Abstract
Treeline responses to climate change ultimately depend on successful seedling recruitment, which requires dispersal of viable seeds and establishment of individual propagules in novel environments. In this study, we evaluated the effects of several abiotic and biotic drivers of early tree seedling recruitment across an alpine treeline ecotone. In two consecutive years, we sowed seeds of low- and high-elevation provenances of Larix decidua (European larch) and Picea abies (Norway spruce) below, at, and above the current treeline into intact vegetation and into open microsites with artificially removed surface vegetation, as well as into plots protected from seed predators and herbivores. Seedling emergence and early establishment in treatment and in control plots were monitored over two years. Tree seedling emergence occurred at and several hundred metres above the current treeline when viable seeds and suitable microsites for germination were available. However, dense vegetation cover at lower elevations and winter mortality at higher elevations particularly limited early recruitment. Post-dispersal predation, species, and provenance also affected emergence and early establishment. This study demonstrates the importance of understanding multiple abiotic and biotic drivers of early seedling recruitment that should be incorporated into predictions of treeline dynamics under climate change.
Introduction
Plant species are responding to recent global temperature increases1 by shifting their ranges as populations track their fundamental niche2,3. There is increasing evidence for climate-induced latitudinal range shifts via increased shrub abundance in circumarctic tundra ecosystems4–6 and elevational shifts of shrubs and trees in mountainous regions7–11. Treeline position, i.e. the range limit of forest ecosystems, is widely considered temperature sensitive and is thus expected to respond to climate warming12–15. However, global treeline dynamics are often modulated by regional-scale drivers such as historical land use changes16 and biotic interactions17. Hence, treeline responses to global warming vary among locations and are often asynchronous with the rate of climate change17–21.
Climate change-induced range expansion of treeline populations also depends on successful recruitment, which requires dispersal of viable seeds followed by successful establishment of individual propagules22. In treeline ecotones, viable seed availability commonly declines with elevation13,23 due to lower abundance of seed bearing trees and less frequent mast years, i.e. synchronous production of large seed crops24–26. Biotic interactions, such as pre-dispersal predation, may further constrain seed productivity at treeline27, impacting future treeline range expansion. Successful recruitment also depends on the availability of suitable microsites that provide the necessary conditions for emergence and establishment of seedlings28,29. Seed bed quality is determined by a complex interplay of abiotic and biotic factors such as microclimatic conditions, the presence of neighbouring vegetation, and herbivory30. Abiotic factors are considered key drivers of seedling recruitment in climatically harsh environments23. Early establishment is particularly limited by temperature and water availability31–33, but other abiotic factors, such as snow cover duration and desiccating winds, may also affect seedling recruitment34–36.
Biotic interactions can be equally or even more important than abiotic factors in determining seed bed conditions37. Microsite cover effects can be highly complex, with neighbouring vegetation positively or negatively affecting tree seedlings depending on vegetation type, species, demographic state, and prevailing weather conditions29,38. On the one hand, neighbouring vegetation can facilitate recruitment by sheltering seedlings from adverse climate effects, seed predators, and herbivores23,28,39,40. On the other hand, a dense vegetation cover can impede seedling emergence and establishment by competing for light, water and nutrients, exerting allelopathic effects, and preventing seeds from reaching a suitable seed bed41–45. The stress-gradient hypothesis predicts that these biotic interactions vary with abiotic conditions46. Therefore, it is expected that competition dominates at lower elevations with relatively low levels of environmental stress, whereas facilitative interactions prevail in more stressful environments at higher elevations40. Furthermore, seed predation and herbivory are other important biotic constraints on seedling recruitment at tree species’ upper range limits47–50. Dense vegetation may additionally promote post-dispersal predation by creating preferred microhabitats and foraging areas for seed predators and herbivores51,52. Thus, a large number of abiotic and biotic factors shape microsite conditions indicating the need for a better understanding of the interplay among these different drivers of seedling recruitment.
Seedling recruitment depends not only on environmental but also on genetic factors acting on seed availability, germination, growth, and survival. Individual tree species are adapted to different elevation ranges, reflecting different temperature sensitivities13. In particular, tree species are adapted to different ranges of germination temperatures31. Recruitment at treeline is thus likely to vary among species. Furthermore, provenance tests have revealed high genetic diversity and site-specific adaptations in conifer species53–55. Physiological and growth adaptations of high-elevation provenances to cold climate conditions are known to exist for later-stage seedlings and adult trees56. Provenance may also be important in early life stages of seedlings but has rarely been evaluated (but see57).
Major bottlenecks to tree seedling recruitment clearly occur in early life stages, yet analysing abiotic and biotic key players in this process remains a scientific challenge. While the influence of individual abiotic or biotic factors on seedling recruitment in treeline ecotones has been tested experimentally in several studies31,32,42,58,59, interactions among several abiotic and biotic factors have rarely been addressed37,50,60. In the European Alps, there exists, to the best of our knowledge, no experimental study testing the relative importance of multiple abiotic and biotic factors on early tree seedling recruitment along an elevation gradient across the treeline ecotone. Here, we investigated seedling emergence and early establishment of two conifer species, Larix decidua MILL. (European larch) and Picea abies (L.) KARST. (Norway spruce), below, at, and above treeline in the Swiss Alps. In two consecutive years, we sowed seeds of low- and high-elevation provenances of the two species into plots with intact vegetation and into plots where surface vegetation was removed to create open microsites to test the influence of seed availability, microsite cover, and provenance on early seedling recruitment. Moreover, herbivore exclosures allowed us to test the influence of seed predators and herbivores. We tested hypotheses addressing biotic and abiotic drivers of early seedling recruitment, specifically, predicting that: (i) emergence would be limited by both seed availability and harsh environmental conditions at high elevation, and thus would decline with increasing elevation and distance from the current treeline; (ii) recruitment would be greater at open microsites than in closed, intact vegetation at lower elevations, but the opposite effect would occur at high elevations where neighbouring vegetation shelters seedlings from adverse environmental conditions; (iii) there would be a negative effect of seed predation and herbivory on seedling recruitment, but this effect would be less pronounced at open microsites; and (iv) at and above treeline, recruitment would be greater for L. decidua than for P. abies and for high-elevation provenances of both species.
