Most staphylococcal species are major causes of health care- and community-associated infections. In particular, Staphylococcus aureus is a common and dangerous pathogen, and Staphylococcus epidermidis is a ubiquitous skin commensal and opportunistic pathogen. Treatment of infections caused by staphylococci has become more difficult because of the emergence of multidrug-resistant strains as well as biofilm formation. In this study, we found that all tested S. aureus, S. epidermidis, Staphylococcus haemolyticus, and Staphylococcus hominis strains were sensitive to the phage lysin LysGH15 (MICs ranging from 8 to 32 μg/ml). More importantly, LysGH15 not only prevented biofilm formation by these staphylococci but also disrupted 24-h and 72-h biofilms. Furthermore, the in vivo efficacy of LysGH15 was demonstrated in a mouse model of S. epidermidis bacteremia. Thus, LysGH15 exhibits therapeutic potential for treating biofilm-related or non-biofilm-related infections caused by diverse staphylococci.
KEYWORDS: phage lysin, LysGH15, Staphylococcus aureus, staphylococci, biofilm
ABSTRACT
Treatment of infections caused by staphylococci has become more difficult because of the emergence of multidrug-resistant strains as well as biofilm formation. In this study, we observed the ability of the phage lysin LysGH15 to eliminate staphylococcal planktonic cells and biofilms formed by Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, and Staphylococcus hominis. All these strains were sensitive to LysGH15, showing reductions in bacterial counts of approximately 4 log units within 30 min after treatment with 20 μg/ml of LysGH15, and the MICs ranged from 8 μg/ml to 32 μg/ml. LysGH15 efficiently prevented biofilm formation by the four staphylococcal species at a dose of 50 μg/ml. At a higher dose (100 μg/ml), LysGH15 also showed notable disrupting activity against 24-h and 72-h biofilms formed by S. aureus and coagulase-negative species. In the in vivo experiments, a single intraperitoneal injection of LysGH15 (20 μg/mouse) administered 1 h after the injection of S. epidermidis at double the minimum lethal dose was sufficient to protect the mice. The S. epidermidis cell counts were 4 log units lower in the blood and 3 log units lower in the organs of mice 24 h after treatment with LysGH15 than in the untreated control mice. LysGH15 reduced cytokine levels in the blood and improved pathological changes in the organs. The broad antistaphylococcal activity exerted by LysGH15 on planktonic cells and biofilms makes LysGH15 a valuable treatment option for biofilm-related or non-biofilm-related staphylococcal infections.
IMPORTANCE Most staphylococcal species are major causes of health care- and community-associated infections. In particular, Staphylococcus aureus is a common and dangerous pathogen, and Staphylococcus epidermidis is a ubiquitous skin commensal and opportunistic pathogen. Treatment of infections caused by staphylococci has become more difficult because of the emergence of multidrug-resistant strains as well as biofilm formation. In this study, we found that all tested S. aureus, S. epidermidis, Staphylococcus haemolyticus, and Staphylococcus hominis strains were sensitive to the phage lysin LysGH15 (MICs ranging from 8 to 32 μg/ml). More importantly, LysGH15 not only prevented biofilm formation by these staphylococci but also disrupted 24-h and 72-h biofilms. Furthermore, the in vivo efficacy of LysGH15 was demonstrated in a mouse model of S. epidermidis bacteremia. Thus, LysGH15 exhibits therapeutic potential for treating biofilm-related or non-biofilm-related infections caused by diverse staphylococci.
INTRODUCTION
Staphylococcus aureus and Staphylococcus epidermidis, two staphylococcal species, are the main causes of nosocomial infections (1, 2). S. aureus is a common and dangerous pathogen that causes both health care- and community-associated infections, including skin and wound infections, pneumonia, severe sepsis, endocarditis, and others (3). S. epidermidis is a ubiquitous skin commensal and opportunistic pathogen, usually causing infection and bacteremia in immunocompromised patients, especially those with implanted medical devices (4, 5). Treatment of infections caused by staphylococci has become more difficult because of the emergence of multidrug-resistant strains, particularly methicillin-resistant staphylococci.
The pathogenicity of both S. aureus and S. epidermidis is also associated with biofilm formation on biotic and abiotic surfaces (6, 7). A biofilm hinders the penetration of antibiotics into its interior, resulting in therapy failure (5, 8). Additionally, the presence of persister cells is also thought to be responsible for the recalcitrance of biofilms to antibiotics (9). Biofilms formed on the surfaces of medical devices act as reservoirs for infection (10). Thus, staphylococcal biofilms are usually responsible for chronic and recurrent infections (11).
It has been reported that S. epidermidis biofilms are more difficult to eliminate than S. aureus biofilms (12). Studies have shown that subinhibitory concentrations of various antibiotics, including nafcillin, tetracycline, and vancomycin, increase biofilm formation by staphylococci (13–15). The internal structure of a biofilm is very firm, making removal difficult (16). Even high concentrations (16× the MIC) of vancomycin do not eliminate staphylococcal biofilms (17).
Lysins encoded by phages are highly evolved peptidoglycan hydrolases that lyse the host cell during the terminal stage of the phage lytic life cycle to ensure the release of phage progeny (18). Other phage virion- or tail-associated muralytic enzymes (VAME or TAME, respectively), such as HydH5, TAME from phage K, and P128, also possess antibacterial properties (19–21). Due to their high efficiency, lack of resistance, lack of neutralizing antibodies, and specificity, lysins hold great therapeutic potential for bacterial infections caused by antibiotic-resistant strains. Additionally, it has been reported that several staphylococcal phage-derived lysins, such as LysH5 (22), LysKΔamidase (23), P128 (10), PlySs2 (24), and ClyF (25), possess the ability to clear biofilms.
Previous studies have proven that LysGH15, a lysin derived from the staphylococcal phage GH15 (26, 27), shows bactericidal activity against methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) (28). The structures of three individual domains of LysGH15 were determined to explore the molecular mechanism underlying its lytic activity (29). LysGH15 did not induce resistance in MRSA or MSSA strains after repeated treatment, and LysGH15-specific antibodies did not affect the killing efficiency of LysGH15 against MRSA in vitro or in vivo (30). LysGH15 showed efficacy in treating mouse models of MRSA bacteremia and pneumonia (28, 31). However, the sensitivity of other staphylococcal species to LysGH15 and the ability of LysGH15 to remove staphylococcal biofilms have not been studied.
In this study, the activity of LysGH15 against both planktonic cells and biofilms of S. aureus, S. epidermidis, Staphylococcus haemolyticus, and Staphylococcus hominis was determined in vitro. The protective effects of LysGH15 against lethal S. epidermidis infection in vivo were also studied.
RESULTS
Bactericidal activity of LysGH15.
All the isolated strains, including 25 S. epidermidis strains, 2 S. haemolyticus strains, 2 S. hominis strains, and 2 S. aureus strains, were confirmed using PCR (see Fig. S1 in the supplemental material) and sequencing. All the staphylococcal strains, with the exception of SE009 and SE020, were mecA gene positive (Fig. S2), indicating that they can be methicillin resistant. The antibiotic resistance phenotypes in most strains were consistent with their gene types, with the exception of R066 and W001, which were mecA gene positive but showed methicillin sensitivity (Table 1).
TABLE 1.
