Abstract
Increased reactive oxygen species (ROS) formation may enhance matrix metalloproteinase (MMP)-2 activity and promote cardiovascular dysfunction. We show for the first time that MMP-2 is upstream of increased ROS formation and activates signaling mechanisms impairing redox balance. Incubation of vascular smooth muscle cells (VSMC) with recombinant MMP-2 increased ROS formation assessed with dihydroethidium (DHE) by flow cytometry. This effect was blocked by the antioxidant apocynin or by polyethylene glycol-catalase (PEG-catalase), and by MMP inhibitors (doxycycline or GM6001). Next, we showed in HEK293 cells that MMP-2 transactivates heparin-binding epidermal growth factor (HB-EGF) leading to EGF receptor (EGFR) activation and increased ROS concentrations. This effect was prevented by the EGFR kinase inhibitor Ag1478, and by phospholipase C (PLC) or protein kinase C (PKC) inhibitors (A778 or chelerythrine, respectively), confirming the involvement of EGFR pathway in MMP-2-induce responses. Next, we showed that intraluminal exposure of aortas to MMP-2 increased vascular MMP-2 levels detected by immunofluorescence and gelatinolytic activity (by in situ zimography) in association with increased ROS formation. This effect was inhibited by MMP inhibitors (phenanthroline or doxycycline) and by apocynin or PEG-catalase. MMP-2 also increased aortic contractility to phenylephrine and this effect was prevented by MMP inhibitor GM6001 and by apocynin or PEG-catalase, showing again that increased ROS formation mediates functional effects of MMP-2. These results show that MMP-2 activates the EGFR and triggers downstream signaling pathways increasing ROS formation and promoting vasoconstriction. These findings may have various implications for cardiovascular diseases.
Keywords: Antioxidant, Epidermal growth factor receptor, Matrix metalloproteinase-2, Smooth muscle cells, Vascular
Graphical abstract

Highlights
-
•
MMP-2 is activated by reactive oxygen species and promotes cardiovascular diseases.
-
•
We show here that MMP-2 is upstream of reactive oxygen species formation.
-
•
This effect involves epidermal growth factor receptor transactivation.
-
•
MMP-2 impairs redox balance and contributes to vascular contraction.
1. Introduction
Imbalanced matrix metalloproteinase (MMP) activity has long been acknowledged as a complex and critical alteration involved in a variety of diseases [1]. Among other MMPs, mounting evidence implicates MMP-2 as a major player in disease processes, particularly in cardiovascular diseases [2], [3]. In fact, increased circulating levels of MMP-2 have been associated with worse prognosis in patients with cardiovascular disease [4] and were suggested to predict mortality [5]. Moreover, acutely increasing circulating MMP-2 levels significantly impaired both cardiac and vascular function [6], although the precise mechanisms explaining these findings have not been fully elucidated.
Current knowledge supports the notion that enhanced tissue levels of reactive oxygen species (ROS) contribute to MMP-2 activation [7], [8] or induce its expression [9] promoting cardiovascular dysfunction [10]. This notion is supported by studies showing that antioxidant drugs attenuate MMP-2 activity and MMP-2-mediated pathophysiological cardiovascular alterations [11], [12] and these findings are very similar to those found with the use of MMP inhibitors [13], [14], [15]. Therefore, both antioxidant drugs and MMP inhibitors have been shown to prevent many of the deleterious effects associated with imbalanced MMP-2 activity. However, the possibility that MMP-2 is upstream of increased ROS formation and activates cell signaling mechanisms that impair redox balance by enhancing ROS formation has not been explored yet. This hypothesis is supported by few studies showing that other MMPs can activate pathways associated with increased ROS production [9], [16], [17], [18]. For example, MMP-7 induced ROS production by activating epidermal growth factor receptor (EGFR) after stimulation of alpha-1 adrenergic receptor in mesenteric arteries from rats [16], [17], [19]. Moreover, increased expression of intracellular cardiac MMP-2 in transgenic mice promoted lipid peroxidation after ischemia reperfusion injury [18].
There is strong evidence that MMP-2 proteolytic activity cleaves the transmembrane protein pro-heparin-binding epidermal growth factor (HB-EGF) into soluble HB-EGF leading to EGF receptor (EGFR) transactivation [16], [20], [21]. In addition, previous studies showed that EGFR stimulation promotes ROS formation in the cardiovascular system [17], [19], [22] and contributes to vasoconstriction [16]. However, there is no evidence linking increased MMP-2 activity and enhanced vascular ROS formation as a result of increased MMP-2 mediated gelatinolytic activity. In this study, we tested the hypothesis that MMP-2-mediated proteolytic activity leads to transactivation of EGFR and triggers pro-oxidant alterations of the vascular biology that contribute to increased vascular contractility. We now provide evidence that MMP-2 is upstream of ROS formation and we show mechanisms directly linking increased MMP-2 activity with impaired redox balance resulting in vascular dysfunction.
2. Material and methods
2.1. Animals
This study complied with the guidelines of the Ribeirao Preto Medical School, University of Sao Paulo (Protocol no. #120/2010), and the animals were handled according to the guiding principles published by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male New Zealand rabbits (2.5–3.0 kg) and male rats (200 ± 10 g) from the colony at University of São Paulo were maintained at room temperature (22–25 °C) on light/dark cycle (12 h) and had free access to standard rat chow and water.
2.2. Materials
Tyrphostin AG 1478, Phenylephrine, Apocynin, Peg-Catalase (PG-Cat), Dihydroethidium (DHE), phenanthroline, Phenylmethylsulfonyl fluoride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). GM6001 was purchased from Merck-Millipore (Tokyo, Japan). MMP-2 polyclonal antibody was purchased from NovusBio (Littleton, CO, USA). DQ Gelatin fluorogenic substrate and Alexa 647-conjugated anti-rabbit secondary antibody was purchased from Molecular Probes (Eugene, OR, USA). The MMP-2 recombinant protein was produced in our laboratory and specific details on its production as well as enzymatic activity data on various lots are described in a previous manuscript [23].
2.3. Cell culture
The Rattus norvegicus vascular smooth muscle cell (VSMC) line A7r5 obtained from American Type Culture Collection (ATCC CRL-1444) (Rockville, MD, USA) was maintained at 37 °C under an atmosphere of 5% CO2 in culture flasks with Dulbecco´s modified Eagle´s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (Life technologies, Cat# 15240112). The cells were used from third to fifth passages after unfreezing.
2.4. Assessment of the effects of MMP-2 and inhibitors on vascular smooth muscle cells ROS production by cell flow cytometry
To assess the effects of MMP-2 on ROS concentrations in vascular smooth muscle cells (VSMC), we incubated VSMC with MMP-2 and assessed ROS concentrations with dihydroethidium (DHE) probe by flow cytometry. Cytofluorographic analysis was performed using a Becton–Dickinson FACS Canto (San Jose, CA, USA) with an argon ion laser tuned to 488 nm. Acquisition was set at 10.000 events. Changes in fluorescence intensity (FI) emitted by DHE were measured in isolated VSMC cells initially analyzed without DHE (Blank) as a control to ensure that there was no interference of DHE emitted fluorescence. After that, the cells were incubated with DHE (10 μM) for 30 min, as previously detailed [24], either in the presence of MMP-2 (16 nmol/l) or vehicle (PBS), which was added immediately after DHE (30 min MMP-2 incubation) or during the last 10 min (10 min MMP-2 incubation).
