Abstract
Objective
To examine associations between clinics’ extent of patient‐centered medical home (PCMH) implementation and improvements in chronic illness care quality.
Data Source
Data from 808 Veterans Health Administration (VHA) primary care clinics nationwide implementing the Patient Aligned Care Teams (PACT) PCMH initiative, begun in 2010.
Design
Clinic‐level longitudinal observational study of clinics that received training and resources to implement PACT. Clinics varied in the extent they had PACT components in place by 2012.
Data Collection
Clinical care quality measures reflecting intermediate outcomes and care processes related to coronary artery disease (CAD), diabetes, and hypertension care were collected by manual chart review at each VHA facility from 2009 to 2013.
Findings
In adjusted models containing 808 clinics, the 77 clinics with the most PACT components in place had significantly larger improvements in five of seven chronic disease intermediate outcome measures (e.g., BP < 160/100 in diabetes), ranging from 1.3 percent to 5.2 percent of the patient population meeting measures, and two of eight process measures (HbA1c measurement, LDL measurement in CAD) than the 69 clinics with the least PACT components. Clinics with moderate levels of PACT components showed few significantly larger improvements than the lowest PACT clinics.
Conclusions
Veterans Health Administration primary care clinics with the most PCMH components in place in 2012 had greater improvements in several chronic disease quality measures in 2009–2013 than the lowest PCMH clinics.
Keywords: Patient‐centered medical home, primary care, chronic disease, quality of care
The patient‐centered medical home (PCMH) model of primary care practice (Kellerman and Kirk 2007; American Academy of Family Physicians 2008) is being implemented in multiple health care settings nationwide (Edwards et al. 2014). The PCMH model focuses on restructuring primary care to increase timely access, continuity of care, and multidisciplinary teamwork, with the goal of making care more comprehensive and patient‐centered, and ultimately to improve the quality of care that patients receive. The PCMH model uses the Chronic Care Model as a framework (Coleman et al. 2009), and it is expected to improve quality of care, especially for patients with common chronic conditions who incur a disproportionate share of health expenditures and health management challenges (Paez, Zhao, and Hwang 2009). However, recent reviews have reported mixed results on the association between PCMH implementation and changes in clinical quality (Jackson et al. 2013; Nielsen et al. 2016). Many of these PCMH studies are limited to a specific geographical area, a short time frame, or focus on a single medical condition.
In 2010, the VHA launched a PCMH initiative called Patient Aligned Care Teams (PACT) in VHA's large, diverse network of over 800 primary care clinics that care for over 5 million enrolled veterans (Rosland et al. 2013). Key components of the PACT model include patient assignment to a “teamlet” (Bodenheimer and Laing 2007) that provides multidisciplinary continuity to a panel of approximately 1,200 patients per full‐time equivalent primary care provider (PCP), increased access through open‐access scheduling and non‐face‐to‐face modes of care, and nurse‐led panel management. All VHA primary care clinics were given similar resources, tools, and access to training to support PACT implementation, but clinics varied in the speed and extent to which they were able to put PACT components in place (Nelson et al. 2014).
It remains unknown whether clinical quality improved following the start of PACT implementation in April 2010. A previous cross‐sectional study that validated a measure of PACT implementation reported that VHA clinics with more PACT components in place had higher levels of chronic disease quality measures in the same year as measurement of PACT implementation (Nelson et al. 2014). However, that study did not examine whether those PACT clinics also had high clinical quality prior to PACT, and how much clinic quality improved with the implementation of PACT. In this study, we examined whether the extent to which clinics had PACT components in place in 2012 was associated with improvements in care quality for patients with chronic conditions from 2009 to 2013.
Methods
Study Design
We conducted a retrospective longitudinal VHA‐wide clinic‐level analysis evaluating clinic‐level associations between extent of PACT medical home components in place in 2012 and changes in chronic disease clinical quality measures from 2009 (pre‐PACT) to 2013 (during PACT implementation). Data on patient characteristics were obtained from the VHA Corporate Data Warehouse (Fihn et al. 2014). Data on clinic‐level performance in chronic disease quality were obtained from the VHA's External Peer Review Program (EPRP). These evaluation efforts are part of ongoing quality improvement programs at the VHA and are not considered research activity; thus, they were exempted from IRB review.
Study Sample
We initially identified 817 VHA clinics reporting clinical quality data in EPRP databases in 2009 and 2013. The quality indicators we examined are mandated by VHA to be collected from most VHA clinics, so there were very few primary care sites in the VHA system that were not represented in this study. Seven clinics were missing a Rural–Urban Continuum Code, due to being located in U.S. territories, and two were mobile clinics and unable to be linked to a particular county's census data; these nine clinics were excluded from analyses.
Clinical Quality Measures
Veterans Health Administration External Peer Review Program clinical quality measures closely resemble NCQA Health care Effectiveness and Data Information Set (HEDIS) measures. Medical records were randomly selected at each VHA clinic from patients assigned to the clinic who meet the quality measure's denominator criteria. Elderly patients (defined as over 75 or 85 years old depending on the measure), those with specific terminal illnesses or enrolled in hospice, and those only receiving behavioral health care in VHA (while explicitly receiving primary care in a non‐VHA setting) are excluded from all measures. Stringent denominator criteria for each measure help to ensure that only patients clinically appropriate to be targeted for that measure are examined. For example, for diabetes measures, patients with gestational diabetes or steroid‐induced hyperglycemia are excluded (see Table S1 for full details on measure numerator and denominator criteria). Medical records are then manually examined by an independent external contractor for adherence to the quality measure, which is calculated as the percent of patients evaluated that received measure‐adherent care or met a clinical outcome threshold. The median number of patients examined per clinic for each measure ranged from 15 to 73 (see Table S3 for details).