Antibiotic resistance of bacteria used in this study
| Strain | Phenotype for resistance to indicated druga |
MIC (μg/ml) of LysGH15 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Vancomycin | Erythromycin | Levofloxacin | Linezolid | Oxacillin | Moxifloxacin | Rifampin | Trimethoprim | Tigecycline | Benzylpenicillin | Tetracycline | Gentamicin | ||
| S. epidermidis | ||||||||||||||
| B021 | R | S | R | R | S | R | R | S | R | S | R | S | R | 16 |
| SE009 | S | S | R | S | S | S | S | S | S | S | R | S | S | 8 |
| SE004 | S | S | R | S | S | R | S | S | R | S | R | R | S | 32 |
| W062 | R | S | S | R | S | R | R | R | R | S | R | S | R | 16 |
| W059 | I | S | R | R | S | R | I | R | R | S | R | S | S | 16 |
| B003 | R | S | R | R | S | R | R | S | R | S | R | S | I | 16 |
| B074 | R | S | S | R | S | R | I | S | R | S | R | R | S | 16 |
| W004 | R | S | S | R | S | R | R | S | R | S | R | R | S | 16 |
| B052 | I | R | S | R | R | R | S | R | I | R | R | R | S | 8 |
| SE020 | I | S | R | R | S | S | I | S | R | S | S | S | S | 8 |
| B020 | R | S | R | R | S | R | I | S | S | S | R | R | S | 16 |
| W073 | I | S | R | R | S | R | I | S | S | S | R | R | S | 32 |
| B032 | I | S | R | R | S | R | I | R | R | S | R | S | R | 8 |
| SE008 | S | S | R | S | S | R | S | S | R | S | R | S | S | 32 |
| W068 | R | S | R | R | S | R | I | S | S | S | R | S | S | 8 |
| B073 | R | S | R | R | S | R | R | S | S | S | R | S | S | 8 |
| B070 | S | S | R | S | S | R | S | S | R | S | R | S | S | 32 |
| B002 | R | S | R | R | S | R | I | S | R | S | R | S | S | 32 |
| W049 | R | S | S | R | S | R | I | S | R | S | R | S | S | 32 |
| W039 | R | S | R | R | S | R | R | S | R | S | R | S | R | 16 |
| 6W059 | R | S | R | R | S | R | I | S | R | S | R | S | S | 16 |
| 8B046 | S | S | R | S | S | R | S | S | S | S | R | S | S | 16 |
| 1W037 | R | S | R | R | S | R | I | S | R | S | R | S | S | 8 |
| 5W062 | R | S | S | R | S | R | I | S | R | S | R | S | S | 8 |
| 8W045 | R | S | R | R | S | R | I | S | S | S | R | S | S | 16 |
| S. hominis | ||||||||||||||
| SWS11 | S | S | R | S | S | R | S | S | R | S | R | R | S | 8 |
| SWS12 | S | S | R | S | S | R | S | S | R | S | R | R | S | 16 |
| S. haemolyticus | ||||||||||||||
| SW053 | R | S | R | R | S | R | R | S | R | S | R | S | R | 8 |
| SW054 | R | S | R | R | S | R | R | S | R | S | R | S | R | 16 |
| S. aureus | ||||||||||||||
| R066 | S | S | R | S | S | S | S | S | S | S | R | S | S | 16 |
| W001 | S | S | S | S | S | S | S | S | S | S | R | S | S | 16 |
R, resistant; S, sensitive; I, intermediate.
These staphylococci were used to detect the bactericidal activity of purified LysGH15 (Fig. S3). As shown in Fig. 1, all the S. aureus, S. epidermidis, S. haemolyticus, and S. hominis strains were sensitive to LysGH15. The overall bacterial numbers decreased from 7 log units to approximately 3 log units after 30 min of treatment with LysGH15 (20 μg/ml). In addition, all the remaining bacteria were sensitive to LysGH15. The MICs of LysGH15 against these strains ranged from 8 to 32 μg/ml, as shown in Table 1.
FIG 1.
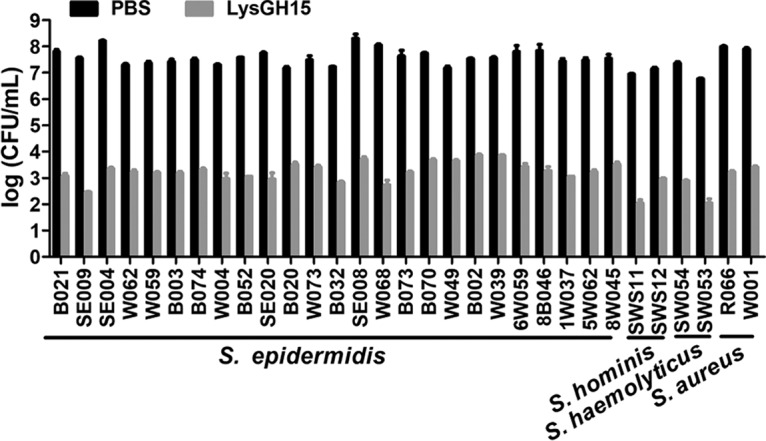
Bactericidal activity of LysGH15. Different strains (approximately 108 CFU/ml) were exposed to LysGH15 (20 μg/ml) or PBS for 30 min at 37°C. Bactericidal activity is displayed as the number of viable bacteria after treatment. The values represent the means ± SD (n = 3).
No potential resistance.
Both the vancomycin-resistant S. epidermidis (VRSE) strain B052 and the vancomycin-sensitive S. epidermidis (VSSE) strain B020 were used to determine the potential resistance to LysGH15. When S. epidermidis was exposed to serial dilutions of LysGH15, no spontaneous resistance mutants (the VRSE strain B052 and VSSE strain B020) were recovered. The cells collected from every generation showed similar sensitivities (Fig. 2). The MICs of the strains that underwent subculturing were the same as those of the parent cells.
FIG 2.
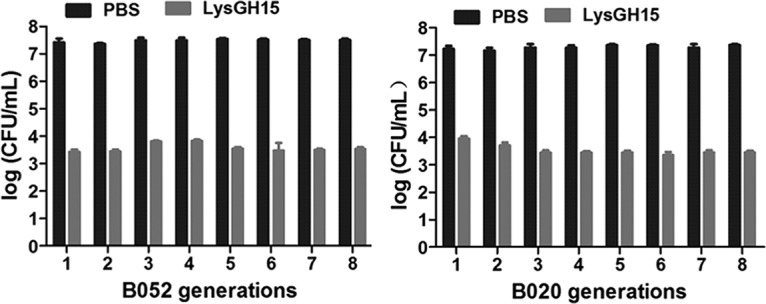
LysGH15 sensitivity of parent S. epidermidis cells and subcultures. The decrease in CFU per milliliter was used to evaluate the bactericidal activity of LysGH15 (20 μg/ml) against different generations of S. epidermidis cells (approximately 107 CFU/ml), and PBS was used as a control. The values represent the means ± SD (n = 3).
Antibiofilm activity of LysGH15.
