Graphical abstract
Keywords: Coccidiosis, Eimeria maxima, Broiler, Genetic selection, Resistance, Tolerance, Growth rate, Bone mineralisation
Highlights
-
•
We measured tolerance and resistance to an Eimeria maxima infection of 2 broiler lines differing in their growth rate.
-
•
Infection induced typical symptoms of coccidiosis in relation to tolerance accompanied by parasite replication in the jejunum.
-
•
Lines did not differ in their resistance or tolerance.
-
•
Bone mineralisation was penalised by infection with effects being more pronounced at the recovery stage.
Abstract
Chickens exhibit varied responses to infection with Eimeria parasites. We hypothesise that broilers selected for increased growth rate will show lower resistance and tolerance to a coccidian challenge. 288 chickens of fast (F) or slow (S) growing lines were inoculated with 0 (control), 2500 (low-dose), or 7000 (high-dose) sporulated E. maxima oocysts at 13 days of age in two consecutive rounds. Gain and Intake were measured daily and their values relative to BW at the point of infection were calculated over the pre-patent (days 1–4 post-infection), acute (d5–8 pi), and recovery (d9–12 pi) phases of infection to assess the impact of infection. Levels of plasma carotenoids, vitamins E and A, long bone mineralisation, caecal microbiota diversity indices, and histological measurements were assessed at the acute (d6 pi) and recovery stage (d13 pi). In addition, we measured the levels of nitric oxide metabolites and the number of parasite genome copies in the jejunumat d6pi. In absolute terms F birds grew 1.42 times faster than S birds when not infected. Infection significantly reduced relative daily gain and intake (P < 0.001), with the effects being most pronounced during the acute phase (P < 0.001). Levels of all metabolites were significantly decreased, apart from NO which increased (P < 0.001) in response to infection on d6pi, and were accompanied by changes in histomorphometric features and the presence of E. maxima genome copies in infected birds, which persisted to d13pi. Furthermore, infection reduced tibia and femur mineralisation, which also persisted to d13pi. Reductions in measured variables were mostly independent of dose size, as was the level of parasite replication. The impact of infection was similar for S and F-line birds for all measured parameters, and there were no significant interactions between line x dose size on any of these parameters. In conclusion, our results suggest that line differences in productive performance do not influence host responses to coccidiosis when offered nutrient adequate diets.
1. Introduction
Genetic selection for production traits, to meet increased requirements for chicken meat, has been applied to broiler chickens at an unprecedented rate (Siegel, 2014; Tixier-Boichard et al., 2012; Zuidhof et al., 2014). Such an emphasis on productive traits may have compromised the ability of modern broilers to cope with metabolic and skeletal disorders (Dawkins and Layton, 2012; Julian, 1998) and infectious pathogens (Cheema et al., 2003; Yunis et al., 2000). This raises concerns amongst the general public and have led, for example, the Dutch Organisation of Retailers to take the strategic decision that they will only sell chicken meat from slow-growing animals. Similar trends appear in other parts of the European Union (van der Aar et al., 2016).
The hypothesis is that when resources are limited, as is in the case of most health challenges, birds from lines selected for productivity will continue to direct these resources to productive rather than functional traits, such as the ability to cope with disease (Coop and Kyriazakis, 1999). This is a consequence of the genetic drive for greater productivity (Rauw, 2012). Here, we used two modern broiler lines that have been selected for different growth rates to test the hypothesis that selection for growth will penalise bird resistance to parasite infection to a greater extend (Coop and Kyriazakis, 1999). A lower level of resistance could potentially affect markers of tolerance, such as the magnitude and duration of pathogen induced anorexia, in such a way that less resistant hosts could show a delayed induction of anorexia, which is of longer duration and of smaller magnitude (Doeschl-Wilson et al., 2009; Lough et al., 2015). Host resistance has been defined as the mechanism by which the entry and/or the replication of pathogens within the host is restricted, with tolerance defined as the host’s ability to limit the detrimental effect of pathogens on performance without necessarily affecting pathogen burden (Doeschl-Wilson and Kyriazakis, 2012; Lough et al., 2015; Rauw, 2012). Although in broilers monospecific coccidian infections rarely occur in the field, a controlled coccidial infection is a good model, to test our hypothesis as the main effects are a reduction in food intake (Kipper et al., 2013; Preston-Mafham and Sykes, 1970) and absorption of nutrients (Persia et al., 2006; Preston-Mafham and Sykes, 1970; Su et al., 2014), leading to reduced availability of nutrient resources. We used infection with Eimeria maxima to test our hypothesis, one of the most commonly encountered coccidia spp. The magnitude of its effects depends on the degree of tissue damage and inflammation (Lillehoj and Trout, 1996; Williams, 2005), typically occuring around the period of maximum parasite schizogony and gametogony (Hein, 1968), which coincides with shortening of the villi and enlargement of crypts.
To assess tolerance we measured performance over the course of infection. In addition, we implemented two sampling points, one at the acute ((d6 post-infection (pi)) and one at the recovery stage of infection (d13pi) to measure plasma levels of lutein and zeaxanthin, which are the major carotenoids in cereal grains (Humphries and Khachik, 2003) and fat-soluble vitamins retinol (vitamin A) and α-tocopherol (vitamin E). Reduced plasma levels of both carotenoids and fat-soluble vitamins may serve as indicators of intestinal epithelial damage and may be used as markers of severity for coccidial infections (Allen et al., 2004; Allen and Fetterer, 2002a; Singh and Donovan, 1973). Furthermore, histological measurements were carried to directly assess the level of damage induced to the intestinal mucosa. Fast and slow growing broilers may differ on the level of long bone mineralisation (Williams et al., 2004) and coccidiosis has been shown to affect aspects of bone development (Fetterer et al., 2013). To that end we also assessed long bone mineralisation at both d6 and d13pi. Plasma levels of nitric oxide (NO) metabolites were also assessed at the acute stage of infection as they constitute a marker of the severity of coccidial infections (Allen, 1997a,b). They facilitate parasite killing (Lillehoj and Li, 2004), but their excessive production contributes to the pathology of E. maxima (Allen and Fetterer, 2002b) infections due to oxidative damage and their concentration is negatively correlated with average daily gain (ADG) and carotenoid concentration at d6pi (Zhu et al., 2000). Even though E. maxima does not replicate in the caeca it was hypothesised that infection in the small intestine might impact the caecal microbiota due to reduced nutrient absorption resulting in increased nutrients in the caeca, whilst differences between genetic lines of chicken have been previously observed (Schokker et al., 2015). In assessing the differences in resistance of our treatment groups, we estimated the number of parasite genome copies in the jejunum, the primary site of E. maxima colonisation and replication, at the peak of parasite replication (i.e. d6pi; (Blake et al., 2006)) and by proxy accounting for all possible underlying immune responses.
2. Materials and methods
2.1. Chicken management
All procedures were conducted under the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63/EU for animal experiments and carried out under Home Office authorization (P441ADF04). The experiment was conducted over two rounds, separated by 6 weeks. Each round consisted of 72 male day-old chicks of a fast-growing line (Ross 308, F), and an equal number of a slow growing line (Ross Ranger Classic, S). All birds were obtained from the same hatchery and had parents subjected to the same husbandry regime. Furthermore, the same parent stock flocks were used for each of the two lines which aged 37 and 43 weeks of age for round A and B, respectively. The growth potential of these lines differs by approximately 25%, according to the performance objectives of the breeding company. Lines F and S originate from the same paternal lines but different maternal lines; growth rate is not part of the selection criteria for the maternal lines of the S line.
Birds were housed in a windowless, thermostatically controlled room in 24 circular pens with a diameter of 1.2 m (1.13 m2). Pens were equipped with tube feeders and bell-drinkers, and wood shavings were used as litter to a depth of 5 cm. Birds had ad libitum access to feed and water throughout the trial. The temperature within the pen was monitored daily and maintained to meet recommendations for spot brooding (Aviagen, 2014b), starting at 34 °C at chick placement and was gradually reduced to 20 °C by 25 days of age. Light intensity at pen level ranged from 180 to 220 lux, while a lighting schedule of 23L:1D was applied for the first 7 days of age and switched to 18L:6D for the remainder of the trial.
Starter (d0–10) and grower (d11–26) diets were manufactured according to Aviagen nutrition specifications (Aviagen, 2014a) and were offered to both lines (Table 1). The starter diet was offered in crumb form and the grower in pelleted form.
Table 1.
Ingredient and calculated chemical composition of the starter (d0–10) and grower (d11–26 post-hatch) diets.
| Item | Starter | Grower |
|---|---|---|
| Ingredient (%) | ||
| Wheat | 47.8 | 51.5 |
| Soybean meal (48 % CP) | 32 | 25.2 |
| Corn | 10 | 10 |
| Soybean full fat | 4.0 | 7.0 |
| Dicalcium phosphate | 1.89 | 1.66 |
| Soy crude oil | 1.84 | 2.32 |
| Limestone | 0.64 | 0.59 |
| Vitamin and mineral premix1 | 0.4 | 0.4 |
| DL methionine | 0.33 | 0.30 |
| L-Lysine | 0.27 | 0.24 |
| Sodium bicarbonate (27 %) | 0.21 | 0.19 |
| Sodium chloride (39 %) | 0.19 | 0.20 |
| L-Threonine | 0.14 | 0.12 |
| Choline chloride (60 %) | 0.05 | 0.05 |
| L-Valine | 0.03 | 0.02 |
| Xylanase3 | 0.02 | 0.02 |
| Calculated nutrient composition (%) | ||
| ME (kcal/kg) | 3,000 | 3,100 |
| Crude protein | 23.5 | 21.7 |
| Crude fat | 4.37 | 5.41 |
| Calcium | 0.96 | 0.87 |
| Phophorus | 0.76 | 0.70 |
| Available phosphorus | 0.48 | 0.44 |
| Ash | 5.23 | 4.78 |
1,2Provided per kilogram of feed vitamins, minerals and digestible AA according to Aviagen Nutrient specifications (Aviagen, 2014a).
3Ronozyme® WX, DSM Nutritional Products Ltd.
2.2. Experimental design and inoculations
This experiment followed a 3 × 2 factorial design with coccidian infection and bird line as the independent variables, while the experimental round was treated as a blocking factor. Upon arrival, day-old chicks of each line were randomly assigned to one of three treatment groups. Each group consisted of 8 replicate pens, and initial stocking density was 6 birds per pen. Birds were orally inoculated at 13 days of age (experimental day 0) with a single 0.5 ml dose of H2O (control group, C), 2500 (low-dose group, L) or 7000 (high-dose group, H) sporulated E. maxima oocysts of the Weybridge laboratory reference strain. Bird weight was measured at placement and bird weight and feed intake at 13 days of age (d0pi) and daily from day 1–13 pi.
