Abstract
Neisseria gonorrhoeae is an exclusive human pathogen that evades the host immune system through multiple mechanisms. We have shown that N. gonorrhoeae suppresses the capacity of antigen-presenting cells to induce CD4+ T cell proliferation. In this study, we sought to determine the gonococcal factors involved in this adaptive immune suppression. We show that suppression of the capacity of antigen-pulsed dendritic cells to induce T cell proliferation is recapitulated by administration of a high-molecular-weight fraction of conditioned medium from N. gonorrhoeae cultures, which includes outer membrane vesicles that are shed during growth of the bacteria. N. gonorrhoeae PorB is the most abundant protein in N. gonorrhoeae–derived vesicles, and treatment of dendritic cells with purified recombinant PorB inhibited the capacity of the cells to stimulate T cell proliferation. This immunosuppressive feature of purified PorB depended on proper folding of the protein. PorB from N. gonorrhoeae, as well as other Neisseria species and other Gram-negative bacterial species, are known to activate host Toll-like receptor 2 (TLR2) signaling. Published studies have demonstrated that purified Neisseria PorB forms proteinacious nanoparticles, termed proteosomes, when detergent micelles are removed. Unlike folded, detergent-solubilized PorB, PorB proteosomes stimulate immune responses. We now demonstrate that the formation of PorB proteosomes from structurally intact PorB eliminates the immunosuppressive property of the protein while enhancing TLR2 stimulation. These findings suggest that gonococcal PorB present in shed outer membrane vesicles plays a role in suppression of adaptive immune responses to this immune-evasive pathogen.
Keywords: dendritic cell, immunosuppression, Toll-like receptor (TLR), outer membrane, T cell, Neisseria gonorrhoeae, outer membrane vesicle, porin, Toll-like receptor 2 (TLR2)
Introduction
Gonorrhea, the urethritis caused by Neisseria gonorrhoeae, has been recognized as a human infection for thousands of years and, to date, remains one of the most prevalent sexually transmitted bacterial infections, with over 78 million new cases reported worldwide in 2012 (1). Treatment of gonorrhea relies on antibiotic therapy, but there is growing concern about resistance to currently approved antimicrobial agents (2, 3). Prevention of gonococcal infections through vaccine development is an appealing strategy to address this problem but is hampered by the lack of known protective immunologic correlates (4).
Most individuals infected with N. gonorrhoeae do not develop protective adaptive immune responses, and thus repeated infections are common, including reinfection by the same gonococcal strain (5–7). Multiple mechanisms have been attributed to the ineffective adaptive immune response to these bacteria. N. gonorrhoeae major surface molecules, including pili, opacity proteins (Opa),4 and lipooligosaccharide (LOS), undergo phase and antigenic variation at high frequency (8). Furthermore, N. gonorrhoeae can manipulate host immune responses through interaction with local mucosal immune cells. It has been reported that Opa52 binds to carcinoembryonic antigen–related cellular adhesion molecule 1 (CEACAM-1) on CD4+ T cells (9, 10), resulting in down-regulation of T cell proliferation in response to antigens (11). Studies of gonococcal infections in the female mouse infection model have shown that N. gonorrhoeae elicits T helper type 17 (Th17) responses through the induction of host transforming growth factor β. Further, the Th17 response drives induction of localized inflammation, including recruitment of host neutrophils (to which N. gonorrhoeae is relatively resistant) (12). Recent studies in a mouse model of gonorrhea have shown that N. gonorrhoeae induces production of interleukin 10 (IL-10) and regulatory T cells (Tr1) that suppress Th1- and Th2-dependent adaptive immune responses (13).
Our previous studies have shown that N. gonorrhoeae suppresses the capacity of dendritic cells, professional antigen-presenting cells that play a key role in promoting pathogen-specific adaptive immune responses, to stimulate antigen-specific T cell proliferation (14). N. gonorrhoeae causes this suppression in part by promoting secretion of inhibitory factors, such as IL-10, and expression of cell-autonomous factors, including up-regulation of programmed death ligand 1, but the key molecular component(s) of N. gonorrhoeae that engage in this process were not identified. In this study, we determined that gonococcus-conditioned medium carries factors that recapitulate the suppression of dendritic cell–induced T cell proliferation observed with whole bacteria. We show that properly folded, recombinant PorB, a major protein component found in conditioned medium, has similar suppressive properties. Surprisingly, prior studies of PorB from N. gonorrhoeae and other Neisseria species have demonstrated that the protein can act as an immune-stimulating adjunct. We further demonstrate that the stimulatory properties of PorB result from a loss of immune-suppressive activity as a consequence of the loss of the properly folded PorB protein structure that occurs when detergent is removed from this integral membrane protein. Taken together, our results suggest that, although the native PorB trimer from N. gonorrhoeae can stimulate signaling in some immune cells through activation of Toll-like receptor 2, PorB overcomes this stimulation by profoundly inhibiting dendritic cell–promoted T cell proliferation when presented to cells in its native, properly folded state.
Results
N. gonorrhoeae–conditioned medium inhibits dendritic cell–induced, antigen-specific T cell proliferation
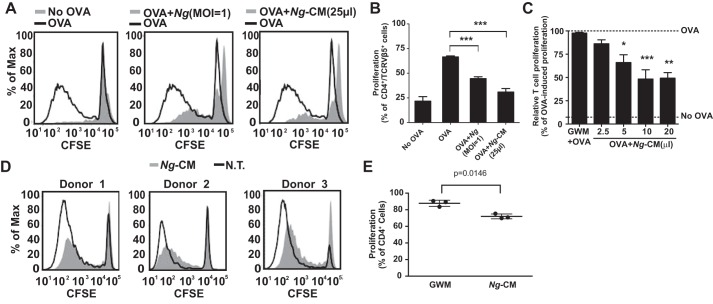
To determine whether N. gonorrhoeae releases factors responsible for inhibition of antigen-pulsed dendritic cell–induced T cell proliferation, conditioned medium from N. gonorrhoeae cultures (Ng-CM) or live N. gonorrhoeae was added to dendritic cells during ovalbumin (OVA) exposure for 24 h, and then the dendritic cells were washed and co-cultured with carboxyfluorescein succinimidyl ester (CFSE)–labeled T cells from OT-II mice. After 7 days, T cell proliferation in each co-culture was evaluated by quantifying the dilution of CFSE fluorescence. Treatment of dendritic cells with either N. gonorrhoeae or Ng-CM resulted in a dose-dependent reduction of proliferation of co-cultured T cells compared with OVA-treated dendritic cells (Fig. 1, A–C). Ng-CM also inhibited the capacity of human monocyte-derived dendritic cells to induce proliferation of allogeneic T lymphocytes (Fig. 1, D and E), demonstrating that Ng-CM-mediated suppression occurs in both human and mouse dendritic cells.
Figure 1.
N. gonorrhoeae cells and N. gonorrhoeae conditioned medium inhibit OVA-primed dendritic cell–induced T cell proliferation. Dendritic cells were exposed to N. gonorrhoeae or Ng-CM in the presence of OVA for 24 h and then co-cultured with CFSE-loaded OT-II T cells for 7 days. T cell proliferation mediated by OVA stimulation was assessed by flow cytometry as described under “Experimental procedures.” A, representative overlay histograms of OT-II T cell proliferation induced by dendritic cells, with the indicated treatment in filled gray. No OVA treatment is defined as GWM without OVA (left panel), Ng (m.o.i. = 1) plus OVA (center panel), or Ng-CM (25 μl) plus OVA (right panel). The OVA-pulsed dendritic cell control is plotted as a black line in each panel. B, the percentage of cultured OT-II T cells that underwent at least 1 generation of proliferation relative to all OT-II T cells in the culture is plotted after co-culture with dendritic cells without OVA antigen (no OVA) or treated with OVA, Ng (m.o.i. = 1) plus OVA, or Ng-CM (25 μl) plus OVA. Statistical significance was determined by one-way ANOVA with a post hoc Tukey analysis for multiple comparisons. ***, p < 0.001. C, T cell proliferation after co-culture with dendritic cells exposed to OVA and GWM or the indicated amounts of Ng-CM was measured as in B. The percentage of OT-II T cell proliferation for each condition was normalized to that observed after co-culture with OVA-pulsed dendritic cells. The top dashed line indicates the normalized proliferation induced by OVA, and the bottom dashed line marks the percentage of proliferation in the absence of antigen (no OVA). Data are mean ± S.E. (n = 11). Statistical significance was determined by one-way ANOVA with a post hoc Tukey analysis for multiple comparisons. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with dendritic cells treated with GWM. D, human dendritic cells from three separate donors were left untreated (N.T.) or treated with Ng-CM (25 μl) and co-cultured with CFSE-labeled allogeneic lymphocytes, and proliferation was assessed by flow cytometry after 7 days in culture. E, the percentage of cultured allogeneic T lymphocytes that underwent at least one generation of proliferation relative to all T lymphocytes in the culture after co-culture with untreated or Ng-CM-treated dendritic cells from the three donors shown in D. The p value was determined by ratio paired t test.