Results
Seedling emergence
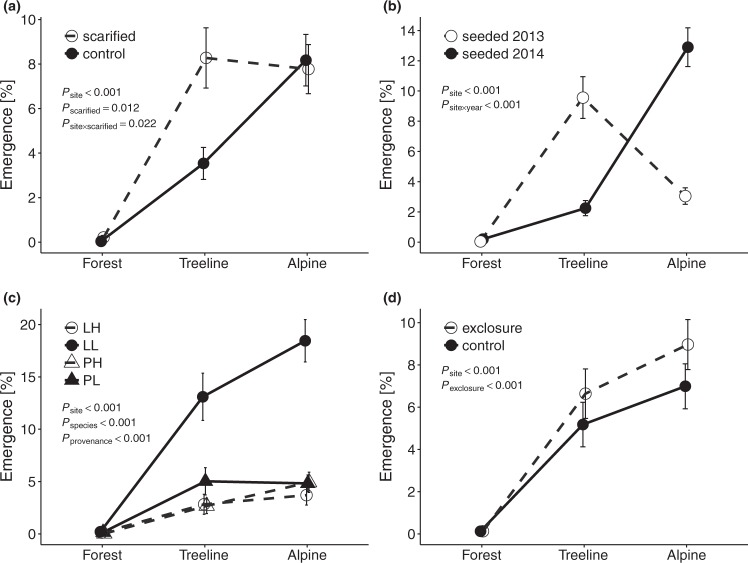
Naturally emerged seedlings were found only at the lowest study site (referred to as forest site) and only during the baseline census in June 2013, when 15 L. decidua seedlings and 7 P. abies seedlings were observed. Emergence in seeded plots was greater than in unseeded control plots (n = 960; t = −11.36; P < 0.001). Germination of experimentally sown seeds was highest at the uppermost site (alpine site), where 981 seedlings emerged (8.0 ± 0.8%, values represent mean ± 1 standard error of the percentage of viable seeds per seeded subplot, for absolute numbers see Supplementary Table S1a), followed by the mid-elevation site (treeline site) with 734 seedlings (5.8 ± 0.8%), and lowest at the forest site, where only 12 seedlings emerged (0.1 ± 0.04%; Psite < 0.001; Table 1; Fig. 1). Seedling emergence was greater in scarified plots than in plots with intact vegetation (5.4 ± 0.6% vs. 3.9 ± 0.5%; Pscarified = 0.012; Fig. 1a), in particular because more seedlings emerged in scarified plots than in plots with intact vegetation at the treeline site (8.0 ± 1.4% vs. 3.5 ± 0.7%) but not at the alpine site (8.0 ± 1.2% vs. 8.0 ± 1.5%) and at the forest site (0.2 ± 0.1% vs. 0.0 ± 0.0%; Psite × scarified = 0.022; Fig. 1a). Moreover, the positive effect of scarification on emergence was more pronounced in 2013 (5.2 ± 1.0% vs. 3.1 ± 0.5%) than in 2014 (5.6 ± 0.9% vs. 4.6 ± 0.8%; Pscarified × year = 0.041). Total emergence was similar in both years of seeding, 4.2 ± 0.6% in 2013 and 5.1 ± 0.6% in 2014 (Pyear = 0.703). In 2013, emergence was more than three times as high at the treeline than at the alpine site (9.5 ± 1.4% vs. 3.0 ± 0.5%), whereas in 2014, emergence at the alpine site was more than six times as high than at the treeline site (13.1 ± 1.4% vs. 2.0 ± 0.5%; Psite× year < 0.001; Fig. 1b). Emergence was about twice as high for L. decidua than for P. abies (6.3 ± 0.7% vs. 2.9 ± 0.4%; Pspecies < 0.001) and almost three times as high for low-elevation provenances than for high-elevation provenances (6.9 ± 0.7% vs. 2.4 ± 0.3%; Pprovenance < 0.001). Emergence of L. decidua seedlings from low-elevation provenance (10.6 ± 1.3%) was three to five times as high than emergence of high-elevation provenance seedlings (2.1 ± 0.4%) and from both P. abies provenances (3.3 ± 0.6% vs. 2.5 ± 0.4% for low- vs. high-elevation provenances; Pspecies × provenance < 0.001; Fig. 1c). Protection against seed predators and herbivores resulted in an overall increase in emergence from 4.0 ± 0.5% to 5.2 ± 0.6% (Pexclosure < 0.001; Fig. 1d). This effect was slightly stronger in 2013 than in 2014 (Pyear × exclosure = 0.037) and the increase was more pronounced for low- than for high-elevation provenance seedlings (Pprovenance × exclosure = 0.005).
Table 1.
Effects of experimental site, scarification, seeding year, species, provenance, and herbivore exclosure treatment (exclosure), as well as their interactions, on seedling emergence, first and second winter survival, and seedling height.
| Seedling emergence (n = 1,727) | 1st winter survival (n = 408) | 2nd winter survival (n = 236) | Seedling height (n = 54) | |
|---|---|---|---|---|
| Site | 75.898*** | 26.408*** | 10.689** | 21.219*** |
| Scarified | 6.308* | 18.638*** | 12.460*** | 2.678 |
| Year | 0.146 | 0.521 | — | — |
| Species | 24.687*** | 2.412 | 3.416 | — |
| Provenance | 38.326*** | 3.779 | 0.367 | — |
| Exclosure | 15.490*** | 0.253 | — | — |
| Site × Scarified | 7.623* | 2.398 | 1.021 | 1.975 |
| Site × Year | 92.573*** | 29.399*** | — | — |
| Site × Species | 1.515 | 10.249** | 0.002 | — |
| Site × Provenance | 0.724 | 0.001 | 0.002 | — |
| Site × Exclosure | 0.033 | 0.134 | — | — |
| Scarified × Year | 4.175* | 0.019 | — | — |
| Scarified × Species | 0.730 | 0.264 | — | — |
| Scarified × Provenance | 0.471 | 6.909** | — | — |
| Scarified × Exclosure | 0.061 | 1.262 | — | — |
| Year × Species | 1.584 | 2.333 | — | — |
| Year × Provenance | 1.021 | 4.199* | — | — |
| Year × Exclosure | 4.371* | 4.695* | — | — |
| Species × Provenance | 16.473*** | 0.148 | — | — |
| Species × Exclosure | 1.069 | 1.051 | — | — |
| Provenance × Exclosure | 7.896** | 0.404 | — | — |
Values and symbols are χ2-values and significances, respectively, from likelihood ratio tests of mixed-effects models. Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001. Degrees of freedom: df = 1 for all factors except for site and its interactions in seedling emergence (df = 2). The forest site was excluded from survival and growth trait models because of very low seedling recruitment.
Figure 1.
Effects of scarification treatment (a), seeding year (b), provenance and species (c), and herbivore exclosure treatment (d) on seedling emergence at the forest, treeline, and alpine sites. LL: low-elevation provenance of L. decidua; LH: high-elevation provenance of L. decidua; PL: low-elevation provenance of P. abies; PH: high-elevation provenance of P. abies. P-values indicate significant effects and interactions from likelihood ratio tests of mixed-effects models. Error bars indicate ± 1 standard error of trait means.
Winter survival and growth
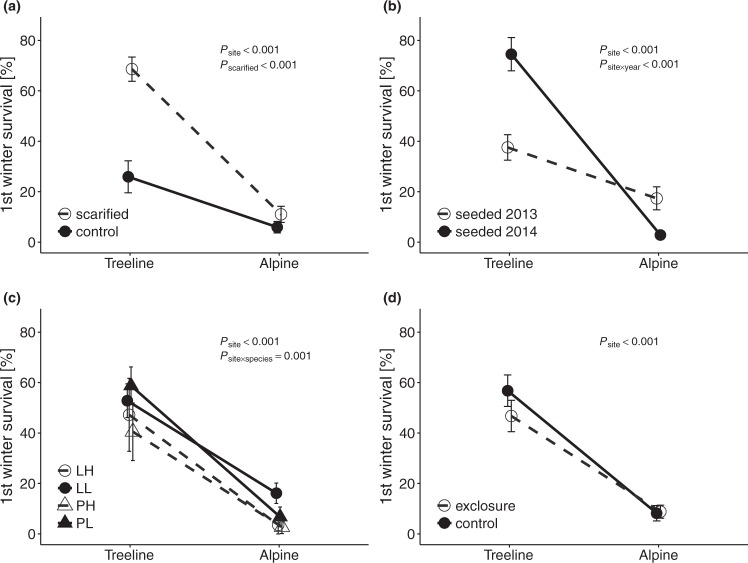
The fraction of seedlings that survived the first winter was lower at the alpine site (8.5 ± 2.0%) than at the treeline site (51.5 ± 4.4%, Psite < 0.001; Table 1; Supplementary Table S1b; Fig. 2). First winter survival was generally higher in scarified plots than in plots with intact vegetation (Pscarified < 0.001; Fig. 2a). Furthermore, first winter survival at the treeline site was higher in winter 2014/2015 than in winter 2013/2014 (Psite × year < 0.001; Fig. 2b). More L. decidua than P. abies seedlings survived the first winter at the alpine site, whereas there was no difference in survival at treeline (Psite × species = 0.001; Fig. 2c). Slightly more low-elevation provenance seedlings of both species survived the first winter compared to high-elevation provenance seedlings (Pprovenance = 0.052; Fig. 2c). The difference in first winter survival between scarified plots and plots with intact vegetation was greater for seedlings from high-elevation provenances than for seedlings from low-elevation provenances (Pscarified × provenance = 0.009). First winter survival was higher for seedlings from low- than from high-elevation provenances seeded in 2014, whereas there were no provenance differences for seedlings seeded in 2013 (Pyear × provenance = 0.040). Slightly more seedlings from 2013 outside of herbivore exclosures than inside exclosures survived the first winter, whereas there was no difference for seedlings from 2014 (Pyear × exclosure = 0.030). However, herbivore exclosure alone did not influence first winter survival (P > 0.1; Fig. 2d).