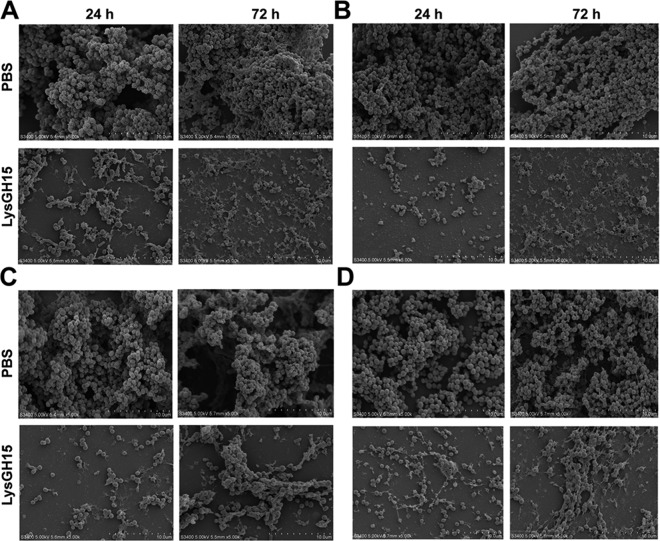
The ability of all the staphylococcal strains to form biofilms was determined. As shown in Fig. S4, S. epidermidis SE009, S. haemolyticus SW053, S. hominis SWS11, and S. aureus R066 formed the strongest biofilms (4 × ODc ≤ OD, where ODc is the cutoff of the optical density [OD] measurement obtained three times in the negative control) at both 24 h and 72 h among all the strains of these four staphylococcal species. LysGH15 degraded the biofilms of all the other strains at a concentration of 150 μg/ml, as shown in Table 2. Furthermore, micrographs obtained via scanning electron microscopy (SEM) clearly revealed the structurally complex and dynamic multilayered matrix architecture of the biofilms (Fig. 3).
TABLE 2.
Elimination of preformed biofilms by LysGH15 (150 μg/ml)
| Strain | % elimination of biofilm (mean ± SD) |
|
|---|---|---|
| 24-h biofilm | 72-h biofilm | |
| B021 | 64.47 ± 5.03 | 82.90 ± 5.95 |
| SE009 | 81.53 ± 3.06 | 68.51 ± 4.71 |
| SE004 | 51.02 ± 14.89 | 47.17 ± 17.80 |
| W062 | 46.83 ± 12.36 | 64.92 ± 10.30 |
| W059 | 48.03 ± 7.59 | 42.86 ± 16.23 |
| B003 | 64.59 ± 3.28 | 82.28 ± 11.42 |
| B074 | 62.34 ± 3.51 | 65.86 ± 9.13 |
| W004 | 56.79 ± 5.56 | 53.49 ± 11.64 |
| B052 | 69.16 ± 3.34 | 68.80 ± 14.02 |
| SE020 | 63.79 ± 15.37 | 54.50 ± 55.31 |
| B020 | 77.36 ± 5.13 | 81.18 ± 3.86 |
| W073 | 62.63 ± 9.64 | 79.88 ± 3.85 |
| B032 | 44.04 ± 9.01 | 75.75 ± 12.83 |
| SE008 | 53.09 ± 10.48 | 35.79 ± 15.42 |
| W068 | 74.12 ± 2.09 | 53.82 ± 4,85 |
| B073 | 78.86 ± 3.15 | 85.51 ± 4.15 |
| B070 | 57.00 ± 9.30 | 67.24 ± 13.85 |
| B098 | 69.28 ± 2.81 | 56.36 ± 8.60 |
| B002 | 54.10 ± 6.64 | 65.84 ± 19.24 |
| W039 | 77.22 ± 2.38 | 64.42 ± 2.91 |
| 6W059 | 50.35 ± 2.90 | 54.11 ± 3.54 |
| 8B046 | 56.38 ± 1.44 | 58.16 ± 3.99 |
| 1W037 | 53.20 ± 2.73 | 56.38 ± 5.92 |
| 5W062 | 48.69 ± 6.85 | 53.64 ± 19.60 |
| 8W045 | 39.68 ± 6.20 | 44.13 ± 3.10 |
| SW053 | 57.89 ± 1.49 | 51.70 ± 7.29 |
| SW054 | 45.63 ± 10.41 | 44.42 ± 4.88 |
| SWS12 | 41.93 ± 4.18 | 39.14 ± 2.11 |
| SWS11 | 72.29 ± 2.95 | 58.58 ± 20.17 |
| R066 | 73.38 ± 3.1 | 64.08 ± 3.24 |
| W001 | 74.17 ± 4.57 | 70.88 ± 8.15 |
FIG 3.
Micrographs of biofilms taken by scanning electron microscopy. The micrographs show biofilm formation by S. epidermidis SE009 (A), S. aureus R066 (B), S. haemolyticus SW053 (C), and S. hominis SWS11 (D) after incubation for 24 h and 72 h, followed by further treatment with LysGH15 (150 μg/ml) or PBS for 1 h. These images were obtained using SEM. The bars represent 10 μm.
LysGH15 prevented biofilm formation by these four strains to differing degrees. The values for OD at 590 nm (OD590) of the three groups treated with different doses of LysGH15 were much lower than those of the untreated group (P < 0.01 [Fig. S5A to D]). The rates of inhibition of biofilm formation by LysGH15 were calculated and are shown in Table 3. Significant decreases in biofilm mass, from 43% to 89%, were observed after LysGH15 treatment (50 μg/ml). The ability of LysGH15 to prevent biofilm formation was dose dependent.
TABLE 3.
Inhibition of biofilm formation by LysGH15
| Strain | % inhibition (mean ± SD) of biofilm formation by indicated concn of LysGH15 |
|||||
|---|---|---|---|---|---|---|
| 24 h |
72 h |
|||||
| 50 μg/ml | 100 μg/ml | 150 μg/ml | 50 μg/ml | 100 μg/ml | 150 μg/ml | |
| SE009 | 73.29 ± 4.41 | 85.95 ± 8.99 | 86.00 ± 6.50 | 89.47 ± 2.49 | 91.13 ± 4.35 | 90.78 ± 1.11 |
| SW053 | 50.16 ± 0.42 | 60.97 ± 2.95 | 63.78 ± 5.51 | 43.21 ± 8.71 | 51.18 ± 3.28 | 63.32 ± 5.82 |
| SWS11 | 47.13 ± 3.94 | 50.55 ± 2.28 | 60.57 ± 6.02 | 44.69 ± 7.74 | 59.38 ± 7.51 | 55.85 ± 7.07 |
| R066 | 58.75 ± 2.14 | 75.79 ± 9.38 | 76.76 ± 7.52 | 67.60 ± 6.98 | 64.99 ± 2.48 | 81.41 ± 3.77 |
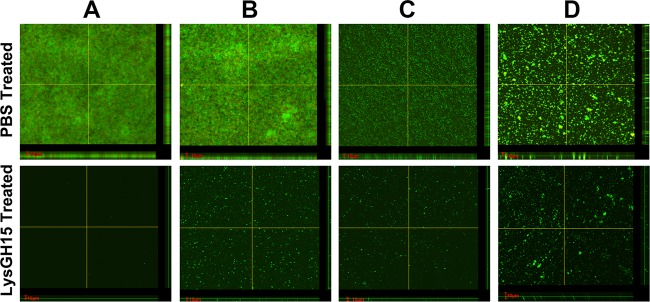
Additionally, preformed young biofilms (24 h) and old biofilms (72 h) were treated with different concentrations of LysGH15 (50 μg/ml, 100 μg/ml, and 150 μg/ml). The strong young and old biofilms (4 × ODc ≤ OD) of the SE009, SW053, SWS11, and R066 strains were destroyed by LysGH15 (Fig. S5E to H). The OD590 values of the two groups treated with high doses of LysGH15 (100 μg/ml and 150 μg/ml) were much lower than those of the untreated groups. The rates of biofilm clearance by LysGH15 were calculated and are shown in Table 4. LysGH15 exhibited dose-dependent activity. The biofilms produced by all four strains underwent significant removal by LysGH15 at a concentration of 150 μg/ml, and all the clearance rates reached 51% to 81%, although clearance was not significant at other doses; these results were also evident in SEM micrographs (Fig. 3). The use of confocal laser scanning microscopy (CLSM) also showed that the biofilm thickness, including that produced by S. epidermidis SE009, S. epidermidis B020, S. epidermidis W039, S. aureus R066, S. hominis SWS11, and S. haemolyticus SW053, was significantly lower after treatment with LysGH15 (150 μg/ml) than after treatment with phosphate-buffered saline (PBS) (Fig. 4; see also Fig. S6). As shown in Fig. S7, the number of live cells (green) in the PBS treatment group was much higher than in the LysGH15 treatment group, which is consistent with the SEM results. Additionally, LysGH15 degraded the biofilms of all the other strains at a concentration of 150 μg/ml, as shown in Fig. S4 and Table 2. Thus, LysGH15 not only prevents the formation of biofilms but also removes previously formed biofilms.