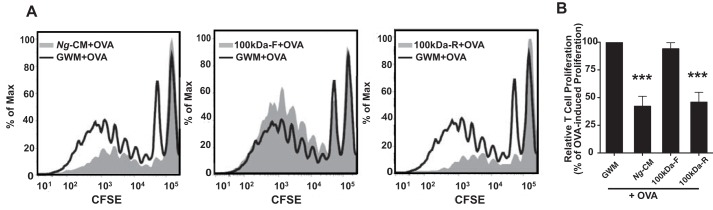
To further characterize the components released from N. gonorrhoeae that are responsible for inhibition of T cell proliferation, Ng-CM was subjected to ultrafiltration through a 100-kDa cutoff filter, and the retentate was restored to its original volume by addition of Graver–Wade medium (GWM) (15). The filtrate and retentate fractions were then assessed for their capacity to inhibit dendritic cell–induced T cell proliferation. The 100-kDa retentate from Ng-CM demonstrated inhibition of T cell proliferation that was indistinguishable from unfiltered Ng-CM, whereas the 100-kDa filtrate from Ng-CM exhibited no detectable inhibition of T cell proliferation (Fig. 2, A and B). Although N. gonorrhoeae does not secrete known protein exotoxins, N. gonorrhoeae and other pathogenic Neisseria species prolifically shed outer membrane vesicles (OMVs, also called membrane blebs) into the environment during growth (16, 17). Ng-CM, Ng-CM 100-kDa filtrate, and Ng-CM 100-kDa retentate were analyzed by SDS-PAGE and silver staining, which revealed that the majority of proteins present in Ng-CM were retained by the 100-kDa filter (Fig. S1A). The finding that the majority of the proteins found in Ng-CM and the inhibitory factor are retained by a 100-kDa filter suggests that the immunosuppressive effect of Ng-CM is due to OMVs or proteins in the OMVs.
Figure 2.
The >100-kDa retentate of ultrafiltered Ng-CM contains all of the inhibitory activity on OVA-pulsed dendritic cell–induced T cell proliferation. Ng-CM was separated into two fractions, retentate (>100 kDa) and filtrate (<100 kDa), by ultrafiltration through a 100-kDa cutoff filter. Dendritic cells were pulsed with OVA plus GWM, Ng-CM, Ng-CM filtrate (100kDa-F), or Ng-CM retentate (100kDa-R) and co-cultured with CFSE-labeled OT-II T cells, and the proliferation of T cells after 7 days in culture was assessed by flow cytometry. A, representative overlay histograms of OT-II T cell proliferation induced by OVA-pulsed dendritic cells treated with the indicated stimulation in gray: Ng-CM + OVA (left panel), Ng-CM 100kDa-F + OVA (center panel), or 100kDa-R + OVA (right panel). The GWM + OVA–pulsed dendritic cell control is plotted as a black line in each panel. B, the percentage of T cells proliferating relative to all of the T cells in the well induced by dendritic cells treated with OVA + GWM, Ng-CM, Ng-CM filtrate, or Ng-CM retentate were normalized to the OVA-pulsed dendritic cell control as described in Fig. 1C. Data are mean ± S.E. (n = 6). Statistical significance was determined by one-way ANOVA with a post hoc Tukey analysis for multiple comparisons. ***, p < 0.001 compared with dendritic cells treated with GWM.
N. gonorrhoeae PorB1B, but not LOS, inhibits OVA-pulsed dendritic cell–induced T cell proliferation
OMVs from N. gonorrhoeae contain large quantities of LOS as well as outer membrane proteins, primarily PorB (17, 18). We therefore sought to determine whether LOS or gonococcal proteins abundant in OMVs present in Ng-CM were responsible for inducing inhibition of dendritic cell–directed, OVA-induced T cell proliferation. The amount of LOS in Ng-CM was estimated following SDS-PAGE and silver staining by comparing the staining intensities to a titration series of purified LOS (Fig. S1A). The quantity of Ng-CM that conferred maximal inhibition of dendritic cell–mediated T cell proliferation (25 μl, Fig. 1C) was estimated to contain ∼100 ng of LOS. When dendritic cells were treated with 100 ng of purified LOS in the presence of OVA prior to co-culture with CD4+ T cells, no inhibition of T cell proliferation was observed (Fig. S1B). When 10-fold more purified LOS (1 μg) was used, a slight inhibition of T cell proliferation was observed, but this trend did not meet statistical significance (Fig. S1C). These data indicate that purified LOS was unable to recapitulate the inhibition of dendritic cell-induced T cell proliferation that was observed with Ng-CM and suggest that outer membrane proteins may be responsible for the inhibition.
N. gonorrhoeae porin (PorB) is the most abundant gonococcal protein in both outer membranes and OMVs, constituting ∼50% of the total protein content (19, 20), and has been shown to have pleotropic effects on host cells through a variety of mechanisms (21–23). N. gonorrhoeae has two PorB serotypes, PorB1A and PorB1B; the strain used in these experiments, FA1090, expresses the PorB1B serotype. As anticipated, immunoblot analysis demonstrated that PorB1B was present in Ng-CM and that all detectable PorB1B was retained by the 100-kDa cut-off filter (Fig. 3A). To examine the effects of PorB1B on dendritic cell–mediated T cell proliferation, PorB1B lacking its signal sequence and containing a N-terminal hexahistidine tag (HT) followed by a tobacco etch virus protease recognition site was expressed as inclusion bodies in E. coli and refolded into an active state in the presence of the detergent LDAO as described under “Experimental procedures.” For some experiments, the hexahistidine tag was removed using tobacco etch virus protease (Fig. 3B). Refolded HT-PorB1B and PorB1B in LDAO micelles were highly pure and eluted as homogenous single peaks in size exclusion chromatography. The estimated molecular mass of purified, refolded PorB1B was ∼250 kDa (Fig. 3C), consistent with two PorB trimers (∼114 kDa), the known tertiary structure for native N. gonorrhoeae PorB1B, and an LDAO detergent micelle (∼25 kDa). Functional activity of refolded and purified PorB1B was demonstrated by its capacity to promote size-dependent permeation of a series of saccharides following reconstitution into liposomes (Fig. 3D) (24).
Figure 3.
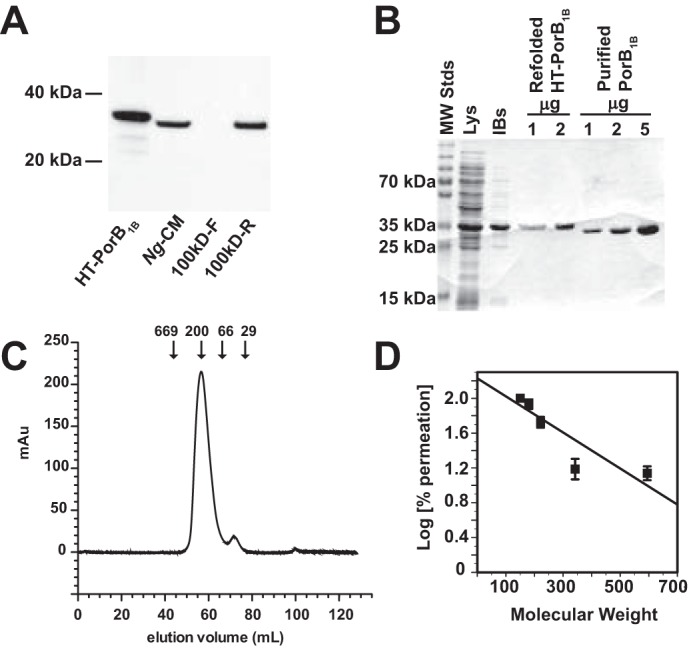
Refolded, purified, recombinant PorB1B forms functional trimeric porin complexes. A, Ng-CM, 100kDa-F, and 100kDa-R from FA1090 were prepared as described under “Experimental procedures” and analyzed by SDS-PAGE and Western blotting using an antibody against PorB1B. A lane containing recombinant HT-PorB1B was run as a positive control for antibody reactivity. B, recombinant PorB1B was prepared as described under “Experimental procedures,” and fractions from the indicated steps of the preparation were analyzed by SDS-PAGE after staining with Coomassie Brilliant Blue R-250. Lys, bacterial lysate; IBs, inclusion bodies. C, recombinant, refolded PorB1B was run on a Sephacryl S300 column, and the A260 was plotted versus the volume of eluent. The elution position for protein standards of known molecular mass (in kilodaltons) are indicated by arrows. D, the log of the ratio of the rate of swelling of liposomes containing purified recombinant PorB1B promoted by saccharides to the rate induced by arabinose (defined as 100%) is plotted against the molecular weight of the saccharide.