Figure 2.
Effects of scarification treatment (a), seeding year (b), provenance and species (c), and herbivore exclosure treatment (d) on first winter survival of seedlings at the treeline and alpine sites. Abbreviations: see Fig. 1. P-values indicate significant effects and interactions from likelihood ratio tests of mixed-effects models. Error bars indicate ± 1 standard error of trait means.
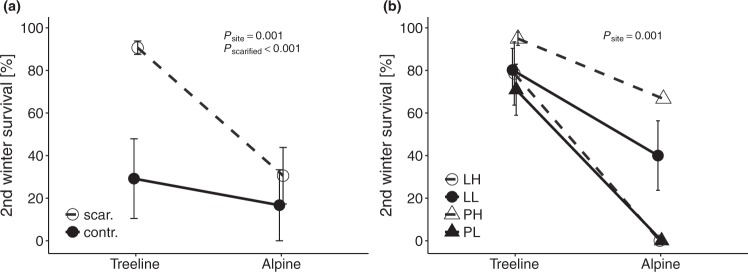
Similar to first winter survival, fewer seedlings survived the second winter at the alpine site (25.9 ± 10.3%) than at the treeline site (79.2 ± 6.0%; Psite = 0.001; Table 1; Supplementary Table S1c; Fig. 3). Second winter survival was generally higher in scarified plots than in plots with intact vegetation (Pscarified < 0.001; Fig. 3a). The survival of P. abies seedlings during the second winter was slightly greater than that of L. decidua seedlings (Pspecies = 0.065), whereas provenance did not influence survival (Pprovenance > 0.1; Fig. 3b). After the second growing season, seedlings were significantly taller at treeline (3.8 ± 0.1 cm) than at the alpine site (2.6 ± 0.1 cm; Psite < 0.001; Table 1; Fig. 4). However, there were no differences in seedling height with respect to scarification treatment (P > 0.1).
Figure 3.
Effects of scarification treatment (a) and provenance and species (b) on second winter survival of seedlings at the treeline and alpine sites. Abbreviations: see Fig. 1. P-values indicate significant effects and interactions from likelihood ratio tests of mixed-effects models. Error bars indicate ± 1 standard error of trait means.
Figure 4.
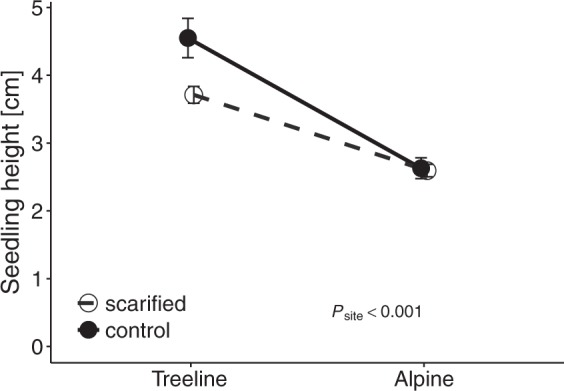
Effects of scarification treatment on seedling height at the treeline and alpine sites. P-values indicate significant effects and interactions from likelihood ratio tests of mixed-effects models. Error bars indicate ± 1 standard error of trait means.
Climate and soil temperatures
In 2013, mean summer air temperature (JJA, i.e. June, July, and August) at the Stillberg climate station was 1.2 °C above average, but in 2014 it was 0.8 °C below the 30-year average (1981–2010) of 9.5 °C (Supplementary Fig. S2). Summer 2015 was the second warmest summer on record, with a mean summer temperature 2.3 °C above average (Supplementary Fig. S2). In the summers of 2013 and 2015, precipitation was 79% and 75% of the average precipitation sum of 448 mm in the 1981 to 2010 period. Conversely, the precipitation sum in summer 2014 was 39% above average. Mean snow depth between 1 November and 30 April was similar for both winters (106% and 108%, respectively, of the 1981 to 2010 average of 78 cm; Supplementary Fig. S3).
Over the three years, summer soil temperatures (JJA) logged at each site were consistently lowest at the forest site and highest at the alpine site (Supplementary Table S2). Furthermore, mean summer soil temperatures in scarified plots were 0.4 °C higher than in plots with intact vegetation at the treeline site, whereas the corresponding temperature difference at the alpine site was 0.2 °C. Based on soil temperature measurements, the forest and treeline sites were snow free at the beginning and middle of May, respectively, in both years, whereas the alpine site was snow free at the beginning of June in 2014 and in mid-May in 2015 (Supplementary Table S3). The growing season started within two to three days after snowmelt and ended in mid-October in all three years (Supplementary Table S3).
Discussion
In our study, tree seedlings emerged at and well above the current treeline but only when viable seeds were sown in suitable microsites for germination. Seedling emergence and early establishment were reduced in plots with intact vegetation below and at treeline but not at the alpine site. Species, provenance, and post dispersal predation further affected emergence. Winter survival and growth of seedlings were lower at the alpine than at the treeline site.
Seed availability and microsite conditions determined recruitment
Observations of naturally emerged seedlings at the forest site in 2013 and the fact that this was a good seed crop year in the region for both species (personal communication A. Burkart, 2016) suggested that seeds were naturally available and able to emerge, as has been previously shown for other nearby subalpine forest stands25. The complete lack of natural recruitment at the treeline and alpine sites, and pronounced experimental seeding effects, however, indicated that emergence was limited by viable seed availability at and above treeline. Reduced viable seed availability in treeline ecotones may be a consequence of lower quality of high elevation seeds or increasing distance to seed bearing trees24.
After experimental seed addition, more L. decidua and P. abies seeds successfully germinated at the alpine site than at the treeline and forest sites (Table 1; Fig. 1), indicating that seedling emergence is possible several hundred metres above the current treeline and thus is not limited by the environmental conditions at high elevation if viable seeds are available. In contrast to air temperature that generally decreases with elevation, our records of summer soil temperatures showed the highest averages and largest fluctuations at the alpine site and in scarified plots at treeline (Supplementary Table S2 and Fig. S4). These higher soil temperatures in open microsites, due to enhanced surface heating by direct insolation61, may have promoted emergence at higher elevations.
The lack of emergence at the forest site (Table 1; Fig. 1a) was likely due to the tall and large-leafed herbaceous Adenostylion understorey vegetation, which may have directly competed for light, water, and nutrients, but probably also reduced seed bed temperatures. Although our experimental design did not allow us to disentangle the contributing factors, the proliferating growth of this understorey vegetation over the summer season likely impeded survival of the few naturally emerged seedlings and germination of sown seeds in both control and scarified plots. Indeed, a dense cover of understorey vegetation has been considered an important recruitment-limiting factor in other subalpine conifer forests62,63 as well as in boreal forests37. These understorey limitations are particularly pronounced where favourable microsites on rotten logs, stumps, and root-soil-plates are absent64.
