Abstract
Podocyte injury is a primary contributor to proteinuria. Triptolide is a major active component of Tripterygium wilfordii Hook F that exhibits potent antiproteinuric effects. We used our previously developed in vivo zebrafish model of inducible podocyte-target injury and found that triptolide treatment effectively alleviated oedema, proteinuria and foot process effacement. Triptolide also inhibited podocyte apoptosis in our zebrafish model and in vitro. We also examined the mechanism of triptolide protection of podocyte. Whole-genome expression profiles of cultured podocytes demonstrated that triptolide treatment downregulated apoptosis pathway-related GADD45B expression. Specific overexpression of gadd45b in zebrafish podocytes abolished the protective effects of triptolide. GADD45B is a mediator of podocyte apoptosis that contains typical NF-κB binding sites in the promoter region, and NF-κB p65 primarily transactivates this gene. Triptolide inhibited NF-κB signalling activation and binding of NF-κB to the GADD45B promoter. Taken together, our findings demonstrated that triptolide attenuated proteinuria and podocyte apoptosis via inhibition of NF-κB/GADD45B signalling, which provides a new understanding of the antiproteinuric effects of triptolide in glomerular diseases.
Introduction
Podocytes are specialized epithelial cells of the glomerulus that crucially contribute to the filtration apparatus of the kidney. Podocyte damage inevitably leads to proteinuria and glomerular dysfunction, which are relevant to acute and chronic glomerular diseases, including focal segmental glomerulosclerosis (FSGS), diabetic kidney disease (DKD) and HIV-related nephropathy1,2. However, treatments targeting podocytes are still very limited.
The GADD45 gene family is involved in the regulation of cell survival and apoptosis, DNA damage repair, and cell cycle arrest. Shi et al. found that GADD45B was upregulated in glomeruli of podo-Dicer−/− mice, which indicated the involvement of GADD45B in podocyte damage3. We previously found that GADD45B was a critical mediator of podocyte apoptosis and that this gene was significantly upregulated in podocytes of patients with FSGS. Podocyte-specific overexpression of gadd45b in zebrafish podocytes aggravated proteinuria and foot process effacement as well as promoted podocyte apoptosis via activation of the p38 MAPK pathway4. These results suggest that GADD45B is a potent therapeutic target for the management of podocyte injury.
Triptolide is a small biologically active molecule and key component isolated from the Chinese medicinal herb Tripterygium wilfordii Hook F. Triptolide exhibits multiple pharmacological benefits, including anti-inflammatory, immunosuppressive, and antineoplastic effects5,6. Triptolide obviously decreases proteinuria levels in patients with minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS) and membranous nephropathy (MN)7. Triptolide reduces proteinuria in rats with puromycin-induced nephropathy (PAN) and passive Heymann nephritis via inhibition of ROS production and the p38 MAPK signalling pathway as well as restoration of RhoA activity8,9. However, the key targets of triptolide in podocytes are not known.
Zebrafish (Danio rerio) emerged as an ideal in vivo model for podocyte research10,11 and small molecule screening12,13. We used an inducible podocyte-target injury model in transgenic zebrafish as an in vivo model system and cultured human podocytes as an in vitro model to examine the protective mechanism of triptolide in podocyte injury. This transgenic zebrafish model is a podocyte-specific ablation model that exhibits no toxic effects on any other cells compared to the PAN nephrosis rat model. Our results indicated that triptolide attenuated proteinuria and podocyte apoptosis in zebrafish. These effects were associated with inhibition of GADD45B expression. We also found that triptolide inhibited NF-κB p65 phosphorylation, which binds to the GADD45B promoter and activates GADD45B transcription in vitro.
Results
Triptolide attenuates MTZ-induced oedema and proteinuria in zebrafish
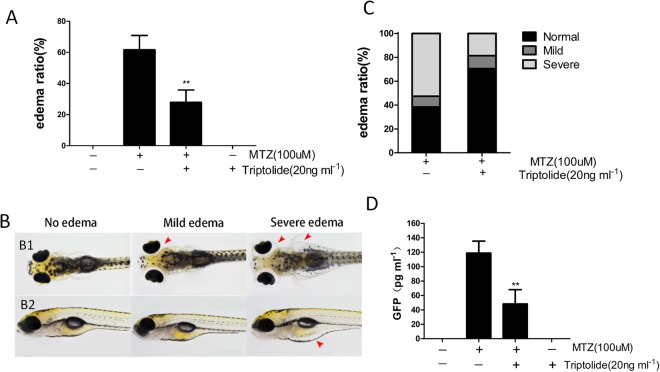
We initially evaluated the effects of triptolide at 0, 20, 40, 60, 80, 100, 120 and 200 ng ml−1 via treatment of wild-type zebrafish embryos at 60 hpf for 72 h. We found that the embryos exhibited several development abnormalities, including body curve, growth delay and axis shortening at triptolide concentrations greater than 80 ng ml−1 (see Supplement Fig. 1). None of the embryos survived in 200 ng ml−1 triptolide. Triptolide at 20, 40 and 60 ng ml−1 produced no obvious toxic effects on embryos. The bacterial nitroreductase (NTR) in the Tg(pod:gal4;UAS:NTR-mCherry) is specifically expressed in podocytes of zebrafish pronephros and mesonephros, which convert metronidazole (MTZ) into a cytotoxin to induce only podocytes injury14. The Tg(pod:Gal4;UAS:NTR-mCherry) embryos (84 hpf) were exposed to 100 μM MTZ for 48 h to induce podocyte injury. The transgenic larvae exhibited periorbital oedema (POE) and sac yolk oedema (SE) (Fig. 1B). MTZ treatment induced a total oedema ratio of 61.5 ± 18.4%. We categorized the embryos with POE alone as mild oedema and embryos with POE and SE as severe oedema (Fig. 1B). The mild and severe oedema ratios were 8.9 ± 4.1% and 52.6 ± 25.3%, respectively. We pre-treated the embryos (60 hpf) with 20 ng ml−1 triptolide for 24 h prior to MTZ treatment to investigate the effect of triptolide on transgenic zebrafish carrying NTR-mCherry. The total oedema ratio decreased significantly to 27.8% ± 15.9% compared to the MTZ group (Fig. 1A). The mild and severe oedema ratios were 10.1 ± 5.9% and 18.5 ± 10.8%, respectively (Fig. 1C). We performed a proteinuria assay in Tg((pod:Gal4;UAS:NTR-mCherry;lfabp:VDBP-GFP) embryos (120 hpf)10 using ELISA against GFP after MTZ treatment to examine whether triptolide attenuated proteinuria. Cotreatment with triptolide significantly reduced MTZ-induced proteinuria (Fig. 1D). Therefore, triptolide treatment significantly reduced the percentage of larvae that developed oedema and proteinuria.