To confirm that cell incubation with MMP-2 affects ROS concentrations, we carried out control experiments to examine the effects of antioxidant agents including apocynin (a ROS scavenger; 100 µmol/l) [25], diphenyl iodonium (DPI; a flavoprotein inhibitor) 10 µmol/l, or polyethylene glycol-catalase (PEG-catalase, which catalyzes the breakdown of intracellular H2O2 into H2O and O2; 3000 U/ml). These experiments were carried out as described above, either in the presence of MMP-2 (30 min MMP-2 incubation) or vehicle.
To confirm that MMP-2 proteolytic activity affects ROS concentrations in VSMC, we examined the effects of MMP inhibitors (doxycycline 100 µmol/l or GM6001 1 µmol/l) on MMP-2-induced changes in ROS concentrations using the same conditions as described above, either in the presence of MMP-2 (30 min MMP-2 incubation) or vehicle.
2.5. Effects of MMP-2-induced EGFR transactivation on cellular ROS concentrations
MMP-2 proteolytic activity is known to promote EGFR transactivation [16], [20], [21], [26], which activates cell signaling. To examine whether MMP-2-induced cleavage of HB-EGF results in increased ROS concentrations and the mechanism involved in this effect, we designed a series of cell experiments.
Firstly, we examined the cleavage of HB-EGF by MMP-2 using a reporter protein in cell culture conditions. A plasmid encoding HB-EGF-AP, a chimeric protein used for alkaline phosphatase (AP) reporter assay, was kindly provided by Dr. Michael R. Freeman (Department of Surgery, Harvard Medical School, Boston) [27], [28], [29], [30]. HEK293 cells were stably transfected with the HB-EGF-alkaline phosphatase (AP) plasmid and seeded (1 × 104) into 96-well plates (Corning) for 24 h. The following day, the cells were starved for 4 h in a DMEM FBS-free medium. After 4 h, the cells were treated in a phenol-free medium with MMP-2 (16 nM) for 10 and 30 min. Briefly, 100 µl of conditioned media were collected from each well and added for 30 min to other individual well of a 96-well plate containing 100 µL of AP buffer (0.5 mol/l Tris-HCl, pH 9.5, containing 5 mmol/l p-nitrophenyl phosphate disodium, 1 mmol/l diethanolamine, 50 µmol/l MgCl2, 150 mmol/l NaCl, 5 mmol/l EDTA) and the absorbance was measured at 405 nm. Three independent experiments were performed in duplicates.
Secondly, to further validate the idea that MMP-2 proteolytic activity promotes EGFR transactivation, we assessed the effects of MMP-2 on ROS concentrations in VSMC by incubating VSMC with MMP-2 in the presence of AG1478 (an EGFR kinase inhibitor) 3 or 10 µmol/l (or vehicle) as described above. ROS concentrations were assessed with DHE probe by flow cytometry, as described above.
Thirdly, given that EGF binding to its receptor activates downstream cell signaling mediated by phospholipase C (PLC) [31], [32] and protein kinase C (PKC) [31], [32], we assessed the effects of MMP-2 on ROS concentrations in VSMC by incubating VSMC with MMP-2 in the presence of A778 (a PLC inhibitor) 10 µmol/l, or Chelerythrine (a PKC inhibitor) 10 µmol/l. Heparin binding-EGF (HB-EGF) and phorbol 12-myristate 13-acetate (PMA) were also used as positive controls. Again, ROS concentrations were assessed with DHE probe by flow cytometry, as described above.
2.6. Assessment of the effects of MMP-2 and inhibitors on ROS production in vascular ex vivo studies
After carrying out a number of cell experiments to show the effects of MMP-2 on ROS formation in VSMC and the mechanism involved, we examined whether the incubation of rabbit aortas with MMP-2 could result in vascular biochemical responses similar to those found with isolated cells. Therefore, we used freshly isolated aortas from rabbits which were exposed to MMP-2 (16 nM) and inhibitors. The thoracic aortas were isolated from rabbits after anesthesia with Ketamine (50 mg/kg) and Xylasine (10 mg/kg) i.m. and inhaled isoflurane as required. Isolated aortas were divided into two 2 cm long segments. The first one was filled with Krebs solution (Vehicle; in mM: NaCl 118, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, KCl 4.8, NaHCO3 25 and glucose 5.5) or Krebs solution containing MMP-2 at 16 nM. Both ends of each vascular segment were clipped and maintained in a Krebs solution saturated with a carbogenic mixture (95% O2/5% CO2), pH 7.4 at 37 °C, for 30 min. After incubation, the aortic segments were embedded in Tissue-tek® and frozen for use to assess gelatinolytic activity (by in situ zymography assay described below) and to measure aortic ROS concentrations by using the DHE technique (described below). The other aortic segment was used to assess ROS formation by using the lucigenin assay (described below) and in vascular reactivity experiments (described below).
2.7. Assessment of vascular MMP activity by in situ zymography and MMP-2 location by immunofluorescence
To determine whether incubation with MMP-2 for 30 min increases proteolytic activity in the aortic rings, we carried out in situ zymography as previously described [33]. Briefly, MMP activity was measured using DQ Gelatin fluorogenic substrate (E12055, Molecular Probes, Eugene, OR, USA) in serial 5 µm section of the vessel, which were cut and incubated in dark wet chambers for 1 h with DQ gelatin concentration 100 µg/ml in Tris CaCl2 buffer (Tris 50 mM, CaCl2 at 10 mM), pH 7,4, in absence or presence of phenanthroline (Phe, an MMP inhibitor) at 100 µM, or doxycycline (Doxy, an MMP inhibitor) at 100 µM, or phenylmethylsulfolnyl fluoride (PMSF, a serine protease inhibitor) at 100 µM. The sections were examined with fluorescent microscopy (Leica Imaging Systems Ltd., Cambridge, England) and the images were captured at a magnification of ×400 and ×200. Proteolytic activity was detected as bright green fluorescence indicating substrate breakdown and evaluated by using ImageJ Program (National Institute of Health).
To evaluate MMP-2 location, aortic sections were incubated with rabbit anti-MMP-2 polyclonal antibody overnight at 4 °C. Red fluorescence was visualized by adding Alexa 647-conjugated anti-rabbit secondary antibody at 1 µg/ml (A31573, Invitrogen) for 1 h. MMP-2 was detected as bright red fluorescence and was evaluated using the ImageJ software. DAPI was used to determine nuclear location in the sections.