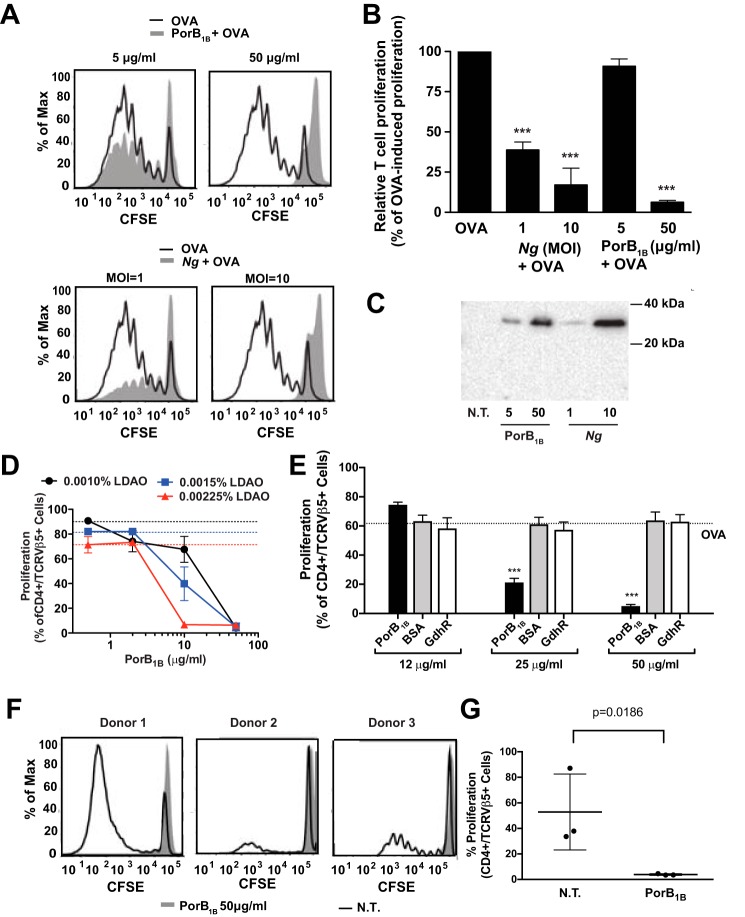
The addition of purified PorB1B (50 μg/ml) to dendritic cells during OVA exposure led to profound inhibition of T cell proliferation in our co-culture system, recapitulating the effects observed with live N. gonorrhoeae treatment (Figs. 4, A and B). The addition of an equivalent volume of LDAO-containing buffer, the vehicle for PorB1B, caused a small, reproducible decrease in dendritic cell–induced T cell proliferation, but this decrease was not statistically significant (Fig. S2). The PorB1B preparations were found to have low levels of endotoxin contamination (between 1.5 and 6 endotoxin units/mg of protein), which, at the highest amount of PorB1B added, corresponds to a maximum concentration of ∼ 2.5 ng of E. coli LPS/ml in the dendritic cell culture. To ensure that E. coli LPS contamination of the recombinant PorB1B was not responsible for the PorB1B-mediated inhibition of T cell proliferation observed, OVA-pulsed dendritic cells were exposed to E. coli LPS at concentrations between 1 and 100 ng/ml and assayed for the ability to stimulate OT-II T cell proliferation. E. coli LPS at these concentrations demonstrated no detectable effect on OVA-pulsed dendritic cell–induced T cell proliferation (Fig. S3).
Figure 4.
Refolded, purified, recombinant PorB1B inhibits OVA-pulsed dendritic cell–induced T cell proliferation. A and B, dendritic cells were pulsed with OVA alone or OVA plus either purified recombinant PorB1B (at 5 and 50 μg/ml) or N. gonorrhoeae (m.o.i. = 1 and 10), and the proliferation of CFSE-labeled OT-II T cells was assessed after 7 days by flow cytometry. A, the dilution of CSFE fluorescence in T cells following incubation with dendritic cells pulsed with the indicated treatments. B, the percentage of T cells undergoing proliferation relative to all of the T cells in the culture is plotted for each of the indicated treatments of the dendritic cells. Data are expressed as mean ± S.E. (n = 4–11 replicates). Statistical significance was determined by one-way ANOVA with a post hoc Tukey analysis for multiple comparisons. ***, p < 0.001 compared with dendritic cells treated with OVA alone. C, dendritic cells were not treated (N.T.) or exposed to either purified recombinant PorB1B (5 and 50 μg/ml) or N. gonorrhoeae (m.o.i. = 1 and 10) for 24 h. The cells were washed, whole-cell lysates were prepared as described under “Experimental Procedures,” and equal amounts of protein were subjected to Western blotting using an antibody against PorB1B. D, dendritic cells were pulsed with OVA in the presence of the indicated final concentration of LDAO (black circles, 0.0010%; blue squares, 0.0015%; red triangles, 0.00225%) and recombinant PorB1B, the pulsed dendritic cells were co-cultured with CFSE-labeled OT-II T cells, and the proliferation of OT-II T cells was determined using flow cytometry. Points represent the mean ± S.E. (n = 4–7 replicates). Dashed lines indicate the proliferation induced by OVA-pulsed dendritic cells treated with the indicated LDAO concentration in the absence of PorB1B. E, dendritic cells were pulsed with OVA in the presence of LDAO (final concentration, 0.0015%) and the indicated proteins at 50 μg/ml: recombinant PorB1B, BSA, or recombinant refolded GdhR, a transcriptional regulator. The pulsed dendritic cells were co-cultured with CFSE-labeled OT-II T cells, and the proliferation of OT-II T cells was determined using flow cytometry. The percentage of T cells undergoing proliferation relative to all of the T cells in the culture is plotted for each of the indicated treatments of the dendritic cells. F and G, human dendritic cells from three separate donors were left untreated or incubated with purified PorB1B (50 μg/ml) for 24 h, and then they were washed and co-cultured with CFSE-labeled allogeneic lymphocytes for 7 days. Lymphocyte proliferation was assessed from the dilution of CSFE fluorescence by flow cytometry. Representative overlay histogram plots of CFSE-labeled allogeneic T cells after co-culture with the indicated dendritic cells are shown for each donor (F), and the percentage of cultured allogeneic T lymphocytes that underwent at least one generation of proliferation relative to all T lymphocytes in the culture is plotted from each of the three donors (G). Data are expressed as mean ± S.E. (n = 3 replicates). The p value was determined by ratio paired t test.
Although these experiments demonstrated an inhibitory effect on dendritic cell–mediated T cell proliferation when recombinant PorB1B was added to the culture, it did so at concentrations much higher than the levels of PorB1B present in either the Ng-CM or live gonococci that produced an equivalent inhibitory effect (data not shown). However, because PorB1B is an integral membrane protein, a substantial percentage of the PorB1B is likely to aggregate or bind to the tissue culture plate when the protein is diluted from its detergent-containing buffer into cell culture medium. Therefore, we sought to determine whether the amounts of dendritic cell-associated PorB1B were similar between cells treated with recombinant purified PorB1B and those treated with live N. gonorrhoeae by performing immunoblot analysis of the dendritic cells prior to their use in the co-culture T cell proliferation assay. The amount of PorB1B associated with dendritic cells after treatment with 50 μg/ml of purified PorB1B was similar to that observed in cells treated with live N. gonorrhoeae at an m.o.i. of 10 (Fig. 4C). We observed more cell-associated PorB1B in cells treated with 5 μg/ml of recombinant porin than with live N. gonorrhoeae at an m.o.i. of 1, even though the former caused little inhibition, whereas the latter decreases T cell proliferation by over 50%. These data indicate that N. gonorrhoeae likely produces additional inhibitory factors that complicate the comparison between live bacteria and a single purified protein. One such factor is the inhibitory interleukin IL-10, which has been shown to be secreted in response to live N. gonorrhoeae (10).
To further assess the loss of the suppressive effect of PorB1B at concentrations lower than 50 μg/ml, we tested the capacity of PorB1B to inhibit dendritic cell–induced T cell proliferation at three different subcytolytic concentrations of LDAO detergent. We observed minor but consistent concentration-dependent decreases in proliferation with detergent alone (∼30% at the highest concentration of detergent). Although complete suppression of T cell proliferation was observed with 50 μg/ml PorB1B regardless of the detergent concentration, we also observed that, as the concentration of LDAO was increased, the inhibitory potency of PorB1B increased (Fig. 4D). These data suggest that, when PorB1B loses association with detergent micelles and presumably loses its native, membrane-associated trimeric structure, it also loses the capacity to inhibit dendritic cell–induced T cell proliferation.