TABLE 4.
Elimination of preformed biofilms by LysGH15
| Strain | % elimination (mean ± SD) of biofilm formation by indicated concn of LysGH15 |
|||||
|---|---|---|---|---|---|---|
| 24 h |
72 h |
|||||
| 50 μg/ml | 100 μg/ml | 150 μg/ml | 50 μg/ml | 100 μg/ml | 150 μg/ml | |
| SE009 | 11.88 ± 28.50 | 58.49 ± 7.55 | 81.53 ± 3.06 | 28.78 ± 19.77 | 57.19 ± 3.71 | 68.51 ± 4.71 |
| SW053 | 47.57 ± 7.61 | 46.57 ± 6.55 | 57.89 ± 1.49 | 40.30 ± 15.43 | 48.28 ± 12.45 | 51.70 ± 7.29 |
| SWS11 | 34.95 ± 4.73 | 51.63 ± 12.30 | 72.29 ± 2.95 | 41.69 ± 19.45 | 52.92 ± 3.05 | 58.58 ± 20.17 |
| R066 | 5.29 ± 6.63 | 53.01 ± 1.78 | 73.38 ± 3.1 | 17.33 ± 4.55 | 42.63 ± 5.00 | 64.08 ± 3.24 |
FIG 4.
CLSM analysis of biofilms. Micrographs show biofilm formation by S. epidermidis SE009 (A), S. aureus R066 (B), S. hominis SWS11 (C), and S. haemolyticus SW053 (D) after incubation for 24 h followed by treatment with LysGH15 (150 μg/ml) or PBS for 1 h. The biofilms produced by the strains were assessed using LIVE/DEAD staining. The bars represent 10 μm. The images were obtained using CLSM.
LysGH15 protects mice against S. epidermidis lethal infection.
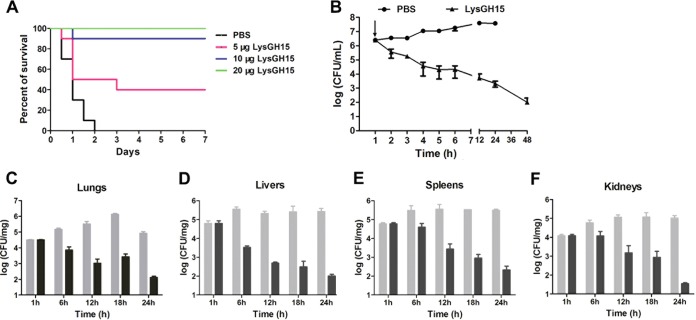
An intraperitoneal injection of 1 × 108 CFU of S. epidermidis B020 per mouse was sufficient to produce 100% mortality within 2 days. The 2× minimum lethal dose (MLD) (2 × 108 CFU) of B020 was determined to be a challenge dose. After infection for 1 h, the bacterial loads reached >106 CFU/ml in the blood. At this time, the mice were treated with LysGH15 at different doses (5, 10, and 20 μg/mouse). As shown in Fig. 5A, all the mice treated with PBS died within 48 h. In contrast, all the mice treated with 20 μg/mouse of LysGH15 recovered, and bacteremia greatly decreased. The bacterial load in the blood was 3.8 log units after 24 h of treatment, which was lower than 7.5 log units after 24 h in the PBS-treated group (Fig. 5B). The bacterial loads in the lungs, livers, spleens, and kidneys of LysGH15-treated mice dropped to 2.1, 2.0, 2.3, and 1.5 log units, respectively, 24 h after treatment, whereas the bacterial loads of PBS-treated mice increased to 4.8, 5.4, 5.3, and 5.0 log units (Fig. 5C to F), respectively. Based on histopathological observations (Fig. S8), the lungs, livers, kidneys, and spleens of the mice treated with LysGH15 did not show severe inflammation or other pathological changes at 48 h. The organ tissues of LysGH15-treated mice at 48 h looked similar to those of normal mice.
FIG 5.
LysGH15 protects mice against S. epidermidis lethal infection. (A) Treatment of a mouse bacteremia model with LysGH15. The mice infected with 2 × 108 CFU of S. epidermidis B020 were divided into four groups (10 mice per group). Groups were treated with different doses of LysGH15 (5, 10, or 20 μg/mouse) or PBS. (B) Bacterial load in the blood after infection. The infected mice were treated with 20 μg/mouse of LysGH15 or PBS at 1 h after the challenge (the arrow indicates the time at which the LysGH15 or PBS was injected). Each group contained six mice. (C) Bacterial load in the lungs. (D) Bacterial load in the livers. (E) Bacterial load in the spleens. (F) Bacterial load in the kidneys. Gray bars, PBS; black bars, LysGH15 (20 μg/mouse). Values are means ± SD (n = 3).
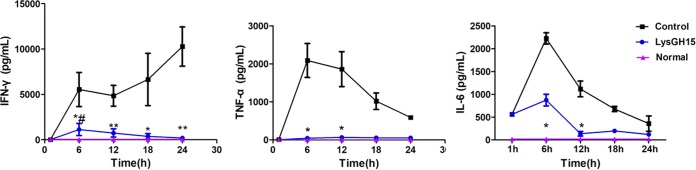
The cytokine levels in the blood of the different treatment groups were measured. In the PBS-treated group, gamma interferon (IFN-γ) levels were increased at all time points; however, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) levels increased rapidly during the first 6 h after the challenge dose and then declined gradually (Fig. 6). In contrast, LysGH15 significantly reduced IFN-γ, TNF-α, and IL-6 levels in the blood at 24 h, resulting in no significant change compared with the blood of normal mice.
FIG 6.