2.8. Measurement of in situ aortic ROS concentrations
DHE was used to evaluate the in situ ROS concentrations production of ROS as previously described [34]. Briefly, serial 5 µm sections of the aortas were cut and incubated in dark wet chambers, at room temperature, with DHE at 100 µM for 30 min in presence or absence of Phe 100 µM, doxy 100 µM, PMSF 100 µM, apocynin 100 µM, tiron 100 µM or PEG-catalase 3000 U/ml. The sections were examined with a fluorescence microscope (Leica Imaging Systems Ltd., Cambridge, England) and the images were captured at a ×200 magnification. Bright red fluorescence represents ROS concentration and was evaluated by using ImageJ Program (NIH).
2.9. Assessment of aortic ROS formation by lucigenin fluorescence assay
To further validate the possible effects of MMP-2 on vascular ROS formation, we carried out the lucigenin fluorescence assay as previously detailed [35]. Briefly, fresh intact aortic rings incubated with MMP-2 or vehicle in absence or presence of the MMP inhibitors doxycycline 100 µM or GM6001 1 µM. Aortic rings were transferred to luminescence vials containing 1 ml of Krebs buffer, pH 7.4. After equilibration and background counts, lucigenin (5 μM) and NADPH (300 μM) were added to the vials and the luminescence count was measured continuously for 15 min in a Berthold FB12 single tube luminometer at 37 °C. Background signals from aortic rings were subtracted from the NADPH-driven signals and the results were normalized for the dry weight and reported as RLU/mg of dry aorta.
2.10. Effects of MMP-2 on the vascular contractility to phenylephrine
Given that ROS levels affect the contractility of smooth muscle cells, we examined whether incubation with MMP-2 could affect the aortic contractility in response to an adrenergic agonist in endothelium-denuded aortas (E-). Thoracic rabbit aortic rings (4 mm) were cut and mounted for isometric tension recording. The rings were placed in chambers of isolated organ bath containing Krebs solution, which was maintained at 37 °C and pH 7.4, bubbled with carbogenic mixture. The system was connected to an isometric force displacement transducer, and the responses were recorded on a computer system using Protowin. The aortic rings were submitted to an equilibration period (2 g for 180 min) and the functionality was tested with Phenylephrine (PE, 0.1 μM). Endothelium-denuded aortas were obtained by slightly rubbing the luminal surface of the aortas and responded to acetylcholine (ACh, 1 µM) with less than 10% of PE-induced contraction. Cumulative concentration-effect curves for PE (0.1 nM to 10 µM) were performed in E- aortic rings in the absence or presence of MMP-2 (16 nM) and GM6001 1 µM, or apocynin 100 µM, or PEG-catalase (250 U/ml) for 30 min before the starting the PE-induced concentration-effect curves. The vascular contraction was represented as tension (g) divided by dry tissue weight (g). The maximum response (Emax) and pD2 value (negative logarithm of the molar concentration of agonist that produces half of Emax) were calculated using a non-linear interactive fitting program (Graph Pad Prism 3.0; Graph Pad Software Inc., La Jolla, CA, USA).
2.11. Statistical analysis
The results are expressed as mean ± S.E.M. Comparisons between groups were assessed by paired or unpaired Student's t-test or by one-way analysis of variance followed by Bonferroni correction when appropriate. A probability value P < 0.05 was considered significant.
3. Results
3.1. MMP-2 increases in vascular smooth muscle cells ROS concentration and this effect is blocked by MMP inhibitors
To examine the possibility that MMP-2 increases ROS in VSMC, we incubated VSMC with MMP-2 for 10 or 30 min and assessed ROS concentrations with DHE by cell flow cytometry. MMP-2 (16 nmol/l) increased DHE fluorescence by approximately 40% and 75% after 10 min and 30 min of incubation, respectively (Fig. 1A; both P < 0.05). To validate the finding that MMP-2 increases ROS concentrations, we assessed the effects of widely accepted antioxidant agents including apocynin, the flavoprotein inhibitor DPI 10 µmol/l, or PEG-catalase. While all antioxidants exerted minor effects in vehicle-treated cells, they fully prevented the increases in DHE fluorescence induced by MMP-2 (Fig. 1B; both P < 0.05).
Fig. 1.
MMP-2-induced increase in ROS concentrations in vascular smooth muscle cells is prevented by MMP inhibitors. (A) ROS concentrations in vascular smooth muscle cells (A7r5 cells) assessed with dihydroethidium (DHE; 10 µmol/l) by flow cytometry after incubation with MMP-2 (16 nmol/l) for 10 or 30 min. (B) Effects of antioxidant agents including apocynin 100 µmol/l (Apo), diphenyl iodonium (DPI; a flavoprotein inhibitor) 10 µmol/l, or PEG-catalase 3000 U/ml (PG-Cat, which catalyzes the breakdown of H2O2) in both vehicle and in MMP-2-treated cells. Panel C shows the effects of MMP inhibitors doxycycline 100 µmol/l (Doxy) or GM6001 1 µmol/l in both vehicle and in MMP-2-treated cells. Data are shown as the mean ± SEM (n = 4/group). * P < 0.05 vs. Vehicle. ** P < 0.05 vs. Vehicle and 10 min. # P < 0.05 vs. Vehicle in MMP-2-treated cells.
Given that doxycycline and GM6001 are MMP inhibitors, we confirmed that MMP-2 increases ROS concentrations in VSMC by examining the effects of both MMP inhibitors on MMP-2-induced increases in ROS concentrations. Interestingly, while both inhibitors did not affect DHE fluorescence in vehicle-treated cells, both MMP inhibitors completely blunted MMP-2-induced increases in DHE fluorescence (Fig. 1C; both P < 0.05), thus indicating that MMP-2-induced increases in ROS concentrations in VSMC depends on MMP proteolytic activity.
Results for control experiments are shown in Supplementary Fig. 1.
3.2. MMP-2-induced EGFR transactivation increases cellular ROS concentrations by mechanisms involving the PLC and PKC pathway
Given that MMP-2 activity may transactivate EGFR and increase ROS concentrations, we carried out experiments to examine this possibility and to define mechanisms involved in this effect. First, we quantified HB-EGF shedding using HEK293 cells transfected with HB-EGF-AP system, which were incubated with MMP-2. We observed that this incubation increased shedding of HB-EGF (measured as AP activity) after 10 min (0.37 ± 0.01, n = 3, P < 0.05, Fig. 2A) or 30 min (0.73 ± 0.06, n = 3, P < 0.05, Fig. 2A) of incubation as compared to Vehicle (0.22 ± 0.01, n = 3), thus confirming that MMP-2 promotes EGFR transactivation.
Fig. 2.