Figure 1.
Triptolide treatment alleviated MTZ-induced oedema and proteinuria. (A,C) Quantification of the oedema ratio (n = 30) and severity of oedema. The embryos were treated with 100 μM MTZ from 84 hpf to 132 hpf. Triptolide pretreatment obviously reduced MTZ-induced oedema percentage and severity. (B) Representative figure shows the severity of oedema (arrowhead) from a dorsal view (B1) and side view (B2). (D) GFP Quantification using ELISA showing that triptolide pretreatment reduced the MTZ-induced proteinuria (n = 3). **P < 0.01. (On the basis of three triplicate tests).
Triptolide protects podocytes from MTZ-induced injury in zebrafish
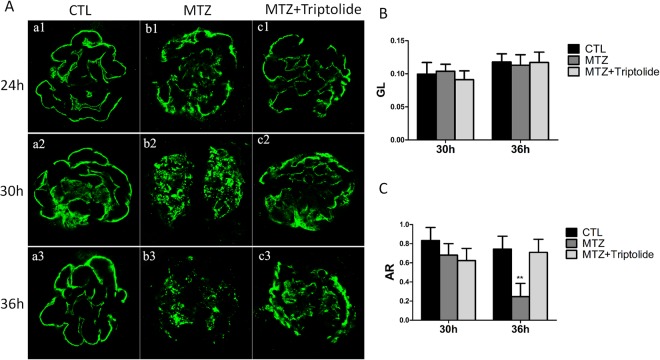
Podocin is a protein component of the filtration slits of podocytes, and it exhibits a very fine linear-like pattern along the capillary loop in normal zebrafish pronephros glomeruli (Fig. 2A(a1–a3). Mutations in NPHS2 or the loss of podocin produce nephritic syndrome and proteinuria15. We created a polyclonal anti-zebrafish podocin antibody and performed whole-mount antibody staining to investigate the effect of triptolide on podocin expression in zebrafish. We divided embryos into an MTZ group and triptolide plus MTZ group. Embryos treated with MTZ for 24 h exhibited a discontinuous distribution of podocin, and the distribution of podocin remained intact in the triptolide + MTZ group (Fig. 2A(b1,c1). Podocin expression was detached in an obvious coarse granular pattern in the MTZ group after 30 h of treatment, but exhibited a linear-like distribution in the triptolide + MTZ group (Fig. 2A(b2,c2). A serious decline in podocin expression was observed in the MTZ group compared to the triptolide + MTZ group after 36 h of MTZ treatment (Fig. 2A(b3,c3). Quantification demonstrated that the immunofluorescence intensity (GL) and stained area to the glomerulus area (AR) was not altered significantly in either group at 30 h (Fig. 2B,C). Podocin AR decreased remarkably at 36 h in the MTZ group (P < 0.01) but did not noticeably change in the triptolide + MTZ group (Fig. 2B,C).
Figure 2.
The effects of triptolide on podocin expression and distribution in MTZ-treated Tg(pod:gal4;UAS:NTR-mCherry) zebrafish. (A) The expression and distribution of podocin in different groups was measured and examined at 24 h, 30 h and 36 h after MTZ treatment in 84 hpf larvae; (a1–a3) normal control (CTL); (b1–b3) MTZ model group; (c1–c3) MTZ + triptolide group. (B) No obvious mean immunofluorescence intensity (GL) differences were observed between the three groups at 30 h and 36 h. (C) Positively stained area to the glomerular area (AR) revealed a remarkably reduction at 36 h in the MTZ model group, but remained strong in the triptolide treatment group. (n = 3), **P < 0.01, (original magnification, x1000).
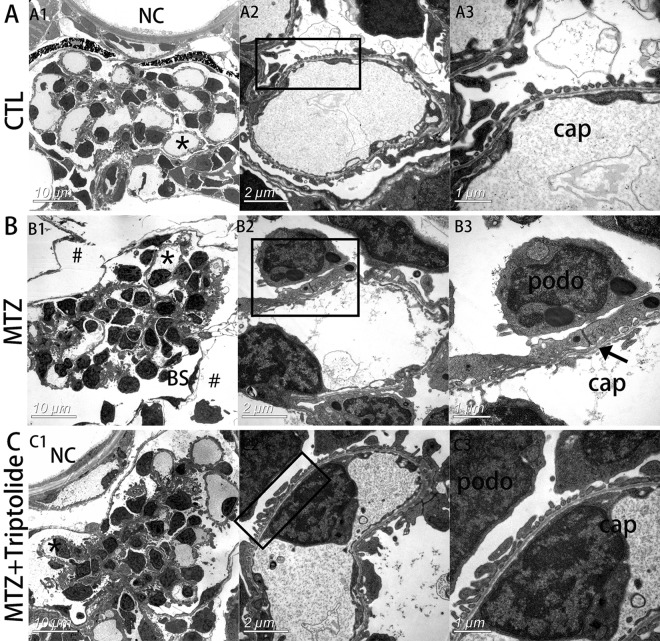
Transmission electron microscopy (TEM) revealed that the space outside of Bowman’s capsule was enlarged in the MTZ group (Fig. 3B1,#), which indicates oedema. Higher magnifications revealed the disappearance of slit diaphragms and severe foot process effacement in the MTZ group (Fig. 3B2,B3). However, the space around glomeruli was normal, and foot process effacement was intact in the triptolide + MTZ group (Fig. 3C). Therefore, we concluded that triptolide pre-treatment protected podocytes from MTZ-induced injury and prevented podocyte loss.