We identified 15 VHA outpatient care quality measures related to chronic disease management that were available from 2009 to 2013, including seven clinical outcome and eight clinical process measures among patients with coronary artery disease (CAD), diabetes mellitus (DM), and hypertension (HTN). Specifically the outcome measures included the following: LDL < 100 among patients with CAD, LDL < 100 among patients with DM, BP < 160/100 and BP < 140/90 among patients with DM, HbA1c < 9 percent among patients with DM, and BP < 160/100 and BP < 140/90 among patients with HTN. The process measures included the following: LDL measured in the last year, and aspirin prescription, among patients with CAD; HbA1c measured in the last year, aspirin prescription, foot exam, retinal exam, renal function testing, and ACE‐inhibitor/ARB prescription among patients with DM (see Table S1 for details on all EPRP measures examined).
PCMH Implementation Measure
The extent of PACT implementation in each primary care clinic by 2012 was measured with the previously developed PACT Implementation Index (Pi2) (Nelson et al. 2014). The Pi2 combines 53 PCMH measures derived from clinical encounter data, patient surveys, and primary care frontline staff surveys. These items represent eight domains that reflect the main components of PACT: access, continuity, coordination, team‐based care, comprehensiveness of care, self‐management support, patient‐centered communication, and shared decision making (see Table S2 for individual items, detailed sources, and response characteristics). To combine data from disparate sources for each clinic, a mean facility‐level response for each Pi2 variable was standardized by subtracting the national level mean from the facility mean and dividing by the standard deviation (SD) for all facilities. Domain scores were created for each site by taking the average of these standardized responses. Sites then received +1 point if they were in the top 25th percentile for that domain or −1 point if they were in the bottom 25th percentile. Therefore, clinic Pi2 scores range from +8 (all domain scores in the top quartile) to −8 (all domain scores in the bottom quartile). Further details on Pi2 scoring and variation among clinics in specific Pi2 domains can be found in Table S2 and have been previously described in the literature (Nelson et al. 2014). For main analyses, clinics were analyzed in five previously used Pi2 categories: +8 to +5, +4 to +2, +1 to −1, −2 to −4, and −5 to −8. In alternate analyses, Pi2 was analyzed as a continuous variable ranging from +8 to −8. The Pi2 score was not able to be calculated prior to 2012, as all component data elements were not available prior to that time.
Analysis
We first describe clinic characteristics and quality scores across categories of PACT implementation. To determine if high‐ and low‐scoring clinics had differing quality pre‐PACT, we used t‐tests to compare group means. To examine the adjusted associations between level of PACT implementation and changes in clinical quality between 2009 and 2013, we used multivariable linear regression models with the 2013 quality indicator as the outcome and the baseline 2009 (pre‐PACT) score as a covariate. This method allowed us to interpret coefficients similarly to a difference model and to account for possible ceiling effects for measures starting at high 2009 levels of quality (Newsom, Jones, and Hofer 2012; Zhang et al. 2014). Multivariable models were adjusted for clinic rurality and type (hospital‐based vs. community‐based). Clinic location was designated as rural or urban based on Rural–Urban Continuum codes from the USDA Economic Research Service (West et al. 2010). We defined “clinic type” as one three‐category variable due to collinearity between rurality and clinic type: urban hospital‐based, urban community, or rural community. The number of patients enrolled in a clinic was collinear with both clinic rurality and type, so it was not included in multivariable models. Reflecting VA‐wide goals to maintain PCP panel sizes at 1,200 patients per full‐time PCP, full‐time PCP panel sizes per varied little across Pi2 category, so panel size was not included in models. We adjusted for the 2012 unemployment rate in the county of the clinic's location, as a measure of clinic area socioeconomic status (SES), because the extent to which a change in health care could lead to changes in chronic disease control may be influenced by the resources of the surrounding community (Kington and Smith 1997; Markovitz et al. 2015). After models were estimated for all 808 clinics with available data, we calculated model‐based predictions of change for the highest (Pi2 = +5 to +8, 77 clinics) and lowest (Pi2 = −8 to −5, 69 clinics) quintiles of Pi2 scores to illustrate the magnitude and direction of quality measure change among the highest and lowest Pi2 clinics.
We conducted two sensitivity analyses. First, we adjusted for 2009 clinic‐level ambulatory care‐sensitive (ACS) (Canadian Institute for Health Information, 2016) hospitalization rates (encompassing VA or fee‐for‐service Medicare billed hospitalizations) for clinic‐assigned patients as a proxy for other unobserved clinic‐level confounding factors that could lead to higher quality of care, such as clinic structure or leadership. Second, the distributions of quality scores for clinical processes were skewed (with many clinics at high performance levels), so we examined regression models using log‐transformed quality indicators. Results were similar to the original results and not shown here (available from authors upon request).
Role of the Funding Source
This study was funded by the VA Office of Primary Care Services and Office of Primary Care Operations. The funders had no role in the design or conduct of the study, or the preparation of the manuscript.
Results
Clinic Characteristics
Among the 808 clinics included in our analyses, 8.5 and 9.5 percent were in the highest (+5 to +8) and lowest (−8 to −5) Pi2 categories, respectively (Table 1). Clinics in the highest and lowest Pi2 categories were less likely to be hospital‐based (10 and 12 percent hospital‐based, respectively) than those in the middle categories. The clinics in the highest Pi2 category had the lowest mean number of patients per clinic (3685) and were most likely to be rural clinics. The clinics in the middle Pi2 categories had the highest mean number of patients and were least likely to be rural. The mean age of clinic patients, proportion of women, and county‐level unemployment rates showed little variation across Pi2 category.
Table 1.
Characteristics of Included Primary Care Clinics by PACT Implementation Index (Pi2) Category
| Characteristic | All Clinics | Clinics by Pi2 Categorya | ||||
|---|---|---|---|---|---|---|
| +5 to +8 | +2 to +4 | −1 to +1 | −4 to −2 | −8 to −5 | ||
| N clinics | 808 | 69 | 190 | 310 | 162 | 77 |
| Clinic type | ||||||
| Hospital‐based | 18% | 10% | 13% | 25% | 15% | 12% |
| Urban CBOC | 54% | 46% | 52% | 54% | 60% | 60% |
| Ruralb CBOC | 28% | 44% | 35% | 21% | 25% | 28% |
| Mean no. of patients assigned to clinicc | 6,419 | 3,685 | 4,773 | 7,936 | 6,965 | 5,725 |
| Mean age (years) of assigned patientsd | 64 | 65 | 65 | 64 | 64 | 64 |
| Mean % female of assigned patients | 5% | 6% | 5% | 6% | 6% | 6% |
| 2012 County unemployment ratee | 7.9% | 7.9% | 7.6% | 7.8% | 8.2% | 8.3% |
Pi2 Category = Patient Aligned Care Teams (PACT) Implementation Index, ranging from +8 (most extensive PACT implementation) to −8 (least extensive PACT implementation).