2.3. Sampling
On d6 and d13pi, a randomly selected bird from each pen was weighed, bled from the wing vein, and then culled by lethal injection with sodium pentobarbital (Euthatal®, Merial Harlow, United Kingdom). Blood was placed in a 5 ml sodium heparin plasma tube (BD Vacutainer, SST II Advance Plus Blood Collection Tubes, Plymouth, United Kingdom). Samples were immediately placed on ice. Within 1.5 h of collection, each sample was centrifuged for 10 min at 1500× g/4 °C. Aliquoted plasma samples were stored at −80 °C, pending analyses.
During necropsy, 5 cm of intestinal tissue centred on Meckel’s diverticulum, the primary site of infection by E. maxima (Long et al., 1976), was excised and opened longitudinally, the digesta carefully removed, and the tissue submerged in 5 ml of RNAlater® (Life Technologies, Carlsbad, CA, USA). Samples were stored at −80 °C, pending further analyses. Also, 2 × 1 cm segments, one from the duodenal loop and one 2.5 cm upstream from Meckel's diverticulum, were sampled from birds dissected on d6 and d13pi and were fixed in 10% buffered formalin for morphometric analysis. Finally, the distal ends of the caeca were cut, and caecal contents were isolated in Eppendorf tubes, immediately stored at -20 °C, and transferred to −80 °C within 1 h from collection. Then, the right tibia and femur were dissected, defleshed and stored in airtight individually labelled polyethylene bags at −20 °C.
2.4. Sample analysis
2.4.1. Quantitative real-time PCR (qPCR)
Using predicted genome sizes of 46.2 Mbp for E. maxima (Reid et al., 2014) and 1.2 Gbp for G. domesticus (Furlong, 2005), and the method of Blake et al. (2008) to extract total genomic DNA (gDNA) from sporulated E. maxima oocysts and uninfected chicken intestinal tissue, ten-fold DNA dilution series were created using previously described methods (Blake et al., 2006; Nolan et al., 2015). For quantifying E. maxima genome copy number, the primers Ema_qPCRf (forward: 5′-TCG TTG CAT TCG ACA GAT TC-3′) and Ema_qPCRr (reverse: 5′-TAG CGA CTG CTC AAG GGT TT-3′), targeting 138 base pairs of the Microneme Protein 1 (MIC1) gene, were used (Blake et al., 2006). For normalization, we used the primers actbF (forward: 5′-GAG AAA TTG TGC GTG ACA TCA-3′) and actbR (reverse: 5′- CCT GAA CCT CTC ATT GCC A -3′), which amplify 152 base pairs of the chicken cytoplasmic beta-actin (actb) gene, according to a previously employed protocol (Nolan et al., 2015). Total gDNA was extracted from excised intestinal tissue using a DNeasy® Blood and Tissue kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. In brief, RNAlater® was removed from the frozen tissue, which was then weighed and immersed in an equal w/v of Qiagen tissue lysis buffer. Each sample was homogenised with a Qiagen TissueRuptor and the equivalent of ≤ 25 mg of the homogenate added to a sterile 1.5 ml microcentrifuge tube. Genomic DNA was then extracted according to manufacturer’s instructions, and stored at -20 °C until analysis.
Quantitative real-time PCR was performed with a CFX96 Touch® Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, California, USA). Amplification of each sample was performed in triplicate in a 20 μl volume containing 1 μl of total gDNA, 300 nM of each primer, 10 μl of SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories), and 8.9 μl of DNase/RNase free water (Gibco™, Life Technologies, Karlsruhe, Germany). Cycling qPCR conditions were 95 °C/2 m (enzyme activation/initial denaturation), followed by 40 cycles of 95 °C/15 s (denaturation), 60 °C/30 s (annealing/extension), followed by melt analysis of 65–95 °C at increments of 0.5 °C/0.5 s. Assays were performed in white hard-shell® 96-well PCR plates (Bio-Rad Laboratories) sealed with Thermo Scientific adhesive sealing sheets and included the respective gDNA dilution series (standards) and no template controls (NTC). Calculation of copy number of each qPCR target was performed with the software CFC Manager v.3.1 (Bio-Rad Laboratories) according to the slope and intercept of the corresponding reference dilution series. Normalization of the predicted parasite genome copy number was performed by comparison to the estimated host genome copy number. Parasite genome copy number was calculated based on the normalised parasite copy number/μl. The average, standard deviation, and relative standard deviation of quantification cycle data derived from triplicate qPCR amplification of each sample were calculated, and the efficiency (E) of each qPCR assay was determined using CFC Manager v.3.1.
2.4.2. Carotenoids, vitamin A and E
Retinyl acetate and echinone were used as internal standards. Retinoid standards (> 95% all-trans isomers) and α-tocopherol were purchased from Sigma-Aldrich while carotenoid standards were from CaroteNature GmbH (Ostermundigen, Switzerland). HPLC-grade acetonitrile, ethanol, methanol, chloroform, hexane and triethylamine were purchased from Fisher Scientific (Loughborough, UK). Butylated hydroxytoluene (BHT) was obtained from Sigma-Aldrich. All procedures were undertaken under orange lighting to avoid analyte degradation. For the preparation of stock solutions, retinol and Vit E were dissolved in ethanol with 0.1% BHT, while lutein and zeaxanthin were dissolved in chloroform with 0.1% BHT. The concentrations of individual calibration standard solutions were confirmed by measuring the absorption in ethanol with a UV spectrophotometer. Internal standards were prepared in ethanol containing 0.01% BHT. One hundred μl of each plasma sample was diluted in 100 μl of water to which 200 μl of internal standard in ethanol was added. Two ml of hexane was added to each sample and samples were vortexed in an orbital shaker for 10 min and then centrifuged at 1500× g for 5 min. Following centrifugation, the upper hexane phase was transferred to clean glass tubes, and samples were re-extracted with a further 2 ml of hexane. Hexane was evaporated under a nitrogen stream, and residues were redissolved in 100 μl of ethanol and transferred to amber glass vials with inserts (Fisher Scientific). Ten μl of sample extract was injected for the analysis using a Shimadzu HPLC System via PDA detection, according to previously described methodology (Liu et al., 2011). The concentrations of vitamin E, lutein, zeaxanthin, and echinenone were quantified at 450 nm, while vitamin A and retinyl acetate were measured at 325 nm.
2.4.3. Nitric oxide metabolites
Plasma concentrations of NO metabolites (NO2− and NO3−), were analysed using previously described methods (Qadir et al., 2013). Spiking solution was prepared from 5 mM sodium nitrate-15N and 0.05 mM sodium nitrite-15N (Cambridge Isotope Laboratories, Inc. Andover, MA, USA) and used as an internal standard. One-hundred microliters of plasma/sample, 100 μl of spiking solution, 20 μl of 2,3,4,5,6-pentafluorobenzyl bromide (Sigma-Aldrich), and 800 μl of acetone (VWR, Lutterworth, Leicestershire, UK) were pipetted into Falcon™ round-bottom polystyrene tubes and placed in a heating block at 50 °C/120 min. Following incubation, acetone was evaporated under a nitrogen stream for 10 min. Samples were then allowed to cool before 2 ml of toluene (Fisher Scientific UK Ltd) was added to each tube and the tubes vortexed for 15 s. Subsequently, 1 ml of distilled H2O was pipetted into each tube, and the samples were re-vortexed twice for 15 s with a rest of 15 s in between. Using glass Pasteur pipettes, the top layer was transferred into amber glass vials and stored at -20 °C pending GCMS-analysis. Other variables such as column type and ionisation temperatures were as described by Tsikas (2000).
2.4.4. Histology
Formalin-fixed intestinal sections from the duodenum and jejunum were dehydrated through a series of graded ethanol baths followed by xylene in a Shandon™ Excelsior™ ES Tissue Processor (Thermo Fisher Scientific Inc., Waltham, Massachusetts), before being embedded in paraffin wax, sectioned at 4 μm and stained with hematoxylin/eosin. Histological sections were examined under a Zeiss Primostar light microscope and images were captured using ZEN imagine software (Zeiss Germany, Oberkochen, Germany). Images were viewed to measure morphometric features of the intestinal structure at 10× magnification. From sections, the villus height and the crypt depths were determined using ImageJ (NIH) software (Schneider et al., 2012). The villus height was estimated by measuring the vertical distance from the villus tip to the villus-crypt junction for 10 villi/section, and the crypt depth by the vertical distance from the villus-crypt junction to the lower limit of the crypt, for 10 corresponding crypts/section.
2.4.5. Microbiota composition
Microbiota composition was determined by sequencing of the V3/V4 variable region of 16S rRNA genes as described previously (Polansky et al., 2016; Varmuzova et al., 2016). The resulting sequences were classified by RDP Seqmatch with an OTU (operational taxonomic units) discrimination level set to 97% using Qiime software. Shannon's and Simpson’s indices for the comparison of microbiota diversity and Chao 1 index were calculated by Qiime.
2.4.6. Bone mineralisation
Defleshed femur and tibia bones were thawed at 4 °C in a walk-in fridge overnight and were equilibrated to room temperature on the following day. Following that, they were subjected to a 3-point break test using an Instron testing machine (Instron 3340 Series, Single Column-Bluehill, Norwood, USA). The testing support consisted of an adjusTable 2-point block jig, spaced at 30 mm for both tibia and femur bones. The crosshead descended at 5 mm/min until a break was determined by measuring a reduction in force of at least 5%. Following breaking strength determination bones were split in two, and the bone marrow was manually removed. Subsequently, bones were soaked in petroleum ether for 48 h for lipid removal and then placed in an oven at 105 °C for 24 h. The dry bone weight was recorded, and samples were ashed for 24 h at 600 °C for the determination of ash weight (g) and ash content (%).
Table 2.
Effects of line, dose and their interaction on performance parameters in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2.5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts over the period post infection (d0-d13pi) (LS means with SEM).
| BW d0 (g) | BW d13 (g) | ADG (g/d) | ADFI (g/d) | FCR | ||
|---|---|---|---|---|---|---|
| Line | ||||||
| Slow | 371 | 1220 | 65.3 | 93.4 | 1.43 | |
| Fast | 479 | 1676 | 92.1 | 122.7 | 1.34 | |
| SEM | 3.4 | 14.4 | 1.01 | 1.17 | 0.009 | |
| Dose | ||||||
| Control | 427 | 1541a | 85.7a | 114.2a | 1.34a | |
| Low | 422 | 1401b | 75.3b | 104.2b | 1.39b | |
| High | 427 | 1402b | 75.0b | 105.7b | 1.42b | |
| SEM | 4.2 | 17.6 | 1.24 | 1.44 | 0.012 | |
| Line × Dose | ||||||
| Slow | Control | 373 | 1296 | 71.0 | 98.6 | 1.39 |
| Low | 369 | 1182 | 62.6 | 90.2 | 1.44 | |
| High | 373 | 1181 | 62.1 | 91.5 | 1.47 | |
| Fast | Control | 481 | 1786 | 100.4 | 129.8 | 1.29 |
| Low | 475 | 1619 | 88.1 | 118.3 | 1.34 | |
| High | 481 | 1624 | 87.9 | 120.0 | 1.37 | |
| SEM | 5.9 | 24.9 | 1.8 | 2.03 | 0.016 | |
| Source | Probabilities | |||||
| Line | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Dose | 0.599 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Line × Dose | 0.972 | 0.522 | 0.476 | 0.716 | 0.981 | |
a-bMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: BW, body weight; AD, Gaverage daily gain; AD, FIaverage daily feed intake; FCR, feed conversion ratio.