Figure 3.
Triptolide reversed podocyte foot process effacement in MTZ-treated Tg(pod:gal4;UAS:NTR-mCherry) zebrafish. (A) Electron micrograph of normal glomerular vascular loop morphology and foot process development at 6 dpf in wild-type larvae. (B) Glomerular morphology of zebrafish treated with MTZ for 24 h. At low magnifications, an enlarged space outside of Bowman’s capsule was observed, indicating oedema (B1, indicated by #). Extensive foot process effacement was observed at higher magnifications (B3, indicated by arrows). (C) Triptolide prevented most foot process effacement. (n = 3). *Capillary that was magnified. podo, podocyte; cap, capillary space; BS, Bowman’s space; NC, notochord. Scale bar = 10 μm (A1,B1,C1), 2 μm (A2,B2,C2), 1 μm (A3,B3,C3).
Triptolide protects podocytes from apoptosis in MTZ-induced zebrafish and PAN- treated podocytes
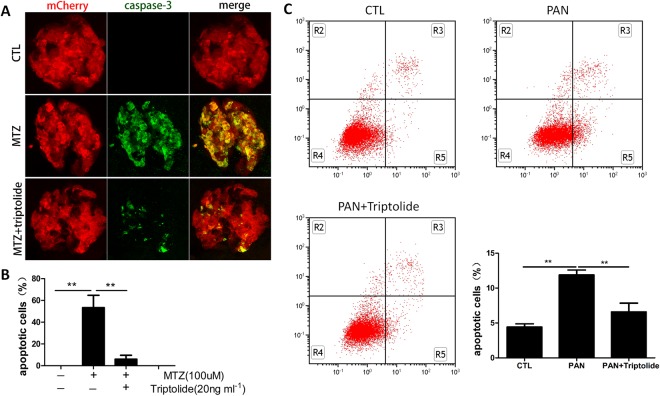
Apoptosis is the process of programmed cell death that occurs in multicellular organisms. Previous studies demonstrated that apoptosis increased in MTZ-treated zebrafish podocytes10. We performed activated caspase-3 staining to examine the protective function of triptolide on podocyte apoptosis. We confirmed extensive apoptosis in podocytes following 24 h of MTZ treatment, and capase-3 signals co-localized with mCherry in pronephros. No activated caspase-3 was observed in the normal group. However, we pre-treated Tg(pod:Gal4;UAS:NTR-mCherry) embryos with triptolide for 24 h prior to MTZ treatment, and few apoptosis signals were detected in zebrafish pronephros (Fig. 4A). We quantified the number of apoptotic podocytes and measured the area ratio of capase-3 to mCherry, which represented all podocytes. The percentage of apoptotic podocytes were 53.2 ± 19.8% in the MTZ group and 5.9 ± 3.6% in triptolide + MTZ group (Fig. 4B). Moreover, in vitro study showed the increase in podocytes apoptosis challenged with PAN was mitigated with triptolide treatment (Fig. 4C). These results suggest the protective effect of triptolide on podocytes directly.
Figure 4.
Triptolide suppressed podocyte apoptosis. (A) 84 hpf larvae treated with MTZ for 24 h exhibit remarkable podocyte apoptosis signalling compared to the untreated group (CTL) on immunostains against cleaved caspase-3. The number of apoptotic cells was significantly reduced in the triptolide treatment group. (B) Quantification of caspase-3-stained area (green) to mCherry (red). Data are expressed as the means ± SEM. The confocal images are shown at a maximum intensity projection. (n = 3), **P < 0.01; *P < 0.05. (Original magnification, x1000). (C) Annexin V-FITC/PI staining followed by FCM showed podocytes apoptosis induced by PAN was alleviated by triptolide. **P < 0.01. (On the basis of three triplicate tests).
Genome-wide transcriptome analysis in triptolide-treated human podocytes
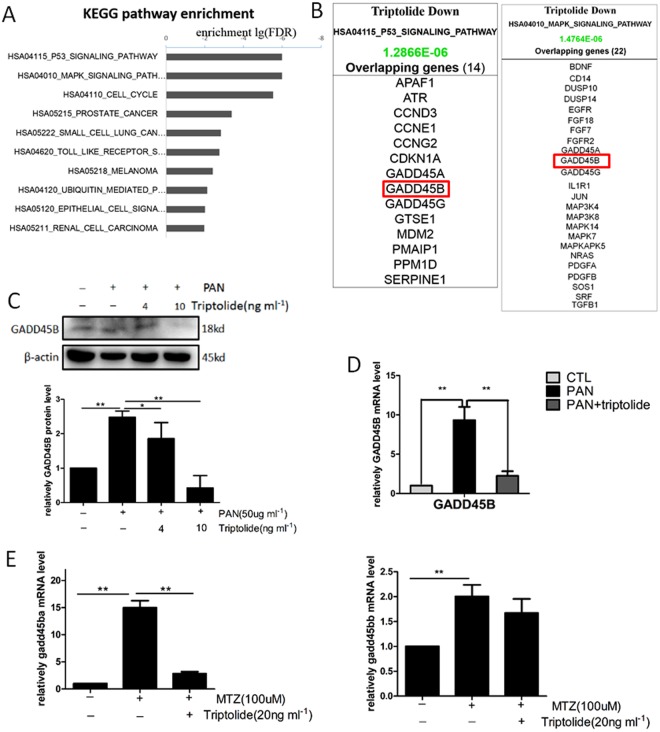
We performed a genome-wide transcriptome microarray on RNA samples extracted from triptolide-treated and non-treated human podocytes to further elucidate the intracellular signalling pathways for the effects of triptolide. We performed KEGG Pathway Analysis to delineate the sensitivity of certain pathways or biological processes to triptolide. The results revealed that the most affected signalling pathways after triptolide treatment to podocytes were the p53 and MAPK signalling pathways (Fig. 5A). Both of these pathways mediate cell apoptosis, which further indicated that triptolide regulated podocyte apoptosis. The downregulation of GADD45B perfectly overlapped with these two signalling pathways (Fig. 5B).