”Rural” defined as nonmetro counties according to Rural–Urban Continuum codes produced by USDA Economic Research Service in 2012.
The VHA assigns patients to a specific Primary Care Provider at a specific VA clinic; “patients assigned to clinic” is the number of unique patients assigned to PCPs at the examined clinic.
Mean age of all patients assigned to the clinic, whether they were included in the clinical quality measure calculation.
2012 Unemployment Rate of the county of the clinic's location, from U.S. Bureau of Labor Statistics.
Clinical Quality Measure Descriptive Characteristics
Mean 2009 (pre‐PACT) clinical outcome quality scores among all clinics (Table 1) ranged from 65 (LDL < 100 mg/dL in CAD) to 96 percent (BP < 160/100 mmHg in diabetes). Clinical process quality scores in 2009 ranged from 75 (aspirin prescription in diabetes) to 97 percent (HbA1c measured annually in diabetes). Pre‐PACT clinical outcome quality scores in 2009 were significantly higher for clinics that later had higher 2012 Pi2 scores compared to clinics that later had lower 2012 Pi2 scores for LDL < 100 mg/dL in CAD and diabetes, and BP < 160/100 in diabetes; and lower for BP < 140/90 in diabetes. Process measures in 2009 were not significantly different between highest and lowest Pi2 clinics.
Mean 2009–2013 overall changes in clinical outcome quality scores among all clinics ranged from a decrease in 3.1 percent points for HbA1c < 9 percent in diabetes to an increase in 3.9 percent for LDL < 100 mg/DL in CAD (Table 2). 2009–2013 changes in process quality scores were most pronounced for timely retinal exams in diabetes (3.1 percent point increase) and ACE‐inhibitor/ARB prescribed in diabetes (1.5 percent point decrease).
Table 2.
Descriptive Characteristics of Clinical Quality Measures Examined, at Baseline (2009) and over time (2009–2013) among 808 Clinics Included in Main Models
| Measure Name | Mean Baseline (2009) Value, among All Clinics | Mean Baseline Value, by 2012 Pi2 Clinic Category | Unadjusted Mean Change (2009–2013), among All Clinics | ||
|---|---|---|---|---|---|
| Lowest† 2012 Pi2 Category Clinics | Highest‡ 2012 Pi2 Category Clinics | p value Lowest vs. Highest Pi2 Clinics§ | |||
| Clinical outcome quality measures | |||||
| CAD | |||||
| LDL level < 100 mg/dL | 65% | 59% | 71% | .001* | 3.9% |
| Diabetes | |||||
| LDL level < 100 mg/dL | 68% | 63% | 69% | .04* | 0.8% |
| Blood pressure < 160/100 mm Hg | 96% | 94% | 97% | .01* | −0.2% |
| Blood pressure < 140/90 mm Hg | 78% | 74% | 82% | .02* | 1.3% |
| HbA1c < 9% | 85% | 82% | 84% | .45 | −3.1% |
| Hypertension | |||||
| Blood pressure < 160/100 mm Hg | 95% | 94% | 95% | .47 | −0.1% |
| Blood pressure < 140/90 mm Hg | 77% | 78% | 79% | .72 | 0.8% |
| Clinical process quality measures | |||||
| CAD | |||||
| LDL cholesterol measured | 95% | 95% | 95% | .77 | 1.7% |
| Aspirin prescription | 91% | 88% | 92% | .15 | 1.5% |
| Diabetes | |||||
| HbA1c measured annually | 97% | 96% | 96% | .95 | 1.0% |
| Aspirin prescription | 75% | 72% | 77% | .25 | 2.3% |
| Foot exam | 88% | 85% | 86% | .69 | 0.9% |
| Retinal exam | 86% | 80% | 84% | .25 | 3.1% |
| Renal function testing | 95% | 93% | 94% | .75 | 0.9% |
| ACE‐inhibitor/ARB prescription | 78% | 80% | 79% | .68 | −1.5% |
Lowest Pi2 category = −5 to −8.
Highest Pi2 category = +5 to +8.
p value calculated with t tests comparing group means.
p < 0.05.
Association between PCMH Implementation and Changes in Clinical Quality Scores
Clinical Outcomes
In multivariable models examining all 808 clinics, the clinics in the category with the most PACT components in place had significantly greater quality improvements for five of seven clinical outcome measures (Table 3, full model results in Table S3), when compared to clinics with the least PACT components. As shown in Figure 1, clinics in the highest Pi2 category (Pi2 +5 to +8, N 77 clinics) had greater adjusted improvement compared to the lowest Pi2 category (Pi2 −5 to −8, N 69 clinics) in LDL < 100 mg/dL in CAD quality scores (7.2 percent point increase in highest Pi2 clinics vs. 2.0 percent points in lowest Pi2 clinics, p = .01), LDL < 100 mg/dL in diabetes (3.2 percent vs. −0.5 percent, p = .03), BP < 160/100 in diabetes (0.2 percent vs. −1.2 percent, p = .03), BP < 160/100 in HTN (0.8 percent vs. −1.1 percent, p < .001), and BP < 140/90 in HTN (2.5 percent vs. −0.1 percent, p = .04). Highest and lowest Pi2 clinics did not significantly differ in change in HbA1c < 9 percent or BP < 140/90 in DM. Only LDL < 100 in CAD showed a statistically significant difference when comparing middle Pi2 clinic categories to the lowest category. Results for alternative models analyzing Pi2 as a continuous variable (see Table S3) showed that increasing Pi2 was significantly associated at p < .05 with increased improvements in three of the same five quality measures whose improvement was significantly different in highest and lowest Pi2 categories. For the other two measures that differed by highest vs. lowest category, continuous Pi2 was associated with increased improvement at the p = .10 statistical significance level. For one measure which did not differ when comparing highest vs. lowest Pi2 category, BP < 140/90 in DM, continuous Pi2 was statistically significantly associated with 0.2 percent additional improvement per each increase in Pi2 score.