2.5. Calculations and statistics
All statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC). For all statistical assessments pen was considered the experimental unit. Average daily feed intake (ADFI), average daily gain (ADG) (g/d) and feed conversion ratio (FCR, ADFI/ADG) were calculated over the period post-infection and were analysed with dose, line and trial as fixed factors and the interaction between line and dose with the general linear model procedure (PROC GLM) (Table 2). To account for the a priori differences in performance between the broiler lines, ADFI, and ADG data were expressed a proportion of BW on d0pi (ADG/BW and ADFI/BW in g/d/g). These were analysed with the repeated measurements mixed procedure (PROC MIXED). The model included dose, line, day and round as fixed factors, the 2-way interactions between dose and line, dose and day and the three-way interaction between dose, day and line. Covariance structures were chosen based on the lowest value for the Akaike and Bayesian information criteria. Based on dpi that a reduction of ADFI was observed (Fig. 1) (see below), effects of infection on ADFI/BW, ADG/BW and FCR were calculated over the pre-patent (d1–4), acute (d5–8), and recovery (d9–12 pi) periods of infection with PROC GLM using the same model as performance data over the whole period pi (Table 3). Single time point data included plasma concentrations of zeaxanthin, lutein, vitamins E and A, histological and bone measurements, Shannon, Simpson and Chao 1 indices deriving from one bird per pen dissected on d6 or d13pi, as well as E. maxima genome copy numbers and nitric oxide metabolites (NO) obtained at d6pi. Histological and bone measurements, apart from ash percentage and villi length/crypt depth ratio (VCR), were expressed as a proportion of BW of dissected birds. This was done to account for the size difference between F and S growing birds and between control and infected birds. Expressing bone variables as a proportion of BW has been previously used in studies comparing genotypes differing in their growth potential (Shim et al., 2012). These were analysed with PROC GLM with dose, line and round as fixed factors and the interaction between dose and line. For E. maxima genome copy numbers control birds were excluded from the model as their value was effectively 0. For all statistical procedures, the normality of the residuals was assessed with the Shapiro-Wilk test. Predicted E. maxima genome copy numbers were log transformed and the Simpson index values were arcsine transformed before analysis to obtain a normal distribution of the residuals. When significant differences were detected, treatment means were separated and compared by the Tukey’s multiple comparison test. Significance was determined at P < 0.05. All data are expressed as model-predicted least square means with the SEM.
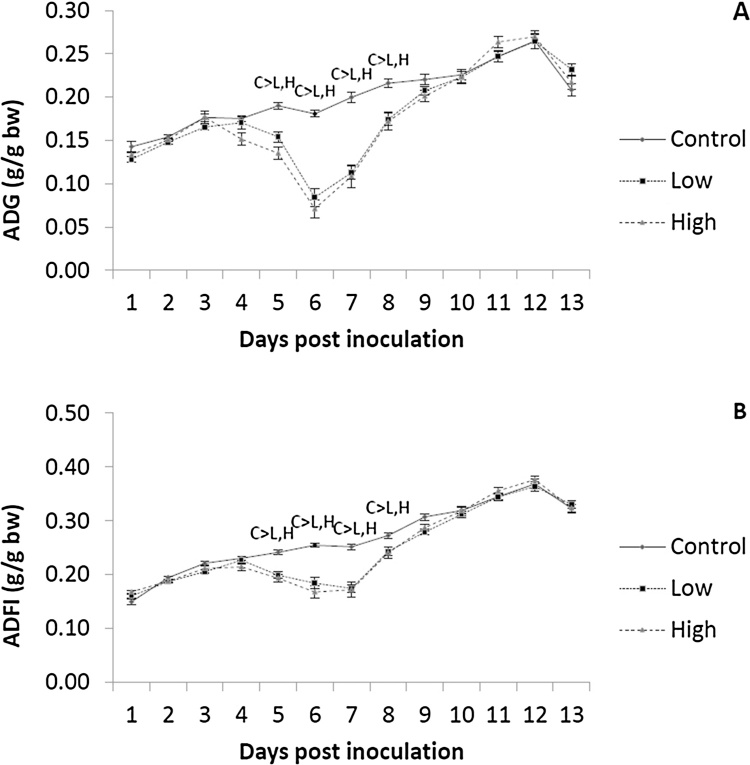
Fig. 1.
Daily ADG (A) and ADFI (B) as a proportion of BW (g/g) at d0 post inoculation (pi) with 0 (Control), 2,5 × 103 (L) or 7 × 103 (H) sporulated E. maxima oocysts over d1-13pi. Superscript values indicate significant differences between means of the control (C), low (L), and (H) doses (P < 0.05).
Table 3.
Effects of line, dose and their interaction on ADG/BW (g/d/g) and ADFI/BW(g/d/g) and FCR, during the pre-patent (d1–4), acute (d5–8), or recovery (d8–12) period of infection in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2.5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| Pre-patent |
Acute |
Recovery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADFI/BW | ADG/BW | FCR | ADFI/BW | ADG/BW | FCR | ADFI/BW | ADG/BW | FCR | ||
| Line | ||||||||||
| Slow | 0.194 | 0.149 | 1.30 | 0.217 | 0.144 | 1.57 | 0.326 | 0.226 | 1.44 | |
| Fast | 0.199 | 0.163 | 1.22 | 0.217 | 0.160 | 1.39 | 0.338 | 0.251 | 1.34 | |
| SEM | 0.0015 | 0.0018 | 0.011 | 0.0033 | 0.0035 | 0.029 | 0.0039 | 0.0030 | 0.009 | |
| Dose | ||||||||||
| Control | 0.199 | 0.162a | 1.23a | 0.255a | 0.197a | 1.30a | 0.335 | 0.239 | 1.40 | |
| Low | 0.195 | 0.153b | 1.28b | 0.200b | 0.131b | 1.54b | 0.325 | 0.235 | 1.38 | |
| High | 0.195 | 0.153b | 1.28b | 0.196b | 0.127b | 1.60b | 0.336 | 0.241 | 1.40 | |
| SEM | 0.0019 | 0.0022 | 0.014 | 0.0040 | 0.0042 | 0.036 | 0.0048 | 0.0037 | 0.012 | |
| Line × Dose | ||||||||||
| Slow | Control | 0.197 | 0.156 | 1.26 | 0.253 | 0.190 | 1.33 | 0.331 | 0.226 | 1.47 |
| Low | 0.195 | 0.145 | 1.32 | 0.206 | 0.126 | 1.65 | 0.318 | 0.225 | 1.41 | |
| High | 0.191 | 0.147 | 1.32 | 0.193 | 0.115 | 1.72 | 0.329 | 0.227 | 1.45 | |
| Fast | Control | 0.201 | 0.168 | 1.19 | 0.257 | 0.204 | 1.27 | 0.339 | 0.253 | 1.34 |
| Low | 0.199 | 0.161 | 1.24 | 0.199 | 0.137 | 1.44 | 0.332 | 0.255 | 1.35 | |
| High | 0.195 | 0.159 | 1.23 | 0.195 | 0.139 | 1.48 | 0.342 | 0.246 | 1.34 | |
| SEM | 0.0025 | 0.0031 | 0.019 | 0.0057 | 0.0060 | 0.051 | 0.0068 | 0.0053 | 0.017 | |
| Source | Probabilities | |||||||||
| Line | 0.045 | <0.001 | <0.001 | 0.972 | 0.003 | <0.001 | 0.045 | <0.001 | <0.001 | |
| Dose | 0.269 | 0.008 | 0.020 | <0.001 | <0.001 | <0.001 | 0.210 | 0.546 | 0.479 | |
| Line × Dose | 0.314 | 0.707 | 0.801 | 0.295 | 0.522 | 0.222 | 0.892 | 0.769 | 0.154 | |
a-bMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: BW, body weight; ADG, average daily gain (g/d/g of BW at d0pi); ADFI, average daily feed intake (g/d/g of BW at d0pi); FCR, feed conversion ratio.
3. Results
3.1. Performance variables over the infection period
Main effects of line, dose and their interaction on ADG, ADFI, and FCR are presented in Table 2. Line and parasite dose did not significantly interact for any of the performance parameters (P > 0.1). Parasite dose significantly affected BW (P < 0.001) at the end of the trial period (d13 pi) and ADG and ADFI over the period pi (P < 0.001), with H and L dosed birds showing smaller values in comparison to C birds and similar to each other, whilst the opposite was the case for FCR. Line significantly affected (P < 0.001) all performance parameters over the period post infection with F line birds being heavier than S line birds at inoculation (d0pi) and the end of the trial (d13pi).
3.2. Repeated measurements on daily ADFI/BW and ADG/BW
There were no significant interactions between line and dose on ADG/BW or ADFI/BW (g/d/g) (P > 0.1). Even when expressing values as a proportion of BW at infection F line birds continued having greater ADG (P < 0.001) and ADFI (P = 0.003) than S line birds (0.194 vs 0.176; SEM = 0.001 and 0.257 vs 0.252; SEM = 0.001, respectively). Dose affected ADG/BW and ADFI/BW (P < 0.001); in comparison to uninfected birds, L and H dosed birds showed significantly smaller ADG/BW (0.200 vs 0.177 vs 0.177; SEM = 0.02) and ADFI/BW (0.268 vs 0.247 vs 0.249; SEM = 0.02 for C, L, and H birds, respectively). ADG/BW and ADFI/BW were affected by the interaction between dose and day (P < 0.001); H and L dosed birds showed significantly smaller ADG/BW and ADFI/BW between d4 and d8 pi compared to the controls. Similar effects were observed for ADFI (P < 0.001) (Fig. 1). For this reason, the experimental period was divided into three equal periods that roughly equated to the pre-patent (d1–4pi), acute (d5–8pi), and recovery (d9–12pi) periods of infection.