Figure 5.
Triptolide downregulates podocyte GADD45B expression in vitro and in vivo. (A) KEGG pathway enrichment analysis of triptolide-treated human podocytes. The most significant pathways regulated by triptolide were the p53 and MAPK signalling pathways. (B) Triptolide downregulated GADD45B expression, which is involved in p53 and MAPK signalling. (C,D) Western blots and qRT-PCR demonstrated that GADD45B expression was upregulated after PAN (50 µg ml−1) treatment for 12 h and inhibited by pretreatment with 10 ng ml−1 triptolide. (E) Measurement of GADD45B expression in isolated zebrafish glomeruli following MTZ induction of podocyte injury. Triptolide significantly decreased gadd45ba expression but had no effect on gadd45bb. Data are expressed as the means ± SEM. (n = 3), **P < 0.01; *P < 0.05. (On the basis of three triplicate tests). Each blot was cropped at the position of the blotted protein, and high-contrast was not used.
Triptolide suppresses podocyte GADD45B expression in vivo and in vitro
Recent studies demonstrated that specific over-expression of gadd45ba/b in zebrafish podocytes increased MTZ-induced apoptosis, and knockdown of gadd45ba/b inhibited apoptosis4. We examined podocyte GADD45B expression at the protein and mRNA level in vitro and in vivo to validate the results of the genome-wide transcriptome microarray. PAN induced podocyte injury in vitro, and GADD45B mRNA and protein expression in the PAN-treated group increased significantly compared to the untreated group. Triptolide remarkably decreased GADD45B expression at 10 ng ml−1 in PAN-cultured podocytes (Fig. 5C and D). We treated Tg(pod:Gal4;UAS:NTR-mCherry) embryos with MTZ and MTZ + triptolide and isolated glomeruli from both groups. Triptolide remarkably downregulated gadd45ba expression compared to the MTZ group, but there was no effect on gadd45bb (Fig. 5E).
Overexpression of gadd45ba in zebrafish podocytes abolishes the protective effects of triptolide
We previously generated a transgenic zebrafish model of podocyte-specific gadd45ba/b overexpression4. We treated this triple transgenic zebrafish Tg(pod:Gal4;UAS:NTR-mCherry;UAS:gadd45ba/b,cmlc2:GFP) with MTZ after a 24 h triptolide pretreatment to further investigate whether triptolide protected podocytes from injury via inhibiting GADD45B expression. We counted oedema ratios after a 48 h exposure. The percentage of oedema was not decreased in podocytes overexpressing gadd45ba compared with the effect of triptolide in the Tg(pod:Gal4;UAS:NTR-mCherry) group. However, gadd45bb overexpression did not produce this result (Fig. 6). These results clearly indicate that triptolide regulation of GADD45B is a critical mechanism that confers the anti-proteinuria function of triptolide.
Figure 6.
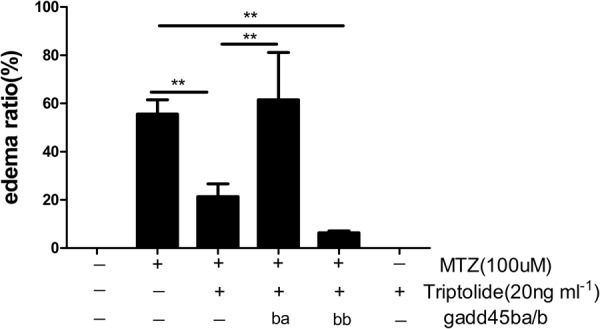
Overexpression of gadd45ba in zebrafish podocyte abolished the protective effects of triptolide. 84 hpf Tg(pod:Gal4;UAS:NTR-mCherry;UAS:gadd45ba/b,cmlc2:GFP) larvae, which overexpress gadd45ba/b specifically in podocytes, were exposed to MTZ (100 µM) following pretreatment with triptolide (20 ng ml−1) for 48 h. The oedema ratio revealed that triptolide significantly decreased the total oedema ratio, but this effect was abolished by overexpression gadd45ba in podocytes. gadd45bb overexpression did not exhibit this function. (n = 3). **P < 0.01.