Table 3.
Multivariablea Linear Regression Models of Association between Clinic Pi2 Score and Mid‐PACT (2013) Clinical Quality Measure Scores
| Pi2 Category | Coefficient (95% CI)b | ||||
|---|---|---|---|---|---|
| Pi2 +5 to +8 | Pi2 +2 to +4 | Pi2 −1 to +1 | Pi2 −2 to −4 | Pi2 −5 to −8 | |
| Clinical outcome quality measures | |||||
| CAD | |||||
| LDL level < 100 mg/dL | Ref | −1.69% (−4.41%, 1.03%) | −1.87% (−5.11%, 1.36%) | −4.15% (−7.64%, −0.66%)a | −5.13% (−9.12%, −1.13%)a |
| Diabetes | |||||
| LDL level < 100 mg/dL | Ref | −1.22% (−3.82%, 1.38%) | −1.22% (−3.82%, 1.38%) | −1.98% (−4.78%, 0.83%) | −3.65% (−6.88%, −0.42%)a |
| Blood pressure < 160/100 mm Hg | Ref | −0.07% (−1.15%, 1.01%) | −0.25% (−1.28%, 0.79%) | −0.72% (−1.84%, 0.39%) | −1.40% (−2.67%, −0.12%)a |
| Blood pressure < 140/90 mm Hg | Ref | −0.59% (−3.11%, 1.93%) | −0.62% (−3.04%, 1.79%) | −2.36% (−4.96%, 0.24%) | −1.87% (−4.86%, 1.12%) |
| HbA1c < 9% | Ref | 0.07% (−1.92%, 2.07%) | 0.17% (−1.73%, 2.08%) | 0.09% (−1.96%, 2.14%) | −0.76% (−3.11%, 1.59%) |
| Hypertension | |||||
| Blood pressure < 160/100 mm Hg | Ref | −0.45% (−1.35%, 0.46%) | −0.73% (−1.59%, 0.14%) | −0.63% (−1.56%, 0.30%) | −1.91% (−2.98%, −0.85%)a |
| Blood pressure < 140/90 mm Hg | Ref | −1.06% (−3.13%, 1.00%) | −0.49% (−2.48%, 1.49%) | −1.18% (−3.31%, 0.94%) | −2.59% (−5.02%, −0.15%)a |
| Clinical process quality measures | |||||
| CAD | |||||
| LDL cholesterol measured | Ref | 0.42% (−1.01%, 1.84%) | −0.33% (−1.68%, 1.03%) | −0.57% (−2.04%, 0.89%) | −2.27% (−3.94%, −0.60%)a |
| Aspirin prescription | Ref | −1.01% (−4.39%, 2.36%) | −1.06% (−4.28%, 2.15%) | −3.84% (−7.31%, −0.38%)a | 0.46% (−3.45%, 4.38%) |
| Diabetes | |||||
| HbA1c measured annually | Ref | −0.13% (−0.8%, 0.54%) | −0.36% (−1.00%, 0.28%) | −0.06% (−0.75%, 0.63%) | −0.82% (−1.61%, −0.03%)a |
| Aspirin prescription | Ref | 2.30% (−1.46%, 6.05%) | 0.84% (−2.75%, 4.43%) | −2.17% (−6.04%, 1.69%) | −0.89% (−5.31%, 3.53%) |
| Foot exam | Ref | −1.05% (−3.86%, 1.76%) | −1.17% (−3.85%, 1.51%) | −3.18% (−6.06%, −0.29%)a | −1.54% (−4.83%, 1.76%) |
| Retinal exam | Ref | 1.50% (−0.57%, 3.57%) | 1.31% (−0.67%, 3.29%) | 1.58% (−0.55%, 3.71%) | 0.04% (−2.40%, 2.49%) |
| Renal function testing | Ref | 0.38% (−0.90%, 1.66%) | 0.08% (−1.14%, 1.31%) | −0.42% (−1.74%, 0.90%) | 0.71% (−0.80%, 2.22%) |
| ACE‐inhibitor/ARB prescription | Ref | 0.43% (−1.97%, 2.82%) | 0.91% (−1.38%, 3.2%) | 1.47% (−0.99%, 3.93%) | 2.73% (−0.09%, 5.54%) |
All models adjusted for 2009 Clinic Quality Measure Score, Clinic Type, Clinic Area Unemployment Level. See Table S3 for full model results.
Clinical quality measures used as model outcomes, formatted as 0–100, where 100 = 100%.
Figure 1.
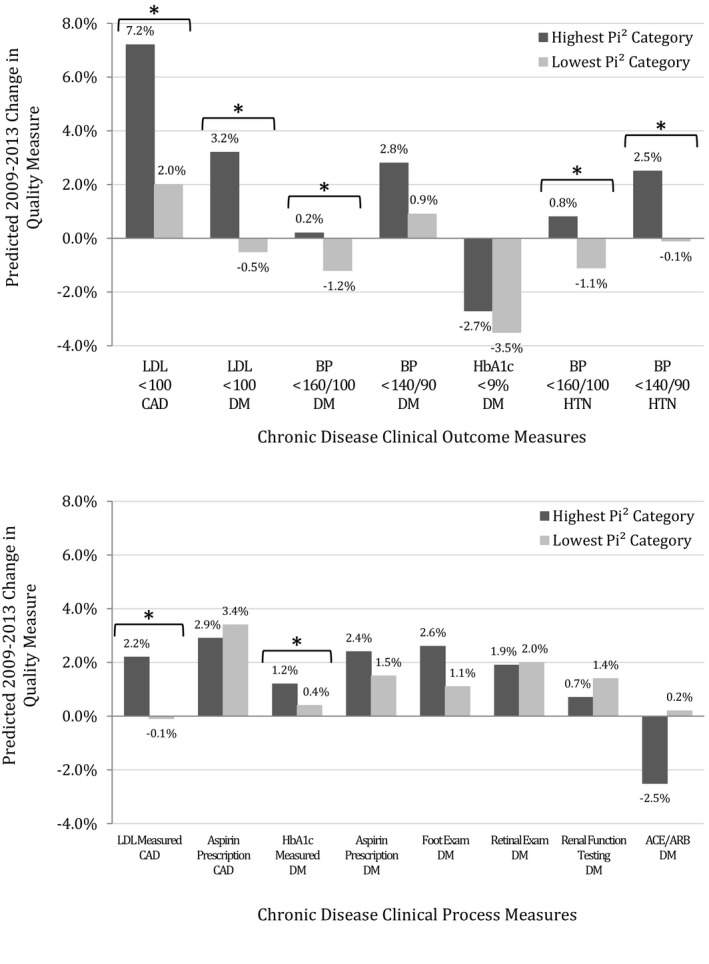
- aAll Models adjusted for 2009 clinic‐level quality of examined quality variable, clinic type (rural/urban, hospital‐based vs. community clinic), and 2009 clinic area unemployment rate.