To ensure that PorB1B was not simply competing with OVA for proteolytic digestion or loading into antigen-presenting MHC molecules, we tested whether other proteins, either BSA or an isolated recombinant gonococcal protein (the transcriptional regulator GdhR) expressed in Escherichia coli, could inhibit dendritic cell–induced CD4 T cell proliferation. In contrast to recombinant PorB1B, neither protein impacted the capacity of OVA-pulsed dendritic cells to induce OT-II T cell proliferation (Fig. 4E) (25).
Because N. gonorrhoeae is an exclusive human pathogen, we also assessed whether addition of PorB1B would inhibit T cell proliferation in a model of human dendritic cell–induced T cell proliferation. As shown previously for treatment with live N. gonorrhoeae and with Ng-CM (Fig. 1, D and E), T cell proliferation induced by co-culture with allogeneic dendritic cells was inhibited when the dendritic cells were first treated with recombinant PorB1B (Fig. 4, F and G). Moreover, neither BSA nor GdhR were capable of inhibiting T cell proliferation when added at the same concentration of purified PorB1b.
PorB1B suppression of dendritic cell–mediated T cell proliferation requires properly folded PorB1B and is not mediated through TLR2 signaling
Purified Neisseria PorB proteins have been shown previously to have immunostimulatory activity in both cultured immune cells and when injected into whole animals (23, 26–28). In these reports, the protein, which was either purified from N. gonorrhoeae or refolded from E. coli inclusion bodies, had the detergent used to solubilized this integral membrane protein removed through a process of ethanol precipitation and dialysis during the final steps of preparation. The resulting detergent-free protein aggregates have been termed PorB “proteosomes” (29). We sought to test whether the suppressive effect on dendritic cell-induced T cell proliferation we observed with PorB1B treatment of dendritic cells was independent of the folded structure of PorB1B. Unfolded PorB1B was prepared by dialysis into PBS of urea-solubilized recombinant PorB1B inclusion bodies, whereas PorB1B proteosomes were prepared from our refolded, detergent-containing PorB1B preparations by ethanol precipitation and dialysis using the methods of Massari et al. (29) (Fig. 5A). Dynamic light scattering performed on refolded and proteosome preparations of recombinant PorB1B clearly demonstrated that refolded PorB1B in LDAO micelles exists as a single homogenous species with an estimated Stokes radius of 10 nm, whereas proteosome PorB1B preparations without detergent formed much larger particles with a Stokes radius of ∼700 nm (Fig. 5B). Based on the crystal structure of Neisseria meningitidis PorB, the size estimate generated by dynamic light scattering for the refolded protein is consistent with two PorB1B trimers sitting within an LDAO micelle, which is also consistent with estimates generated by retention time in size exclusion chromatography (Fig. 5C). Each of these protein preparations resulted in highly purified PorB1B, as determined by SDS-PAGE (Fig. 5C). These preparations were then tested for their effects on OVA-pulsed dendritic cell stimulation of OT-II T cell proliferation. As we had observed previously, refolded PorB1B demonstrated suppression of T cell proliferation, but neither the unfolded nor the proteosome preparation of PorB1B suppressed T cell proliferation in this system (Fig. 5D). These data suggest that the suppressive properties of PorB are disrupted when the structure of the protein is not maintained.
Figure 5.
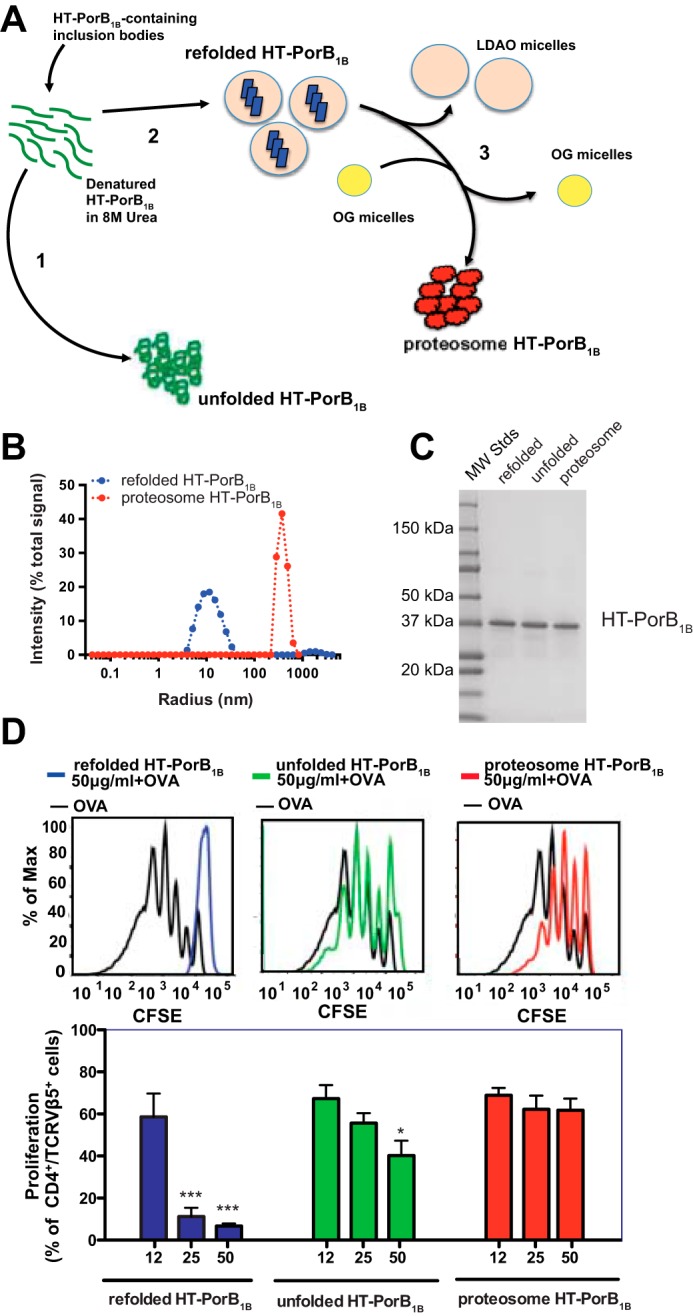
Recombinant PorB1B must be properly folded to inhibit dendritic cell–induced T cell proliferation. A, schematic of the preparation of unfolded, refolded, and proteosome recombinant HT-PorB1B. from urea-solubilized recombinant HT-PorB1B–containing inclusion bodies. 1, urea removed from unfolded HT-PorB1B by dialysis. 2, refolded HT-PorB1B generated by dilution of urea-solubilized protein into buffer containing LDAO detergent micelles, followed by size exclusion chromatography. 3, HT-PorB1B proteosomes were prepared by ethanol precipitation of refolded HT-PorB1B, resuspension in octyl glucoside (OG), followed by removal of octyl glucoside by dialysis. B, the Stokes radii determined from dynamic light scattering of refolded and proteosome HT-PorB1B. C, equal quantities (2 μg) of unfolded, refolded, and proteosomal HT-PorB1B were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250. D, dendritic cells were pulsed with OVA in the presence of LDAO (final concentration, 0.0015%) and the indicated concentrations of recombinant unfolded, refolded, or proteosomal HT-PorB1B. The pulsed dendritic cells were co-cultured with CFSE-labeled OT-II T cells, and the proliferation of OT-II T cells was determined using flow cytometry. The top panels show representative flow cytometry histograms from a single experiment in which the dendritic cells were treated with 50 μg/ml of the indicated protein preparation. The bottom panels show the percentage of T cells undergoing proliferation relative to all of the T cells in the culture plotted for each of the indicated treatments of the dendritic cells. Data are expressed as mean ± S.E. (n = 3 replicates). Statistical significance was determined by one-way ANOVA with a post hoc Tukey analysis for multiple comparisons. *, p < 0.05; ***, p < 0.001 compared with dendritic cells treated with OVA alone.