Although a considerable number of sown seeds germinated at treeline, emergence and winter survival were reduced in plots with intact vegetation cover compared with in scarified plots (Table 1; Figs 1a, 2a, 3a). Whereas many other studies reported positive effects of neighbouring vegetation at treeline (e.g.29,45,65,66), our results indicate predominantly negative effects on early seedling recruitment at the treeline site. This site is characterised by a dense dwarf shrub layer, a vegetation type that has been shown to impair tree seedling recruitment by competing for water, nutrients, and light43,67. Although we did not measure resource levels, and thus cannot determine the underlying mechanisms, seedlings in control plots tended to grow taller (Fig. 4), suggesting increased competition for light in vegetation-covered plots68. In line with our findings, microhabitat comparisons in a Pyrenean alpine treeline ecotone revealed that microsites with dense dwarf shrub layers were not suitable for the recruitment of shade-intolerant Pinus uncinata seedlings28. Moreover, in alpine treeline ecotones in southern Norway and the French Alps, competition by herbaceous neighbouring vegetation has been suggested to reduce seedling emergence of P. abies37 and L. decidua38. These dense subalpine grassland vegetation types – as well as dwarf-shrub layers – in the Alps are very different from the low-stature alpine tundra vegetation at more continental treelines in the Rocky Mountains38, where positive biotic interactions prevail29,69,70. Thick and dense moss layers below the dwarf-shrub canopy at our treeline site may have further constrained seedling emergence by preventing seeds from reaching the soil surface and by soil moisture deficits during drier periods71. However, depending on moss thickness, moisture content, and moss species, moss seed beds can also facilitate seedling recruitment72–74. Furthermore, negative allelopathic effects of dwarf shrubs and mosses cannot be completely ruled out44,75–77.
While removal of vegetation cover enhanced early seedling recruitment at treeline, there was no difference in emergence between scarified and vegetation-covered plots at the alpine site (Table 1; Fig. 1a), indicating that there was no net effect of neighbouring vegetation on recruitment at this elevation. Indeed, the absence of tall dwarf shrubs, the scarce low-stature vegetation, and a relatively high proportion of bare mineral soil in this alpine meadow may have provided suitable seed beds for germination. Similarly, Munier, et al.50 related the reduced positive effects of substrate disturbance on tree seedling recruitment at alpine sites to the habitat-specific high proportion of disturbed ground and moss seed beds. Although in our treeline ecotone net negative biotic interactions seem to have decreased with elevation, we did not detect facilitative interactions at the alpine site. This finding, which is in contrast to those of other studies (e.g.69) and to predictions of the stress-gradient hypothesis40, may be due to the structure of the particular alpine plant community, the specific susceptibilities of the studied seedling species38, or the specific location of the study sites on a northeast-facing mountain slope, where heat stress and desiccation may be less important factors78.
Besides microsite, prevailing weather conditions can also influence seedling recruitment29. Indeed, the reduced emergence at the alpine site in the warm and dry summer 2013 compared to in 2014 (Table 1; Fig. 1b) may have been due to excessive soil warming and desiccation of seed beds. These effects have been shown to inhibit germination and cause damage to freshly emerged seedlings23,31,65,79,80. This suggests that extreme weather patterns, such as summer droughts, which are expected to become more frequent under future climate change, might strongly affect seedling recruitment. As our observations were based on a short experimental period, longer-term monitoring may improve our understanding of seed source and recruitment mechanisms and their impacts on treeline dynamics. In the long run, population modelling81,82 suggests that other effects, such as dispersal distance and differences in recruitment success, may be more important in determining future treeline position. Nevertheless, the pronounced emergence after experimental seed addition in a warm and dry year as well as in a cool and wet year suggests that a recruitment pulse is possible once viable seeds reach suitable microsites for emergence at higher elevation, opening the potential for treeline expansion.
Seed source and post-dispersal predation modified recruitment success
Larix decidua had greater emergence success at and above treeline than P. abies (Table 1), which is in line with its higher upper range limit and greater tolerance for low temperatures31,83. In contrast to our hypothesis, more L. decidua seedlings from low- than from high-elevation provenance emerged at and above treeline (Table 1). This may be explained by its 16% greater seed mass compared to that of the high-elevation provenance (Table 2a), which might indicate a maternal effect or genetic differentiation among provenances84. The better performance of low-elevation provenances persisted over the first winter but diminished over the second year, with only 15% of the seedlings surviving for another full year (Supplementary Table S1). The role of provenance is thus likely to change over time. Similarly, a study on early seedling recruitment of Picea engelmannii showed that low-elevation provenances were selected for better initial survival and high-elevation provenances for tolerating harsher climate conditions in later stages of seedling establishment57,60. Likewise, a study with transplanted four-year-old P. abies seedlings indicated higher growth rates and frost tolerance levels for seedlings from high- compared to those from low-elevation provenances near treeline85. The similar responses of seedlings from high-elevation provenances and from the low-elevation P. abies provenance in our study suggested similar environmental sensitivities in early stages of seedling establishment. However, elevation-specific adaptations, such as different tolerance levels for frost or snow breakage86, may become more apparent in later stages of seedling establishment.
Table 2.
Locations of the seed provenances (a) and experimental sites (b).
| Longitude [°N] | Latitude [°E] | Elevation [m a.s.l.] | Aspect | Slope [°] | Collection year | TGW [g] | Viability [%] | Elevation range of species [m a.s.l.] | |
|---|---|---|---|---|---|---|---|---|---|
| (a) Provenance | |||||||||
| LL | 46.699 | 9.709 | 1350 | SW | — | 1995 | 8.5 | 28 | 600–2100 |
| LH | 46.509 | 9.849 | 1760 | SE | — | 1970 | 7.3 | 11 | 600–2100 |
| PL | 46.917 | 9.785 | 1000 | S | — | 1985 | 6.8 | 74 | 500–1800 |
| PH | 46.734 | 9.849 | 1960 | SW | — | 1983 | 6.8 | 61 | 500–1800 |
| (b) Experimental site | |||||||||
| Forest | 46.777 | 9.868 | 1930 | NE | 25–30 | — | — | — | — |
| Treeline | 46.774 | 9.866 | 2090 | NE | 35–40 | — | — | — | — |
| Alpine | 46.769 | 9.862 | 2410 | NE | 25–30 | — | — | — | — |
TGW: seed mass in thousand grain weight. Viability: seed viability. LL: low-elevation provenance of L. decidua; LH: high-elevation provenance of L. decidua; PL: low-elevation provenance of P. abies; PH: high-elevation provenance of P. abies.
The overall positive effect of herbivore exclosures on emergence is in line with our hypothesis and other studies, which showed that post-dispersal predation and herbivory can constrain seedling recruitment in treeline ecotones48,49. Although we did not directly observe seed and seedling predation and cannot disentangle the two, empty seed coats in experimental plots as well as seedlings damaged by herbivory indicated that both forms of predation were present. Moreover, damage to seedlings inside herbivore exclosures suggests that invertebrate predation also played a role, as has been described for other forest and treeline ecosystems50,74,87. The observed preference of seed predators for the low-elevation L. decidua provenance (Table 1) may be explained by its greater seed weight (Table 2a), as predators have been shown to prefer heavier, nutrient-rich seeds23,88. Contrary to our expectation, predation had similar effects on open and vegetation-covered microsites, suggesting that either vegetation did not influence predation, or different groups of predators profited equally from both microsite types. Furthermore, the effects of predation and herbivory may be overestimated in seeding experiments, like the one presented here, because the high density of seeds or seedlings may attract herbivory89,90, whereas natural seedlings at low density would be less affected69. Thus, additional studies of natural seedlings are needed to quantify the importance of post-dispersal seed predation and herbivory effects for treeline dynamics.
Seedling survival and growth declined with elevation
In contrast to emergence, winter survival was lower at the alpine than at the treeline site (Table 1; Figs 2, 3) and thus was possibly limited by adverse climate conditions at high elevations during snow free periods between the fall census and the spring census of the following year. Indeed, records of soil temperatures were lower at the alpine than at the treeline site in these periods (Supplementary Fig. S4). Similarly, other studies showed that winter survival of tree seedlings is primarily determined by climate conditions during snow-free periods in early and late winter91. While low temperature may have directly impacted survival, the combination with bright sunlight causing photoinhibition may have additionally increased seedling mortality at the high elevation site. Both effects can impose limitations on early tree seedling survival at alpine treelines28,35,69,92. The considerably lower first winter survival at treeline in winter 2013/2014 than in the following winter (Table 1; Fig. 2b) may have been a consequence of the slow formation of an insulating snow cover (Supplementary Fig. S3) leaving seedlings not well protected against freezing events in late fall. Similarly, Batllori, et al.28 observed high mortality of P. uncinata seedlings in a winter with a shallow snow cover. Besides its strong influence on seedling survival, snow cover duration may also have influenced seedling growth. The observed decline in seedling height with increasing elevation (Table 1; Fig. 4) is in line with the observation of reduced growth rates due to shorter growing seasons at higher elevation13,86. In addition, Zurbriggen, et al.58 suggested that declining tree seedling growth at higher elevation may be due to reduced soil nutrient availability.