- *p < .05.
- ACE/ARB, angiotensin‐converting‐enzyme inhibitor/angiotensin receptor blocker prescription; BP, blood pressure; CAD,coronary artery disease; DM, diabetes mellitus; HTN, hypertension; LDL, low density lipoprotein, measured in mg/dL. Pi2, PACT Implementation Index.
Clinical Processes
Highest Pi2 clinics had significantly greater improvement than the lowest Pi2 clinics for only two of eight clinical process measures (Figure 1): (1) LDL measurement in CAD (2.2 percent points vs. −0.1 percent points, p < .01) and (2) HbA1c measurement in diabetes (1.2 percent vs. 0.4 percent, p = .04). As shown in Table 3 (full model results in Table S3), there were few statistically significant differences when comparing the high Pi2 category to the middle three Pi2 categories for process measures. The exceptions were aspirin prescription in CAD and foot exams in diabetes, which showed significantly more improvement in the highest Pi2 category compared to the second lowest Pi2 category (n = 162). No Pi2 clinic categories significantly differed in change in aspirin and ACE‐inhibitor/ARB prescription in diabetes, aspirin prescription in CAD, and foot exams, retinal exams, or renal functional testing in diabetes. Results for alternative models analyzing Pi2 as a continuous variable (see Table S3) showed similar results to categorical Pi2 models for five of eight process quality measures. For HbA1c checked annually in DM, which significantly differed when comparing highest vs. lowest Pi2 category, continuous Pi2 was associated with increased improvement at a p = .12 statistical significance level. For two measures which did not differ when comparing highest vs. lowest Pi2 category, aspirin prescription in DM and ACE‐inhibitor/ARB prescription in DM, continuous Pi2 was statistically significantly associated with small changes in quality (0.3 percent more improvement and 0.2 percent less improvement per Pi2 point, respectively).
Discussion
In this study of a PCMH program with several hundred participating clinics in geographically diverse areas, we found that the clinics with the most PACT components in place in 2012 had greater 4‐year improvements in seven of 15 chronic disease clinical quality measures examined, ranging from 0.8 percent to 5.2 percent more of the clinic population meeting measures, compared to clinics with the least PACT components in place. There were very few statistically significant differences in quality measure changes between clinics with moderate levels of PACT components and clinics with the least PACT components.
Significant differences among highest and lowest PACT clinics were more likely to be seen in intermediate clinical outcome metrics (e.g., HbA1c levels or blood pressure levels) than clinical process metrics, possibly because clinical outcomes had more room for improvement from baseline. Clinics may have had limited opportunity for process measure improvement due to ceiling effects, thereby making it difficult to improve or to detect small differences in change across clinic categories. However, the measures with the highest pre‐PACT levels were among those whose further improvement showed significant differences between highest and lowest PACT implementation clinics.
Over the examined 4‐year period, the additional percentages of the patient population meeting intermediate clinical outcome quality measures ranged from 1.3 percent to 5.2 percent. Few prior studies of PCMH implementation have found evidence of impact on clinical outcomes. Many past studies of the impact of PCMH implementation on clinical quality indicators have been limited to clinical process measures, cross‐sectional analyses, or certain medical conditions (e.g., diabetes only), and they have had varying results (Jaén et al. 2010; Reid et al. 2010; Jackson et al. 2013; Solberg et al. 2013; Rosenthal et al. 2015a,b,c). Three larger studies in Medicaid (Phillips et al. 2014) and multipayer PCMH programs (Jones et al. 2016; Lemak et al. 2015) found statistically significant improvements in diabetes care processes (such as HbA1c measurement and diabetes complication screening) among PCMH practices; while two other PCMH studies found no improvements in diabetes care processes in multipayer (Rosenthal et al. 2013) and Medicare‐(Dale et al. 2016) supported programs. Friedberg and colleagues (Friedberg et al. 2014) examined four diabetes care processes as well as the intermediate clinical outcomes of HbA1c and LDL cholesterol levels among 32 PCMH and 29 matched practices in Pennsylvania and found that only nephropathy screening was significantly higher among PCMH practices by year 3. However, HbA1c testing, LDL testing, and HbA1c levels showed trends toward significantly better values (5–7 percent point higher performance). A recent study of 1000 Community Health Centers found that PCMH certification was cross‐sectionally associated with 1–3 higher percentage point performance in diabetes glycemic control and receipt of appropriate asthma therapy, but not with blood pressure control in hypertension (Shi et al. 2016).
Our study notably differs from these prior studies in the use of several clinical outcome measures across multiple chronic conditions, its longitudinal nature, and in the length of follow‐up (4 years). While comparing results from one PCMH program to another is difficult due to the varying nature of the programs themselves, and variation in their measures of PCMH implementation, PACT model changes in structures and processes of care closely map to National Committee for Quality Assurance (NCQA) PCMH certification metrics and other widely used models of PCMH (Rosland et al. 2013; Nelson et al. 2014). While magnitudes of changes in some of the intermediate clinical outcomes are modest in percentage, if the changes seen in the highest PACT clinics were extended to the full VA primary care population, an additional 38,000 VA patients with hypertension would meet the BP < 160/100 measure (equivalent to 1.9 percent of the denominator population), and an additional 37,000 VA patients with diabetes would meet the LDL < 100 measure (3.7 percent of the population).