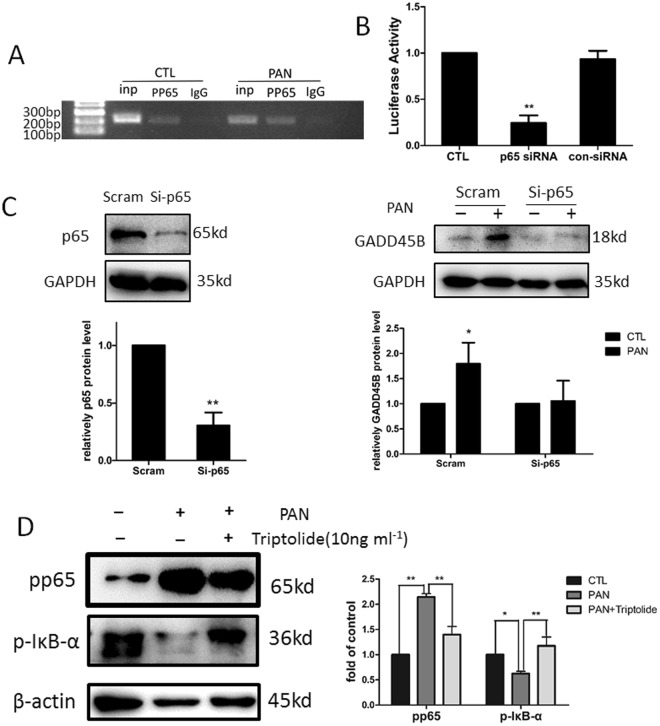
Triptolide inhibits GADD45B transcriptional activation via regulation of NF-κB
In silico analysis identified several NF-κB consensus sequences within the GADD45B gene (http://www.sabiosciences.com/)). P65 (RelA) is the only TAD-domain-containing subunit that activates the transcriptional expression of GADD45B via binding to three κB elements on the promoter region of the GADD45B gene16. Triptolide is an effective inhibitor that is involved in the activation of NF-κB signalling17–20. Therefore, we determined whether triptolide regulated GADD45B expression via suppression of NF-κB in podocytes. Chromatin immunoprecipitation analysis was performed to determine whether phosphorylated NF-κB p65 bound to the GADD45B promoter region in chromatin of podocytes with or without PAN treatment. The results demonstrated that phosphorylated NF-κB p65 directly bound to the +255 to +455 region downstream of the transcription start site. Phosphorylated NF-κB p65 antibodies immunoprecipitated more chromatin in cells incubated with PAN than untreated cells (Fig. 7A). A double luciferase reporter assay was also performed in podocytes. We constructed a human GADD45B promoter luciferase plasmid and transfected it into human podocytes cultured at 33 °C. Reporter luciferase activity was significantly decreased in cultured podocytes co-transfected with NF-κB p65 siRNA (Fig. 7B). We transfected podocytes with NF-κB p65 siRNA to knockdown its expression for 48 h prior to PAN treatment (50 µg ml−1) for 12 h to further investigate the effect of NF-κB inhibition on GADD45B expression. NF-κB p65 knockdown using siRNA effectively prevented PAN-induced upregulation of GADD45B (Fig. 7C). These results indicated that NF-κB p65 transactivated GADD45B expression in podocytes. We examined whether triptolide inhibited NF-κB signalling in podocytes using Western blotting. Our data demonstrated higher protein levels of phosphorylated NF-κB p65 and lower phosphorylated IκB-α levels in the PAN-treated group in vitro compared to the normal group. Triptolide treatment significantly reduced phosphorylated NF-κB p65 protein levels and enhanced phosphorylated IκB -αlevels (Fig. 7D). These results indicate that triptolide regulates GADD45B expression via inhibition of NF-κB signalling.
Figure 7.
Triptolide downregulates GADD45B expression via inhibition of NF-κB signalling. (A) Chromatin immunoprecipitation (ChIP) analysis in podocytes using an antibody to phospho-NF-κB p65, followed by polymerase chain reaction (PCR) using the GADD45B gene promoter-specific primer. The amplified sequence is designed +255 to +455 base pairs downstream of the transcription start site (chr19: 2476721-2476731). More NF-κB p65 bound directly to the GADD45B promoter region after exposure to PAN for 24 h compared to normal control. (B) The luciferase assay confirmed that GADD45B promoter activity was significantly decreased by inhibition of NF-κB p65. (C) Human podocytes transfected with NF-κB p65 siRNA and scramble control for 48 h followed by PAN stimulation (50 µg ml−1) for 12 h. NF-κB p65 knockdown prevented PAN-induced upregulation of GADD45B. (D) Human podocytes were incubated with 50 µg ml−1 PAN and 10 ng ml−1 triptolide for 6 h, and immunoblotting shows a marked reduction in NF-κB activity in the podocytes treated with triptolide and PAN compared to podocytes treated with PAN only. (n = 3). **P < 0.01; *P < 0.05. Each blot was cropped at the position of the blotted protein, and high-contrast was not used.
Discussion
The present study demonstrated several novel results. First, triptolide attenuated proteinuria and protected podocytes from apoptosis in a zebrafish model. Second, this effect was associated with inhibition of GADD45B expression, which mediates podocyte apoptosis via activation of the p38 MAPK signalling pathway. Third, triptolide regulated GADD45B expression via suppression of NF-κB activation, which bound to the GADD45B promoter and activated its transcription.
The mechanism of triptolide protection of podocytes was investigated previously8,9,21,22. However, the definitive pharmacological target of triptolide in podocytes remained controversial. The present study provided a novel mechanism. We discovered that triptolide suppressed podocyte apoptosis. Many stimulating factors induce podocyte apoptosis, including inflammation and ROS production. Triptolide also exhibits strong anti-inflammatory activities23 and inhibits ROS generation8,9. Our previous research demonstrated that p38 MAPK signalling was the key regulatory pathway for triptolide efficacy. The present study elucidated the underlying mechanism of triptolide alleviation of podocyte apoptosis and the upstream signalling of the p38 MAPK pathway. We recently demonstrated that GADD45B exacerbated apoptosis in zebrafish podocytes and in vitro. GADD45B was confirmed as an upstream target protein of the p38 MAPK signalling pathway in the mediation of apoptosis. Triptolide and GADD45B exhibit many opposite properties, and microarray analysis indicated that triptolide downregulated GADD45B expression. Therefore, we validated the potential connection between triptolide podocyte protection and its regulation of GADD45B in an inducible zebrafish podocyte injury model. Our results confirmed that triptolide treatment inhibited GADD45B expression, and gadd45ba overexpression in zebrafish podocytes abolished the beneficial effect of triptolide. Therefore, we suggest that GADD45B is an essential molecule that, at least partially, mediates the triptolide-podocyte protective function.