First winter survival was considerably lower than second winter survival at the treeline site (Supplementary Table S1), whereas a similar comparison for the alpine site was impossible due to high first winter mortality. Seedlings that survived the first winter mostly also survived the following summer at both sites (Supplementary Fig. S5). These results confirm the common observation that the first winter is an important bottleneck for seedling recruitment, see Körner13 and references therein. Thus, high winter mortality in alpine environments can strongly affect overall recruitment and contributes to the complex puzzle of multiple abiotic and biotic factors determining regeneration in treeline ecotones.
Conclusions
This study provides experimental evidence for the successful emergence and early establishment of tree seedlings at and above the current treeline when viable seeds reach suitable microsites for germination. While dense understorey and dwarf-shrub vegetation may prevent infilling of open subalpine forests below and at treeline, recruitment above treeline is spatially and temporally restricted to suitable microsites and climatically favourable years. Our findings demonstrate the importance of multiple abiotic and biotic drivers of early seedling recruitment in the treeline ecotone that should be considered when predicting treeline dynamics under climate change.
Methods
Study species and provenances
The two study species, L. decidua and P. abies, are among the most important treeline-forming conifers in the European Alps, with L. decidua mostly restricted to the European Alps and P. abies common in the subalpine and boreal zones of Eurasia93. In Switzerland, the elevation range of L. decidua is 600 to 2,100 m a.s.l., whereas P. abies has a slightly lower elevation range of 500 to 1,800 m a.s.l.83. For each species, we used seeds from a low- and a high-elevation provenance, located within 30 km of the study area (Table 2a). Seeds were procured from the Swiss Federal Institute for Forest, Snow and Landscape Research WSL (Birmensdorf, Switzerland) and stored at 5 °C prior to sowing. Seed viability was determined by direct germination tests under laboratory conditions.
Experimental design
In summer 2013, three experimental sites were established along an elevational gradient across an alpine treeline ecotone located on a northeast-exposed slope in the Dischma valley, Davos, Switzerland (Table 2b; Supplementary Fig. S1). The lowest study site (forest site) is located at 1,930 m a.s.l., below the treeline and close to the regional upper range limit of P. abies and L. decidua, in a subalpine larch-spruce forest (Larici-Picetum) with tall and large-leafed herbaceous understorey vegetation (predominantly Adenostylion; canopy height approx. 50–100 cm). The mid-elevation site (treeline site) is located at 2,090 m a.s.l., at the current treeline, and is dominated by dense dwarf shrub vegetation (predominantly Rhododendro-Vaccinietum; canopy height approx. 50 cm) interspersed with low-tree islands of Pinus cembra and Pinus mugo. The soil was covered by a 10–15 cm thick moss layer at this site. The uppermost site (alpine site) is located at 2,410 m a.s.l., approximately 300 m above treeline, in an alpine meadow with a vegetation height of approx. 5–15 cm (Caricetum curvulae).
The three experimental sites were set up following the standard protocol of the global G-TREE initiative94. In a split-plot design, 20 whole plots (224 cm × 45 cm) were established at each site. They were completely randomly assigned to the 2 × 2 treatment combinations of the main factors seeding and scarification (i.e. seeding and scarification, seeding only, scarification only, and full control), resulting in five replications per main treatment combination. Distances between whole plots were at least 0.5 m, but usually between 1 m and 10 m. Each whole plot was divided into 16 split-plots (22.5 cm × 28 cm; referred to as subplots), to which treatment combinations of the four additional two-level factors species, provenance, herbivore exclosure, and seeding year were randomly assigned. Overall, this resulted in 960 split-plots (3 sites × 20 main plots per site × 16 split-plots per main plot). Plot setup and seeding treatment applications were staggered in time at the three sites (forest, then treeline, then alpine) to reflect the natural difference in growing season start at the three elevations. At the beginning of the experiment in summer 2013, natural recruitment and vegetation cover were assessed in each subplot. Thereafter, we applied the scarification treatment by removing vegetation, plant litter, mosses, and lichens from the plot surfaces with a hand-cultivator, but leaving roots and non-organic material in the soil. With this treatment we simulated soil disturbance and created open microsites, which is an established method to study biotic interactions with neighbouring vegetation37,95. While vegetation cover regrew within several weeks at the forest site, we did not observe significant regrowth of vegetation cover over the experiment duration in scarified plots at treeline and at the alpine site although the plots were scarified only once. Immediately after scarification, 200 seeds were spread evenly on each of the respective subplots. A second seeding treatment was applied to a different set of subplots at the beginning of the following growing season in spring 2014. In both years, immediately after seeding, herbivore exclosures made of durable and stable metal cages (45 cm × 28 cm, mesh size 2 × 4 cm) were installed on half of the subplots for the duration of the growing season. Each cage covered two adjacent subplots. We expected that the cages would exclude small mammals and birds, whereas burrowing animals, such as voles, and invertebrates were not prevented from entering the plots (personal communication M. Schütz, 2013). Fences were installed at each site to protect experimental plots from grazing and trampling by cattle and horses.
Seedling recruitment was assessed by counting the seedlings in each subplot at the beginning and end of the growing seasons in 2013, 2014, and 2015. All seedlings were individually marked to avoid double counts. Emergence was defined as the percentage of germinated seeds at the end of the first growing season (i.e. three to four months after sowing). First and second winter survival were defined as the percentage of surviving seedlings between the fall census and the spring census of the following year. Seedling height was measured with a hand ruler as the total length from the original emerging point to the apical meristem of approximately 15-month-old seedlings at the end of the growing seasons 2014 and 2015. In subplots with more than ten seedlings, only ten haphazardly chosen seedlings were measured. Seedling growth was defined as the average seedling height per subplot after two growing seasons. Maximum seedling height per subplot was also tested and showed similar results because of small height variation among seedlings (data not shown).
Daily values of mean air temperature, precipitation, and snow depth were measured at the Stillberg climate station at 2,090 m a.s.l. located at the Stillberg Long-Term Ecosystem Research site96 approx. 50 m from the treeline site. At each site, two to five temperature loggers (iButton; Maxim Integrated Products, Sunnyvale, CA, USA) recorded soil temperatures at a depth of 5 cm in vegetation-covered plots, as well as in plots where surface vegetation was removed. The beginning and end of the growing season were defined as the dates when soil temperature rose for the first time above (beginning) or fell below (end) 3.2 °C for two contiguous days14,97. Additionally, the dates of the first snow cover in autumn and snowmelt in spring were derived from soil temperature measurements and defined as the dates when daily temperature fluctuations stopped and soil temperature remained at 0 °C (snow cover), and when daily temperature fluctuations restarted (snowmelt).
Statistical Analyses
A two-sided t-test was applied to compare emergence in seeded versus non-seeded plots. Linear and generalised linear mixed-effect models (LMMs and GLMMs)98 were used to analyse the response of seedling recruitment (emergence, winter survival, and growth) to the investigated abiotic and biotic factors and their interactions. Due to the almost complete lack of natural germination, non-seeded plots were omitted from these analyses. The forest site was excluded from survival and growth trait models because of very low seedling recruitment. GLMMs for seedling emergence and first winter survival contained site, scarification treatment, seeding year, species, provenance, and herbivore exclosure treatment as fixed effects. Because of the small data set for second winter survival, it was not possible to test the influence of seeding year and herbivore exclosure treatment, as well as their interactions. All three GLMMs used binomial distributions. Seedling height was modelled using a LMM with the fixed effects site and scarification treatment and their interaction. All models accounted for spatial correlation among plots and subplots by including plot and two subplot-structures as random effects. Significance of model factors was determined by likelihood ratio tests, and fixed effects that did not significantly improve the model fit were eliminated. All models were fitted using standard procedures for model diagnostics99 with the lme4-library (version 1.1–13)100 in R 3.3.3101.