3.3. ADG/BW, ADFI/BW and FCR during the pre-patent, acute, and recovery periods
The main effects of line, dose and their interaction on ADG/BW, ADFI/BW, and FCR over the pre-patent, acute and recovery periods post infection are presented in Table 3. Line and parasite dose did not significantly interact for any of the performance parameters on either period post infection (P > 0.1). Dose significantly affected ADG/BW and FCR during the prepatent and acute periods as well as ADFI/BW during the acute period (P < 0.05); in all cases C birds had significantly greater ADFI/BW and ADG/BW and smaller FCR than L and H dosed birds. F line birds had significantly greater ADFI/BW, ADG/BW and smaller FCR than S line birds during all periods apart from the acute period when they had similar ADFI/BW with S line birds.
3.4. Carotenoids, vitamin A, E, and nitric oxide metabolites
The main effects of dose, line and their interaction on the concentration of carotenoids, vitamin A, E (μm/l) and NO (μM) are presented in Table 4. Dose significantly affected the concentration (P < 0.001) of lutein, zeaxanthin, and vitamins A and E at d6pi, being significantly greater in control birds compared to L and H dose birds. Similar effects were observed at d13pi for lutein (P < 0.001), zeaxanthin (P = 0.007), and vitamin E (P = 0.030). In contrast, dose induced an opposite effect on NO at d6pi (P < 0.001). Bird line did not affect the concentration of any of the measured metabolites at d6 and d13pi (P > 0.1), apart from vitamin A (P < 0.001), which was greater on d13pi for S than for F line birds. Line and dose interacted for plasma vitamin E (P = 0.050); at d13pi control birds of the line S had significantly greater values than those of the L and H dose birds of line S and H dose birds of line F.
Table 4.
Effects of line, dose and their interaction on plasma metabollite concentration (μm/l) in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2,5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| d6 post-infection |
d13 post-infection |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| lutein | zeaxanthin | vitamin E | vitamin A | NO (μM) | lutein | zeaxanthin | vitamin E | vitamin A | ||
| Line | ||||||||||
| Slow | 1.12 | 0.269 | 42.8 | 3.16 | 48.6 | 1.93 | 0.412 | 69.9 | 4.63 | |
| Fast | 1.19 | 0.285 | 41.9 | 2.95 | 42.9 | 1.86 | 0.402 | 69.2 | 3.84 | |
| SEM | 0.052 | 0.0117 | 1.91 | 0.117 | 3.10 | 0.064 | 0.0149 | 2.56 | 0.110 | |
| Dose | ||||||||||
| Control | 2.27a | 0.515a | 93.0a | 4.19a | 28.7a | 2.16a | 0.456a | 76.1a | 3.97 | |
| Low | 0.65b | 0.169b | 17.8b | 2.65b | 54.2b | 1.77b | 0.387b | 68.4b | 4.34 | |
| High | 0.55b | 0.146b | 16.3b | 2.32b | 54.4b | 1.75b | 0.377b | 64.1b | 4.39 | |
| SEM | 0.064 | 0.014 | 2.34 | 0.143 | 3.79 | 0.079 | 0.0182 | 3.14 | 0.134 | |
| Line × Dose | ||||||||||
| Slow | Control | 2.25 | 0.505 | 97.0 | 4.23 | 31.9 | 2.27 | 0.476 | 82.3a | 4.46 |
| Low | 0.64 | 0.173 | 16.9 | 2.78 | 62.1 | 1.70 | 0.372 | 63.3b | 4.64 | |
| High | 0.47 | 0.130 | 14.6 | 2.47 | 51.8 | 1.82 | 0.389 | 64.1b | 4.79 | |
| Fast | Control | 2.30 | 0.526 | 88.9 | 4.15 | 25.5 | 2.05 | 0.436 | 70.0ab | 3.49 |
| Low | 0.66 | 0.166 | 18.7 | 2.52 | 46.2 | 1.85 | 0.402 | 73.5ab | 4.03 | |
| High | 0.62 | 0.163 | 18.0 | 2.18 | 57.0 | 1.68 | 0.366 | 64.1b | 4.00 | |
| SEM | 0.090 | 0.0203 | 3.31 | 0.203 | 5.36 | 0.113 | 0.0258 | 4.44 | 0.190 | |
| Source | Probabilities | |||||||||
| Line | 0.326 | 0.342 | 0.735 | 0.224 | 0.204 | 0.417 | 0.621 | 0.838 | <0.001 | |
| Dose | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | 0.031 | 0.066 | |
| Line × Dose | 0.758 | 0.601 | 0.184 | 0.865 | 0.164 | 0.225 | 0.370 | 0.050 | 0.622 | |
a-bMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: NO, Nitric oxide metabolites.
3.5. qPCR and histology
The main effects of dose, line and their interaction on histological measurements villi length and crypt depth (um/kg of BW at dissection), VCR and number of E. maxima genome copies for measurements obtained on d6 and 13pi are presented in Table 5, Table 6, respectively. Parasite genomes were not detected in samples collected from control birds at d6pi. Parasite replication on d6pi was not affected by any of the independent variables or the interaction. Dose significantly affected (P < 0.05) all digestive tract morphological parameters at d6pi; infected H and L birds had shorter villi and enlarged crypts and a smaller VCR in comparison to C birds, whilst being similar to each other. At d13pi, duodenal and jejunal villi length were significantly affected by dose (P = 0.016 and P = 0.008; respectively) being significantly longer (P < 0.05) in H and L dose birds in comparison to C birds. Crypt depth of intestinal sections, were significantly affected by dose (P < 0.001) being greater for H and L dose birds compared to C birds. On the other hand, VCR was significantly affected by dose (P < 0.05), being significantly smaller for H dose birds in the duodenum and both and H and L birds in the jejunum than C birds. Line F birds had significantly shorter villi and smaller crypt depth (P < 0.01) at all intestinal sites at both dpi. Line and dose interacted for crypt depth in the jejunum (P = 0.024) at d6pi with S birds receiving the H and L doses displaying significantly (P < 0.05) greater crypt depth than F line L dosed birds.
Table 5.
Effects of line, dose and their interaction on intestinal morphology, log transformed E. Maxima genomy copy number (GC), Shannon, arcsine transformed Simpson and Chao 1 indices at d6 post inoculation in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2,5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| Duodenum |
Jejunum |
Caeca |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VL/BW (μm/kg) |
CD/BW (μm/kg) |
VCR | VL/BW (μm/kg) |
CD/BW (μm/kg) |
VCR | E. maxima GC | Shannon index | Simpson index | Chao 1 index | ||
| Line | |||||||||||
| Slow | 1699 | 396 | 5.45 | 761 | 293 | 3.71 | 5.87 | 6.72 | 1.26 | 9609 | |
| Fast | 1428 | 289 | 6.00 | 574 | 231 | 3.63 | 5.89 | 6.99 | 1.27 | 8502 | |
| SEM | 46.9 | 16.8 | 0.260 | 35.8 | 14.7 | 0.282 | 0.12 | 0.179 | 0.024 | 435 | |
| Dose | |||||||||||
| Control | 1861a | 212a | 9.49 | 757a | 125a | 6.45a | NA | 6.85 | 1.29 | 8847 | |
| Low | 1467b | 380b | 4.26 | 646b | 311b | 2.48b | 5.89 | 6.83 | 1.24 | 9221 | |
| High | 1362b | 436b | 3.42 | 599b | 349b | 2.07b | 5.87 | 6.87 | 1.27 | 9100 | |
| SEM | 57.4 | 20.6 | 0.32 | 43.8 | 18.0 | 0.34 | 0.12 | 0.219 | 0.029 | 532 | |
| Line × Dose | |||||||||||
| Slow | Control | 1957 | 227 | 9.28 | 836 | 136c | 6.63 | NA | 7.08 | 1.29 | 7885 |
| Low | 1669 | 444 | 3.98 | 732 | 385a | 2.19 | 5.91 | 7.21 | 1.22 | 8675 | |
| High | 1471 | 517 | 3.10 | 714 | 359a | 2.29 | 5.87 | 6.68 | 1.31 | 8946 | |
| Fast | Control | 1765 | 196 | 9.71 | 677 | 114c | 6.27 | NA | 6.62 | 1.29 | 9809 |
| Low | 1265 | 317 | 4.54 | 561 | 238b | 2.78 | 5.88 | 6.5 | 1.27 | 9767 | |
| High | 1254 | 354 | 3.74 | 483 | 340ab | 1.85 | 5.87 | 7.08 | 1.23 | 9254 | |
| SEM | 81.2 | 29.1 | 0.451 | 61.9 | 25.5 | 0.487 | 0.17 | 0.310 | 0.041 | 752 | |
| Source | Probabilities | ||||||||||
| Line | <0.001 | <0.001 | 0.146 | 0.001 | 0.005 | 0.858 | 0.940 | 0.302 | 0.777 | 0.080 | |
| Dose | <0.001 | <0.001 | <0.001 | 0.049 | <0.001 | <0.001 | 0.880 | 0.988 | 0.491 | 0.876 | |
| Line × Dose | 0.370 | 0.073 | 0.973 | 0.825 | 0.024 | 0.498 | 0.927 | 0.177 | 0.298 | 0.579 | |
a-cMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: VL, villus length; CD, crypt depth; VCR, villi length: crypt depth ratio; BW, body weight at dissection.
Table 6.
Effects of line, dose and their interaction on intestinal morphology, Shannon, arcsine transformed Simpson and Chao 1 indices at d13 post inoculation in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2,5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| Duodenum |
Jejunum |
Caeca |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VL/BW (μm/kg) |
CD/BW (μm/kg) |
VCR | VL/BW (μm/kg) |
CD/BW (μm/kg) |
VCR | Shannon index | Simpson index | Chao 1 index | ||
| Line | ||||||||||
| Slow | 1241 | 128 | 10.15 | 578 | 97 | 6.33 | 6.79 | 1.26 | 9822 | |
| Fast | 956 | 104 | 9.77 | 467 | 80 | 6.28 | 7.08 | 1.29 | 9886 | |
| SEM | 24.7 | 2.9 | 0.324 | 22.1 | 3.0 | 0.239 | 0.142 | 0.018 | 595 | |
| Dose | ||||||||||
| Control | 1026a | 98a | 10.82a | 448a | 68a | 7.05a | 6.92 | 1.28 | 10245 | |
| Low | 1118b | 120b | 9.76a,b | 552b | 97b | 6.02b | 7.10 | 1.28 | 9557 | |
| High | 1151b | 130b | 9.30b | 566b | 100b | 5.84b | 6.80 | 1.26 | 9761 | |
| SEM | 30.2 | 3.6 | 0.397 | 27.0 | 3.7 | 0.293 | 0.173 | 0.022 | 729 | |
| Line × Dose | ||||||||||
| Slow | Control | 1164 | 110 | 11.02 | 498 | 74 | 6.92 | 7.36 | 1.24 | 9126 |
| Low | 1280 | 137 | 9.63 | 610 | 107 | 6.20 | 6.93 | 1.31 | 10482 | |
| High | 1279 | 136 | 9.80 | 625 | 109 | 5.87 | 6.94 | 1.24 | 9858 | |
| Fast | Control | 888 | 86 | 10.63 | 399 | 62 | 7.19 | 6.48 | 1.32 | 11364 |
| Low | 956 | 102 | 9.88 | 494 | 88 | 5.84 | 6.66 | 1.25 | 8631 | |
| High | 1023 | 124 | 8.79 | 508 | 90 | 5.82 | 7.24 | 1.29 | 9664 | |
| SEM | 42.8 | 5.1 | 0.561 | 38.2 | 5.2 | 0.413 | 0.245 | 0.031 | 1031 | |
| Source | Probabilities | |||||||||
| Line | <0.001 | <0.001 | 0.406 | 0.001 | <0.001 | 0.887 | 0.164 | 0.282 | 0.940 | |
| Dose | 0.016 | <0.001 | 0.029 | 0.008 | <0.001 | 0.013 | 0.489 | 0.809 | 0.792 | |
| Line × Dose | 0.726 | 0.083 | 0.541 | 0.964 | 0.744 | 0.756 | 0.068 | 0.087 | 0.150 | |
a-cMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: VL, villus length; CD, crypt depth; VCR, villi length: crypt depth ratio; BW, body weight at dissection.