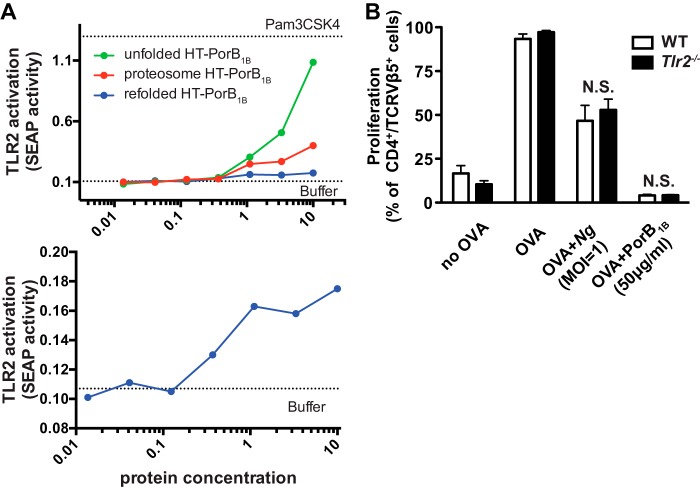
Neisseria PorB proteins have been shown previously to activate host TLR2 (28, 30). Structural and functional studies indicate that the seventh extracellular loop of PorB1B appears to be the PorB1B-derived ligand for TLR2 (31). Unfolded, refolded, and proteosomal HT-PorB1B was applied to a reporter cell line expressing human TLR2. Each PorB1B protein preparation activated TLR2 under these conditions. Refolded PorB1B generated a maximal TLR2 activation of a roughly 1.5-fold increase over baseline reporter activity. In contrast, proteosome PorB1B preparations induced a nearly 7-fold activation, and unfolded PorB1B induced a nearly 15-fold activation (Fig. 6A). These data suggest that high levels of TLR2 activation previously reported for proteosome PorB1B preparations from a number of Neisseria species could be the result of high-binding valency associated with an aggregated protein ligand, whereas actual native PorB1B stimulation of TLR2 is much more modest.
Figure 6.
PorB1B does not inhibit dendritic cell–induced T cell proliferation through TLR2. A, HEK-Blue hTLR2 reporter cells were treated with LDAO-containing buffer (0.0075%) alone or with the indicated concentrations of recombinant unfolded, refolded, and proteosome HT-PorB1B. After 24 h, cell culture supernatants from the treated cells were assayed for the activity of secreted alkaline phosphatase as described under “Experimental procedures.” The top panel shows the dose response for all three PorB1B preparations, whereas the bottom panel shows only refolded PorB1B with an expanded y axis. B, WT or Tlr2−/− dendritic cells were treated with GWM (no OVA), OVA, OVA + Ng (m.o.i. = 1), or OVA + PorB1B (50 μg/ml) and then co-cultured with CSFE-labeled OT-II T cells. T cell proliferation was assessed by flow cytometry. The mean ± S.E. (n = 4) of the percentage of OT-II T cells that underwent proliferation over 7 days is plotted for cells co-cultured with WT dendritic cells (black columns) or Tlr2−/− dendritic cells (white columns) for the indicated treatments. Statistical significance was assessed by two-way ANOVA with a post hoc Bonferroni analysis for multiple comparisons. No statistically significant difference was found between WT and Tlr2−/− cells.
Because TLR2 activation by capsular polysaccharide is known to mediate the immunosuppressive effects of outer membrane vesicles from the gut commensal Bacteroides fragilis, we sought to determine whether TLR2 engagement by PorB1B was activating tolerogenic signaling pathways in dendritic cells by utilizing dendritic cells generated from Tlr2−/− mice (32). T cell proliferation following co-culture with Tlr2−/− dendritic cells was indistinguishable from the proliferation observed when co-cultured with WT dendritic cells (Fig. 6B). Furthermore, N. gonorrhoeae or refolded PorB1B treatment of dendritic cells prior to co-culture with T cells resulted in equivalent inhibition of T cell proliferation regardless of the genotype of the dendritic cell. Taken together, these data suggest that, although TLR2-activating capacity is present in isolated N. gonorrhoeae PorB regardless of the folded structure of the protein, the immunosuppressive effects of the protein, which requires functionally folded PorB, are dominant and overcome any TLR2-activating activity.
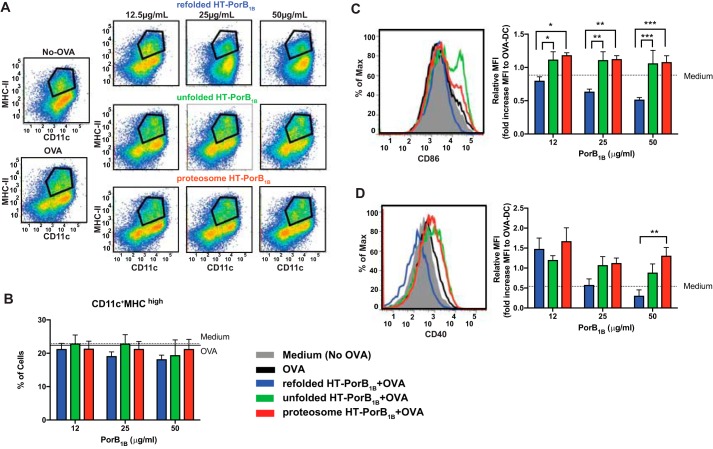
Inhibition of dendritic cell–induced T cell proliferation by N. gonorrhoeae PorB was observed even 3 days into the dendritic cell/T cell co-culture, suggesting that PorB acts directly on the dendritic cells rather than by inducing inhibitory T cell signaling, such as T regulatory cell differentiation, within the co-culture (Fig. S4). To test whether PorB treatment impacted the maturation of dendritic cells exposed to OVA prior to co-culture with T cells, we examined the fraction of cells with high CD11c and MHC class II expression and found no significant differences between cells treated with refolded, unfolded, or proteosome PorB (Fig. 7, A and B). In contrast, we observed that treatment of dendritic cells with refolded PorB resulted in a significant decrease of the co-stimulatory dendritic cell surface protein CD86 compared with treatment with unfolded or proteosome PorB (Fig. 7C). Treatment of dendritic cells at the highest doses of refolded PorB (50 μg/ml) also caused a significant decrease in surface expression of the costimulatory molecule CD40 compared with treatment with proteosome PorB (Fig. 7D). Taken together, these studies support the finding that properly folded N. gonorrhoeae PorB acts directly on dendritic cells to reduce their capacity to stimulate antigen-driven T cell proliferation.
Figure 7.
Properly folded PorB1B causes a decrease in surface expression of costimulatory molecules on dendritic cells. Dendritic cells were pulsed with OVA for 24 h in the presence of LDAO (final concentration, 0.0015%) and the indicated concentrations of recombinant unfolded, refolded, or proteosome HT-PorB1B, as was done prior to co-culture of dendritic cells with T cells in Fig. 5. Cells not pulsed with OVA were provided culture medium with LDAO (final concentration, 0.0015%) as a control. After 24 h, the cells were stained for the indicated dendritic cell surface markers and analyzed by flow cytometry. A, representative scatterplots for MHCII versus CD11c from each treatment group, with the gating used to assess the fraction of cells with high MHC and CD11c indicated by the black lines. B, the columns represent the percentage of dendritic cells that stained positively for high expression of both MHCII and CD11c after the indicated treatments from seven separate dendritic cell cultures. C, surface expression of the co-stimulatory molecule CD86 in the MHCIIhigh,CD11chigh dendritic cell populations was determined. A representative histogram of CD86 expression is shown for cells treated with 25 μg/ml of the indicated PorB preparation (left panel). Also shown is a bar graph representing the mean fluorescence intensity (MFI) of CD86 staining relative to cells pulsed with OVA without addition of PorB for all treatment groups (right panel). D, surface expression of the co-stimulatory molecule CD40 in the MHCIIhigh,CD11chigh dendritic cell populations was determined. A representative histogram of CD40 expression is shown for cells treated with 25 μg/ml of the indicated PorB preparation (left panel). Also shown is a bar graph representing the mean fluorescent intensity of CD86 staining relative to cells pulsed with OVA without addition of PorB for all treatment groups (right panel). Statistical significance was assessed by a two-way ANOVA with a post hoc Bonferroni analysis for multiple comparisons. *, p < 0.05; **, p < 0.01; ***, p < 0.001 for the noted comparisons.
Discussion
N. gonorrhoeae is a highly adapted human pathogen that is closely related to commensal Neisseria species. Like commensal Neisseria that live on human mucosal surfaces without eliciting protective immunologic responses, N. gonorrhoeae has the ability to escape the human immune response. Mechanisms involved in successful evasion of N. gonorrhoeae from human immune responses are multifactorial and complex. Recent findings have suggested that N. gonorrhoeae can actively suppress host immune responses through interaction with different types of immune cells. Antigen-presenting dendritic cells direct host immune responses toward either immunity or tolerance (33, 34). Studies have shown that the human vaginal mucosa contains four major subsets of myeloid-derived dendritic cells, each displaying unique functions that direct different types of immune responses (35). Therefore, understanding how N. gonorrhoeae interacts with dendritic cells to produce an end response is an important step in devising strategies to overcome the immune evasion of this pathogen.