Data availability
Climate data are archived in the EnviDat Digital Repository: 10.16904/envidat.4396 and 10.16904/envidat.42102. All other data generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
We thank M. Heggli, L. Voegele, E.S. Frei, A. Kulonen, Q. Canelles Trabal, G. Lang, S. Burg, C. Vergani, A. Ilari, I. Barbeito, U. Schmid, M. Buchmann, L. Erdle, S. Wipf, and the team of the SLF Werkstatt for helping with setting up the experiment and measuring seedling recruitment. We also thank the Alpgenossenschaft Davos for the permission to work on their land. Furthermore, we thank M. Schütz for support with the herbivore exclosures and J. Caduff-Fiddes for providing climate station data and the ETH Statistik Beratung for statistical advice. E.R.F. is thankful for support by the Basler Stiftung für biologische Forschung.
Author Contributions
E.R.F., P.B., C.D.B., A.J.T., S.D.M., and C.R. conceived the ideas and designed the methodology; E.R.F., E.B., G.B., M.A.D., and C.R. collected the data; E.R.F., E.B., G.B., and M.A.D. analysed the data; E.R.F., P.B., and C.R. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28808-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 1535, www.cambridge.org/9781107661820 (Cambridge University Press, 2013).
- 2.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Sturm M, Douglas T, Racine C, Liston GE. Changing snow and shrub conditions affect albedo with global implications. Journal of Geophysical Research-Biogeosciences. 2005;110:1–13. doi: 10.1029/2005JG000013. [DOI] [Google Scholar]
- 5.Myers-Smith IH, et al. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters. 2011;6:1–15. doi: 10.1088/1748-9326/6/4/045509. [DOI] [Google Scholar]
- 6.Elmendorf SC, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change. 2012;2:453–457. doi: 10.1038/nclimate1465. [DOI] [Google Scholar]
- 7.Theurillat JP, Guisan A. Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change. 2001;50:77–109. doi: 10.1023/A:1010632015572. [DOI] [Google Scholar]
- 8.Devi N, et al. Expanding forests and changing growth forms of Siberian larch at the Polar Urals treeline during the 20th century. Global Change Biology. 2008;14:1581–1591. doi: 10.1111/j.1365-2486.2008.01583.x. [DOI] [Google Scholar]
- 9.Danby RK, Hik DS. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology. 2007;95:352–363. doi: 10.1111/j.1365-2745.2006.01200.x. [DOI] [Google Scholar]
- 10.Kullman L. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology. 2002;90:68–77. doi: 10.1046/j.0022-0477.2001.00630.x. [DOI] [Google Scholar]
- 11.Esper J, Schweingruber FH. Large-scale treeline changes recorded in Siberia. Geophysical Research Letters. 2004;31:1–5. doi: 10.1029/2003GL019178. [DOI] [Google Scholar]
- 12.Holtmeier FK, Broll G. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography. 2005;14:395–410. doi: 10.1111/j.1466-822X.2005.00168.x. [DOI] [Google Scholar]
- 13.Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits (Springer, 2012).
- 14.Körner C, Paulsen J. A world-wide study of high altitude treeline temperatures. Journal of Biogeography. 2004;31:713–732. doi: 10.1111/j.1365-2699.2003.01043.x. [DOI] [Google Scholar]
- 15.Körner, C. Significance of temperature in plant life in Plant Growth and Climate Change (eds Morison, J. I. L. & Morecroft, M. D.) 48-69 (Blackwell, 2007).
- 16.Gehrig-Fasel J, Guisan A, Zimmermann NE. Tree line shifts in the Swiss Alps: Climate change or land abandonment? Journal of Vegetation Science. 2007;18:571–582. doi: 10.1111/j.1654-1103.2007.tb02571.x. [DOI] [Google Scholar]
- 17.Holtmeier FK, Broll G. Treeline advance driving processes and adverse factors. Landscape Online. 2007;1:1–33. doi: 10.3097/LO.200701. [DOI] [Google Scholar]
- 18.Chapin FS, et al. Role of land-surface changes in Arctic summer warming. Science. 2005;310:657–660. doi: 10.1126/science.1117368. [DOI] [PubMed] [Google Scholar]
- 19.Loarie SR, et al. The velocity of climate change. Nature. 2009;462:1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 20.Harsch MA, Hulme PE, McGlone MS, Duncan RP. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters. 2009;12:1040–1049. doi: 10.1111/j.1461-0248.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd AH. Ecological histories from Alaskan tree lines provide insight into future change. Ecology. 2005;86:1687–1695. doi: 10.1890/03-0786. [DOI] [Google Scholar]
- 22.Turnbull LA, Crawley MJ, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. doi: 10.1034/j.1600-0706.2000.880201.x. [DOI] [Google Scholar]
- 23.Holtmeier, F. K. Mountain Timberlines: Ecology, Patchiness, and Dynamics. 2nd edn, Vol. 36 (Springer, 2009).
- 24.Kroiss SJ, HilleRisLambers J. Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology. 2015;96:1286–1297. doi: 10.1890/14-0595.1. [DOI] [PubMed] [Google Scholar]
- 25.Kuoch R. Der Samenanflug 1963/1964 an der oberen Fichtenwaldgrenze im Sertigtal. Mitt. Eidg. Anst. forst. Versuchswesen. 1965;41:63–85. [Google Scholar]
- 26.Mazepa VS. Stand density in the last millennium at the upper tree-line ecotone in the Polar Ural Mountains. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2005;35:2082–2091. doi: 10.1139/x05-111. [DOI] [Google Scholar]
- 27.Jameson RG, Trant AJ, Hermanutz L. Insects can limit seed productivity at the treeline. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2015;45:286–296. doi: 10.1139/cjfr-2014-0385. [DOI] [Google Scholar]
- 28.Batllori E, Camarero JJ, Ninot JM, Gutierrez E. Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line ecotones. Implications and potential responses to climate warming. Global Ecology and Biogeography. 2009;18:460–472. doi: 10.1111/j.1466-8238.2009.00464.x. [DOI] [Google Scholar]
- 29.Germino, M. J., Smith, W. K. & Resor, A. C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecology162, 157–168, 10.1023/A:1020385320738 (2002).
- 30.Leck, M. A., Parker, V. T. & Simpson, R. L. Seedling Ecology and Evolution (Cambridge University Press, 2008).