3.6. Microbiota composition
Main effects of line, dose and their interaction on Shannon, Simpson and Chao 1 indexes are presented in Table 5, Table 6 for birds sampled on d6 and 13pi, respectively. None of the indexes was affected by any of the independent variables or their interaction.
3.7. Bone mineralisation
The main effects of line, dose and their interaction on femur and tibia measurements at d6 and d13 pi are presented in Table 7, Table 8, respectively. There was no significant interaction between line and dose on tibia and femur parameters both on d6pi and d13pi. The impact of infection on d6pi was evident on femur: dose significantly affected BBS/BW (P = 0.019) and ash percentage (P = 0.038), which were significantly greater for C than H and L birds. On the contrary on d13pi, dose significantly reduced all markers of tibia mineralisation; BBS, ash and ash percentage were significantly smaller for H in comparison to C birds (P < 0.05) whereas values of L birds were intermediate and not significantly different to either group. In addition, it affected femur ash (P < 0.05), which was significantly smaller for H in comparison to C birds (P < 0.05). Moreover, there was an effect (P = 0.005) on femur ash percentage being significantly smaller for H and L birds in comparison to C birds. Tibia ash weight was significantly greater for the S in comparison to the F line on both d6 (P < 0.01) and 13pi (P < 0.05), but femur ash weight only on d13pi (P < 0.001). On the other hand, BBS was only significantly larger (P < 0.05) for the femurs of the S growing line at d13pi, while they did not differ in ash percentage.
Table 7.
Effects of line, dose and their interaction on long bone mineralisation parameters at d6 post inoculation, in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2,5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| Tibia |
Femur |
||||||
|---|---|---|---|---|---|---|---|
| BBS/BW (N/kg) |
Ash/BW (g/kg) |
Ash (%) | BBS/BW (N/kg) |
Ash/BW (g/kg) |
Ash (%) | ||
| Line | |||||||
| Slow | 212 | 1.13 | 42.6 | 194 | 0.805 | 44.3 | |
| Fast | 197 | 1.07 | 42.2 | 181 | 0.778 | 44.4 | |
| SEM | 10.0 | 0.018 | 0.45 | 6.2 | 0.0151 | 0.42 | |
| Dose | |||||||
| Control | 213 | 1.09 | 42.8 | 204a | 0.809 | 45.4a | |
| Low | 196 | 1.11 | 42.5 | 172b | 0.786 | 43.6b | |
| High | 204 | 1.09 | 41.8 | 186ab | 0.779 | 43.9ab | |
| SEM | 12.2 | 0.022 | 0.55 | 7.6 | 0.0185 | 0.51 | |
| Line × Dose | |||||||
| Slow | Control | 209 | 1.12 | 43.3 | 209 | 0.813 | 45.4 |
| Low | 205 | 1.15 | 43.0 | 180 | 0.812 | 43.9 | |
| High | 222 | 1.10 | 41.4 | 191 | 0.788 | 43.6 | |
| Fast | Control | 218 | 1.07 | 42.3 | 198 | 0.805 | 45.5 |
| Low | 188 | 1.07 | 42.1 | 163 | 0.759 | 43.4 | |
| High | 185 | 1.07 | 42.1 | 181 | 0.769 | 44.2 | |
| SEM | 17.3 | 0.032 | 0.77 | 10.8 | 0.0262 | 0.72 | |
| Source | Probabilities | ||||||
| Line | 0.296 | 0.030 | 0.540 | 0.147 | 0.216 | 0.889 | |
| Dose | 0.629 | 0.711 | 0.391 | 0.019 | 0.477 | 0.038 | |
| Line × Dose | 0.422 | 0.761 | 0.446 | 0.937 | 0.672 | 0.727 | |
a-cMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: BBS, bone breaking strength; BW, body weight at dissection.
Table 8.
Effects of line, dose and their interaction on long bone mineralisation parameters at d13 post inoculation, in broiler chickens of either fast or slow growing lines, inoculated with 0 (Control), 2,5 × 103 (Low) or 7 × 103 (High) sporulated E. maxima oocysts (LS means with SEM).
| Tibia |
Femur |
||||||
|---|---|---|---|---|---|---|---|
| BBS/BW (N/kg) |
Ash/BW (g/kg) |
Ash (%) | BBS/BW (N/kg) |
Ash/BW (g/kg) |
Ash (%) | ||
| Line | |||||||
| Slow | 191 | 1.15 | 44.9 | 175 | 0.821 | 43.8 | |
| Fast | 194 | 1.08 | 45.1 | 152 | 0.750 | 43.7 | |
| SEM | 7.2 | 0.019 | 0.21 | 6.4 | 0.0118 | 0.42 | |
| Dose | |||||||
| Control | 215a | 1.15a | 45.8a | 168 | 0.809a | 45.1a | |
| Low | 189ab | 1.13ab | 44.7b | 162 | 0.794ab | 42.7b | |
| High | 174b | 1.05b | 44.4b | 160 | 0.754b | 43.4b | |
| SEM | 8.8 | 0.024 | 0.26 | 7.8 | 0.0145 | 0.51 | |
| Line × Dose | |||||||
| Slow | Control | 210 | 1.19 | 45.4 | 180 | 0.858 | 45.5 |
| Low | 200 | 1.19 | 45.2 | 186 | 0.846 | 42.9 | |
| High | 164 | 1.05 | 44.1 | 160 | 0.760 | 42.9 | |
| Fast | Control | 221 | 1.18 | 46.2 | 157 | 0.760 | 44.8 |
| Low | 179 | 1.07 | 44.3 | 139 | 0.743 | 42.4 | |
| High | 183 | 1.05 | 44.7 | 161 | 0.749 | 43.9 | |
| SEM | 12.4 | 0.032 | 0.37 | 10.9 | 0.0205 | 0.72 | |
| Source | Probabilities | ||||||
| Line | 0.764 | 0.021 | 0.593 | 0.014 | <0.001 | 0.924 | |
| Dose | 0.007 | 0.011 | 0.001 | 0.748 | 0.030 | 0.005 | |
| Line × Dose | 0.237 | 0.182 | 0.052 | 0.104 | 0.052 | 0.424 | |
a-cMeans within a column that do not share a common superscript are significantly different (P < 0.05).
Abbreviations: BBS, bone breaking strength; BW, body weight at dissection.
4. Discussion
We tested the hypothesis that resistance and tolerance of modern broiler lines to E. maxima infection would be sensitive to genetic selection for growth rate. This hypothesis was based on the assumption that as resource intake during Eimeria infection is limited, mainly due to pathogen-induced anorexia (Kyriazakis, 2014) and reduced nutrient absorption (Preston-Mafham and Sykes, 1970), fast-growing birds would divert more resources to growth rather than to pathogen-coping functions. Furthermore, the supposition was that at increasing pathogen doses, the concomitant resource limitation would be greater for the fast-growing line. We appreciate that there are potential confounding issues when making comparisons between hosts of different size as has been pointed out previously (Coltherd et al., 2011), which may arise, for example, from the relative nutrition of the host or parasite dose given to large and small size birds. Accounting for all these factors will make for a very complex experimental design and for this reason we have opted for an experiment where the treatments imposed on both bird genotypes were similar. Contrary to our hypothesis, lines did not differ in their resistance or tolerance to E. maxima. In the subsequent segments of the discussion we dissect the effects of dose and line on response variables and we discuss the absence of interactive effects.
To assess variation in parasite replication as a consequence of differential resistance between fast and slow growing commercial lines, we used quantitative real-time PCR to measure parasite genome copy number in tissues surrounding Meckel’s diverticulum, which supports higher throughput analysis than conventional measurement of oocysts per gram. The approach also minimised the impact of variation related to the temporal manner of oocyst excretion (Blake et al., 2006). Quantitative real-time PCR has previously been used to define variation in parasite replication in chicken lines with a known polymorphism in their resistance to E. maxima, revealing the biggest differences five and six days after infection. In this study, predicted parasite copy number at d6pi in line F and S birds was similar, rejecting our hypothesis that a fast-growing line may show reduced resistance. Moreover, there were no significant differences observed among birds from line S and F given L or H doses, likely illustrating the crowding effect on parasite replication (i.e. parasite fecundity is reduced once a ‘threshold’ has been reached) (Williams, 2001).
Infection with E. maxima resulted in reductions in relative ADFI and ADG between d4–8pi. No differences in performance were observed between control and infected birds during the recovery period of infection. Thus, our results do not suggest that infected birds had the capacity for compensatory growth within the period of study. This is in accordance with results obtained in broilers infected with coccidia sp. (Gabriel et al., 2006; Preston-Mafham and Sykes, 1970; Takhar and Farrell, 1979), but on the other hand may be a reflection of the post-recovery environment and especially food composition which may define if a host is able to compensate or not (Kyriazakis and Houdijk, 2007). The observed reductions in performance during the acute stage of infection were coupled with damage to the gastrointestinal mucosa of the duodenum and jejunum at d6pi. At d13pi birds showed compensatory villi development, but the pathological effects of infection persisted as birds also displayed reduced VCR at both intestinal sites. A marked reduction in plasma carotenoid concentration levels was observed during the acute phase of infection, which is characteristic of coccidian infections affecting the proximal intestine (Allen, 1992; Hernández-Velasco et al., 2014; Zhu et al., 2000). Similar reductions were observed for vitamin E, in accordance with previous studies (Allen and Fetterer, 2002a, b). These reductions are attributed to malabsorption caused by the damage to the gastrointestinal mucosa (Allen and Fetterer, 2002a, b), leading to defective fat absorption (Adams et al., 1996; Sharma and Fernando, 1975), and to oxidation by reactive oxygen species (Allen, 1997b). In the present study, these effects persisted to d13pi for carotenoids compared to the effects on vitamin A, which may have increased in concentration as a result of its release by the liver (Harrison, 2005). We also assessed levels of NO metabolites at d6pi that were significantly elevated as a result of infection according to our expectation. Our results confirm the lack of alpha diversity reported previously where birds were inoculated with E. tenella (Macdonald et al., 2017; Zhou et al., 2017) or mixed infection (E. acervulina, E. maxima, E. tenella) (Martynova-Van Kley et al., 2012).