The zebrafish gadd45b gene has two members, gadd45ba and gadd45bb. The present study demonstrated that triptolide inhibited gadd45ba expression but did not affect gadd45bb expression, and gadd45bb overexpression did not affect the protective function of triptolide. Our previous studies revealed that normal zebrafish glomeruli express more gadd45ba than gadd45bb and upregulation of gadd45ba was more prominent than gadd45bb after podocyte injury. Overexpression of gadd45ba in zebrafish podocytes resulted in a more serious phenotype than gadd45bb overexpression. These results suggest that gadd45ba, but not gadd45bb, plays a primary role in zebrafish podocytes. Therefore, triptolide protects zebrafish podocytes from injury primarily via inhibition of gadd45ba expression rather than gadd45bb expression.
The molecular events that lead to GADD45B upregulation after podocyte injury are still not fully understood. LPS24, TNFα24 and interleukin 1(IL-1)25 activate NF-κB signalling and induce GADD45B expression. The GADD45B promoter contains three NF-κB binding sites, and each of these sites is required for optimal transcriptional activation16. SiRNA knockdown of NF-κB p65 reduced GADD45B expression in PAN-induced podocyte injury. ChIP-PCR revealed that phospho-NF-κB p65 bound to the GADD45B promoter in human podocytes, and more phospho-NF-κB P65 bound to the GADD45B promoter after podocyte injury compared to the normal group. The luciferase assay further confirmed that co-transfection with NF-κB p65 siRNA strongly reduced GADD45B promoter activity. Therefore, GADD45B induction by various stimuli in podocytes was partially due to increased NF-κB activity, which is required for GADD45B transcriptional activation. Many previous studies demonstrated that triptolide was a potent inhibitor of NF-κB. We also found that triptolide inhibited NF-κB activation via increasing phospho-IκBα and reducing NF-κB p65 phosphorylation at the protein level following podocyte injury. These findings suggest that triptolide regulates GADD45B transactivation via inhibition of NF-κB signalling.
The present study used the transgenic zebrafish line Tg (pod:Gal4;UAS:NTR-mCherry) to establish an inducible podocyte injury model that exhibits cell death exclusively within NTR+ cells14,26. Triptolide also produced remarkably beneficial effects on zebrafish podocytes, which consistent with the results in a puromycin aminonucleoside nephrosis rat model. Our results provide novel evidence for the clinical application of triptolide. However, this zebrafish podocyte injury model is a podocyte-specific ablation model that exhibits no toxic effect on any other cells, compared to the PAN nephrosis rat model. Many methods are used to estimate glomerular structure and function. We monitored proteinuria in the transgenic zebrafish line Tg(pod:Gal4;UAS:NTR-mCherry;lfabp:vbp-gfp), which expresses a VDBP-GFP fusion protein of a similar molecular weight as mammalian albumin10. In situ hybridization, RT-PCR, and the synthesized antibody examined alterations in nephrin and podocin at the protein level27. We also performed podocin staining to observe its expression and distribution after triptolide treatment. TEM allowed us to observe morphological changes in podocytes directly. Therefore, zebrafish are a promising tool to investigate gene-drug interactions. Our results demonstrated that zebrafish are a suitable model to identify new drug targets on podocytes in future studies. This species are more convenient and costs less than rodents.
In conclusion, our results provide new insights into the potential molecular mechanisms of triptolide protection of podocytes. We demonstrated that triptolide protected podocytes from apoptosis and that this effect was associated with inhibition of the NF-κB/GADD45B/p38 MAPK signalling pathway (Fig. 8). We also demonstrated that zebrafish embryos are a robust experimental vertebrate model that can be used to identify new podocyte-protecting drugs and perform fast and reproducible pharmacological assays with excellent outcomes.
Figure 8.
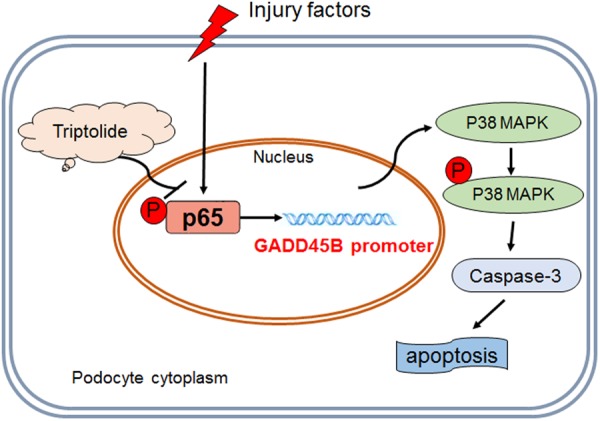
Schematic diagram of triptolide downregulation of GADD45B expression in the protection of podocytes from apoptosis. Podocyte injury produces remarkable GADD45B upregulation due to aberrant phospho-NF-κB p65 induction and GADD45B promoter transactivation. GADD45B induces podocyte apoptosis via activation of the p38 MAPK signalling pathway. Triptolide effectively corrects aberrant NF-κB activation and decreases GADD45B expression to protect podocytes from apoptosis.
Materials and Methods
Reagents
Triptolide (C20H24O6, molecular weight 360, Nanjing Spring & Autumn Biological Engineering Company, Nanjing, China). Metronidazole (MTZ) and PAN (Sigma-Aldrich, St Louis, MO, USA). The anti-zebrafish podocin antibody was synthesized by Genscript Company (Nanjing, China), and the sequence of the synthesized peptides was CDGSDDGTKDSPM.
Fish Breeding and Maintenance
Zebrafish (Danio rerio) were maintained under standard laboratory conditions28. All experiments on zebrafish were performed in accordance with the guidelines for the Care and Use of Laboratory Animals approved by the Nanjing University School of Medicine.
Generation of transgenic zebrafish
Transgenic zebrafish lines Tg(pod:Gal4), Tg(UAS:NTR-mCherry), Tg(l-fabp:VDBP-GFP), Tg(UAS:gadd45ba,cmlc2:gfp) and Tg(UAS:gadd45bb,cmlc2:gfp) were generated as previously described4.
