.. 2011. “Projections of the Cost of Cancer Care in the United States: 2010–2020.” Journal of the National Cancer Institute 103: 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program . 2015a. “SEER Bibliography” [accessed on December 11, 2015]. Available at: http://seer.cancer.gov/pubsearch/
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program . 2015b. “Overview of the SEER Program,” October 15, 2015 [accessed on December 11, 2015]. Available at http://seer.cancer.gov/about/overview.html
- National Cancer Institute: SEER Training Modules . 2016. “Prostate Staging Schemes” [accessed on January 12, 2016]. Available at http://training.seer.cancer.gov/staging/systems/schemes/prostate.html
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program . 2017. “Population Characteristics” [accessed on January 31, 2017]. Available at https://seer.cancer.gov/registries/characteristics.html
- Newcomer, L. N. , Gould B., Page R. D., Donelan S., and Perkins M.. 2014. “Changing Physician Incentives for Affordable, Quality Cancer Care: Results of an Episode Payment Model.” Journal of Oncology Practice 10: 322–6. [DOI] [PubMed] [Google Scholar]
- Polite, B. N. , and Miller H. D.. 2015. “Medicare Innovation Center Oncology Care Model: A Toe in the Water When a Plunge is Needed.” Journal of Oncology Practice [published online on February 17, 2015] Available at http://jop.ascopubs.org/content/early/2015/02/17/JOP.2014.002899.full [DOI] [PubMed] [Google Scholar]
- Riley, G. F. , Potosky A. L., Lubitz J. D., and Kessler L. G.. 1995. “Medicare Payments from Diagnosis to Death for Elderly Cancer Patients by Stage and Diagnosis.” Medical Care 33: 828–41. [DOI] [PubMed] [Google Scholar]
- Taplin, S. H. , Barlow W., Urban N., Mandelson M., Timlin D. J., Ichikawa L., and Nefcy P.. 1995. “Stage, Age, Comorbidity, and Direct Costs of Colon, Prostate, and Breast Cancer Care.” Journal of the National Cancer Institute 87: 417–26. [DOI] [PubMed] [Google Scholar]
- Warren, J. L. , Klabunde C. N., Schrag D., Bach P., and Riley G.. 2002. “Overview of the SEER‐Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population.” Medical Care 40 (8 Suppl): 3–18. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Herrin J., Soulos P. R., Saraf A., Robers K., Killelea B., Wang S., Long J., Wang R., Ma X., and Gross C.. 2016. “The Role of Patient Factors, Cancer Characteristics, and Treatment Patterns in the Cost of Care for Medicare Beneficiaries with Breast Cancer.” Health Services Research 51 (1): 167–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff, R. K. , Borowski L., and Lipscomb J.. 2013. “Economic Studies in Colorectal Cancer: Challenges in Measuring and Comparing Costs.” Journal of the National Cancer Institute Monographs 46: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff, J. R. , Warren J. L., and Brown M. L.. 2007. “Costs of Cancer Care in the USA: A Descriptive Review.” Nature Clinical Practice Oncology 4: 643–56. [DOI] [PubMed] [Google Scholar]
- Yabroff, K. R. , Lamont E. B., Mariotto A., Warren J. L., Topor M., Meekins A., and Brown M.. 2008. “Cost of Care for Elderly Cancer Patients in the United States.” Journal of the National Cancer Institute 100: 630–41. [DOI] [PubMed] [Google Scholar]
- Yabroff, K. R. , Kund J., Kepka D., and Mariotto A.. 2011. “Economic Burden of Cancer in the United States: Estimates, Projections, and Future Research.” Cancer Epidemiology, Biomarkers & Prevention 20: 2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1. Author Matrix.
Figure S1. (a) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer, Extending from the Month Prior to Diagnosis (Month −1) through the 11th Month after Diagnosis (Month 11), by Category of Spending. (b) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer Extending from the 11th Month Prior to Death (Month 11) through the Month of Death (Month 0), by Category of Spending.
Figure S2. Lorenz Curves for Mean Annual Medicare Spending in the Year of Diagnosis and Year of Death, by Type of Cancer.
Figure S3. Mean Annual per Patient Medicare Spending in the Year of Diagnosis, among Patients Who Died of Cancer within 12 Months, Who Died of Another Cause within 12 Months, or Who Survived 12 Months.
Figure S4. Mean Annual per Patient Medicare Spending in the Year of Death, Stratified by Cause of Death (Cancer Versus Non‐Cancer).
Table S1. (a) Mean Monthly per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer, Extending from the Month Prior to Diagnosis (Month −1) Through the 11th Month after Diagnosis (Month 11), by Stage at Diagnosis. (b) Mean Monthly Per Patient Medicare Spending for Breast, Prostate, Lung, and Colorectal Cancer Extending from the 11th Month Prior to Death (Month 11) through the Month of Death (Month 0), by Stage at Diagnosis.