Prolific blebbing of OMVs is a characteristic of Neisseria species; however, the consequences of this process are not fully understood (16, 17). A recent study demonstrated that the polysaccharide capsule associated with OMVs from the commensal bacillus B. fragilis is capable of inducing a tolerogenic phenotype in dendritic cells (32). Our studies demonstrate that an OMV-containing fraction of conditioned medium from N. gonorrhoeae is also capable of inducing immune suppression in dendritic cells. Unlike B. fragilis, N. gonorrhoeae does not produce capsular polysaccharide, which led us to further investigate the key components of Ng-CM that harbor these immunosuppressive properties. Yu et al. (36) recently demonstrated that dendritic cells exposed to N. gonorrhoeae suppress CD4+ T cell responses to HIV-derived antigens presented by those dendritic cells. This suppression appears to be mediated largely by activation of the immune-inhibitory CEACAM1 receptor on dendritic cells by N. gonorrhoeae Opa proteins, which have also been implicated in promoting production of the immune-suppressive cytokines IL-10 and transforming growth factor β, thereby inhibiting T cell proliferation (13). However, because N. gonorrhoeae Opa proteins bind only to human CEACAM-1 but not mouse CEACAM-1 (37), mechanisms exclusive of Opa-CEACAM-1 interactions must exist that are responsible for suppression of antigen-dependent T cell proliferation by mouse dendritic cells exposed to N. gonorrhoeae or Ng-CM. Although OMV-based vaccines have demonstrated that OMVs are capable of inducing immune responses to proteins and polysaccharides associated with OMVs, the relative antigenicity of OMV-associated proteins compared with those of the isolated proteins or proteins in association with bacterial pathogen-associated molecular patterns (PAMPs) that are also found in OMVs is not known. It is possible that physiologic levels of OMV shed by pathogenic and commensal bacteria actually suppress immune responses, as suggested by this study and studies published previously (32).
In addition to PorB, LOS is another major component of OMVs shed by Neisseria species. LOS is a potent inducer of the inflammatory cytokine response, and N. meningitidis LOS has adjuvant properties that presumably are mediated by TLR4 activation by the lipid A moiety of LOS (38). The oligosaccharide component of gonococcal LOS can vary structurally because of phase-variable expression of enzymes in the synthetic pathway (39), and these LOS variants have been reported to alter the cytokine secretion profiles of dendritic cells because of their stimulation of different surface lectin receptors, with changes in the cytokine profile influencing CD4+ T cell subtype differentiation (40). We found that, unlike N. gonorrhoeae cells or OMV-containing Ng-CM, purified gonococcal LOS was unable to elicit inhibition of T cell proliferation by OVA-primed dendritic cells.
PorB is the most abundant outer membrane protein in N. gonorrhoeae. Importantly, we found that purified recombinant PorB1B has the capacity to inhibit dendritic cell-mediated CD4+ T cell proliferation. Neisseria PorB proteins have been shown to activate host TLR2 responses (28, 30, 41), and purified PorBs from several commensal Neisseria species have been shown to stimulate immune responses when injected in mice and to induce dendritic cell maturation when added to cells in culture (26, 28, 42). The major difference between those published studies and this study is the preparation of PorB. All published studies demonstrating immune activation by PorB have been carried out with a PorB preparation in a detergent-free particle termed a proteosome (28, 43). Because PorB is an integral membrane protein, it is unlikely that it maintains its native functional structure under these conditions. The purified recombinant PorB in the nontoxic detergent (LDAO) micelles we use in this study mimics native PorB both structurally and physiologically, as shown by its performance on gel filtration chromatography and its capacity to facilitate sugar permeation when reconstituted into liposomes (Fig. 4C). The potency of detergent-solubilized PorB1B for inhibiting dendritic cell–mediated T cell proliferation increases as the amount of LDAO in the culture medium is increased, which, we suspect, suppresses the tendency of PorB to aggregate when LDAO micelles disperse upon dilution. Importantly, recombinant PorB1B in the absence of refolding or refolded PorB1B taken through the precipitation and detergent removal procedure used to generate PorB proteosomes in other published reports eliminates its immunosuppressive activity. These data strongly suggest that the native micelle/membrane-associated structure of PorB is required for its inhibitory function.
Unfolded, refolded, and proteosome PorB1B all have the capacity to stimulate TLR2, as has been reported previously, but at markedly different levels. Our data suggest that proteosome PorB1B retains its TLR2-stimulating activity while simultaneously eliminating the immunosuppressive activity of PorB1B, which would explain the reported immunologic adjuvant properties of Neisseria species PorB that populate the current biomedical literature. Interestingly, PorB proteosomes have been shown to induce much more robust anti-PorB antibody responses than an equivalent quantity of native gonococcal PorB administered in naturally derived outer membrane vesicles (43). Given that poor immune responses to N. gonorrhoeae in infected humans are well-documented, there is certainly indirect evidence that naturally occurring gonococcal PorB associated with the bacteria or OMVs does not support a robust immune response in the natural host.
Unlike B. fragilis OMV–associated capsular polysaccharide, which induces a tolerogenic dendritic cell phenotype through host TLR2 activation (32), N. gonorrhoeae cells and purified recombinant PorB1B inhibited the capacity of dendritic cells derived from Tlr2−/− or WT mice to induce T cell proliferation. These data demonstrate that properly folded PorB from N. gonorrhoeae present in whole bacteria or in OMVs shed from the bacteria are capable of exerting immunosuppressive effects on dendritic cells. The mechanism underlying this suppression has yet to be determined. Gonococcal PorB has been shown to traffic to host cell mitochondria. In some studies, this interaction has been reported to promote host cell apoptosis, whereas, in others, it has been reported to inhibit host cell apoptosis (21, 44, 45). Some gonococcal PorB proteins have been shown to interact with host cell surface receptors, including a scavenger receptor expressed in endothelial cells and Gp96, both of which have been implicated in immune cell signaling and immune tolerance (46–48). These and potentially novel signaling pathways clearly need to be investigated further for their potential role in mediating immunosuppressive signaling downstream of gonococcal PorB.
Experimental procedures
Ethics statement
All experiments involving the use of mice were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at UNC. Human dendritic cells were obtained from subjects enrolled in a UNC Institutional Review Board–approved study (Study 05-2860) who were deidentified prior to use in this study. The UNC Office of Human Research Ethics reviewed the proposed use of deidentified human subject-derived dendritic cells and determined that the use described (Study 12-0024) does not constitute human subject research as defined under federal regulations (45 CFR 46.102 (d or f) and 21 CFR 56.102(c)(e)(l)) and does not require further IRB approval.
Preparation of N. gonorrhoeae and N. gonorrhoeae conditioned medium
Inocula of a predominantly Opa+ frozen stock of FA1090 of N. gonorrhoeae were prepared as described previously (14). To minimize phase or antigenic variation, passage of FA1090 and its derivatives was kept to a minimum. The bacterial density of each inoculum was estimated by measuring A600 and confirmed by plating of serial dilutions.
To generate Ng-CM, N. gonorrhoeae strain FA1090 was grown overnight from frozen stock streaked on GC medium broth (GCB) plates. The cells were swabbed from the plates, introduced into 10 ml of GWM at an A600 of 0.2, and grown for 4.5 h in a shaking incubator at 37 °C with 5% CO2. Bacteria were removed by centrifugation at 1200 × g for 10 min, followed by filtration through a sterile 0.22-μm filter. The Ng-CM was further fractionated for some experiments by ultrafiltration using a Centriprep device with a 100-kDa cut-off filter (EMD Millipore, Billerica, MA) according to the manufacturer's instructions.
Purification and functional activity of recombinant N. gonorrhoeae PorB1B
Recombinant PorB1B was produced in E. coli as inclusion bodies and refolded using a modification of the method described by Olesky et al. (24). Full details are provided in supporting Materials and Methods, but a brief description is as follows. PorB1B expression was induced in BL21*(DE3) harboring pT7-HTb-por1090, which replaces the PorB signal sequence with a 28-amino acid extension (HT) containing a hexahistidine tag and a tobacco etch virus protease cleavage site at the N terminus of the protein (HT-PorB1B). HT-PorB1B–containing inclusion bodies were isolated and solubilized in 8 m urea. Unfolded HT-PorB was prepared from urea-solubilized inclusion bodies by dialysis into PBS. Refolded HT-PorB1B was prepared by slowly diluting urea-solubilized inclusion bodies into refolding buffer (200 mm 3-(cyclohexylamino)propanesulfonic acid, 400 mm NaCl, 50 mm Tris-HCl, 0.3% LDAO (pH 11)), and after stirring overnight, the solution's pH was lowered to 8.0 with concentrated HCl, and any precipitated protein was removed by centrifugation and filtration. The protein was purified by immobilized Ni2+ chromatography. In most experiments, the protein used had the HT tag removed by digestion with tobacco etch virus protease, and the untagged, properly folded PorB1B was isolated in the flow-through of a nickel-nitrilotriacetic acid column run in the presence of 15 mm imidazole, followed by size exclusion chromatography on a Sephacryl S-300 column in 1× PBS, 0.1% LDAO (PBSL). Preliminary experiments showed no difference between PorB1B and HT-PorB1B for inhibition of dendritic cell–mediated T cell proliferation (Fig. S5); therefore, in later experiments, the HT tag was left attached to the protein. Proteosome HT-PorB1B was prepared from refolded HT-PorB1B by precipitation with ethanol, resuspending the precipitated protein in 8% β-octyl glucoside, and then dialyzing in PBS to remove the detergent. Purified PorB preparations were tested for the presence of LPS using a chromogenic Limulus amebocyte lysate endotoxin assay (ToxinsensorTM, Genscript) and human TLR4-expressing reporter cells (HEK-BlueTM hTLR4, Invivogen) according to the manufacturer's protocol.