Drug administration
The Tg(pod:gal4:UAS:NTR-mCherry) embryos at 60 hpf were selected according to mCherry fluorescence in glomeruli under a stereomicroscope (Nikon SMZ25, Tokyo, Japan) and transferred into 6-well plates. Embryos were divided randomly into normal control, triptolide control, and MTZ model and MTZ + triptolide treatment group. Thirty embryos were pooled together in each well. The triptolide treatment group and control group were pretreated with triptolide (20 ng ml−1) for 24 h prior to MTZ treatment and throughout the experimental process. The normal control and MTZ model groups were incubated in embryo medium containing 0.1% DMSO. MTZ was added to the MTZ model and MTZ + triptolide treatment groups to induce podocyte injury at 84 hpf for the indicated period.
Proteinuria Assay
The l-fabp:VDBP-GFP plasmid was a kind gift from Dr. Weibin Zhou. Triple transgenic embryos Tg(pod:Gal4;UAS:NTR-mCherry;lfabp:VDBP-GFP) were generated by crossing Tg(pod:Gal4;UAS:NTR-mCherry) and Tg(lfabp: VDBP-GFP) fish. Three embryos were pooled in each well of a 96-well plate in 150 µl of E3 medium for each group. MTZ treatment was initiated at 120 hpf. The E3 medium in each group was collected for proteinuria assays using ELISA (Abcam, Cambridge, UK) at 144 hpf10.
Whole-mount Immunofluorescence and Quantitation
Immunohistochemistry was performed as described previously29. The anti-zebrafish podocin antibody (1:10000) and anti-cleaved caspase 3 antibody (cat 559565, 1: 1000; BD Pharmingen, CA, USA) were incubated for 10–15 h at 4 °C. The secondary antibody was donkey anti-rabbit IgG (H + L) conjugated with Alexa Fluor® 488 (cat A-21206, 1:1000, Invitrogen, USA). Confocal images were captured using a Zeiss LSM 710 laser-scanning microscope and Z-stack. We scanned 5–10 glomeruli images in each group using consistent parameters and performed a maximum intensity projection with confocal microscope to quantify fluorescence intensities30. The average value of the glomeruli images from each group was used as the protein expression state. The mean immunofluorescence intensity, which measured as the average grey level (GL), and the area ratio of the stained area to the glomerulus area (AR)31 were examined using Image Pro Plus 6.0.
Transmission Electron Microscopy (TEM)
The TEM was performed according to our previous study32.
Cell culture and reagent treatment
The conditionally immortalized human podocytes (HPCs) were a gift from Dr. Moin Saleem at Bristol University, UK. The cells were cultured in RPMI 1640 medium containing streptomycin, penicillin, insulin, transferrin, selenite (Gibco, USA) and 10% foetal calf serum at 33 °C. Podocytes were switched to a growth temperature to 37 °C for 10–14 days to induce a differentiated phenotype. Podocyte injury was induced with 50 μg ml−1 PAN for the indicated time. Triptolide (10 ng ml−1) was preincubated for 30 min prior to PAN exposure.
Annexin V flow cytometric analysis of apoptosis
Podocytes were collected, washed with ice-cold PBS twice, resuspended in 100 μl of 1 × binding buffer, and then incubated with 5 μl Annexin V-FITC and 5 μl PI staining solution (Vazyme, Nanjing, China) at room temperature for 10 min, followed by analysis with FACScan using Cellquest software (Becton Dickinson, Franklin Lakes, NJ, USA).
Microarray analysis
Total RNA was extracted using TRIzol (Invitrogen) and purified using an RNeasy mini kit (QIAGEN). A NanoDrop spectrophotometer (ND-1000, NanoDrop Technologies) was used to evaluate RNA quality and quantity. RNA integrity was examined using gel electrophoresis. Labelling, hybridization, washing and staining were performed according to manufacturer’s recommendations (Affymetrix, Santa Clara, CA, USA). The microarray analysis was performed using the Affymetrix Human HTA2.0.
RNA interference
NF-κB p65 knockdown was performed using an NF-κB p65-specific siRNA. The sequence of the sense strand of the siRNA oligonucleotides was 5-GCCCUAUCCCUUUACGUCA-3′. Podocytes differentiated well and were cultured to ~90% confluence prior to transfection with NF-κB p65 siRNA using Lipofectamine RNAiMAX (Invitrogen, USA) following the manufacturer’s instructions.
Western blot
Lysates from cultured cells were prepared and subjected to Western blot assays following previously described procedures4. Western blot was performed using the following primary antibodies: anti-GADD45B rabbit polyclonal antibody (Abcam, Cambridge, UK), NF-κB p65, phospho-NF-κB p65 (Ser536) and phospho-IκBα (Beyotime Bio Co., Shanghai, China). Western blots were developed using an ECL plus Western blotting detection system (Vazyme, USA). The protein quantities were analysed using Image J software.
Chromatin immunoprecipitation (ChIP)
The chromatin immunoprecipitation (ChIP) assay was performed using the Zymo Research ChIP Chromatin Immuno-precipitation Kit (CA, USA) following the manufacturer’s instructions. Antibodies (5 μg) specific for phospho-NF-κB p65 or an IgG isoform control were incubated at 4 °C overnight with rotation. PCR amplification was performed after de-crosslinking and extracting the DNA using primer sets that contained the tentative NF-κB p65 binding elements in the GADD45B promoter region (ChIP-pos F/R, +255 to +455). The primer sequences are provided in Supplement Table 1.
Luciferase reporter assay
The 39-UTR of GADD45B was obtained from PCR using human genomic DNA and inserted downstream of the pGL3-promoter (Promega, Madison, WI). Reporter plasmids were transfected into cultured human podocytes. A renilla luciferase plasmid (pRL-SV40, Promega, USA) was used as an internal control. The NF-κB p65 siRNA and con-siRNA were co-transfected with luciferase plasmids using Lipofectamine 2000 (Invitrogen, USA). A dual luciferase assay system (Promega, USA) was used to assess luciferase activity after 24 h.