MMP-2 mediates HB-EGF shedding and induces EGFR-dependent increases in ROS concentrations. (A) MMP-2 promotes HB-EGF shedding, as revealed by quantification of HB-EGF shedding measured by alkaline phosphatase (AP) activity in the supernatant of HEK293 cells after incubation of MMP-2 (16 nmol/l) or Vehicle for 10 and 30 min. Shedding activity is presented as the ratio of the AP activity in the supernatant and in the cell lysate as described in Section 2. (B) Incubation with MMP-2 (16 nmol/l) for 10 or 30 min increases ROS concentrations in vascular smooth muscle cells (A7r5 cells) assessed with dihydroethidium (DHE; 10 µmol/l) by flow cytometry, and this effect is prevented by the EGFR kinase inhibitor (Ag1478; 3 or 10 μmol/l). Data are shown as the mean ± SEM (n = 4/group). * P < 0.05 vs. respective Control (Vehicle). *P < 0.05 vs. respective Control (Vehicle) and vs. MMP-2 10 min. # P < 0.05 vs. Vehicle + Vehicle treated cells. ## P < 0.05 vs. Vehicle + Vehicle treated cells and vs. MMP-2 + Vehicle treated cells for 10 min.
Next, we found that MMP-2 proteolytic activity activates EGFR resulting in increased ROS concentrations in VSMC, and this effect is prevented by the EGFR kinase inhibitor Ag1478, which prevents downstream EGFR activation and increased ROS production. These important results are shown in Fig. 2B, which shows that MMP-2 increases DHE fluorescence after 10 or 30 min of incubation with this protease (Fig. 2B; both P < 0.05). While the lower concentrations of EGFR kinase inhibitor Ag1478 (3 μmol/l) prevented the increases in ROS concentrations after 10 min (but not after 30 min) of incubation with MMP-2, the higher Ag1478 concentration (10 μmol/l) fully prevented MMP-2-induced effects (Fig. 2B; both P < 0.05). These findings show that preventing EGFR-mediated signaling is enough to prevent MMP-2-induced pro-oxidant mechanisms.
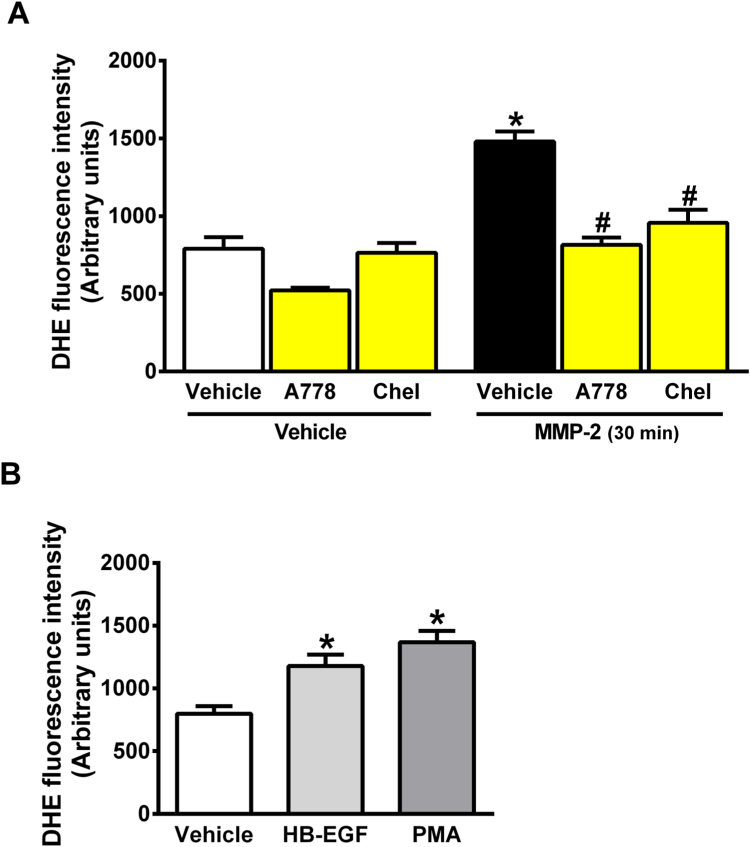
Finally, EGF binds to EGFR and activates PLC and PKC cell signaling, and therefore we examined whether PLC or PKC inhibitors could prevent MMP-2-induced EGFR activation and increased ROS formation in VSMC. Fig. 3 shows that MMP-2-induced increases in ROS concentrations in VSMC are fully prevented by the PLC inhibitor A778 or by the PKC inhibitor Chelerythrine (Fig. 3A, both P < 0.05). Positive control experiments using recombinant HB-EGF as an EGFR agonist or the PKC activator PMA confirmed that activation of EGFR pathway results in similar effects as compared with those found after incubation with MMP-2 (Fig. 3B, both P < 0.05).
Fig. 3.
MMP-2-induced increases in ROS concentrations in vascular smooth muscle cells is abolished by a phospholipase C inhibitor or by a protein kinase C inhibitor. (A) Quantification of ROS production after 30 min of MMP-2 incubation with VSMC in the absence or presence of the phospholipase C inhibitor (A778 10 μM) or the PKC inhibitor (Chelerythrine, Chel, 3 μM). ROS concentrations were assessed with DHE probe by flow cytometry. (B) Control experiments showing quantification of ROS production stimulated by an EGFR agonist (recombinant HB-EGF) or by a PKC activator (phorbol 12-myristate 13-acetate; PMA) after 30 min of incubation with MMP-2. Data are shown as the mean ± SEM (n = 4/group). *P < 0.05 vs. Vehicle + Vehicle treated cells. # P < 0.05 vs. Vehicle + MMP-2 treated cells.
3.3. MMP-2 invades the vascular tissues, increases vascular gelatinolytic activity and exerts pro-oxidant effects
Given that results described above showing that MMP-2 activates cell pathways that promote oxidative stress in isolated VSMC, we decided to further validate these findings in vascular tissues incubated with MMP-2. To reach this goal, we carried out initial experiments in aortas isolated from rabbits to examine whether incubation of these vessels with MMP-2 would increase vascular gelatinolytic activity, and therefore aortic segments were incubated with MMP-2 (16 nM) intraluminally for 30 min. Interestingly, we found that the incubation with recombinant MMP-2 increased aortic gelatinolytic activity assessed by in situ zimography by approximately 50% (Fig. 4A and B; P < 0.05). In agreement with these findings, this procedure was associated with major increases in MMP-2 concentrations detected by immunofluorescence in all layers of the aortas (Fig. 4A and C; P < 0.05).
Fig. 4.
Intraluminal incubation of rabbit aorta with MMP-2 for 30 min increases gelatinolytic activity in all layers of the vessel. (A) Representative confocal photomicrographs of rabbit aortic sections incubated with DAPI, DQ-Gelatin and anti-MMP-2 antibody (Alexa 647 used as secondary antibody). DAPI´s blue fluorescence indicates the nuclei; green fluorescence reflects the total gelatinolytic activity, and red fluorescence indicates the staining with anti-MMP-2 antibody (this antibody reacts only with the recombinant MMP-2 protein and not with endogenous rabbit MMP-2). (B) Aortic gelatinolytic activity quantified by in situ zymography in aortas exposed to intraluminal incubation with MMP-2 or Vehicle (control).(C) Quantification of the Alexa 647 signal (which reflects aortic MMP-2 concentration) in photomicrographs of rabbit aortas that were intraluminally incubated with recombinant MMP-2. Data are shown as the mean ± SEM (n = 4–5/group). * P < 0.05 vs. Vehicle group.