Functional activity of refolded and purified PorB1B trimers was demonstrated by the swelling assay originally described by Nikaido and Rosenberg (49). Permeation rates of arabinose (MW 150), glucose (MW 180), galactose (MW 180), GlcNAc (MW 221), sucrose (MW 342), and raffinose (MW 595) were quantified as a ratio of the rate of permeation of the indicated sugar to the permeation rate of arabinose. Stachyose (MW 660) was used to find the isoosmotic concentration of the liposome preparation as described previously (24, 49).
Generation, infection, and stimulation of bone marrow–derived dendritic cells
Bone marrow–derived dendritic cells (BMDCs) were prepared from 9- to 12-week-old C57BL/6 or C57BL/6 Tlr2−/− mice (The Jackson Laboratory, Bar Harbor, ME) as described previously (14). After 7 days of growth and differentiation, BMDCs were washed, resuspended, and incubated with 100 μg/ml OVA (Sigma-Aldrich, St. Louis, MO) and either N. gonorrhoeae FA1090 at the indicated m.o.i., GWM, Ng-CM, purified gonococcal LOS purified from FA1090, recombinant PorB1B, or PBSL (vehicle). After incubation for 24 h, the cells were collected and washed for co-culture with T cells or downstream assays.
BMDC–T cell co-culture/proliferation assay
T cells were isolated from spleens and lymph nodes of OT-II mice (C57BL/6-Tg(TcraTcrb)425Cbn/J, The Jackson Laboratory) and labeled with CFSE (Life Technologies) as described previously (14). Labeled, enriched T cells (5 × 105 cells/ml) were co-cultured with BMDCs at a density of 5 × 104 cells/ml in 48-well plates or plates containing transwell inserts (Costar, Corning, NY). After 7 days in co-culture, T cell proliferation was assessed by measuring the dilution of CFSE fluorescence in CD4+,TCRVβ5+ lymphocytes using flow cytometry on a BD FACSCanto or a BD LSRII-SOS (BD Biosciences, Palo Alto, CA) as described previously (14). Data from 10,000–100,000 events were acquired for each sample and saved as an FCS 3.0 file that was subsequently analyzed with FlowJo software (Tree Star, Ashland, OR).
Assessment of TLR2 activation
HEK-BlueTM hTLR2 reporter cells were acquired from Invivogen (San Diego, California). The cells were cultured in growth medium (Dulbecco's modified Eagle's medium (Gibco) containing 10% heat-inactivated fetal bovine serum, l-glutamine (2 mm), glucose (4.5 g/liter), penicillin/streptomycin (50 units/ml and 50 μg/ml), and Normocin (100 μg/ml)) and then maintained in selection medium (growth medium supplemented with 100 μg/ml neocin). Cells were seeded in 96-well plates at a density of 5 × 104 cells in 200 μl per well, and after 24 h, they were exposed to vehicle, the indicated concentrations of PorB1B preparations, or 30 ng/ml Pam3CSK4 (Invivogen). Secreted alkaline phosphatase activity was assessed after 24 h of incubation by adding 20 μl of cell culture supernatant to 180 μl of Quanti-blueTM reagent (Invivogen) in a new 96-well plate and measuring optical density at 625 nm after a 2 h incubation at 37 °C.
Statistical analysis
Statistical analyses were performed using Prism 5 or 6 software (GraphPad, La Jolla, CA). Significance of the differences between multiple groups was assessed using one-way or two-way ANOVA with Tukey or Bonferroni as a post hoc test for multiple comparisons. In all cases, p < 0.05 was considered statistically significant.
Author contributions
W. Z., J. T., G. D. S., R. A. N., and J. A. D. conceptualization; W. Z., J. T., K. J. K., and J. E. A. investigation; W. Z., J. T., J. E. A., K. P. M., G. D. S., R. A. N., and J. A. D. methodology; W. Z., J. T., and J. A. D. writing-original draft; J. T., J. E. A., G. D. S., R. A. N., and J. A. D. writing-review and editing; J. E. A., K. P. M., G. D. S., R. A. N., and J. A. D. resources; G. D. S. formal analysis; G. D. S., R. A. N., and J. A. D. funding acquisition; J. A. D. supervision; J. A. D. project administration.
Supplementary Material
Acknowledgments
Antibodies to OpaB and PorB1B were kindly provided by Dr. Janne Cannon. Lipooligosaccharide from N. gonorrhoeae strain FA1090 was provided by Dr. Gary Jarvis (University of California, San Francisco). Flow cytometry was performed in the Duke Human Vaccine Institute Research Flow Cytometry Shared Resource Facility (Durham, NC), and select studies were performed in the Regional Biocontainment Laboratory at Duke University.
This project was supported by the Burroughs Wellcome Fund Career Awards for Medical Scientists (to J. A. D.) and the National Institutes of Health through the following grants and contracts: U19-AI031496 (Southeastern Sexually Transmitted Infection Cooperative Research Center) (to J. A. D. and G. D. S.); U19-AI113170-01 (Atlantic Coast Sexually Transmitted Infection Cooperative Research Center) (to J. A. D., G. D. S., and R. A. N.); UC6-AI058607 (Regional Biocontainment Laboratory at Duke University Medical Center) (to G. D. S.); and NIGMS Grant R01-GM066861 (to R. A. N.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Methods and Figs. S1–S5.
- Opa
- opacity protein
- LOS
- lipooligosaccharide
- IL
- interleukin
- Ng-CM
- Neisseria gonorrhoeae conditioned medium
- CFSE
- carboxyfluorescein succinimidyl ester
- OVA
- ovalbumin
- GWM
- Graver–Wade medium
- OMV
- outer membrane vesicle
- HT
- hexahistidine tag
- LDAO
- L-N,N-dimethylamine-N-oxide
- LPS
- lipopolysaccharide
- m.o.i.
- multiplicity of infection
- MHC
- major histocompatibility complex
- UNC
- University of North Carolina
- MW
- molecular weight
- BMDC
- bone marrow-derived dendritic cell
- ANOVA
- analysis of variance.