As far as bone mineralisation is concerned, older studies using E. acervulina have shown impaired calcium and mineral absorption and retention although a higher absorption efficiency has been observed during the recovery period, depending on infection severity (Takhar and Farrell, 1979; Turk, 1973). Investigations with E. acervulina in starter chicks (infection at d2-d8) have shown reduced bone ash, bone Ca content, or Ca:P ratio (Giraldo et al., 1987; Ward et al., 1990; Watkins et al., 1989; Watson et al., 2005; Willis and Baker, 1981). A recent investigation employing a natural co-infection with E. acervulina and E. tenella, by placing day old chicks in seeded litter has revealed that BBS is adversely affected by 21 days of age (Shaw et al., 2011). However, in that study BBS data were not adjusted for the birds’ reduced BW following infection, while the timing of effects in relation to the parasite cycle is unknown when using a natural model of infection. Finally, single species infections with either E. acervulina or E. maxima have been shown to reduce bone mineral content at d6pi, but their effects have not been investigated at later time points (Fetterer et al., 2013). Ours is the first study to investigate bone mineralisation at the acute and recovery timepoints over the course of a primary infection with an Eimeria sp. in broiler chicks. Effects on bone mineralisation were present across timepoints, but were more pronounced at the recovery stage. Mineralisation of the femur was affected earlier than the tibia post infection; this could be attributed to the faster mineralisation rate of the femur compared to the tibia during the initial stages of broiler growth (Applegate and Lilburn, 2002). By d13pi (d26 post-hatch), although infected birds matched the growth rates of their non-infected counterparts, their bones were less mineralised with effects being present for both long bones. This indicates, that upon recovering from a limitation of nutrient resources as a result of coccidiosis, bone development lags behind tissue accretion.
We hypothesised that a greater level of parasite infection would have a greater impact on line F birds. The observed differences between L and H dose birds were not statistically significantly different. This may be related to the relatively high pathogenicity of E. maxima and the magnitude of the difference between dose sizes. Comparison between sizes of infective doses administered in other studies are not of direct relevance as the age at which birds were infected, the broiler line, and E. maxima strain used are directly related to the pathogenicity of the dose administered (Allen, 1997a,b; Allen et al., 2005; Conway et al., 1993; Idris et al., 1997). Our results are consistent with the suggestion that over a range of infectious doses the extent of pathogen-induced anorexia is similar (Sandberg et al., 2007).
Previous studies comparing broiler genotypes in their resistance to coccidian infections have utilised genetically distant inbred populations (Allen and Lillehoj, 1998; Bumstead et al., 1995; Lillehoj and Ruff, 1987), outbred lines (Pinard-Van Der Laan et al., 1998), or lines selected for different pro-inflammatory response (Swaggerty et al., 2015) to elucidate effector mechanisms contributing to resistance, and their relationship to the magnitude of penalties observed on performance. To our knowledge, the only study which specifically compared lines differing in their performance objectives was that of Swinkels et al. (2007), where genotypes originating from distinctively different lineages of chicken were contrasted (Ross 308 vs Hubbard JA 957). Therefore, the present study is the first to address specifically selection for growth rate on resistance and tolerance to coccidian infections. Over the post-infection period, the two lines differed by 30% in their final body weight at d26 of age while they differed by 7% in their FCR, according to expectations.
In a recent review (Tallentire et al., 2016), summarising effects of selection for performance on the digestive physiology of broilers, it was stated that selection for growth rate has reduced the size of the gastrointestinal tract (GIT), but is accompanied by increased surface area due to greater intestinal villi size. In the present study the relative size of GIT was not assessed. However, we found that F line birds had shorter villi than S line birds when villi length was expressed relative to BW. Coupled with the smaller FCR of F line birds, this finding suggests that these birds have the ability to absorb nutrients more efficiently. In the presence of infection, one would expect a smaller impact on FCR in F than S line birds attributed to the proportionally smaller GIT and the concomitant smaller energetic and nutrient costs which would accompany the repair of damaged intestinal tissue (Sandberg et al., 2007). Although, a statistically significant interaction was not attained for FCR, the percentage increase over the acute period of infection was greater for S than F line birds (≈ 26 vs 15%) in relation to their non-infected counterparts. A larger number of replicates may have supported the aforementioned notion. Irrespective of differences in intestinal architecture, caecal microbiota diversity and richness were not affected by broiler line, or its interaction with dose.
It has long been thought that selection for growth rate negatively affects aspects of bone mineralisation (Williams et al., 2004, 2000). In the present study, tibias and femurs of S line birds yielded more ash weight proportionally to their BW across the two slaughter points apart from femur ash weight which was initially similar between the two lines. These changes likely reflect the influence of the selection criteria of the maternal lines of the F line on body conformation traits. It has been proposed that improved mineralisation is achieved at lower growth rates due to the increased capacity of the skeleton to adapt to the increasing body mass (Brickett et al., 2007; Williams et al., 2004). Comparisons between selected fast-growing broilers with unselected ones demonstrated that several parameters are superior to the former in comparison to the latter when data were expressed in absolute values (McDevitt et al., 2006). However, as bones are components of body weight at increasing body weights concomitant increases in some minerals deposited and on bone breaking strength are expected to increase. More recently, comparison of fast and slow growing subpopulations from a randomised population indicated that almost all bone mineralisation measurements were greater in the slow-growing population when expressed per unit of BW at dissection at six weeks of age (Shim et al., 2012). Furthermore, phenotypic correlations within the same subpopulation indicate that growth rate is negatively associated with BBS and ash percentage (González-Cerón et al., 2015). Herein, percentage tibia and femur ash were similar among the two genotypes indicating similar rates of hydroxyapatite formation over the grower phase, which is indicative of bone maturation. On the other hand, femur BBS was smaller for birds of the F line at d26 of age which bears implications on their ability to tolerate the stresses applied to their long bones under the influence of high growth rates.
There are several reasons why differences between lines were absent under the hypothesis tested in relation to resistance and tolerance. There is a consensus that single trait selection in earlier genetic schemes focused on high productivity, such as increased weight gain and egg production, may have lead to unwanted consequences for traits that were not selected for, leading to the creation of less robust phenotypes with altered immune functions and decreased resistance and tolerance to infections (Havenstein et al., 2003; Hocking, 2014; van der Most et al., 2011). This would explain, to a large extent, previously described negative correlations between selection for performance and disease susceptibility (Rauw et al., 1998). In addition, experimental genetic selection in poultry for disease resistance has been based on immunity to a specific infectious agent, such as Marek’s disease or avian leukosis virus, or to a vaccine (Zekarias et al., 2002). This may have resulted in the formulation of inbred populations resistant to specific pathogens exhibiting lower resistance to others (Swaggerty et al., 2009). Attempt to draw comparisons in inbred lines, concerning the effect of growth rate, is therefore problematic. In addition, natural variation in antibody response, or different immune status of the heritage lines, could be driving the immune response observed when comparing different commercial broiler lines rather than selection for production traits per se. In the present study, the two genotypes originated from the same paternal lines, bred under identical husbandry conditions to account for these factors of variation. Our focus was not to look at immune pathways, but at the impact of a given pathogen on resistance and tolerance, and by proxy to account for their immunocompetence. It has been previously shown that selection for cellular and humoral immune responsiveness is possible without compromising growth, which may be related to the fact that relative energetic and protein requirements of immune responses are smaller than the ones of growth (van der Most et al., 2011). In the modern poultry industry, multi-trait selection is followed, encompassing functional traits in the selection programmes (Hocking, 2014) which allows for improvements in performance and health-related traits as part of a balanced breeding program (Kapell et al., 2012). On the other hand, the absence of a trade-off between growth rate and resistance may be related to the nature of the immune response evoked in the present host-pathogen model. The acute stage of a primary infection with E.maxima is predominated by Th-1 type immune responses (Cornelissen et al., 2009; Hong et al., 2006) which may be more preserved under different growth rates than Th-2 type immune responses evoked by nematode infections, where resistance has been shown to be sensitive to selection for growth rate (Coltherd et al., 2009; Zaralis et al., 2008). In the former case the immune response may be prioritized over growth due to the more severe pathology induced by intracellular pathogens and the necessity to effectively eliminate the infection to ensure host survival (Klasing, 2004). However, currently there is no experimental evidence to support this conjecture.
To summarise, faster growth rates did not lead to reduced resistance or tolerance to infection with E. maxima. Ranger Classic is a relatively new genotype destined for slow-growing broiler markets (Aviagen personal communication). The nutritional specifications for this line are not different from Ross 308, albeit they are expected to be lower due to the lower growth rate. Pathogen-induced anorexia may be sensitive to dietary nutrient adequacy, and this has implications for the differential response of fast vs slow growing genotypes (Kyriazakis, 2010). In addition, future studies should look into different coccidial species, such as E. acervulina and E. tenella, which evoke differential immune responses to that of E. maxima (Cornelissen et al., 2009).
Acknowledgments
This work was conducted under the PROHEALTH project. PRO-HEALTH received funding from the European Union 7th Framework Programme for Research, Technological development and Demonstration under grant agreement no 613574. The work was supported in part by BBSRC project BB/L004046. The authors express their thanks to Sheralyn Smith for carrying the bone measurements and assisting in the organisation and execution of the experiment and to Othman Qadir for the training and guidance he kindly provided for the analysis of Nitric oxide metabolites.