Next, we examined whether the incubation of aortas with MMP-2 would result in vascular pro-oxidant responses that could be attributable to MMP-2-induced increases in gelatinolytic activity. Therefore, we measured gelatinolytic activity and ROS levels (by DHE technique) in aortas incubated with MMP-2 in the presence of classical MMP inhibitors (phenanthroline or doxycycline) or PMSF (a serine-protease inhibitor), or vehicle. We found that incubation of aortic segments with MMP-2 increased vascular gelatinolytic activity and this effect was totally inhibited by both MMP inhibitors (phenanthroline and doxycycline) (Fig. 5A and B, P < 0.05), but not by PMSF (Fig. 5A and B, P > 0.05). Consistent with our cell experiments, we found that incubation of aortic segments with MMP-2 increased DHE signal by approximately 50% (Fig. 5C and D, P < 0.05), and this response was totally abolished by both MMP inhibitors (phenanthroline and doxycycline) (Fig. 5C and D, P < 0.05), but not by PMSF (Fig. 5C and D, P > 0.05).
Fig. 5.
MMP inhibitors prevent the increases in vascular gelatinolytic activity, in ROS concentrations, and in lucigenin chemiluminescence after intraluminal incubation of rabbit aorta with MMP-2. (A) Representative photomicrographs of aortic in situ zymography experiments showing that MMP-2 gelatinolytic activity was totally inhibited by phenanthroline (Phe, 100 µM) or doxycycline (Doxy, 100 µM), but not by phenylmethylsulfonyl fluoride (PMSF, 100 µM). The arteries were incubated with the inhibitors together with MMP-2 (16 nM) intraluminally for 30 min. (B) Quantification of aortic gelatinolytic activity measured in experiments described in A. (C) Representative photomicrographs of aortic sections incubated with DHE. Red fluorescence reflects ROS production and shows that incubation with MMP-2 (16 nM) intraluminally for 30 min increased ROS production, and this effect was totally inhibited by phenanthroline (Phe, 100 µM) or doxycycline (Doxy, 100 µM), but not by phenylmethylsulfonyl fluoride (PMSF, 100 µM). The experiments were carried out as described in A. (D), Quantification of aortic fluorescence of DHE measured in experiments described in C. (E), Quantification of lucigenin chemiluminescence measured in fresh aortas after intraluminal incubation with MMP-2 (16 nM) or vehicle for 30 min in absence or presence of MMP inhibitors, doxycycline (Doxy 100 µM) or GM6001 (10 µM). Data are shown as the mean ± SEM (n = 4–5/group). * P < 0.05 vs. Vehicle + Vehicle group. # P < 0.05 vs. Vehicle+MMP-2 group.
To further validate the pro-oxidant effects of MMP-2, we used the lucigenin chemiluminescence assay to examine whether MMP-2 stimulates the vascular formation of ROS. Therefore, we examined the vascular responses to MMP-2 in the presence of MMP inhibitors (doxycycline or GM6001) or vehicle. In agreement with the DHE results, we found that incubation with MMP-2 increased vascular lucigenin chemiluminescence signal by approximately 50% (Fig. 5E, P < 0.05) and both MMP inhibitors completely blunted this effect (Fig. 5E, both P < 0.05).
The results described above show that MMP-2 increases vascular gelatinolytic activity and promotes pro-oxidant responses that are prevented by MMP inhibitors. These finding are further supported by the observation that both ROS levels (measured by DHE technique) and lucigenin activity are strongly correlated with gelatinolytic activity in aortic segments exposed to MMP-2 (Fig. 6A and B, respectively), either in the absence or in the presence of MMP inhibitors, thus strongly indicating that MMP-induced gelatinolytic activity is a major determinant of vascular ROS concentrations.
Fig. 6.
Markers of increased aortic oxidative stress increase in proportion to the increases in aortic MMP-2 gelatinolytic activity. (A) Aortic concentrations of reactive oxygen species (ROS) measured by DHE technique increase in proportion to the increases in gelatinolytic activity. This association analysis was carried out using data reported in Fig. 5D. (B), Aortic lucigenin chemiluminescence increase in proportion to the increases in gelatinolytic activity. This association analysis was carried out using data reported in Fig. 5E. The regression line and the 95% confidence interval are plotted.
3.4. Pro-oxidant effects of MMP-2 are prevented by the antioxidant apocynin or by PEG-catalase
Experiments were carried out with vessels to examine whether antioxidant compounds including apocynin and PEG-catalase could attenuate MMP-2 induced vascular oxidative stress. We found that both apocynin and PEG-catalase blunted MMP-2 induced increases in DHE signal in aortas (Fig. 7A and B; all P < 0.05). These findings are consistent with the idea that MMP-2 impairs vascular redox biology.
Fig. 7.
The antioxidant apocynin or PEG-catalase prevent pro-oxidant effects of MMP-2. (A) Representative photomicrographs of aortic sections incubated with MMP-2 (16 nM) intraluminally for 30 min. ROS production assessed by DHE was totally inhibited by apocynin (Apo 100 µM) or by PEG-catalase (PG-CAT 2500 U/mL). (B) Quantification of aortic fluorescence stimulated by DHE. Data are shown as the mean ± SEM (n = 4–5/group). *P < 0.05 vs. Vehicle+Vehicle group. # P < 0.05 vs. Vehicle+MMP-2 group.
3.5. Pro-oxidant effects of intraluminal MMP-2 enhances adrenergic receptor-mediated vasoconstriction
In order to explore possible functional implications of the previous findings reported here, we examined whether MMP-2-induced increases in vascular gelatinolytic activity could enhance the vasoconstriction induced by phenylephrine (an alpha-adrenergic agonist) in endothelium-denuded aortic rings. Interestingly, we found that MMP-2 significantly increased the Emax response of aortic rings to phenylephrine (from 1485 ± 77 mg/g to 2004 ± 116 mg/g; P < 0.05; n = 7; Fig. 8A and B). This effect was totally prevented by the MMP inhibitor GM6001 (Emax response to phenylephrine = 1552 ± 77 mg/g; P < 0.05 vs. MMP-2 group; n = 7; Fig. 8C and D), thus strongly implicating MMP-2 mediated gelatinolytic activity in this effect.
Fig. 8.