Cytokine levels. The BALB/c mice were injected intraperitoneally with S. epidermidis B020 (2 × 108 CFU/mouse). One hour later, the mice were treated with LysGH15 (20 μg/mouse) or PBS. At the indicated times, IFN-γ, TNF-α, and IL-6 levels in the serum were determined. * and **, P values of <0.05 and <0.01, respectively, compared with the buffer-treated control; #, P < 0.05 compared with the uninfected control. There were 6 mice in every group (PBS, LysGH15, and normal), and this experiment was repeated three times. Values are means ± SD (n = 3).
DISCUSSION
In this study, not only S. aureus but also S. epidermidis, S. haemolyticus, and S. hominis were sensitive to LysGH15. In general, S. aureus strains are more sensitive to lysins than S. epidermidis strains (32). However, our study demonstrated that LysGH15 showed similar bactericidal activity against the above-named species of staphylococci. We also found that S. epidermidis did not develop resistance to LysGH15 after repeated exposure, and this was consistent with the results obtained in previous studies of phage lysins, such as ClyS and PlySs2 (33, 34).
Many reports noted that both S. aureus and S. epidermidis could be killed by lysins of S. aureus phages, which included LysH5 (22), LysKΔamidase (23), P128 (10), PlyGRCS (35), SAL-2 (36), and ClyF (25), as well as the lysins of phages 80α, Φ11, K, P68, Twort, PhiSH2, and WMY (37). Additionally, S. epidermidis phage-derived lysins, such as Andhra_gp14 and Andhra_gp10 (38), also showed bactericidal activity against S. aureus and S. epidermidis. Interestingly, PlySs2 (also known as CF-301), the lysin derived from Streptococcus suis phage, showed lytic activity against staphylococci all the same (24). In comparison, the activity of LysGH15 (MIC ranged from 8 to 32 μg/ml) against diverse staphylococci in vitro was similar to or better than those of most other lysins, at least based on these limited studies.
LysGH15 effectively inhibited the biofilm formation of S. aureus, S. epidermidis, S. haemolyticus, and S. hominis. The ability of LysGH15 to inhibit biofilm formation is most likely attributable to its rapid bactericidal activity before biofilm formation. However, a few biofilms still formed even when a high concentration of LysGH15 was used. LysH5 and P128 also showed similar results (10, 39). This phenomenon should be attributed to the method used to test the biofilm. Crystal violet (CV) was used to stain the biofilms, and then the OD590 was determined. All of the denatured protein (lysin), dead bacteria, bacteria debris, live bacteria, and extracellular polymeric substances (EPS) contributed to the value of OD590. LysGH15 also could destroy preformed biofilms at higher doses. The role of phage lysin is to hydrolyze the cell wall of the host so that phages are released. Therefore, lysin should not destroy the matrix of biofilms unless it kills the cells. However, cells are the main component of biofilms. Once the cells in the biofilms are lysed, the integrity of the biofilm is destroyed, resulting in clearance (11). Thus, the biofilm removal range of a lysin should be consistent with its lytic spectrum, and the results of this study support this hypothesis. The dose of LysGH15 required to clear biofilms was higher than that required to lyse planktonic staphylococci and inhibit biofilm formation, potentially because the matrix of the biofilms, including the exopolysaccharide, environmental DNA (eDNA), and proteins, hinders the penetration of LysGH15. The results obtained by SEM, CLSM, and OD590 detection indicated the continued presence of biofilms, which could be attributed to LysGH15 insufficiency because the remaining cells and biofilms were also sensitive to LysGH15 (data not shown). Thus far, several lysins, including LysKΔamidase (23), LysH5 (22), LysSA97 (40), and ClyF (25), have been reported to be able to remove biofilms formed by S. aureus and/or S. epidermidis. LysGH15 showed biofilm elimination ability similar to or better than those of these lysins. It is worth mentioning that PlySs2, PlyGRCS, and P128 were most likely more effective at removing biofilm (10, 24, 35).
In the animal experiments, a lower dose (20 μg) of LysGH15 was needed to protect mice against lethal S. epidermidis bacteremia than the dose of LysGH15 (50 μg) to treat mouse bacteremia caused by MRSA (28). This indicated that LysGH15 showed superior elimination against S. epidermidis than against S. aureus in vivo. The levels of cytokine significantly increased at 6 h after treatment with LysGH15, which could be attributed to the lysis of S. epidermidis. This phenomenon was similar to the results obtained in S. aureus infection after treatment with LysGH15 (41), and the reason could be that the rapid release of abundant S. epidermidis cell debris and contents induced the innate immune response (42, 43). For revealing the antibiofilm ability of LysGH15 in vivo, further research is necessary.
This study supports the use of phage-encoded lysin to prevent and disperse staphylococcal biofilms. The broad antistaphylococcal activity of LysGH15 on planktonic cells and biofilms makes LysGH15 a valuable treatment option for biofilm-related and non-biofilm-related staphylococcal infections, including infections with S. aureus, S. epidermidis, S. haemolyticus, and S. hominis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All the strains used in this study were isolated from clinical specimens from patients at the First Hospital of Jilin University (Changchun, Jilin Province, China). The bacterial types and antibiotic susceptibilities of these isolates were evaluated using the Vitek2 Compact microbial identification system (bioMérieux, Boston, MA). All the isolated strains were confirmed by identifying the gap gene. The primers Gap1-for (ATGGTTTTGGTAGAATTGGTCGTTTA) and Gap2-rev (GACATTTCGTTATCATACCAAGCTG) were used to amplify the 931-bp fragment of the gap gene as described previously (44). The primers MRS1 (5′-TAGAAATGACTGAACGTCCG-3′) and MRS2 (5′-TTGCGATCAATGTTACCGTAG-3′) were used to detect a 154-bp fragment of the mecA gene (methicillin resistance) (45). All the isolates were routinely cultured using tryptic soy broth (TSB; Becton, Dickinson and Company, Franklin Lakes, NJ) at 37°C with shaking at 180 rpm.
Animals.
Female BALB/c mice, 18 g to 20 g (6 to 8 weeks of age), were obtained from the Experimental Animal Company of Yisi (Changchun, China) and kept under specific-pathogen-free conditions. The mice were maintained in individually ventilated cages (five animals per cage) with a 12-h light/dark cycle. The animals were treated humanely, and all possible efforts were made to minimize suffering. The mice had access to food and water ad libitum. The researchers did not know which test group the mice were allocated to during the experiment or when assessing the outcome.
All the animal experiments were conducted in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People's Republic of China (46) and approved by the Animal Welfare and Research Ethics Committee at Jilin University.
Preparation of LysGH15.
An Escherichia coli BL21(DE3) strain that expressed LysGH15 was constructed and stored by our laboratory in a previous study. LysGH15 was expressed, purified, and prepared according to the previous description (29).
Bactericidal range of LysGH15 among staphylococcal strains.
All the staphylococcal strains were cultured in TSB medium until the optical density at 600 nm (OD600) reached 0.4 to 0.5, and then the cultures were washed three times with phosphate-buffered saline (PBS) by centrifugation (8,000 × g for 1 min at 4°C). LysGH15 was added to the staphylococcal suspensions, and the final concentration of LysGH15 was adjusted to 20 μg/ml. Then the mixtures were incubated at 37°C, and the bacteria were counted 30 min after incubation began. As a negative control, PBS was used to treat the staphylococcal isolates under the same conditions.