- 31.Loranger H, Zotz G, Bader MY. Early establishment of trees at the alpine treeline: idiosyncratic species responses to temperature-moisture interactions. AoB PLANTS. 2016;8:plw053. doi: 10.1093/aobpla/plw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyes AB, Germino MJ, Kueppers LM. Moisture rivals temperature in limiting photosynthesis by trees establishing beyond their cold-edge range limit under ambient and warmed conditions. New Phytologist. 2015;207:1005–1014. doi: 10.1111/nph.13422. [DOI] [PubMed] [Google Scholar]
- 33.Richter S, et al. Phenotypic plasticity facilitates resistance to climate change in a highly variable environment. Oecologia. 2012;169:269–279. doi: 10.1007/s00442-011-2191-x. [DOI] [PubMed] [Google Scholar]
- 34.Wipf S, Rixen C. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research. 2010;29:95–109. doi: 10.1111/j.1751-8369.2010.00153.x. [DOI] [Google Scholar]
- 35.Renard SM, McIntire EJB, Fajardo A. Winter conditions - not summer temperature - influence establishment of seedlings at white spruce alpine treeline in Eastern Quebec. Journal of Vegetation Science. 2016;27:29–39. doi: 10.1111/jvs.12347. [DOI] [Google Scholar]
- 36.McIntire EJB, Piper FI, Fajardo A. Wind exposure and light exposure, more than elevation-related temperature, limit tree line seedling abundance on three continents. Journal of Ecology. 2016;104:1379–1390. doi: 10.1111/1365-2745.12599. [DOI] [Google Scholar]
- 37.Tingstad L, Olsen SL, Klanderud K, Vandvik V, Ohlson M. Temperature, precipitation and biotic interactions as determinants of tree seedling recruitment across the tree line ecotone. Oecologia. 2015;179:599–608. doi: 10.1007/s00442-015-3360-0. [DOI] [PubMed] [Google Scholar]
- 38.Loranger H, Zotz G, Bader MY. Competitor or facilitator? The ambiguous role of alpine grassland for the early establishment of tree seedlings at treeline. Oikos. 2017;126:1625–1636. doi: 10.1111/oik.04377. [DOI] [Google Scholar]
- 39.D’Odorico P, et al. Vegetation-microclimate feedbacks in woodland-grassland ecotones. Global Ecology and Biogeography. 2013;22:364–379. doi: 10.1111/geb.12000. [DOI] [Google Scholar]
- 40.Callaway RM, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- 41.Thrippleton T, Bugmann H, Kramer-Priewasser K, Snell RS. Herbaceous Understorey: An Overlooked Player in Forest LandscapeDynamics? Ecosystems. 2016;19:1240–1254. doi: 10.1007/s10021-016-9999-5. [DOI] [Google Scholar]
- 42.Hobbie SE, Chapin FS. An experimental test of limits to tree establishment in Arctic tundra. Journal of Ecology. 1998;86:449–461. doi: 10.1046/j.1365-2745.1998.00278.x. [DOI] [Google Scholar]
- 43.Schönenberger W. Standortseinflüsse auf Versuchsaufforstungen an der alpinen Waldgrenze (Stillberg, Davos) Mitt. Eidg. Anst. forst. Versuchswesen. 1975;51:359–428. [Google Scholar]
- 44.Pellissier F. Allelopathic inhibition of spruce germination. Acta Oecologica-International Journal of Ecology. 1993;14:211–218. [Google Scholar]
- 45.Moir WH, Rochelle SG, Schoettle AW. Microscale patterns of tree establishment near upper treeline, Snowy Range, Wyoming, USA. Arctic Antarctic and Alpine Research. 1999;31:379–388. doi: 10.2307/1552586. [DOI] [Google Scholar]
- 46.Bertness MD, Callaway R. Positive interactions in communities. Trends in Ecology & Evolution. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 47.Brown CD, Vellend M. Non-climatic constraints on upper elevational plant range expansion under climate change. Proc. R. Soc. B. 2014;281:20141779. doi: 10.1098/rspb.2014.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cairns DM, Lafon C, Moen J, Young A. Influences of animal activity on treeline position and pattern: Implications for treeline responses to climate change. Physical Geography. 2007;28:419–433. doi: 10.2747/0272-3646.28.5.419. [DOI] [Google Scholar]
- 49.Cairns DM, Moen J. Herbivory influences tree lines. Journal of Ecology. 2004;92:1019–1024. doi: 10.1111/j.1365-2745.2004.00945.x. [DOI] [Google Scholar]
- 50.Munier A, Hermanutz L, Jacobs JD, Lewis K. The interacting effects of temperature, ground disturbance, and herbivory on seedling establishment: implications for treeline advance with climate warming. Plant Ecology. 2010;210:19–30. doi: 10.1007/s11258-010-9724-y. [DOI] [Google Scholar]
- 51.Royo AA, Carson WP. Direct and indirect effects of a dense understory on tree seedling recruitment in temperate forests: habitat-mediated predation versus competition. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2008;38:1634–1645. doi: 10.1139/X07-247. [DOI] [Google Scholar]
- 52.Pellissier F, Trosset L. Difficulty of natural regeneration of sub-alpine forests - seed consumption and humic inhibition. Annales Des Sciences Forestieres. 1992;49:383–388. doi: 10.1051/forest:19920406. [DOI] [Google Scholar]
- 53.Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2009;39:1259–1269. doi: 10.1139/X09-054. [DOI] [Google Scholar]
- 54.Turesson G. The genotypical response of the plant species to the habitat. Hereditas. 1922;3:211–350. doi: 10.1111/j.1601-5223.1922.tb02734.x. [DOI] [Google Scholar]
- 55.Rehfeldt GE, Ying CC, Spittlehouse DL, Hamilton DA. Genetic responses to climate in Pinus contorta: Niche breadth, climate change, and reforestation. Ecological Monographs. 1999;69:375–407. doi: 10.1890/0012-9615(1999)069[0375:GRTCIP]2.0.CO;2. [DOI] [Google Scholar]
- 56.Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. 2nd edn (Springer, 2003).
- 57.Castanha C, Torn MS, Germino MJ, Weibel B, Kueppers LM. Conifer seedling recruitment across a gradient from forest to alpine tundra: effects of species, provenance, and site. Plant Ecology & Diversity. 2013;6:307–318. doi: 10.1080/17550874.2012.716087. [DOI] [Google Scholar]
- 58.Zurbriggen N, Hättenschwiler S, Frei ES, Hagedorn F, Bebi P. Performance of germinating tree seedlings below and above treeline in the Swiss Alps. Plant Ecology. 2013;214:385–396. doi: 10.1007/s11258-013-0176-z. [DOI] [Google Scholar]
- 59.Danby RK, Hik DS. Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Global Change Biology. 2007;13:437–451. doi: 10.1111/j.1365-2486.2006.01302.x. [DOI] [Google Scholar]
- 60.Kueppers LM, et al. Warming and provenance limit tree recruitment across and beyond the elevation range of subalpine forest. Global Change Biology. 2017;23:2383–2395. doi: 10.1111/gcb.13561. [DOI] [PubMed] [Google Scholar]
- 61.Scherrer D, Körner C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Global Change Biology. 2010;16:2602–2613. [Google Scholar]
- 62.Imbeck H, Ott E. Verjüngungsökologische Untersuchungen in einem hochstaudenreichen subalpinen Fichtenwald, mit spezieller Berücksichtigung der Schneeablagerung und der Lawinenbildung. Mitt. Eidg. Inst. Schnee- u. Lawinenforschung. 1987;42:202 p. [Google Scholar]
- 63.Ott E, Lüscher F, Frehner M, Brang P. Verjüngungsökologische Besonderheiten im Gebirgsfichtenwald im Vergleich zur Bergwaldstufe. Schweiz Z Forstwes. 1991;142:879–904. [Google Scholar]
- 64.Bace R, Svoboda M, Janda P. Density and height structure of seedlings in subalpine spruce forests of Central Europe: logs vs. stumps as a favourable substrate. Silva Fennica. 2011;45:1065–1078. doi: 10.14214/sf.87. [DOI] [Google Scholar]
- 65.Maher EL, Germino MJ, Hasselquist NJ. Interactive effects of tree and herb cover on survivorship, physiology, and microclimate of conifer seedlings at the alpine tree-line ecotone. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2005;35:567–574. doi: 10.1139/x04-201. [DOI] [Google Scholar]
- 66.Bader MY, van Geloof I, Rietkerk M. High solar radiation hinders tree regeneration above the alpine treeline in northern Ecuador. Plant Ecology. 2007;191:33–45. doi: 10.1007/s11258-006-9212-6. [DOI] [Google Scholar]
- 67.Weih M, Karlsson PS. The nitrogen economy of mountain birch seedlings: implications for winter survival. Journal of Ecology. 1999;87:211–219. doi: 10.1046/j.1365-2745.1999.00340.x. [DOI] [Google Scholar]
- 68.Berntson GM, Wayne PM. Characterizing the size dependence of resource acquisition within crowded plant populations. Ecology. 2000;81:1072–1085. doi: 10.1890/0012-9658(2000)081[1072:CTSDOR]2.0.CO;2. [DOI] [Google Scholar]
- 69.Maher EL, Germino MJ. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience. 2006;13:334–341. doi: 10.2980/i1195-6860-13-3-334.1. [DOI] [Google Scholar]
- 70.Bansal S, Reinhardt K, Germino MJ. Linking carbon balance to establishment patterns: comparison of whitebark pine and Engelmann spruce seedlings along an herb cover exposure gradient at treeline. Plant Ecology. 2011;212:219–228. doi: 10.1007/s11258-010-9816-8. [DOI] [Google Scholar]
- 71.Brang, P. Experimentelle Untersuchungen zur Ansamungsökologie der Fichte im zwischenalpinen Gebirgswald PhD thesis, ETH Zürich, 10.3929/ethz-a-001513475 (1995).