Statistical analysis
Statistical analyses were performed using SPSS software (version 20). All results are expressed as the means ± SD. We used the unpaired t-test to compare two groups and one-way ANOVA to compare three or more groups. Statistical significance was accepted at P < 0.05.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Approval
All sampling and experimental procedures involving animals were performed according to the experimental animal use guidelines and approved by the Institutional Animal Care and Use Committee at Jinling Hospital (Nanjing, Jiangsu, China). Approved number: 2014GJJ-110.
Electronic supplementary material
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 81470943, No. 81100490), grants from the National Key Technology Research and Development Program (2015BAI12B05, 2015BAI12B02) and a grant from Jinling Hospital, Nanjing University School of Medicine (2016036).
Author Contributions
L.W. performed most of the experimental work and wrote the manuscript. L.Z. performed podocyte culture and treatment. Q.H. generated the transgenic zebrafish and provided funding. X.Z. performed TEM. Z.C. and Z.L. designed the experiments, provided funding and revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29203-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhaohong Chen, Email: rin@nju.edu.cn.
Zhihong Liu, Email: liuzhihong@nju.edu.cn.
References
- 1.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clinical and experimental nephrology. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 2.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney international. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 3.Shi S, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. Journal of the American Society of Nephrology: JASN. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, et al. GADD45B mediates podocyte injury in zebrafish by activating the ROS-GADD45B-p38 pathway. Cell death & disease. 2016;7:e2068. doi: 10.1038/cddis.2015.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q. Triptolide and its expanding multiple pharmacological functions. International immunopharmacology. 2011;11:377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs in R&D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Leishi L. Clinical study of Tripterygium wilfordii Hook in treating glomerulonephritis. Chin J Intern Med. 1981;20:216–220. [PubMed] [Google Scholar]
- 8.Zheng CX, et al. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney international. 2008;74:596–612. doi: 10.1038/ki.2008.203. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZH, et al. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney international. 2010;77:974–988. doi: 10.1038/ki.2010.41. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. Journal of the American Society of Nephrology: JASN. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan X, et al. Loss of Epithelial Membrane Protein 2 Aggravates Podocyte Injury via Upregulation of Caveolin-1. Journal of the American Society of Nephrology: JASN. 2016;27:1066–1075. doi: 10.1681/ASN.2014121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groh ED, et al. Inhibition of Histone Deacetylase Expands the Renal Progenitor Cell Population. Journal of the American Society of Nephrology. 2010;21:794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cianciolo Cosentino C, et al. Histone deacetylase inhibitor enhances recovery after AKI. Journal of the American Society of Nephrology: JASN. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nature protocols. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boute N, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nature genetics. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 16.Jin R, et al. Regulation of the gadd45beta promoter by NF-kappaB. DNA and cell biology. 2002;21:491–503. doi: 10.1089/104454902320219059. [DOI] [PubMed] [Google Scholar]
- 17.Geng Y, et al. Triptolide down-regulates COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and MAP kinases in lipopolysaccharide-treated PC12 cells. Phytotherapy research: PTR. 2012;26:337–343. doi: 10.1002/ptr.3538. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Hong Y, Huang H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-kappaB Signaling Pathway. Kidney & blood pressure research. 2016;41:901–910. doi: 10.1159/000452591. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, et al. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-kappaB downregulation. International journal of cancer. 2014;134:2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, et al. Triptolide simultaneously induces reactive oxygen species, inhibits NF-kappaB activity and sensitizes 5-fluorouracil in colorectal cancer cell lines. Cancer letters. 2010;291:200–208. doi: 10.1016/j.canlet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Jiang CB, et al. Triptolide Attenuates Podocyte Injury by Regulating Expression of miRNA-344b-3p and miRNA-30b-3p in Rats with Adriamycin-Induced Nephropathy. Evidence-based complementary and alternative medicine: eCAM. 2015;2015:107814. doi: 10.1155/2015/107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma R, et al. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Experimental and therapeutic medicine. 2013;6:649–656. doi: 10.3892/etm.2013.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XJ, Jiang ZZ, Zhang LY. Triptolide: progress on research in pharmacodynamics and toxicology. Journal of ethnopharmacology. 2014;155:67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, et al. NF-kappaB and not the MAPK signaling pathway regulates GADD45beta expression during acute inflammation. The Journal of biological chemistry. 2005;280:21400–21408. doi: 10.1074/jbc.M411952200. [DOI] [PubMed] [Google Scholar]
- 25.Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1991;6:165–167. [PubMed] [Google Scholar]
- 26.Pisharath H, Parsons MJ. Nitroreductase-mediated cell ablation in transgenic zebrafish embryos. Methods in molecular biology (Clifton, N. J.) 2009;546:133–143. doi: 10.1007/978-1-60327-977-2_9. [DOI] [PubMed] [Google Scholar]
- 27.Fukuyo Y, et al. Nephrin and Podocin functions are highly conserved between the zebrafish pronephros and mammalian metanephros. Molecular medicine reports. 2014;9:457–465. doi: 10.3892/mmr.2013.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5th edn. (Univ. of Oregon Press, 2007).
- 29.Ichimura K, et al. Developmental localization of nephrin in zebrafish and medaka pronephric glomerulus. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2013;61:313–324. doi: 10.1369/0022155413477115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caster AH, Kahn RA. Computational method for calculating fluorescence intensities within three-dimensional structures in cells. Cellular logistics. 2012;2:176–188. doi: 10.4161/cl.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan N, Ding J, Deng J, Zhang J, Yang J. Key molecular events in puromycin aminonucleoside nephrosis rats. Pathology international. 2004;54:703–711. doi: 10.1111/j.1440-1827.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, et al. Ultrastructural characterization of the pronephric glomerulus development in zebrafish. Journal of morphology. 2016;277:1104–1112. doi: 10.1002/jmor.20560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.