Determination of MIC.
The MICs of LysGH15 against the different strains were determined according to previous studies (33). Briefly, all the strains were suspended in TSB (5 × 105 CFU/ml) and subsequently distributed into each well of a 96-well microplate. Then different concentrations of LysGH15 (ranging from 512 μg/ml to 0.25 μg/ml) or the control buffer were added to challenge the cells. The mixtures were incubated for 48 h at 37°C. Cell pellets were obtained after centrifugation (5,000 × g for 10 min at 4°C), and the viable cells in the pellets were detected using an alamarBlue vital dye (Invitrogen, Carlsbad, CA).
Determination of S. epidermidis resistance to LysGH15.
The bacterial resistance of S. epidermidis strains B052 and B020 to LysGH15 was determined by repeated exposure in plate lysis assays as previously described, with some modifications (47). Twofold serial dilutions of LysGH15 (concentrations ranged from 160 μg/ml to 10 μg/ml) were spotted (10 μl) onto a freshly plated lawn of a vancomycin-sensitive strain (B020) or a vancomycin-resistant strain (B052) on TSB plates and grown overnight at 37°C. The cells from spots that did not fully clear the lawn (sublethal) were scraped, inoculated in 5 ml of TSB, and grown to an OD600 of 0.4 to generate a new lawn for the next round of plating and LysGH15 exposure; this process was repeated for eight cycles. The cells obtained from each generation were tested for their sensitivity to LysGH15 (20 μg/ml).
Biofilm assay.
The ability of staphylococcal strains to form biofilms was evaluated using a 96-well microtiter plate method described previously (48), with some modifications. Briefly, the staphylococcal strains were inoculated into 5 ml of sterile TSB medium and grown for 16 h at 37°C. A 1:100 dilution of each staphylococcal culture was transferred into fresh TSB medium, and 200 μl (106 CFU/ml) of each diluted culture was added to three wells in untreated 96-well microtiter plates (NEST Biotechnology, Wuxi, China). The wells that contained only broth were used as the negative controls. The plates were incubated at 37°C for 24 h and 72 h without agitation. The nonadherent cells were removed by removing the cultures with a pipette, and the wells were then washed three times with sterile PBS. The biofilms were treated with 200 μl of LysGH15 (150 μg/ml) for 1 h at 37°C. The bacteria that remained attached to the well walls were fixed with 200 μl of methanol (Destilacija, Teslić, Bosnia and Herzegovina) for 30 min, and then the wells were emptied and dried at room temperature. Subsequently, each well was stained for 30 min with 200 μl of 1% (wt/vol) crystal violet (CV) stain at room temperature. The wells containing the biofilm total biomass were washed gently with sterile deionized water and dried at room temperature. Then 200 μl of 33% (vol/vol) glacial acetic acid was added to each well to solubilize the bound CV from the stained S. epidermidis biofilms. The OD590 was measured using a Synergy 2 multimode microplate reader (BioTek, Winooski, VT). The adherence capabilities of the isolates were classified as one of four levels categorized in a previous study (49): strong (4 × ODc ≤ OD), moderate (2 × ODc < OD ≤ 4 × ODc), weak (ODc < OD ≤ 2 × ODc), or non-biofilm forming (OD ≤ ODc). ODc is the cutoff of the OD measurement obtained three times in the negative control. The experiments were performed in triplicate.
Activity of LysGH15 against staphylococcal biofilms.
R066, SE009, SW053, and SWS11 formed the strongest biofilms among the S. aureus, S. epidermidis, S. haemolyticus, and S. hominis isolates, respectively. These strains were used to detect the LysGH15-mediated inhibition of biofilm formation, as described previously (48), with some modifications. Briefly, the strains were inoculated into 5 ml of sterile TSB medium and grown for 16 h at 37°C. A 1:100 dilution of each strain culture was transferred into sterile TSB medium and then cultivated to an OD600 of 0.4. The cultures were then centrifuged, resuspended in equal volumes of TSB medium, and mixed, and 100 μl of culture was added to each well of a 96-well microtiter plate. The wells were then divided into five groups. A volume of 100 μl of LysGH15 diluted in TSB was added to different groups at a final concentration of 50 μg/ml, 100 μg/ml, or 150 μg/ml. Sterile TSB medium (100 μl) was added into staphylococcal cultures as a control. Sterile TSB medium (200 μl) was used as a negative control. Each group contained triplicate samples. The plate was covered, and the bacteria were left to adhere and grow at 37°C for 24 h and 72 h without agitation. After incubation, the residual biofilms were stained with CV, and the OD590 was measured.
The effects of LysGH15 on the removal of young (24 h) and old (72 h) biofilms from the wells were also tested. S. aureus R066, S. epidermidis SE009, S. haemolyticus SW053, and S. hominis SWS11 were cultured in a 96-well microtiter plate to form young biofilms (24 h) and old biofilms (72 h). The nonadherent cells were removed by pipetting out the remaining culture and washing the wells three times with sterile PBS. The biofilms were treated with 200 μl of LysGH15 (50 μg/ml, 100 μg/ml, or 150 μg/ml) diluted in PBS and incubated for 1 h at 37°C. As a negative control, 200 μl of sterile PBS was added to other wells. Each group contained triplicate samples. After incubation, the residual biofilms were stained with CV, and the OD590 was measured.
SEM of biofilms.
S. aureus R066, S. epidermidis SE009, S. haemolyticus SW053, and S. hominis SWS11 were cultivated in 5 ml of TSB to an OD600 of 0.4 at 37°C with shaking at 180 rpm. The cultures were then centrifuged and resuspended in equal volumes of TSB medium. Next, 200 μl of bacterial culture and 200 μl of fresh TSB were added to each well of a 24-well microtiter plate; the wells were covered with sterile 14-mm-diameter glass sheets pretreated with polylysine. Then the 24-well microtiter plate was incubated overnight at 37°C without shaking. After incubation for 24 h and 72 h, the nonadherent cells were removed by removing the cultures with a pipette and washing the wells three times with sterile PBS. The biofilms were treated with 150 μg/ml of LysGH15 diluted in buffer for 1 h at 37°C. PBS was used as a negative control. The biofilm lysates were immobilized with 5% glutaraldehyde, dehydrated with different concentrations (20%, 50%, 70%, 90%, and 100%) of ethanol, and then freeze-dried before performing scanning electron microscopy (SEM) (Hitachi S-3400N; Hitachi High-Technologies Europe GmbH, Krefeld, Germany).
CLSM.
The effects of LysGH15 on the biofilms were assessed using a LIVE/DEAD BacLight staining kit (catalog number L-13152; Invitrogen, Carlsbad, CA) and visualized using confocal laser scanning microscopy (CLSM) (50). Young biofilms (24 h) of S. epidermidis SE009, S. aureus R066, S. hominis SWS11, S. haemolyticus SW053, S. epidermidis B020, and S. epidermidis W039 were cultured. The nonadherent cells were removed and the wells were washed three times with sterile PBS. Then the biofilms were treated with 150 μg/ml of LysGH15 or PBS for 1 h at 37°C, and the wells were washed once with 0.85% sodium chloride. SYTO9 and propidium iodide (PI), which stain live cells and dead cells, respectively, were used to stain the biofilms by following the manufacturer's protocol. Finally, the biofilms were visualized using CLSM (FV300; Olympus, Tokyo, Japan).