There are two possible contributors to the positive associations observed in this study of the PACT program. First, it is possible that clinics with more components of PACT in place had overarching features, such as committed leadership or a culture promoting quality improvement, which would have allowed them to increase clinical quality scores more effectively than other clinics, whether the PACT initiative had been put into place. Clinics that had achieved high PACT implementation in 2012 did already have higher pre‐PACT (2009) quality in some outcome quality measures, but not in process measures. Therefore, unmeasured clinic‐level characteristics of high Pi2 clinics may have allowed them to achieve better control of outcome measures during the years examined in this study as well. However, models adjusted for pre‐PACT levels of another quality metric, patient ambulatory care‐sensitive admission rates, had similar results. While this analysis issue could be partly addressed by including a measure of change in PACT implementation overtime, the Pi2 score of PACT implementation could not be measured prior to 2012 due to the use of key data elements not available in prior years.
Second, it is plausible that having extensive features of PACT in place contributed to increased chronic disease clinical outcome improvement, through several mechanisms. Starting in 2010, the PACT initiative focused attention on measures of overall nonchronic disease‐specific care delivery processes, such as decreased waiting times for primary care appointments, increased availability of same‐day care, increased continuity of care, and increased use of phone appointments across all patients. Metrics of these PACT care delivery processes started to show improvements nationally about 6 months after the start of PACT (Rosland et al. 2013), and they may have contributed to subsequent increases in quality of care for patients with chronic health conditions. PACT expanded PCP‐ and nurse‐patient ratios significantly, and it assigned patients to multidisciplinary primary care teams that include clinical pharmacists and social workers (Helfrich et al. 2014). This team structure may have helped PC teams provide more consistent and comprehensive chronic disease management and self‐management support that may have allowed patients to benefit from ongoing relationships with specific team members. PACT also calls for clinical staff training in patient communication techniques that may facilitate patient decision making and self‐management in chronic disease; in fact, the Pi2 domains with the most variation between high and low Pi2 clinics were Patient‐Centeredness, Self‐Management Support, and Shared Decision Making (as shown in Table S2B). PACT also provides teamlets with population management resources, such as databases that identify clinical care gaps for assigned patients, which they may have used to focus on gaps for chronically ill patients at a patient panel level. While most PACT resources were directed at all primary care patients, two in particular—telehealth monitoring and shared medical appointments—were specifically focused on patients with certain chronic conditions (such as diabetes or heart disease). Subsequent studies can further investigate which PACT components may have played key roles in chronic disease quality improvement; however, patient‐centered medical homes are conceived of as an integrated approach to care, and it is likely that multiple individual components work together to help patients receive increased self‐management support and quality of care.
Analyses comparing clinics with relatively moderate levels of PACT in place to those of low PACT clinics, and analyses of Pi2 scores as continuous, did not indicate many statistically significant changes in clinical processes and outcomes among the moderate level PACT clinics. It is possible that clinical quality is only significantly impacted when PACT components are in place at a high level across most or all PACT domains. A second possibility is that PACT implementation had not yet reached the point at which its mechanisms were affecting chronic disease quality. PACT implementation often proceeded in a step‐wise fashion (Forman et al. 2013), with clinics first focusing on hiring and training new staff and creating new teamlet structures, then on implementing new PACT processes (such as new scheduling systems or teamlet huddles), and only then did many clinics start to apply new PACT processes toward quality improvement in chronic disease care. Therefore, it can take months to years to work through these steps carefully to the point where PCMH is implemented to the extent that it can start to affect even clinical processes (such as checking HbA1c regularly). Then, there are several subsequent changes that need to happen (e.g., prescription algorithms, or patient behavior changes) in order for changed clinic processes to lead to clinical outcomes (such as lower HbA1c). Furthermore, implementing a large multifaceted program such as PACT can consume much of the energy and resources of local leaders and staff (Forman et al. 2013); in some clinics still in earlier implementation phases, this could inadvertently draw attention away from chronic disease quality improvement goals during initial PACT startup.
This study should be interpreted in the context of data limitations. There was no control group of clinics that were not asked to implement PACT, so we conducted a within‐clinic analysis of change controlling for pre‐PACT quality levels. We analyzed 15 separate regression models, so it is statistically likely that at least one model had significant results by chance alone. While the Pi2 represents a significant advance in measuring PCMH implementation in a multifaceted and objective way (Baron 2014), Pi2 scores are calculated in relative terms and combine clinic performance from multiple domains into one score. Therefore, Pi2 scores do not reflect which components of PACT are well or poorly implemented, and they do not allow for a detailed examination of the extent to which implementation of each component is associated with the outcome. In addition, Pi2 scores are derived from standardized component scores, so the highest and lowest Pi2 categories have by design a lower number of clinics than the middle Pi2 category, which may have limited our power when comparing across categories. Some of the quality measures included in this study have been more recently updated—in particular, therapy targeted to specific LDL levels has been replaced with guidelines emphasizing cardiac risk‐based statin therapy. However, reductions in LDL levels reflect increased adherence to the clinical guidelines in effect at the time of the study, and likely reflect an increased use of statin medications among appropriate populations. Finally, this study focused solely on improvement in chronic disease quality, which is only one of several desirable goals of PCMH. In previous studies, VA PACT implementation has been linked to lower than expected costs and ambulatory care‐sensitive hospitalizations (Hebert et al. 2014), and to higher patient satisfaction ratings (Nelson et al. 2014).
In this study of one of the largest U.S. PCMH programs to date, we found that clinics with the most PCMH components in place had greater improvements over 4 years in seven of 15 chronic disease intermediate clinical outcome and process quality measures examined, when compared to clinics with the least PCMH components in place. Health systems and clinics may be able to achieve improved care quality for patients with common chronic conditions through PCMH‐aligned changes in care delivery across all patients if those changes are extensively implemented.
Supporting information
Appendix SA1: Author Matrix.
Table S1: EPRP Quality‐of‐Care Indicators and Sampling Frame Used for Veterans Affairs (VA).