Intraluminal incubation of rabbit aorta with MMP-2 increases the vascular contractility to phenylephrine (PE). (A) Cumulative concentration-effect curves to PE were performed in endothelium denuded (E-) aortic rings obtained from vessels filled with MMP-2 (16 nM) or vehicle. (B) Quantification of maximum effect (Emax) induced by PE in E- aortic rings filled with MMP-2 or vehicle. (C) Cumulative concentration-effect curves to PE in E- aortic rings in presence or absence of the MMP inhibitor GM6001 (10 µM), or apocyanin (Apo 100 µM), or PEG-Catalase (PG-Cat 3000 U/mL). (D) Quantification of maximum effect (Emax) induced by PE in E- aortic rings filled with MMP-2 in presence or absence of GM6001, Apocyanin or PEG-catalase. Data are shown as the mean ± SEM (n = 7–9/group). * P < 0.05 vs. Vehicle group. # P < 0.05 vs. MMP-2 group.
Given that MMP-2 increased vascular adrenergic receptor-mediated vasoconstriction, we examined whether this effect is mediated by MMP-2 induced increases in vascular ROS production. Therefore the effects of the antioxidant apocynin or PEG-catalase on MMP-2 induced increases in vascular contractility to phenylephrine were examined in aortic rings. Consistent with the previous findings reported here, we found that both apocynin and PEG-catalase completely abolished the increases in vascular contractility induced by MMP-2 (Emax response to phenylephrine = 1632 ± 60 mg/g, n = 7, for MMP-2 + apocynin group, and 1542 ± 57 mg/g, n = 7, for MMP-2 + PEG-catalase, n = 7; both P < 0.05 vs. MMP-2 group; Fig. 8C and D). No significant differences were found in pD2 values. Together, these results show that MMP-2-induced increases in vascular contractility are mediated by increased ROS formation.
4. Discussion
We show evidence for the first time that MMP-2 activates downstream signaling pathways inducing vascular ROS formation in VSMC and in aortas. The increased ROS production induced by MMP-2 depends on EGFR activation, which mediates PLC and PKC activation, thus enhancing vascular ROS production and facilitating phenylephrine-induced aorta contraction (Fig. 9).
Fig. 9.
MMP-2 exerts pro-oxidant effects in vascular smooth muscle cell. MMP-2 induces shedding of HB-EGF which activates the EGF receptor (EGFR) activating phospholipase C (PLC-y) leading to diacylglycerol (DAG) formation and protein kinase C (PKC) activation. PKC enhances NADPH oxidase activity and increases ROS production.
Increased MMP-2 activity has been widely shown in disease conditions associated with redox imbalance, and it has usually been accepted that increased ROS production is upstream of MMP activation. While this is an important mechanism explaining enhanced MMP-2 expression in these conditions [3], [10], [11], [12], [36], [37], [38], [39], we now provide evidence contrary to this current notion. We show that MMP-2 is upstream of ROS production. This mechanism may be similar to those described for other MMPs [16], [17], [18]. To show this new perspective of MMP-2 promoting redox imbalance, we carried out a series of experiments starting with VSMC to demonstrate that MMP-2 promotes ROS production, an effect that is attributable to its proteolytic activity, as MMP inhibitors fully inhibited MMP-2-induced oxidative stress.
Our results provide evidence that MMP-2 increases ROS production by mechanisms that involve MMP-2-mediated cleavage of pro-HB-EGF leading to EGFR transactivation, as previously shown by others [16], [20], [21]. In fact, we showed here that MMP-2 induced HB-EGF-mediated EGFR activation is abrogated by EGFR kinase inhibitor Ag 1478 in VSMC, which totally prevented MMP-2-induced oxidative stress. Consistent with our findings, previous studies showed that activation of EGFR simulates ROS production under other experimental conditions [16], [19], [22]. Indeed, EGFR is a tyrosine kinase receptor that, following binding of its ligand, receptor dimerization occurs leading to increased phosphorylation and activation of intracellular proteins including ERK, AKT and PLC [19], [40], [41], which are intracellular kinases known to increase ROS formation [42], [43]. Again, our results confirmed the activation of pro-oxidant signaling pathways stimulated by MMP-2 as drugs (A778 or chelerythrine) inhibiting two proteins (PLC or PKC, respectively) activated in EGFR cascade abrogated redox imbalance induced by MMP-2. Our findings implicating EGFR transactivation in the mechanisms promoted by MMP-2 are similar to those previously described for MMP-7 in an interesting study showing that stimulation with phenylephrine activates MMP-7 and HB-EGF sheddases with subsequent increases in ROS production, thus contributing to maintain the vascular contraction [16], [17], [19]. Together, these findings suggest that MMPs may share common mechanisms to increase vascular ROS production and contribute to disease conditions.
To further validate our results obtained with cell experiments, we examined the pro-oxidant effects of MMP-2 in vessels exposed to MMP-2. This approach could mimic in vivo conditions associated with increased circulating MMP-2 levels, which could directly interact with vascular cells. Interestingly, we found that intraluminal incubation of aortas with MMP-2 increased both MMP-2 levels and gelatinolytic activity detected in all vascular layers. This response was associated with increased ROS levels and lucigenin activity in vascular tissue, with strong correlation between markers of redox imbalance and gelatinolytic activity. These findings consistently support the conclusions made from our previous cell experiments indicating that MMP-2 promotes pro-oxidant alterations. Again, further supporting this idea, we found that various MMP inhibitors (phenanthroline, doxycycline, or GM6001) completely prevented MMP-2 induced pro-oxidant effects. Next, we further confirmed MMP-2 mediated pro-oxidant effects by showing that antioxidant compounds including apocyanin, DPI, and PEG-catalase prevent such effects. Together, these vascular experiments fully support the findings with cells and confirmed pro-oxidant effects of MMP-2.
Next, we examined functional implications of the biochemical alterations induced by MMP-2. Interestingly, we found that the pro-oxidant effects of intraluminal incubation with MMP-2 enhanced adrenergic receptor-mediated vasoconstriction. Both an MMP inhibitor (GM6001) and antioxidant compounds (apocynin or PEG-catalase) abolished MMP-2-induced increases in vascular contractility to phenylephrine. These results strongly suggest that the effects of MMP-2 on vascular contractility are explained by MMP-2-mediated pro-oxidant effects. Our findings with MMP-2 are similar to those reported by Hao et al. [17], which showed that activation of alpha-1 adrenergic receptors increases ROS formation by MMP-7 and contributes to maintain the vasoconstriction induced by phenylephrine in mesenteric arteries of rats. In parallel with our findings, the MMP inhibitors GM6001 or doxycycline attenuated ROS production and vascular contraction. These results are consistent with other studies showing that intraluminal infusion of MMP-7, MMP-9 or the combination of both resulted in vasoconstriction of arterioles and venules from rats [44]. It should be clear that the effects of MMPs on vascular contractility are complex and involve other vascular receptors that may be degraded by MMPs, including beta-adrenergic receptors [6], [44].
Mitochondrial activity is a major source of ROS, and Lovett et al. [9] showed that overexpression of a mitochondrial isoform of MMP-2 (NTT-MMP-2) in cardiomyocytes downregulates BcL-xL and HSPD1, which are genes associated with resistance to oxidative stress. These findings are consistent with pro-oxidant effects of MMP-2, and may help to explain increased susceptibility to cardiac lipid peroxidation shown in a transgenic mice model with increased MMP-2 activity [18].