LysGH15 activity against S. epidermidis in vivo.
A mouse model of bacteremia caused by S. epidermidis B020 was constructed according to a previous study, with some modifications (28). The mice in three groups (each group contained 10 mice) were inoculated intraperitoneally with different doses of a bacterial suspension (1 × 107, 1 × 108, and 1 × 109 CFU/mouse) to determine the minimum dose that caused 100% mortality over 7 days of follow-up (minimum lethal dose [MLD]). The number of dead mice was recorded every day. Once the MLD was determined, 2× MLD was used as the infective inoculum (challenge dose). The procedure for this experiment was carried out as described previously, with modifications (51).
The mice were treated with LysGH15 (0, 5, 10, or 20 μg/mouse) intraperitoneally 1 h after injection with 2× MLD (2 × 108 CFU) B020 (n = 10 in each group). The control group was treated with an equal volume of PBS under the same conditions. The number of dead mice was recorded every day for 30 days.
The bacterial loads in the blood and vital organs were measured. At predetermined intervals, the bacterial counts were determined in 10 μl of peripheral blood obtained from the caudal veins of mice treated with either LysGH15 or PBS. To determine the bacterial loads in the organs, the lungs, livers, spleens, and kidneys were collected from euthanized mice (ketamine and xylazine, 100 mg/kg of body weight) at 1, 6, 12, 18, or 24 h following B020 challenge. Then the organs were weighed and homogenized in sterile PBS on ice. The bacterial loads in the tissues were measured using serial dilution and plating techniques.
Histopathology was performed for the main organ tissues. The mice were euthanized at 24 h and 48 h after B020 challenge, and the lungs, livers, spleens, and kidneys were removed and immediately placed in 4% formalin. Then, the fixed tissues were stained with hematoxylin and eosin (H&E) using conventional staining methods, and the tissue cells were examined using microscopy (30).
The cytokine levels in the blood were measured in different groups at 1, 6, 12, 18, or 24 h after challenge. The blood samples were collected from mice receiving different treatments, separated overnight at 4°C, and then centrifuged (5,000 × g) for 10 min at 4°C. The cytokines, including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6), were quantified using an enzyme-linked immunosorbent assay (ELISA) (eBioscience, San Diego, CA) according to the manufacturer's instructions (52).
Statistical analysis.
SPSS Statistics version 19.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis of the experimental data. A one-way analysis of variance (ANOVA) was performed for normally distributed data. A P value of <0.05 was considered statistically significant. Error bars in figures represent standard deviations (SD).
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported through grants from the National Key Research and Development Program of China (no. 2017YFD0501000) and the National Natural Science Foundation of China (no. 31502103 and 31572553).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00886-18.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumfitt W, Hamilton-Miller J. 1989. Methicillin-resistant Staphylococcus aureus. N Engl J Med 320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 4.Hogan S, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. 2015. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr Pharm Des 21:100–113. doi: 10.2174/1381612820666140905123900. [DOI] [PubMed] [Google Scholar]
- 5.Rogers KL, Fey PD, Rupp ME. 2009. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am 23:73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Qin Z, Zhang J, Xu B, Chen L, Wu Y, Yang X, Shen X, Molin S, Danchin A, Jiang H, Qu D. 2006. Structure-based discovery of inhibitors of the YycG histidine kinase: new chemical leads to combat Staphylococcus epidermidis infections. BMC Microbiol 6:96. doi: 10.1186/1471-2180-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziebuhr W, Hennig S, Eckart M, Kranzler H, Batzilla C, Kozitskaya S. 2006. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents 28(Suppl 1):S14–S20. doi: 10.1016/j.ijantimicag.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Otto M. 2013. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 9.Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. [DOI] [PubMed] [Google Scholar]
- 10.Poonacha N, Nair S, Desai S, Tuppad D, Hiremath D, Mohan T, Vipra A, Sharma U. 2017. Efficient killing of planktonic and biofilm-embedded coagulase-negative staphylococci by bactericidal protein P128. Antimicrob Agents Chemother 61:e00457-. doi: 10.1128/AAC.00457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutiérrez D, Briers Y, Rodriguez-Rubio L, Martinez B, Rodriguez A, Lavigne R, Garcia P. 2015. Role of the pre-neck appendage protein (Dpo7) from phage vB_SepiS-phiIPLA7 as an anti-biofilm agent in staphylococcal species. Front Microbiol 6:1315. doi: 10.3389/fmicb.2015.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sass P, Bierbaum G. 2007. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol 73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank KL, Reichert EJ, Piper KE, Patel R. 2007. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51:888–895. doi: 10.1128/AAC.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CY, Lin MH, Chen CC, Chien SC, Cheng YH, Su IN, Shu JC. 2011. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol Med Microbiol 63:236–247. doi: 10.1111/j.1574-695X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuehl R, Al-Bataineh S, Gordon O, Luginbuehl R, Otto M, Textor M, Landmann R. 2009. Furanone at subinhibitory concentrations enhances staphylococcal biofilm formation by luxS repression. Antimicrob Agents Chemother 53:4159–4166. doi: 10.1128/AAC.01704-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claessens J, Roriz M, Merckx R, Baatsen P, Van Mellaert L, Van Eldere J. 2015. Inefficacy of vancomycin and teicoplanin in eradicating and killing Staphylococcus epidermidis biofilms in vitro. Int J Antimicrob Agents 45:368–375. doi: 10.1016/j.ijantimicag.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Sivaranjani M, Prakash M, Gowrishankar S, Rathna J, Pandian SK, Ravi AV. 2017. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl Microbiol Biotechnol 101:3349–3359. doi: 10.1007/s00253-017-8231-7. [DOI] [PubMed] [Google Scholar]