References
- Adams C., Vahl H.A., Veldman A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: development of an experimental infection model. Br. J. Nutr. 1996;75:867–873. doi: 10.1079/bjn19960192. [DOI] [PubMed] [Google Scholar]
- Allen P.C. Effect of coccidiosis on the distribution of dietary lutein in the chick. Poult. Sci. 1992;71:1457–1463. doi: 10.3382/ps.0711457. [DOI] [PubMed] [Google Scholar]
- Allen P.C. Nitric oxide production during Eimeria tenella infections in chickens. Poult. Sci. 1997;76:810–813. doi: 10.1093/ps/76.6.810. [DOI] [PubMed] [Google Scholar]
- Allen P.C. Production of free radical species during Eimeria maxima infections in chickens. Poult. Sci. 1997;76:814–821. doi: 10.1093/ps/76.6.814. [DOI] [PubMed] [Google Scholar]
- Allen P.C., Fetterer R.H. Effects of dietary vitamin E on chickens infected with Eimeria maxima: observations over time of primary infection. Avian Dis. 2002;46:839–846. doi: 10.1637/0005-2086(2002)046[0839:EODVEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Allen P.C., Fetterer R.H. Interaction of dietary vitamin E with Eimeria maxima infections in chickens. Poult. Sci. 2002;81:41–48. doi: 10.1093/ps/81.1.41. [DOI] [PubMed] [Google Scholar]
- Allen P.C., Lillehoj H.S. Genetic influence on nitric oxide production during Eimeria tenella infections in chickens. Avian Dis. 1998;42:397–403. [PubMed] [Google Scholar]
- Allen P.C., Danforth H.D., Vinyard B.L. Development of a protective index to rank effectiveness of multiple treatments within an experiment: application to a cross-protection study of several strains of Eimeria maxima and a live vaccine. Avian Dis. 2004;48:370–375. doi: 10.1637/7010. [DOI] [PubMed] [Google Scholar]
- Allen P.C., Jenkins M.C., Miska K.B. Cross protection studies with Eimeria maxima strains. Parasitol. Res. 2005;97:179–185. doi: 10.1007/s00436-005-1423-6. [DOI] [PubMed] [Google Scholar]
- Applegate T.J., Lilburn M.S. Growth of the femur and tibia of a commercial broiler line. Poult. Sci. 2002;81:1289–1294. doi: 10.1093/ps/81.9.1289. [DOI] [PubMed] [Google Scholar]
- Aviagen . Aviagen Ltd.; Scotland, UK: 2014. Ross 308: Nutrition Specifications. [Google Scholar]
- Aviagen . Aviagen Ltd.; Scotland, UK: 2014. Ross Broiler Management Handbook. [Google Scholar]
- Blake D.P., Hesketh P., Archer A., Shirley M.W., Smith A.L. Eimeria maxima: the influence of host genotype on parasite reproduction as revealed by quantitative real-time PCR. Int. J. Parasitol. 2006;36:97–105. doi: 10.1016/j.ijpara.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Qin Z., Cai J., Smith A.L. Development and validation of real-time polymerase chain reaction assays specific to four species of Eimeria. Avian Pathol. 2008;37:89–94. doi: 10.1080/03079450701802248. [DOI] [PubMed] [Google Scholar]
- Brickett K.E., Dahiya J.P., Classen H.L., Annett C.B., Gomis S. The impact of nutrient density, feed form, and photoperiod on the walking ability and skeletal quality of broiler chickens. Poult. Sci. 2007;86:2117–2125. doi: 10.1093/ps/86.10.2117. [DOI] [PubMed] [Google Scholar]
- Bumstead J.M., Bumstead N., Rothwell L., Tomley F.M. Comparison of immune responses in inbred lines of chickens to Eimeria maxima and Eimeria tenella. Parasitology. 1995;111:143–151. doi: 10.1017/s003118200006488x. [DOI] [PubMed] [Google Scholar]
- Cheema M.A., Qureshi M.A., Havenstein G.B. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–1529. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- Coltherd J.C., Bunger L., Kyriazakis I., Houdijk J.G. Genetic growth potential interacts with nutrition on the ability of mice to cope with Heligmosomoides bakeri infection. Parasitology. 2009;136:1043–1055. doi: 10.1017/S0031182009006428. [DOI] [PubMed] [Google Scholar]
- Coltherd J.C., Babayan S.A., Bünger L., Kyriazakis I., Allen J.E., Houdijk J.G.M. Interactive effects of protein nutrition, genetic growth potential and Heligmosomoides bakeri infection pressure on resilience and resistance in mice. Parasitology. 2011;138:1305–1315. doi: 10.1017/S0031182011000990. [DOI] [PubMed] [Google Scholar]
- Conway D.P., Sasai K., Gaafar S.M., Smothers C.D. Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens. Avian Dis. 1993;37:118–123. [PubMed] [Google Scholar]
- Coop R.L., Kyriazakis I. Nutrition-parasite interaction. Vet. Parasitol. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Cornelissen J.B.W.J., Swinkels W.J.C., Boersma W.A., Rebel J.M.J. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet. Parasitol. 2009;162:58–66. doi: 10.1016/j.vetpar.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Dawkins M.S., Layton R. Breeding for better welfare: genetic goals for broiler chickens and their parents. Anim. Welf. 2012;21:147–155. [Google Scholar]
- Doeschl-Wilson A.B., Kyriazakis I. Should we aim for genetic improvement in host resistance or tolerance to infectious pathogens? Front. Genet. 2012;3:272. doi: 10.3389/fgene.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeschl-Wilson A.B., Brindle W., Emmans G., Kyriazakis I. Unravelling the relationship between animal growth and immune response during micro-parasitic infections. PLoS ONE. 2009;4:e7508. doi: 10.1371/journal.pone.0007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterer R.H., Miska K.B., Mitchell A.D., Jenkins M.C. The use of dual-energy X-ray absorptiometry to assess the impact of Eimeria infections in broiler chicks. Avian Dis. 2013;57:199–204. doi: 10.1637/10392-092812-Reg.1. [DOI] [PubMed] [Google Scholar]
- Furlong R.F. Insights into vertebrate evolution from the chicken genome sequence. Genome Biol. 2005;6:207. doi: 10.1186/gb-2005-6-2-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel I., Mallet S., Leconte M., Fort G., Naciri M. Effects of whole wheat feeding on the development of coccidial infection in broiler chickens until market age. Anim. Feed Sci. Technol. 2006;129:279–303. doi: 10.1093/ps/82.11.1668. [DOI] [PubMed] [Google Scholar]
- Giraldo C.A., Brown D.R., Watkins K.L., Southern L.L. Responses to excess dietary magnesium as affected by experimental Eimeria acervulina infection or by dietary ammonium chloride ingestion in the chick. J. Nutr. 1987;117:1053–1059. doi: 10.1093/jn/117.6.1053. [DOI] [PubMed] [Google Scholar]
- González-Cerón F., Rekaya R., Aggrey S.E. Genetic analysis of bone quality traits and growth in a random mating broiler population. Poult. Sci. 2015;94:883–889. doi: 10.3382/ps/pev056. [DOI] [PubMed] [Google Scholar]
- Harrison E.H. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Hein H. The pathogenic effects of Eimeria acervulina in young chicks 1. Exp. Parasitol. 1968;22:1–11. doi: 10.1016/0014-4894(68)90072-6. [DOI] [PubMed] [Google Scholar]
- Hernández-Velasco X., Chapman H.D., Owens C.M., Kuttappan V.A., Fuente-Martínez B., Menconi A., Latorre J.D., Kallapura G., Bielke L.R., Rathinam T., Hargis B.M., Tellez G. Absorption and deposition of xanthophylls in broilers challenged with three dosages of Eimeria acervulina oocysts. Brit. Poult. Sci. 2014;55:167–173. doi: 10.1080/00071668.2013.879095. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. Unexpected consequences of genetic selection in broilers and turkeys: problems and solutions. Brit. Poult. Sci. 2014;55:1–12. doi: 10.1080/00071668.2014.877692. [DOI] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lillehoj E.P., Lee S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:259–272. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Humphries J.M., Khachik F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003;51:1322–1327. doi: 10.1021/jf026073e. [DOI] [PubMed] [Google Scholar]
- Idris A.B., Bounous D.I., Goodwin M.A., Brown J., Krushinskie E.A. Quantitative pathology of small intestinal coccidiosis caused by Eimeria maxima in young broilers. Avian Pathol. 1997;26:731–747. doi: 10.1080/03079459708419249. [DOI] [PubMed] [Google Scholar]
- Julian R.J. Rapid growth problems: ascites and skeletal deformities in broilers. Poult. Sci. 1998;77:1773–1780. doi: 10.1093/ps/77.12.1773. [DOI] [PubMed] [Google Scholar]
- Kapell D.N., Hill W.G., Neeteson A.M., McAdam J., Koerhuis A.N., Avendano S. Genetic parameters of foot-pad dermatitis and body weight in purebred broiler lines in 2 contrasting environments. Poult. Sci. 2012;91:565–574. doi: 10.3382/ps.2011-01934. [DOI] [PubMed] [Google Scholar]
- Kipper M., Andretta I., Lehnen C.R., Lovatto P.A., Monteiro S.G. Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet. Parasitol. 2013;196:77–84. doi: 10.1016/j.vetpar.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Klasing K. The costs of immunity. Acta Zool. Sin. 2004;50:961–969. [Google Scholar]
- Kyriazakis I. Is anorexia during infection in animals affected by food composition? Anim. Feed Sci. Technol. 2010;156:1–9. [Google Scholar]
- Kyriazakis I. Pathogen-induced anorexia: a herbivore strategy or an unavoidable consequence of infection? Anim. Prod. Sci. 2014;54:1190–1197. [Google Scholar]
- Kyriazakis I., Houdijk J.G.M. Food intake and performance of pigs during health disease and recovery. In: J Wiseman J., Varley M.A., McOrist S., Kemp B., editors. Paradigms in Pig Science. Nottingham University Press; Nottingham: 2007. pp. 493–513. [Google Scholar]
- Lillehoj H.S., Li G.X. Nitric oxide production by macrophages stimulated with coccidia sporozoites, lipopolysaccharide, or interferon-gamma, and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Dis. 2004;48:244–253. doi: 10.1637/7054. [DOI] [PubMed] [Google Scholar]
- Lillehoj H.S., Ruff M.D. Comparison of disease susceptibility and subclass-specific antibody response in Sc and Fp chickens experimentally inoculated with Eimeria Tenella, Eimeria Acervulina, or Eimeria Maxima. Avian Dis. 1987;31:112–119. [PubMed] [Google Scholar]
- Lillehoj H.S., Trout J.M. Avian gut associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996;9:349–360. doi: 10.1128/cmr.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Lee H.-J., Garofalo F., Jenkins D.J.A., El-Sohemy A. Simultaneous measurement of three tocopherols, all-trans-retinol, and eight carotenoids in human plasma by isocratic liquid chromatography. J. Chromatogr. Sci. 2011;49:221–227. [Google Scholar]
- Long P.L., Millard B.J., Joyner L.P., Norton C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976;6:201–217. [PubMed] [Google Scholar]