This study has some limitations that should be mentioned. Recent findings have shown evidence that may limit our interpretation of data obtained with the DHE technique. It is possible that the signal obtained with DHE may not be selective for ROS as DHE could react with other reactive sulfide species (RSS) as shown with other methods used to detect ROS [45]. Moreover, catalase may act as an sulfide oxidase or sulfur reductase and therefore affect RSS concentrations [46]. However, we used both DHE and lucigenin assay in isolated cell and vascular tissue experiments to examine ROS production with consistent results in the present study. Another limitation is that the antioxidant compounds used in the present study (apocynin, DPI and PEG-catalase) are not selective inhibitors of specific pro-oxidant mechanisms.
Our findings may have several implications for our understanding and therapy of cardiovascular diseases. Of major importance, developing new MMP inhibitors may be of greater importance than previously anticipated, especially in disease conditions associated with increased oxidative stress such as hypertension and other cardiovascular diseases [47]. This suggestion is based on the idea that MMP inhibitors may prevent deleterious alterations associated with imbalanced redox conditions in addition to their expected effect on abnormal MMP activity. Our findings reported here may also help to explain antioxidant responses previously reported with the use of MMP inhibitors [10], [13], [14], [15], [36], [37], [48].
In conclusion, our results show consistent evidence that MMP-2 activates downstream signaling pathways involving the EGFR which results in PLC and PKC activation and pro-oxidant mechanisms in VSMC. These MMP-2-mediated effects enhance the vascular responses to alpha-1 adrenergic receptor stimulation and may have various long-term implications for cardiovascular diseases.
Acknowledgements
The authors thank Sandra de Oliveira Conde for excellent technical support. This work was supported by Fundação de Aparo a Pesquisa do Estado de São Paulo (FAPESP Grant no. 2014/23888-0), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nivel Superior).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.07.005.
Appendix A. Supplementary material
Supplementary material
References
- 1.Cui N., Hu M., Khalil R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman M.R., Teerlink J.R., Mahimkar R., Li L., Zhu B.Q., Nguyen A., Dahi S., Karliner J.S., Lovett D.H. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 3.Ceron C.S., Rizzi E., Guimaraes D.A., Martins-Oliveira A., Cau S.B., Ramos J., Gerlach R.F., Tanus-Santos J.E. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 2012;31:261–270. doi: 10.1016/j.matbio.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki T., Lee J.D., Shimizu H., Uzui H., Ueda T. Circulating matrix metalloproteinase-2 is elevated in patients with congestive heart failure. Eur. J. Heart Fail. 2004;6:41–45. doi: 10.1016/j.ejheart.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 5.George J., Patal S., Wexler D., Roth A., Sheps D., Keren G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am. Heart J. 2005;150:484–487. doi: 10.1016/j.ahj.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Ferraz K.C., Sousa-Santos O., Neto-Neves E.M., Rizzi E., Muniz J.J., Gerlach R.F., Tanus-Santos J.E. Recombinant human matrix metalloproteinase-2 impairs cardiovascular beta-adrenergic responses. Basic Clin. Pharmacol. Toxicol. 2013;112:103–109. doi: 10.1111/bcpt.12001. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto T., Akaike T., Sawa T., Miyamoto Y., van der Vliet A., Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 8.Viappiani S., Nicolescu A.C., Holt A., Sawicki G., Crawford B.D., Leon H., van Mulligen T., Schulz R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009;77:826–834. doi: 10.1016/j.bcp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Lovett D.H., Mahimkar R., Raffai R.L., Cape L., Maklashina E., Cecchini G., Karliner J.S. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One. 2012;7:e34177. doi: 10.1371/journal.pone.0034177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonio R.C., Ceron C.S., Rizzi E., Coelho E.B., Tanus-Santos J.E., Gerlach R.F. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol. Cell. Biochem. 2014;386:99–105. doi: 10.1007/s11010-013-1848-7. [DOI] [PubMed] [Google Scholar]
- 11.Castro M.M., Rizzi E., Rodrigues G.J., Ceron C.S., Bendhack L.M., Gerlach R.F., Tanus-Santos J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Rizzi E., Castro M.M., Ceron C.S., Neto-Neves E.M., Prado C.M., Rossi M.A., Tanus-Santos J.E., Gerlach R.F. Tempol inhibits TGF-beta and MMPs upregulation and prevents cardiac hypertensive changes. Int. J. Cardiol. 2013;165:165–173. doi: 10.1016/j.ijcard.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Castro M.M., Rizzi E., Ceron C.S., Guimaraes D.A., Rodrigues G.J., Bendhack L.M., Gerlach R.F., Tanus-Santos J.E. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide. 2012;26:162–168. doi: 10.1016/j.niox.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Castro M.M., Rizzi E., Figueiredo-Lopes L., Fernandes K., Bendhack L.M., Pitol D.L., Gerlach R.F., Tanus-Santos J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes D.A., Rizzi E., Ceron C.S., Oliveira A.M., Oliveira D.M., Castro M.M., Tirapelli C.R., Gerlach R.F., Tanus-Santos J.E. Doxycycline dose-dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin. Pharmacol. Toxicol. 2011;108:318–325. doi: 10.1111/j.1742-7843.2010.00656.x. [DOI] [PubMed] [Google Scholar]
- 16.Hao L., Du M., Lopez-Campistrous A., Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ. Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 17.Hao L., Nishimura T., Wo H., Fernandez-Patron C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2006;26:819–825. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H.Z., Ma X., Gray M.O., Zhu B.Q., Nguyen A.P., Baker A.J., Simonis U., Cecchini G., Lovett D.H., Karliner J.S. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2007;358:189–195. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagareddy P.R., Chow F.L., Hao L., Wang X., Nishimura T., MacLeod K.M., McNeill J.H., Fernandez-Patron C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc. Res. 2009;84:368–377. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 20.Lucchesi P.A., Sabri A., Belmadani S., Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 21.Smiljanic K., Obradovic M., Jovanovic A., Djordjevic J., Dobutovic B., Jevremovic D., Marche P., Isenovic E.R. Thrombin stimulates VSMC proliferation through an EGFR-dependent pathway: involvement of MMP-2. Mol. Cell. Biochem. 2014;396:147–160. doi: 10.1007/s11010-014-2151-y. [DOI] [PubMed] [Google Scholar]