- 72.Motta R, Brang P, Frehner M, Ott E. Copertura muscinale e rinnovazione di abete rosso (Picea abies L.) nella pecceta subalpina di Sedrun (Grigioni, Svizzera) Monti e Boschi. 1994;45:49–56. [Google Scholar]
- 73.Hunziker U, Brang P. Microsite patterns of conifer seedling establishment and growth in a mixed stand in the southern Alps. Forest Ecology and Management. 2005;210:67–79. doi: 10.1016/j.foreco.2005.02.019. [DOI] [Google Scholar]
- 74.Wheeler, J. A., Hermanutz, L. & Marino, P. M. Feathermoss seedbeds facilitate black spruce seedling recruitment in the forest–tundra ecotone (Labrador, Canada) Oikos120, 1263–1271, 10.1111/j.1600-0706.2010.18966.x (2011).
- 75.Nilsson MC, Wardle DA. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Frontiers in Ecology and the Environment. 2005;3:421–428. doi: 10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2. [DOI] [Google Scholar]
- 76.Nilsson MC, Zackrisson O, Sterner O, Wallstedt A. Characterisation of the differential interference effects of two boreal dwarf shrub species. Oecologia. 2000;123:122–128. doi: 10.1007/s004420050997. [DOI] [PubMed] [Google Scholar]
- 77.Dufour-Tremblay G, De Vriendt L, Lévesque E, Boudreau S. The importance of ecological constraints on the control of multi-species treeline dynamics in Eastern Nunavik, Québec. American Journal of Botany. 2012;99:1638–1646. doi: 10.3732/ajb.1200279. [DOI] [PubMed] [Google Scholar]
- 78.Brang P. Early seedling establishment of Picea abies in small forest gaps in the Swiss Alps. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 1998;28:626–639. doi: 10.1139/x98-035. [DOI] [Google Scholar]
- 79.Moyes AB, Castanha C, Germino MJ, Kueppers LM. Warming and the dependence of limber pine (Pinus flexilis) establishment on summer soil moisture within and above its current elevation range. Oecologia. 2013;171:271–282. doi: 10.1007/s00442-012-2410-0. [DOI] [PubMed] [Google Scholar]
- 80.Lucas-Borja ME, et al. Pinus nigra Arn. ssp salzmannii seedling recruitment is affected by stand basal area, shrub cover and climate interactions. Annals of Forest Science. 2016;73:649–656. doi: 10.1007/s13595-016-0550-9. [DOI] [Google Scholar]
- 81.Conlisk E, et al. Seed origin and warming constrain lodgepole pine recruitment, slowing the pace of population range shifts. Global Change Biology. 2018;24:197–211. doi: 10.1111/gcb.13840. [DOI] [PubMed] [Google Scholar]
- 82.Conlisk E, et al. Declines in low-elevation subalpine tree populations outpace growth in high-elevation populations with warming. Journal of Ecology. 2017;105:1347–1357. doi: 10.1111/1365-2745.12750. [DOI] [Google Scholar]
- 83.Brändli, U. B. Die häufigsten Waldbäume der Schweiz - Ergebnisse aus dem Landesforstinventar 1983–1985. Ber. Eidg. Forschungsanst. WSL342, 278 p, http://www.dora.lib4ri.ch/wsl/islandora/object/wsl:14554 (1998).
- 84.Castro J. Seed mass versus seedling performance in Scots pine: a maternally dependent trait. New Phytologist. 1999;144:153–161. doi: 10.1046/j.1469-8137.1999.00495.x. [DOI] [Google Scholar]
- 85.Ryter U. Hochlagenaufforstungen in Lawinenverbauungen im Berner Oberland. Schweiz Z Forstwes. 2014;165:259–267. doi: 10.3188/szf.2014.0259. [DOI] [Google Scholar]
- 86.Tranquillini, W. Physiological Ecology of the Alpine Timberline - Tree Existence at High Altitudes with Special Reference to the European Alps (Springer, 1979).
- 87.Côté M, Ferron J, Gagnon R. Invertebrate predation of postdispersal seeds and juvenile seedlings of black spruce (Picea mariana) in the boreal forest of eastern Canada. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière. 2005;35:674–681. doi: 10.1139/x05-001. [DOI] [Google Scholar]
- 88.Maron JL, Pearson DE, Potter T, Ortega YK. Seed size and provenance mediate the joint effects of disturbance and seed predation on community assembly. Journal of Ecology. 2012;100:1492–1500. doi: 10.1111/j.1365-2745.2012.02027.x. [DOI] [Google Scholar]
- 89.Janzen, D. H. Seed predation by animals. Annual Review of Ecology and Systematics2, 465-492, 10.1146/annurev.es.02.110171.002341 (1971).
- 90.Price MV, Heinz KM. Effects of body size, seed density, and soil characteristics on rates of seed harvest by heteromyid rodents. Oecologia. 1984;61:420–425. doi: 10.1007/BF00379646. [DOI] [PubMed] [Google Scholar]
- 91.Bansal S, Germino MJ. Variation in ecophysiological properties among conifers at an ecotonal boundary: comparison of establishing seedlings and established adults at timberline. Journal of Vegetation Science. 2010;21:133–142. doi: 10.1111/j.1654-1103.2009.01127.x. [DOI] [Google Scholar]
- 92.Germino MJ, Smith WK. Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant Cell and Environment. 1999;22:407–415. doi: 10.1046/j.1365-3040.1999.00426.x. [DOI] [Google Scholar]
- 93.Ellenberg, H. Vegetation Mitteleuropas mit den Alpen in ökologischer Sicht. 2nd edn (Ulmer, 1978).
- 94.Brown, C. D., Johnstone, J. F., Mamet, S. D. & Trant, A. J. Global Treeline Range Expansion Experiment Field Protocols, http://www.treelineresearch.com (2013).
- 95.Aarssen LW, Epp GA. Neighbor manipulations in natural vegetation - a review. Journal of Vegetation Science. 1990;1:13–30. doi: 10.2307/3236049. [DOI] [Google Scholar]
- 96.Bebi, P. Long-term meteorological and snow station at 2090 m a.s.l., Stillberg, Davos, Switzerland (1975 - present). WSL Institute for Snow and Avalanche Research SLF, 10.16904/envidat.43 (2016).
- 97.Rixen C, Dawes MA, Wipf S, Hagedorn F. Evidence of enhanced freezing damage in treeline plants during six years of CO2 enrichment and soil warming. Oikos. 2012;121:1532–1543. doi: 10.1111/j.1600-0706.2011.20031.x. [DOI] [Google Scholar]
- 98.Faraway, J. J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models (Chapman & Hall/CRC, 2006).
- 99.Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Springer, 2009).
- 100.Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 101.R Core Team R: A language and environment for statistical computing, https://www.R-project.org (R Foundation for Statistical Computing, Vienna, 2017).
- 102.Frei, E. R., Bebi, P., Dawes, M. A. & Rixen C. G-TREE: Global Treeline Range Expansion Experiment Davos, Switzerland. WSL Institute for Snow and Avalanche Research SLF, 10.16904/envidat.42 (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Climate data are archived in the EnviDat Digital Repository: 10.16904/envidat.4396 and 10.16904/envidat.42102. All other data generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.