Table S2: PACT Implementation Index (Pi2) Calculation and Component Details.
Table S3: Full Model Results: Multivariate Linear Regression Models of Mid‐PACT (2013) Clinical Quality Measure Scores at Clinic Level.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was supported by the VA PACT Demonstration Laboratory Coordinating Center and funded by VHA Office of Primary Care Operations and Office of Primary Care Services. Donna Zulman is a VA Career Development Awardee and Matthew Maciejewski is a VA Senior Research Scientist. The views expressed in this paper are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Disclosures: None.
Disclaimer: None.
References
- American Academy of Family Physicians . 2008. “Joint Principles of the Patient‐Centered Medical Home.” Delaware Medical Journal 80 (1): 21–2. [PubMed] [Google Scholar]
- Baron, R. J. 2014. “Not All (Medical) Homes Are Built Alike: Some Work Better Than Others.” JAMA Internal Medicine 174 (8): 1358–9. [DOI] [PubMed] [Google Scholar]
- Bodenheimer, T. , and Laing B. Y.. 2007. “The Teamlet Model of Primary Care.” Annals of Family Medicine 5 (5): 457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information . 2016. “Ambulatory Care Sensitive Conditions,” Canadian Institute for Health Information. Indicator Library: General Methodology Notes — Clinical Indicators, February 2016. Ottawa, ON: CIHI. [Google Scholar]
- Coleman, K. , Austin B. T., Brach C., and Wagner E. H.. 2009. “Evidence on the Chronic Care Model in the New Millennium.” Health Affairs 28 (1): 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, S. B. , Ghosh A., Peikes D. N., Day T. J., Yoon F. B., Taylor E. F., Swankoski K., O'Malley A. S., Conway P. H., Rajkumar R., and Press M. J.. 2016. “Two‐Year Costs and Quality in the Comprehensive Primary Care Initiative.” New England Journal of Medicine 374: 2345–56. Available at http://www.nejm.org/doi/full/10.1056/NEJMsa1414953 [DOI] [PubMed] [Google Scholar]
- Edwards, S. T. , Bitton A., Hong J., and Landon B. E.. 2014. “Patient‐Centered Medical Home Initiatives Expanded in 2009–13: Providers, Patients, and Payment Incentives Increased.” Health Affairs 33 (10): 1823–31. [DOI] [PubMed] [Google Scholar]
- Fihn, S. D. , Francis J., Clancy C., Nielson C., Nelson K., Rumsfeld J., Cullen T., Bates J., and Graham G. L.. 2014. “Insights from Advanced Analytics at the Veterans Health Administration.” Health Affairs 33 (7): 1203–11. [DOI] [PubMed] [Google Scholar]
- Forman, J. , Harrod M., Robinson C., Annis‐Emeott A., Ott J., Saffar D., and Krein S. L.. 2013. “First Things First: Foundational Requirements for a Medical Home in an Academic Medical Center.” Journal of General Internal Medicine 29 (Suppl 2): S640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, M. W. , Schneider E. C., Rosenthal M. B., Volpp K. G., and Werner R. M.. 2014. “Association between Participation in a Multipayer Medical Home Intervention and Changes in Quality, Utilization, and Costs of Care.” Journal of the American Medical Association 311 (8): 815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, P. L. , Liu C.‐F., Wong E. S., Hernandez S. E., Batten A., Lo S., Lemon J. M., Conrad D. A., Grembowski D., Nelson K., and Fihn S. D.. 2014. “Patient‐Centered Medical Home Initiative Produced Modest Economic Results for Veterans Health Administration, 2010–12.” Health Affairs 33 (6): 980–7. [DOI] [PubMed] [Google Scholar]
- Helfrich, C. D. , Dolan E. D., Fihn S. D., Rodriguez H. P., Meredith L. S., Rosland A.‐M., Lempa M., Wakefield B. J., Joos S., Lawler L. H., Harvey H. B., Stark R., Schectman G., and Nelson K. M.. 2014. “Association of Medical Home Team‐Based Care Functions and Perceived Improvements in Patient‐Centered Care at VHA Primary Care Clinics.” Healthcare (Amsterdam, Netherlands) 2 (4): 238–44. [DOI] [PubMed] [Google Scholar]
- Jackson, G. L. , Powers B. J., Chatterjee R., Prvu Bettger J., Kemper A. R., Hasselblad V., Dolor R. J., Irvine R. J., Heidenfelder B. L., Kendrick A. S., Gray R., John J., and Williams W.. 2013. “The Patient‐Centered Medical Home: A Systematic Review.” Annals of Internal Medicine 158 (3): 169–78. [DOI] [PubMed] [Google Scholar]
- Jaén, C. R. , Ferrer R. L., Miller W. L., Palmer R. F., Wood R., Davila M., Stewart E. E., Crabtree B. F., Nutting P. A., and Stange K. C.. 2010. “Patient Outcomes at 26 Months in the Patient‐Centered Medical Home National Demonstration Project.” Annals of Family Medicine 8 (Suppl 1): S57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C. , Finison K., McGraves‐Lloyd K., Tremblay T., Mohlman M. K., Tanzman B., Hazard M., Maier S., and Samuelson J.. 2016. “Vermont's Community‐Oriented All‐Payer Medical Home Model Reduces Expenditures and Utilization While Delivering High‐Quality Care.” Population Health Management 19 (3): 196–205. 10.1089/pop.2015.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman, R. , and Kirk L.. 2007. “Principles of the Patient‐Centered Medical Home.” American Family Physician 76 (6): 774–5. [PubMed] [Google Scholar]
- Kington, R. S. , and Smith J. P.. 1997. “Socioeconomic Status and Racial and Ethnic Differences in Functional Status Associated with Chronic Diseases.” American Journal of Public Health 87 (5): 805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemak, C. H. , Nahra T. A., Cohen G. R., Erb N. D., Paustian M. L., Share D., and Hirth R. A.. 2015. “Michigan's Fee‐for‐Value Physician Incentive Program Reduces Spending and Improves Quality in Primary Care.” Health Affairs 34 (4): 645–52. [DOI] [PubMed] [Google Scholar]