- 22.Bae Y.S., Kang S.W., Seo M.S., Baines I.C., Tekle E., Chock P.B., Rhee S.G. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 23.Goncalves A.N., Meschiari C.A., Stetler-Stevenson W.G., Nonato M.C., Alves C.P., Espreafico E.M., Gerlach R.F. Expression of soluble and functional full-length human matrix metalloproteinase-2 in Escherichia coli. J. Biotechnol. 2012;157:20–24. doi: 10.1016/j.jbiotec.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.S., Basalyga D.M., Simionescu A., Isenburg J.C., Simionescu D.T., Vyavahare N.R. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am. J. Pathol. 2006;168:490–498. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schroder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 26.Razandi M., Pedram A., Park S.T., Levin E.R. Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 27.Aragao A.Z., Nogueira M.L., Granato D.C., Simabuco F.M., Honorato R.V., Hoffman Z., Yokoo S., Laurindo F.R., Squina F.M., Zeri A.C., Oliveira P.S., Sherman N.E., Paes Leme A.F. Identification of novel interaction between ADAM17 (a disintegrin and metalloprotease 17) and thioredoxin-1. J. Biol. Chem. 2012;287:43071–43082. doi: 10.1074/jbc.M112.364513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Raab G., Deutsch U., Zhang J., Ezzell R.M., Klagsbrun M. Induction of heparin-binding EGF-like growth factor expression during myogenesis. Activation of the gene by MyoD and localization of the transmembrane form of the protein on the myotube surface. J. Biol. Chem. 1995;270:18285–18294. doi: 10.1074/jbc.270.31.18285. [DOI] [PubMed] [Google Scholar]
- 29.Dethlefsen S.M., Raab G., Moses M.A., Adam R.M., Klagsbrun M., Freeman M.R. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J. Cell. Biochem. 1998;69:143–153. doi: 10.1002/(sici)1097-4644(19980501)69:2<143::aid-jcb5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Kim J., Lin J., Adam R.M., Lamb C., Shively S.B., Freeman M.R. An oxidative stress mechanism mediates chelerythrine-induced heparin-binding EGF-like growth factor ectodomain shedding. J. Cell. Biochem. 2005;94:39–49. doi: 10.1002/jcb.20276. [DOI] [PubMed] [Google Scholar]
- 31.Cha B., Chen T., Sarker R., Yang J., Raben D., Tse C.M., Kovbasnjuk O., Donowitz M. Lysophosphatidic acid stimulation of NHE3 exocytosis in polarized epithelial cells occurs with release from NHERF2 via ERK-PLC-PKCdelta signaling. Am. J. Physiol. Cell Physiol. 2014;307:C55–C65. doi: 10.1152/ajpcell.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9 doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cau S.B., Guimaraes D.A., Rizzi E., Ceron C.S., Souza L.L., Tirapelli C.R., Gerlach R.F., Tanus-Santos J.E. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodelling in renovascular hypertension. Br. J. Pharmacol. 2011;164:372–381. doi: 10.1111/j.1476-5381.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro L.C., Oliveira-Paula G.H., Portella R.L., Guimaraes D.A., de Angelis C.D., Tanus-Santos J.E. Omeprazole impairs vascular redox biology and causes xanthine oxidoreductase-mediated endothelial dysfunction. Redox Biol. 2016;9:134–143. doi: 10.1016/j.redox.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins-Oliveira A., Guimaraes D.A., Ceron C.S., Rizzi E., Oliveira D.M.M., Tirapelli C.R., Casarini D.E., Fernandes F.B., Pinheiro L.C., Tanus-Santos J.E. Direct renin inhibition is not enough to prevent reactive oxygen species generation and vascular dysfunction in renovascular hypertension. Eur. J. Pharmacol. 2018;821:97–104. doi: 10.1016/j.ejphar.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Cau S.B., Barato R.C., Celes M.R., Muniz J.J., Rossi M.A., Tanus-Santos J.E. Doxycycline prevents acute pulmonary embolism-induced mortality and right ventricular deformation in rats. Cardiovasc. Drugs Ther. 2013;27:259–267. doi: 10.1007/s10557-013-6458-9. [DOI] [PubMed] [Google Scholar]
- 37.Neto-Neves E.M., Sousa-Santos O., Ferraz K.C., Rizzi E., Ceron C.S., Romano M.M., Gali L.G., Maciel B.C., Schulz R., Gerlach R.F., Tanus-Santos J.E. Matrix metalloproteinase inhibition attenuates right ventricular dysfunction and improves responses to dobutamine during acute pulmonary thromboembolism. J. Cell. Mol. Med. 2013;17:1588–1597. doi: 10.1111/jcmm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santa-Cecilia F.V., Socias B., Ouidja M.O., Sepulveda-Diaz J.E., Acuna L., Silva R.L., Michel P.P., Del-Bel E., Cunha T.M., Raisman-Vozari R. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox. Res. 2016 doi: 10.1007/s12640-015-9592-2. [DOI] [PubMed] [Google Scholar]
- 39.Azevedo A., Prado A.F., Antonio R.C., Issa J.P., Gerlach R.F. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin. Pharmacol. Toxicol. 2014;115:301–314. doi: 10.1111/bcpt.12282. [DOI] [PubMed] [Google Scholar]
- 40.Asakura M., Kitakaze M., Takashima S., Liao Y., Ishikura F., Yoshinaka T., Ohmoto H., Node K., Yoshino K., Ishiguro H., Asanuma H., Sanada S., Matsumura Y., Takeda H., Beppu S., Tada M., Hori M., Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 41.Saifeddine M., El-Daly M., Mihara K., Bunnett N.W., McIntyre P., Altier C., Hollenberg M.D., Ramachandran R. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharmacol. 2015;172:2493–2506. doi: 10.1111/bph.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makki N., Thiel K.W., Miller F.J., Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 2013;14:20597–20613. doi: 10.3390/ijms141020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S.Y., Lee H.S., Kim E.K., Ha J.M., Kim Y.W., Bae S. Reactive oxygen species and PI3K/Akt signaling in cancer. Free Radic. Biol. Med. 2014;75(Suppl. 1):S34–S35. doi: 10.1016/j.freeradbiomed.2014.10.773. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues S.F., Tran E.D., Fortes Z.B., Schmid-Schonbein G.W. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H25–H35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeLeon E.R., Gao Y., Huang E., Arif M., Arora N., Divietro A., Patel S., Olson K.R. A case of mistaken identity: are reactive oxygen species actually reactive sulfide species? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R549–R560. doi: 10.1152/ajpregu.00455.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson K.R., Gao Y., DeLeon E.R., Arif M., Arif F., Arora N., Straub K.D. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS) Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro M.M., Tanus-Santos J.E. Inhibition of matrix metalloproteinases (MMPs) as a potential strategy to ameliorate hypertension-induced cardiovascular alterations. Curr. Drug Targets. 2013;14:335–343. doi: 10.2174/1389450111314030005. [DOI] [PubMed] [Google Scholar]
- 48.Lai H.C., Yeh Y.C., Ting C.T., Lee W.L., Lee H.W., Wang L.C., Wang K.Y., Lai H.C., Wu A., Liu T.J. Doxycycline suppresses doxorubicin-induced oxidative stress and cellular apoptosis in mouse hearts. Eur. J. Pharmacol. 2010;644:176–187. doi: 10.1016/j.ejphar.2010.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material