- 18.Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channabasappa S, Durgaiah M, Chikkamadaiah R, Kumar S, Joshi A, Sriram B. 2018. Efficacy of novel antistaphylococcal ectolysin P128 in a rat model of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 62:e01358-. doi: 10.1128/AAC.01358-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul VD, Rajagopalan SS, Sundarrajan S, George SE, Asrani JY, Pillai R, Chikkamadaiah R, Durgaiah M, Sriram B, Padmanabhan S. 2011. A novel bacteriophage tail-associated muralytic enzyme (TAME) from phage K and its development into a potent antistaphylococcal protein. BMC Microbiol 11:226. doi: 10.1186/1471-2180-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez L, Martinez B, Zhou Y, Rodriguez A, Donovan DM, Garcia P. 2011. Lytic activity of the virion-associated peptidoglycan hydrolase HydH5 of Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88. BMC Microbiol 11:138. doi: 10.1186/1471-2180-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez D, Ruas-Madiedo P, Martinez B, Rodriguez A, Garcia P. 2014. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Zhang H, Bao H, Wang X, Wang R. 2017. The lytic activity of recombinant phage lysin LysKΔamidase against staphylococcal strains associated with bovine and human infections in the Jiangsu province of China. Res Vet Sci 111:113–119. doi: 10.1016/j.rvsc.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. 2017. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother 61:e02666-. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Zhang H, Wang J, Yu J, Wei H. 2017. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci Rep 7:40182. doi: 10.1038/srep40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu J, Liu X, Yang M, Li Y, Sun C, Lu R, Song J, Zhang Q, Lei L, Feng X, Du C, Yu H, Yang Y, Han W. 2013. Genomic characterization of lytic Staphylococcus aureus phage GH15: providing new clues to intron shift in phages. J Gen Virol 94:906–915. doi: 10.1099/vir.0.049197-0. [DOI] [PubMed] [Google Scholar]
- 27.Gu J, Liu X, Lu R, Li Y, Song J, Lei L, Sun C, Feng X, Du C, Yu H, Yang Y, Han W. 2012. Complete genome sequence of Staphylococcus aureus bacteriophage GH15. J Virol 86:8914–8915. doi: 10.1128/JVI.01313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu J, Xu W, Lei L, Huang J, Feng X, Sun C, Du C, Zuo J, Li Y, Du T, Li L, Han W. 2011. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol 49:111–117. doi: 10.1128/JCM.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu J, Feng Y, Feng X, Sun C, Lei L, Ding W, Niu F, Jiao L, Yang M, Li Y, Liu X, Song J, Cui Z, Han D, Du C, Yang Y, Ouyang S, Liu ZJ, Han W. 2014. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-hand-like” calcium-binding phage lysin. PLoS Pathog 10:e1004109. doi: 10.1371/journal.ppat.1004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Li D, Li X, Hu L, Cheng M, Xia F, Gong P, Wang B, Ge J, Zhang H, Cai R, Wang Y, Sun C, Feng X, Lei L, Han W, Gu J. 2016. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci Rep 6:29344. doi: 10.1038/srep29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia F, Li X, Wang B, Gong P, Xiao F, Yang M, Zhang L, Song J, Hu L, Cheng M, Sun C, Feng X, Lei L, Ouyang S, Liu ZJ, Li X, Gu J, Han W. 2016. Combination therapy of LysGH15 and apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus. Appl Environ Microbiol 82:87–94. doi: 10.1128/AEM.02581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Rubio L, Martinez B, Rodriguez A, Donovan DM, Garcia P. 2012. Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 HydH5 virion-associated peptidoglycan hydrolase: fusions, deletions, and synergy with LysH5. Appl Environ Microbiol 78:2241–2248. doi: 10.1128/AEM.07621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jado I, Lopez R, Garcia E, Fenoll A, Casal J, Garcia P, Spanish Pneumococcal Infection Study Network. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother 52:967–973. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- 35.Linden SB, Zhang H, Heselpoth RD, Shen Y, Schmelcher M, Eichenseher F, Nelson DC. 2015. Biochemical and biophysical characterization of PlyGRCS, a bacteriophage endolysin active against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol 99:741–752. doi: 10.1007/s00253-014-5930-1. [DOI] [PubMed] [Google Scholar]
- 36.Son JS, Lee SJ, Jun SY, Yoon SJ, Kang SH, Paik HR, Kang JO, Choi YJ. 2010. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl Microbiol Biotechnol 86:1439–1449. doi: 10.1007/s00253-009-2386-9. [DOI] [PubMed] [Google Scholar]
- 37.Schmelcher M, Shen Y, Nelson DC, Eugster MR, Eichenseher F, Hanke DC, Loessner MJ, Dong S, Pritchard DG, Lee JC, Becker SC, Foster-Frey J, Donovan DM. 2015. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother 70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cater K, Dandu VS, Bari SM, Lackey K, Everett GF, Hatoum-Aslan A. 2017. A novel staphylococcus podophage encodes a unique lysin with unusual modular design. mSphere 2(2):e00040-. doi: 10.1128/mSphere.00040-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández L, Gonzalez S, Campelo AB, Martinez B, Rodriguez A, Garcia P. 2017. Downregulation of autolysin-encoding genes by phage-derived lytic proteins inhibits biofilm formation in Staphylococcus aureus. Antimicrob Agents Chemother 61:e02724-16. doi: 10.1128/AAC.02724-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Y, Ryu S. 2017. Characterization of a novel cell wall binding domain-containing Staphylococcus aureus endolysin LysSA97. Appl Microbiol Biotechnol 101:147–158. doi: 10.1007/s00253-016-7747-6. [DOI] [PubMed] [Google Scholar]
- 41.Gu J, Zuo J, Lei L, Zhao H, Sun C, Feng X, Du C, Li X, Yang Y, Han W. 2011. LysGH15 reduces the inflammation caused by lethal methicillin-resistant Staphylococcus aureus infection in mice. Bioeng Bugs 2:96–99. doi: 10.4161/bbug.2.2.14883. [DOI] [PubMed] [Google Scholar]
- 42.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. doi: 10.1128/CMR.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larkin EA, Carman RJ, Krakauer T, Stiles BG. 2009. Staphylococcus aureus: the toxic presence of a pathogen extraordinaire. Curr Med Chem 16:4003–4019. doi: 10.2174/092986709789352321. [DOI] [PubMed] [Google Scholar]
- 44.Ghebremedhin B, Layer F, Konig W, Konig B. 2008. Genetic classification and distinguishing of Staphylococcus species based on different partial gap, 16S rRNA, hsp60, rpoB, sodA, and tuf gene sequences. J Clin Microbiol 46:1019–1025. doi: 10.1128/JCM.02058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iorio NL, Azevedo MB, Frazao VH, Barcellos AG, Barros EM, Pereira EM, de Mattos CS, dos Santos KR. 2011. Methicillin-resistant Staphylococcus epidermidis carrying biofilm formation genes: detection of clinical isolates by multiplex PCR. Int Microbiol 14:13–17. [DOI] [PubMed] [Google Scholar]
- 46.State Council of the People's Republic of China. 1988. Regulations for the administration of affairs concerning experimental animals. State Council of the People's Republic of China, Beijing, China. [Google Scholar]
- 47.Pastagia M, Schuch R, Fischetti VA, Huang DB. 2013. Lysins: the arrival of pathogen-directed anti-infectives. J Med Microbiol 62:1506–1516. doi: 10.1099/jmm.0.061028-0. [DOI] [PubMed] [Google Scholar]
- 48.Saising J, Dube L, Ziebandt AK, Voravuthikunchai SP, Nega M, Gotz F. 2012. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 56:5804–5810. doi: 10.1128/AAC.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Zhao X, Zhu C, Xia X, Qin W, Li M, Wang T, Chen S, Xu Y, Hang B, Sun Y, Jiang J, Richard LP, Lei L, Zhang G, Hu J. 2017. Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of Actinobacillus pleuropneumoniae strain L20. Vet Microbiol 203:202–210. doi: 10.1016/j.vetmic.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Park E, Kim GD, Go MS, Kwon D, Jung IK, Auh JH, Kim JH. 2017. Anti-inflammatory effects of Nelumbo leaf extracts and identification of their metabolites. Nutr Res Pract 11:265–274. doi: 10.4162/nrp.2017.11.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmeck B, Moog K, Zahlten J, van Laak V, N′Guessan PD, Opitz B, Rosseau S, Suttorp N, Hippenstiel S. 2006. Streptococcus pneumoniae induced c-Jun-N-terminal kinase- and AP-1-dependent IL-8 release by lung epithelial BEAS-2B cells. Respir Res 7:98. doi: 10.1186/1465-9921-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.