- Markovitz, A. R. , Alexander J. A., Lantz P. M., and Paustian M. L.. 2015. “Patient‐Centered Medical Home Implementation and Use of Preventive Services: The Role of Practice Socioeconomic Context.” JAMA Internal Medicine 175 (4): 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, K. M. , Helfrich C., Sun H., Hebert P. L., Liu C.‐F., Dolan E., Taylor L., Wong E., Maynard C., Hernandez S. E., Sanders W., Randall I., Curtis I., Schectman G., Stark R., and Fihn S. D.. 2014. “Implementation of the Patient‐Centered Medical Home in the Veterans Health Administration: Associations with Patient Satisfaction, Quality of Care, Staff Burnout, and Hospital and Emergency Department Use.” JAMA Internal Medicine 174 (8): 1350–8. [DOI] [PubMed] [Google Scholar]
- Newsom, J. , Jones R. N., and Hofer S. M.. 2012. “Chapter 5: Basic Longitudinal Analysis Approaches for Continuous and Categorical Variables, Multivariate Applications Series, Routledge In Longitudinal Data Analysis: A Practical Guide for Researchers in Aging, Health, and Social Sciences, edited by Jason Newsom, Jones Richard N., and Hofer Scott M., pp. 143–180. New York: Routledge. [Google Scholar]
- Nielsen M., Buelt L., Patel K., and Nichols L. 2016. “The Patient Centered Medical Home's Impact on Cost and Quality, Review of Evidence,” Patient‐Centered Primary Care Collaborative [accessed on March 31, 2016]. Available at https://www.pcpcc.org/resource/patient-centered-medical-homes-impact-cost-and-quality-2014-2015
- Paez, K. A. , Zhao L., and Hwang W.. 2009. “Rising out‐of‐Pocket Spending for Chronic Conditions: A Ten‐Year Trend.” Health Affairs 28 (1): 15–25. [DOI] [PubMed] [Google Scholar]
- Phillips, R. L. , Han M., Petterson S. M., Makaroff L. A., and Liaw W. R.. 2014. “Cost, Utilization, and Quality of Care: An Evaluation of Illinois’ Medicaid Primary Care Case Management Program.” Annals of Family Medicine 12 (5): 408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, R. J. , Coleman K., Johnson E. A., Fishman P. A., Hsu C., Soman M. P., Trescott C. E., Erikson M., and Larson E. B.. 2010. “The Group Health Medical Home at Year Two: Cost Savings, Higher Patient Satisfaction, and Less Burnout for Providers.” Health Affairs 29 (5): 835–43. [DOI] [PubMed] [Google Scholar]
- Rosenthal, M. B. , Friedberg M. W., Singer S. J., Eastman D., Li Z., and Schneider E. C.. 2013. “Effect of a Multipayer Patient‐Centered Medical Home on Health Care Utilization and Quality: The Rhode Island Chronic Care Sustainability Initiative Pilot Program.” JAMA Internal Medicine 173 (20): 1907. [DOI] [PubMed] [Google Scholar]
- Rosenthal, M. B. , Alidina S., Friedberg M. W., Singer S. J., Eastman D., Li Z., and Schneider E. C.. 2015a. “A Difference‐in‐Difference Analysis of Changes in Quality, Utilization and Cost Following the Colorado Multi‐Payer Patient‐Centered Medical Home Pilot.” Journal of General Internal Medicine 31 (3): 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, M. B. , Alidina S., Friedberg M. W., Singer S. J., Eastman D., Li Z., and Schneider E. C.. 2015b. “Impact of the Cincinnati Aligning Forces for Quality Multi‐Payer Patient Centered Medical Home Pilot on Health Care Quality, Utilization, and Costs.” Medical Care Research and Review 23 (5): 247–254. 10.1177/1077558715618566 [DOI] [PubMed] [Google Scholar]
- Rosenthal, M. B. , Sinaiko A. D., Eastman D., Chapman B., and Partridge G.. 2015c. “Impact of the Rochester Medical Home Initiative on Primary Care Practices, Quality, Utilization, and Costs.” Medical Care 53 (11): 967–73. [DOI] [PubMed] [Google Scholar]
- Rosland, A. M. , Nelson K., Haili S., Dolan E., Maynard C., Bryson C., Stark R., Shear J., Kerr E. A., Fihn S. D., and Schectman G.. 2013. “The Patient Centered Medical Home in the Veterans Health Administration.” American Journal of Managed Care 19 (7): e263–72. [PubMed] [Google Scholar]
- Shi, L. , Lee D.‐C., Chung M., Liang H., Lock D., and Sripipatana A.. 2016. “Patient‐Centered Medical Home Recognition and Clinical Performance in U.S. Community Health Centers.” Health Services Research 52 (3): 984–1004. 10.1111/1475-6773.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg, L. I. , Crain A. L., Tillema J., Scholle S. H., Fontaine P., and Whitebird R.. 2013. “Medical Home Transformation: A Gradual Process and a Continuum of Attainment.” Annals of Family Medicine 11 (Suppl 1): S108–14. 10.1370/afm.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A. N. , Lee R. E., Shambaugh‐Miller M. D., Bair B. D., Mueller K. J., Lilly R. S., Kaboli P. J., and Hawthorne K.. 2010. “Defining ‘Rural’ for Veterans’ Health Care Planning.” Journal of Rural Health 26 (4): 301–9. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Paul J., Nantha‐Aree M., Buckley N., Shahzad U., Cheng J., DeBeer J., Winemaker M., Wismer D., Punthakee D., Avram V., and Thabane L.. 2014. “Empirical Comparison of Four Baseline Covariate Adjustment Methods in Analysis of Continuous Outcomes in Randomized Controlled Trials.” Clinical Epidemiology 6: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Table S1: EPRP Quality‐of‐Care Indicators and Sampling Frame Used for Veterans Affairs (VA).
Table S2: PACT Implementation Index (Pi2) Calculation and Component Details.
Table S3: Full Model Results: Multivariate Linear Regression Models of Mid‐PACT (2013) Clinical Quality Measure Scores at Clinic Level.