- Lough G., Kyriazakis I., Bergmann S., Lengeling A., Doeschl-Wilson A.B. Health trajectories reveal the dynamic contributions of host genetic resistance and tolerance to infection outcome. Proc. R. Soc. Lond. B. Biol. Sci. 2015:282. doi: 10.1098/rspb.2015.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S.E., Nolan M.J., Harman K., Boulton K., Hume D.A., Tomley F.M., Stabler R.A., Blake D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynova-Van Kley M.A., Oviedo-Rondon E.O., Dowd S.E., Hume M., Nalian A. Effect of Eimeria infection on cecal microbiome of broilers fed essential oils. Int. J. Poult. Sci. 2012;11:747–755. [Google Scholar]
- McDevitt R.M., McEntee† G.M., Rance† K.A. Bone breaking strength and apparent metabolisability of calcium and phosphorus in selected and unselected broiler chicken genotypes. Br. Poult. Sci. 2006;47:613–621. doi: 10.1080/00071660600963560. [DOI] [PubMed] [Google Scholar]
- Nolan M.J., Tomley F.M., Kaiser P., Blake D.P. Quantitative real-time PCR (qPCR) for eimeria tenella replication—implications for experimental refinement and animal welfare. Parasitol. Int. 2015;64:464–470. doi: 10.1016/j.parint.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persia M.E., Young E.L., Utterback P.L., Parsons C.M. Effects of dietary ingredients and eimeria acervulina infection on chick performance, apparent metabolizable energy, and amino acid digestibility. Poult. Sci. 2006;85:48–55. doi: 10.1093/ps/85.1.48. [DOI] [PubMed] [Google Scholar]
- Pinard-Van Der Laan M.H., Monvoisin J.L., Pery P., Hamet N., Thomas M. Comparison of outbred lines of chickens for resistance to experimental infection with coccidiosis (Eimeria tenella) Poult. Sci. 1998;77:185–191. doi: 10.1093/ps/77.2.185. [DOI] [PubMed] [Google Scholar]
- Polansky O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., Rychlik I. Characterisation of the most important metabolic pathways and biological processes expressed in chicken caecal microbiota. Appl. Environ. Microbiol. 2016;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston-Mafham R.A., Sykes A.H. Changes in body weight and intestinal absorption during infections with Eimeria acervulina in the chicken. Parasitology. 1970;61:417–424. doi: 10.1017/s0031182000041263. [DOI] [PubMed] [Google Scholar]
- Qadir O.K., Teh J., Siervo M., Seal C.J., Brandt K. Method using gas chromatography mass spectrometry (GC-MS) for analysis of nitrate and nitrite in vegetables. In: D’Haene K., Vandecasteele B., De Vis R., Crappé S., Callens D., Mechant E., editors. NUTRIHORT: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality. Institute for Agricultural and Fisheries Research; Ghent, Belgium: 2013. [Google Scholar]
- Rauw W.M. Immune response from a resource allocation perspective. Front. Genet. 2012;3:267. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw W.M., Kanis E., Noordhuizen-Stassen E.N., Grommers F.J. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest. Prod. Sci. 1998;56:15–33. [Google Scholar]
- Reid A.J., Blake D.P., Ansari H.R., Billington K., Browne H.P., Bryant J., Dunn M., Hung S.S., Kawahara F., Miranda-Saavedra D., Malas T.B., Mourier T., Naghra H., Nair M., Otto T.D., Rawlings N.D., Rivailler P., Sanchez-Flores A., Sanders M., Subramaniam C., Tay Y.L., Woo Y., Wu X., Barrell B., Dear P.H., Doerig C., Gruber A., Ivens A.C., Parkinson J., Rajandream M.A., Shirley M.W., Wan K.L., Berriman M., Tomley F.M., Pain A. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014;24:1676–1685. doi: 10.1101/gr.168955.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg F.B., Emmans G.C., Kyriazakis I. The effects of pathogen challenges on the performance of naive and immune animals: the problem of prediction. Animal. 2007;1:67–86. doi: 10.1017/S175173110765784X. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D., Veninga G., Vastenhouw S.A., Bossers A., de Bree F.M., Kaal-Lansbergen L.M.T.E., Rebel J.M.J., Smits M.A. Early life microbial colonization of the gut and intestinal development differ between genetically divergent broiler lines. BMC Genom. 2015;16:418. doi: 10.1186/s12864-015-1646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.D., Fernando M.A. Effect of Eimeria acervulina infection on nutrient retention with special reference to fat malabsorption in chickens. Can. J. Comp. Med. 1975;39:146–154. [PMC free article] [PubMed] [Google Scholar]
- Shaw A.L., van Ginkel F.W., Macklin K.S., Blake J.P. Effects of phytase supplementation in broiler diets on a natural Eimeria challenge in naive and vaccinated birds. Poult. Sci. 2011;90:781–790. doi: 10.3382/ps.2010-01158. [DOI] [PubMed] [Google Scholar]
- Shim M.Y., Karnuah A.B., Anthony N.B., Pesti G.M., Aggrey S.E. The effects of broiler chicken growth rate on valgus, varus, and tibial dyschondroplasia. Poult. Sci. 2012;91:62–65. doi: 10.3382/ps.2011-01599. [DOI] [PubMed] [Google Scholar]
- Siegel P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014;2:375–385. doi: 10.1146/annurev-animal-022513-114132. [DOI] [PubMed] [Google Scholar]
- Singh S.P., Donovan G.A. A relationship between coccidiosis and dietary vitamin a levels in chickens. Poult. Sci. 1973;52:1295–1301. doi: 10.3382/ps.0521295. [DOI] [PubMed] [Google Scholar]
- Su S., Miska K.B., Fetterer R.H., Jenkins M.C., Wong E.A. Expression of digestive enzymes and nutrient transporters in Eimeria acervulina challenged layers and broilers. Poult. Sci. 2014;93:1217–1226. doi: 10.3382/ps.2013-03807. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Pevzner I.Y., He H., Genovese K.J., Nisbet D.J., Kaiser P., Kogut M.H. Selection of broilers with improved innate immune responsiveness to reduce on-farm infection by foodborne pathogens. Foodborne Pathog. Dis. 2009;6:777–783. doi: 10.1089/fpd.2009.0307. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Pevzner I.Y., Kogut M.H. Selection for pro-inflammatory mediators produces chickens more resistant to Eimeria tenella. Poult. Sci. 2015;94:37–42. doi: 10.3382/ps/peu053. [DOI] [PubMed] [Google Scholar]
- Swinkels W.J.C., Post J., Cornelissen J.B., Engel B., Boersma W.J.A., Rebel J.M.J. Immune responses to an Eimeria acervulina infection in different broilers lines. Vet. Immunol. Immunopathol. 2007;117:26–34. doi: 10.1016/j.vetimm.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Takhar B.S., Farrell D.J. Energy and nitrogen metabolism of chickens subjected to infection and reinfection with Eimeria acervulina. Brit. Poult. Sci. 1979;20:213–224. doi: 10.1080/00071667908416570. [DOI] [PubMed] [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron. Sustain. Dev. 2016;36:66. [Google Scholar]
- Tixier-Boichard M., Leenstra F., Flock D.K., Hocking P.M., Weigend S. A century of poultry genetics. World’s Poult. Sci. J. 2012;68:307–321. [Google Scholar]
- Tsikas D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Phy/mass spectrometry. Anal. Chem. 2000;72:4064–4072. doi: 10.1021/ac9913255. [DOI] [PubMed] [Google Scholar]
- Turk D.E. Calcium absorption during coccidial infections in chicks. Poult. Sci. 1973;52:854–857. doi: 10.3382/ps.0520854. [DOI] [PubMed] [Google Scholar]
- van der Aar P., Doppenberg J., Kwakernaak C., Burton E., Gatcliffe J., O’Neill H., Scholey D. In: Sustainable Poultry Production in Europe. CABI, Oxon. Burton E., Gatcliffe J., O’Neill H.M., Scholey D., editors. 2016. pp. 103–111. [Google Scholar]
- van der Most P.J., de Jong B., Parmentier H.K., Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct. Ecol. 2011;25:74–80. [Google Scholar]
- Varmuzova K., Kubasova T., Davidova-Gerzova L., Sisak F., Havlickova H., Sebkova A., Faldynova M., Rychlik I. Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella enteritidis infection. Front. Microbiol. 2016;7:957. doi: 10.3389/fmicb.2016.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T.L., Watkins K.L., Southern L.L. Interactive effects of sodium zeolite a (ethacal) and monensin in uninfected and Eimeria acervulina infected chicks. Poult. Sci. 1990;69:276–280. doi: 10.3382/ps.0690276. [DOI] [PubMed] [Google Scholar]
- Watkins K.L., Vagnoni D.B., Southern L.L. Effect of dietary sodium zeolite A and excess calcium on growth and tibia calcium and phosphorus concentration in uninfected and Eimeria acervulina-infected chicks. Poult. Sci. 1989;68:1236–1240. doi: 10.3382/ps.0681236. [DOI] [PubMed] [Google Scholar]
- Watson B.C., Matthews J.O., Southern L.L., Shelton J.L. The interactive effects of Eimeria acervulina infection and phytase for broiler chicks. Poult. Sci. 2005;84:910–913. doi: 10.1093/ps/84.6.910. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Williams B., Solomon S., Waddington D., Thorp B., Farquharson C. Skeletal development in the meat-type chicken. Brit. Poult. Sci. 2000;41:141–149. doi: 10.1080/713654918. [DOI] [PubMed] [Google Scholar]
- Williams B., Waddington D., Murray D.H., Farquharson C. Bone strength during growth: influence of growth rate on cortical porosity and mineralization. Calcif. Tissue Int. 2004;74:236–245. doi: 10.1007/s00223-002-2124-0. [DOI] [PubMed] [Google Scholar]
- Willis G.M., Baker D.H. Phosphorus utilization during Eimeria acervulina infection in the chick. Poult. Sci. 1981;60:1960–1962. doi: 10.3382/ps.0601960. [DOI] [PubMed] [Google Scholar]
- Yunis R., Ben-David A., Heller E.D., Cahaner A. Immunocompetence and viability under commercial conditions of broiler groups differing in growth rate and in antibody response to Escherichia coli vaccine. Poult. Sci. 2000;79:810–816. doi: 10.1093/ps/79.6.810. [DOI] [PubMed] [Google Scholar]
- Zaralis K., Tolkamp B.J., Houdijk J.G.M., Wylie A.R.G., Kyriazakis I. Changes in food intake and circulating leptin due to gastrointestinal parasitism in lambs of two breeds1. J. Anim. Sci. 2008;86:1891–1903. doi: 10.2527/jas.2007-0698. [DOI] [PubMed] [Google Scholar]
- Zekarias B., Ter Huurne A.A.H.M., Landman W.J.M., Rebel J.M.J., Pol J.M.A., Gruys E. Immunological basis of differences in disease resistance in the chicken. Vet. Res. 2002;33:109–125. doi: 10.1051/vetres:2002001. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Nie K., Huang Q., Li K., Sun Y., Zhou R., Wang Z., Hu S. Changes of cecal microflora in chickens following Eimeria tenella challenge and regulating effect of coated sodium butyrate. Exp. Parasitol. 2017;177:73–81. doi: 10.1016/j.exppara.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Zhu J.J., Lillehoj H.S., Allen P.C., Yun C.H., Pollock D., Sadjadi M., Emara M.G. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 2000;79:619–625. doi: 10.1093/ps/79.5.619. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]