References
- 1. Newman L., Rowley J., Vander Hoorn S., Wijesooriya N. S., Unemo M., Low N., Stevens G., Gottlieb S., Kiarie J., and Temmerman M. (2015) Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 10, e0143304 10.1371/journal.pone.0143304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolan G. A., Sparling P. F., and Wasserheit J. N. (2012) The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 366, 485–487 10.1056/NEJMp1112456 [DOI] [PubMed] [Google Scholar]
- 3. Unemo M., and Nicholas R. A. (2012) Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 7, 1401–1422 10.2217/fmb.12.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jerse A. E., and Deal C. D. (2013) Vaccine research for gonococcal infections: where are we? Sex Transm. Infect. 89, Suppl. 4, iv63–iv8 10.1136/sextrans-2013-051225 [DOI] [PubMed] [Google Scholar]
- 5. Hobbs M. M., Alcorn T. M., Davis R. H., Fischer W., Thomas J. C., Martin I., Ison C., Sparling P. F., and Cohen M. S. (1999) Molecular typing of Neisseria gonorrhoeae causing repeated infections: evolution of porin during passage within a community. J. Infect Dis. 179, 371–381 10.1086/314608 [DOI] [PubMed] [Google Scholar]
- 6. Fox K. K., and Knapp J. S. (1999) Antimicrobial resistance in Neisseria gonorrhoeae. Curr. Opin. Urol. 9, 65–70 10.1097/00042307-199901000-00011 [DOI] [PubMed] [Google Scholar]
- 7. Katz A. R., Komeya A. Y., Soge O. O., Kiaha M. I., Lee M. V., Wasserman G. M., Maningas E. V., Whelen A. C., Kirkcaldy R. D., Shapiro S. J., Bolan G. A., and Holmes K. K. (2012) Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin. Infect. Dis. 54, 841–843 10.1093/cid/cir929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson J. E., Hobbs M. M., Biswas G. D., and Sparling P. F. (2003) Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 48, 1325–1337 10.1046/j.1365-2958.2003.03496.x [DOI] [PubMed] [Google Scholar]
- 9. Chen T., Grunert F., Medina-Marino A., and Gotschlich E. C. (1997) Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 185, 1557–1564 10.1084/jem.185.9.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T., Bolland S., Chen I., Parker J., Pantelic M., Grunert F., and Zimmermann W. (2001) The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J. Biol. Chem. 276, 17413–17419 10.1074/jbc.M010609200 [DOI] [PubMed] [Google Scholar]
- 11. Boulton I. C., and Gray-Owen S. D. (2002) Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3, 229–236 10.1038/ni769 [DOI] [PubMed] [Google Scholar]
- 12. Liu Y., and Russell M. W. (2011) Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti-transforming growth factor β antibody generates immunological memory and protective immunity. MBio 2, e00095-000111, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y., Liu W., and Russell M. W. (2014) Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 7, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu W., Ventevogel M. S., Knilans K. J., Anderson J. E., Oldach L. M., McKinnon K. P., Hobbs M. M., Sempowski G. D., and Duncan J. A. (2012) Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T cell proliferation. PLoS ONE 7, e41260 10.1371/journal.pone.0041260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wade J. J., and Graver M. A. (2007) A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol. Lett. 273, 35–37 10.1111/j.1574-6968.2007.00776.x [DOI] [PubMed] [Google Scholar]
- 16. Ellis T. N., and Kuehn M. J. (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol. Biol. Rev. 74, 81–94 10.1128/MMBR.00031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Post D. M., Zhang D., Eastvold J. S., Teghanemt A., Gibson B. W., and Weiss J. P. (2005) Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 280, 38383–38394 10.1074/jbc.M508063200 [DOI] [PubMed] [Google Scholar]
- 18. Dorward D. W., Garon C. F., and Judd R. C. (1989) Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171, 2499–2505 10.1128/jb.171.5.2499-2505.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston K. H., and Gotschlich E. C. (1974) Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J. Bacteriol. 119, 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lappann M., Otto A., Becher D., and Vogel U. (2013) Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J. Bacteriol. 195, 4425–4435 10.1128/JB.00625-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kozjak-Pavlovic V., Dian-Lothrop E. A., Meinecke M., Kepp O., Ross K., Rajalingam K., Harsman A., Hauf E., Brinkmann V., Günther D., Herrmann I., Hurwitz R., Rassow J., Wagner R., and Rudel T. (2009) Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS Pathog. 5, e1000629 10.1371/journal.ppat.1000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faulstich M., Hagen F., Avota E., Kozjak-Pavlovic V., Winkler A. C., Xian Y., Schneider-Schaulies S., and Rudel T. (2015) Neutral sphingomyelinase 2 is a key factor for PorB-dependent invasion of Neisseria gonorrhoeae. Cell Microbiol. 17, 241–253 10.1111/cmi.12361 [DOI] [PubMed] [Google Scholar]
- 23. Massari P., Henneke P., Ho Y., Latz E., Golenbock D. T., and Wetzler L. M. (2002) Cutting edge: Immune stimulation by Neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168, 1533–1537 10.4049/jimmunol.168.4.1533 [DOI] [PubMed] [Google Scholar]
- 24. Olesky M., Zhao S., Rosenberg R. L., and Nicholas R. A. (2006) Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 188, 2300–2308 10.1128/JB.188.7.2300-2308.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rouquette-Loughlin C. E., Zalucki Y. M., Dhulipala V. L., Balthazar J. T., Doyle R. G., Nicholas R. A., Begum A. A., Raterman E. L., Jerse A. E., and Shafer W. M. (2017) Control of gdhR expression in Neisseria gonorrhoeae via autoregulation and a master repressor (MtrR) of a drug efflux pump operon. MBio. 8, e00449–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X., Wetzler L. M., and Massari P. (2008) The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 26, 786–796 10.1016/j.vaccine.2007.11.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosaheb M. M., Reiser M. L., and Wetzler L. M. (2017) Toll-like receptor ligand-based vaccine adjuvants require intact MyD88 signaling in antigen-presenting cells for germinal center formation and antibody production. Front Immunol. 8, 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singleton T. E., Massari P., and Wetzler L. M. (2005) Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174, 3545–3550 10.4049/jimmunol.174.6.3545 [DOI] [PubMed] [Google Scholar]
- 29. Massari P., King C. A., MacLeod H., and Wetzler L. M. (2005) Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 44, 136–146 10.1016/j.pep.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 30. Toussi D. N., Carraway M., Wetzler L. M., Lewis L. A., Liu X., and Massari P. (2012) The amino acid sequence of Neisseria lactamica PorB surface-exposed loops influences Toll-like receptor 2-dependent cell activation. Infect. Immun. 80, 3417–3428 10.1128/IAI.00683-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kattner C., Toussi D. N., Zaucha J., Wetzler L. M., Rüppel N., Zachariae U., Massari P., and Tanabe M. (2014) Crystallographic analysis of Neisseria meningitidis PorB extracellular loops potentially implicated in TLR2 recognition. J. Struct. Biol. 185, 440–447 10.1016/j.jsb.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen Y., Giardino Torchia M. L., Lawson G. W., Karp C. L., Ashwell J. D., and Mazmanian S. K. (2012) Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 10.1016/j.chom.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinman R. M., Hawiger D., and Nussenzweig M. C. (2003) Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- 34. Steinman R. M., and Hemmi H. (2006) Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 311, 17–58 [DOI] [PubMed] [Google Scholar]
- 35. Duluc D., Gannevat J., Anguiano E., Zurawski S., Carley M., Boreham M., Stecher J., Dullaers M., Banchereau J., and Oh S. (2013) Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 6, 626–638 10.1038/mi.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu Q., Chow E. M., McCaw S. E., Hu N., Byrd D., Amet T., Hu S., Ostrowski M. A., and Gray-Owen S. D. (2013) Association of Neisseria gonorrhoeae Opa(CEA) with dendritic cells suppresses their ability to elicit an HIV-1-specific T cell memory response. PLoS ONE 8, e56705 10.1371/journal.pone.0056705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Voges M., Bachmann V., Kammerer R., Gophna U., and Hauck C. R. (2010) CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol. 10, 117 10.1186/1471-2180-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zughaier S., Agrawal S., Stephens D. S., and Pulendran B. (2006) Hexa-acylation and KDO(2)-glycosylation determine the specific immunostimulatory activity of Neisseria meningitidis lipid A for human monocyte derived dendritic cells. Vaccine 24, 1291–1297 10.1016/j.vaccine.2005.09.039 [DOI] [PubMed] [Google Scholar]
- 39. Shafer W. M., Datta A., Kolli V. S., Rahman M. M., Balthazar J. T., Martin L. E., Veal W. L., Stephens D. S., and Carlson R. (2002) Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J. Endotoxin Res. 8, 47–58 10.1177/09680519020080010501 [DOI] [PubMed] [Google Scholar]
- 40. van Vliet S. J., Steeghs L., Bruijns S. C., Vaezirad M. M., Snijders Blok C., Arenas Busto J. A., Deken M., van Putten J. P., and van Kooyk Y. (2009) Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 5, e1000625 10.1371/journal.ppat.1000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Massari P., Visintin A., Gunawardana J., Halmen K. A., King C. A., Golenbock D. T., and Wetzler L. M. (2006) Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176, 2373–2380 10.4049/jimmunol.176.4.2373 [DOI] [PubMed] [Google Scholar]
- 42. Platt A., MacLeod H., Massari P., Liu X., and Wetzler L. (2013) In vivo and in vitro characterization of the immune stimulating activity of the Neisserial porin PorB. PLoS ONE 8, e82171 10.1371/journal.pone.0082171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wetzler L. M., Barry K., Blake M. S., and Gotschlich E. C. (1992) Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 60, 39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Müller A., Günther D., Düx F., Naumann M., Meyer T. F., and Rudel T. (1999) Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18, 339–352 10.1093/emboj/18.2.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Massari P., King C. A., Ho A. Y., and Wetzler L. M. (2003) Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 5, 99–109 10.1046/j.1462-5822.2003.00257.x [DOI] [PubMed] [Google Scholar]
- 46. Murshid A., Borges T. J., and Calderwood S. K. (2015) Emerging roles for scavenger receptor SREC-I in immunity. Cytokine 75, 256–260 10.1016/j.cyto.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rechner C., Kühlewein C., Müller A., Schild H., and Rudel T. (2007) Host glycoprotein Gp96 and scavenger receptor SREC interact with PorB of disseminating Neisseria gonorrhoeae in an epithelial invasion pathway. Cell Host Microbe 2, 393–403 10.1016/j.chom.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y., Ansa-Addo E., and Li Z. (2015) GP96: safeguarding Treg. Oncotarget 6, 19936–19937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nikaido H., and Rosenberg E. Y. (1983) Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J. Bacteriol. 153, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.