No vaccine is licensed for enterotoxigenic Escherichia coli (ETEC) strains, which remain a leading cause of diarrhea in children from developing countries and international travelers. GM1-binding heat-labile toxin (LT) which is a key virulence factor of ETEC diarrhea is a strong vaccine antigen and a self-adjuvant. LT can also serve a backbone or platform for MEFA (multiepitope fusion antigen), a newly developed structural vaccinology technology, to present heterogeneous epitopes (by replacing LT epitopes) and to mimic epitope antigenicity for development of broadly protective vaccines. Data from this study identified neutralizing LT epitopes and demonstrated that substitution of LT epitopes eliminated LT enterotoxicity without altering GM1-binding activity, suggesting LT is potentially a versatile MEFA platform to present heterogeneous epitopes for multivalent vaccines against ETEC and other pathogens.
KEYWORDS: ETEC (enterotoxigenic Escherichia coli), LT (heat-labile toxin), epitope, vaccine platform
ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) strains producing heat-labile toxin (LT) and/or heat-stable toxin (STa) are a top cause of children's diarrhea and travelers' diarrhea. Holotoxin-structured GM1-binding LT is a strong immunogen and an effective adjuvant, and can serve a carrier or a platform for multivalent vaccine development. However, the significance of peptide domains or epitopes of LT particularly enzymatic LTA subunit in association with LT enterotoxicity and immunogenicity has not been characterized. In this study, we identified B-cell epitopes in silico from LTA subunit and examined epitopes for immunogenicity and association with LT enterotoxicity. Epitopes identified from LTA subunit were individually fused to a modified chicken ovalbumin carrier protein, and each epitope-ovalbumin fusion was used to immunize mice. Data showed all 11 LTA epitopes were immunogenic; epitope 7 (105SPHPYEQEVSA115) induced greater titers of anti-LT antibodies which neutralized LT enterotoxicity more effectively. To examine these epitopes for the significance in LT enterotoxicity, we constructed LT mutants by substituting each of 10 epitopes at the toxic A1 domain of LTA subunit with a foreign epitope and examined LT mutants for enterotoxicity and GM1-binding activity. Data showed that LT mutants exhibited no enterotoxicity but retained GM1-binding activity. The results from this study indicated that while not all immunodominant LTA epitopes were neutralizing, LT mutants with an individual epitope substituted lost enterotoxicity but retained GM1-binding activity. These results provided additional information to understand LT immunogenicity and enterotoxicity and suggested the potential application of LT platform for multivalent vaccines against ETEC diarrhea and other diseases.
IMPORTANCE No vaccine is licensed for enterotoxigenic Escherichia coli (ETEC) strains, which remain a leading cause of diarrhea in children from developing countries and international travelers. GM1-binding heat-labile toxin (LT) which is a key virulence factor of ETEC diarrhea is a strong vaccine antigen and a self-adjuvant. LT can also serve a backbone or platform for MEFA (multiepitope fusion antigen), a newly developed structural vaccinology technology, to present heterogeneous epitopes (by replacing LT epitopes) and to mimic epitope antigenicity for development of broadly protective vaccines. Data from this study identified neutralizing LT epitopes and demonstrated that substitution of LT epitopes eliminated LT enterotoxicity without altering GM1-binding activity, suggesting LT is potentially a versatile MEFA platform to present heterogeneous epitopes for multivalent vaccines against ETEC and other pathogens.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) strains continue to be a leading cause of children's diarrhea and travelers' diarrhea (1–4). Currently, there is no vaccine licensed for ETEC diarrhea (4–7). ETEC bacteria produce colonization factor antigen (CFA) or coli surface antigen (CS) adhesins and enterotoxins. CFA and CS adhesins facilitate ETEC bacteria to attach to host cell receptors and to colonize small intestines; enterotoxins, including heat-labile toxin (LT) and heat-stable type 1b toxin (STa), elevate intracellular cyclic AMP or cGMP levels in host epithelial cells to cause water and fluid hypersecretion and watery diarrhea (8–10).
LT (LT-I) produced by ETEC is a typical AB5 holotoxin and binds GM1 receptor (9, 11–13). The enzymatic A subunit (LTA) consists of an A1 domain and an A2 domain. The A1 peptide is ADP-ribosylating; the A2 peptide noncovalently associates the A subunit to the GM1-binding B subunit (LTB) pentamer to form the AB5 holotoxin structure. LT, detoxified LT mutants, and the nontoxic B subunit are strong immunogens to induce anti-LT antibodies; thus, they are often targeted as antigens for ETEC vaccine development (14–16). Interestingly, LT and LT mutants are also effective mucosal and parenteral adjuvants (17–23). In addition, LT, LTB, and derivatives can serve carrier proteins to facilitate or to up-immunoregulate immunogenicity of carrying proteins and peptides (24–30).
Recently, MEFA (multiepitope fusion antigen) technology has been developed to design structure-based multivalent vaccines (31–35). Aided with protein computational modeling and molecular dynamics stimulation (35), this MEFA technology applies a special backbone protein to present multiple foreign epitopes and to mimic epitope native antigenicity, thus having a single protein to induce protective antibodies against multiple heterogeneous virulence factors. The strongly immunogenic, GM1-binding, and self-adjuvant LT or LT mutants become an ideal backbone or platform for the MEFA technology. By applying a nontoxic and GM1-binding LT backbone to present a STa toxoid (19 amino acids in length) and epitopes of the most important CFA adhesins (i.e., the other ETEC virulence determinants), ideally through substitution of LTA subunit epitopes, we can develop a broadly protective mucosal vaccine against ETEC diarrhea.
However, before LT is applied as a MEFA backbone or platform for ETEC vaccine development, epitopes of LT particularly the enzymatic LTA subunit domain A1 (LTA1) need to be characterized for the significance associated with LT immunogenicity and enterotoxicity. While antigenic elements from the toxic LTA subunit need to be included in an ETEC vaccine to induce antibodies neutralizing LT enterotoxicity, a nontoxic GM1-binding LT is preferred as a safe antigen to induce systemic and mucosal immunity against ETEC diarrhea. If this safe GM1-binding LT backbone can also induce neutralizing antibodies against LT enterotoxicity, it becomes ideal for ETEC mucosal vaccine development. In this study, we designed experiments to identify immunodominant and neutralizing B-cell epitopes from LTA subunit and to investigate the significance of each epitope in LT enterotoxicity and GM1-binding activity. The results from this study should improve our understanding of LT immunogenicity and enterotoxicity and provide useful information to assess the application of LT as a versatile MEFA backbone or platform for multivalent mucosal vaccine development.
RESULTS
Eleven continuous B-cell epitopes were in silico identified from LTA subunit.
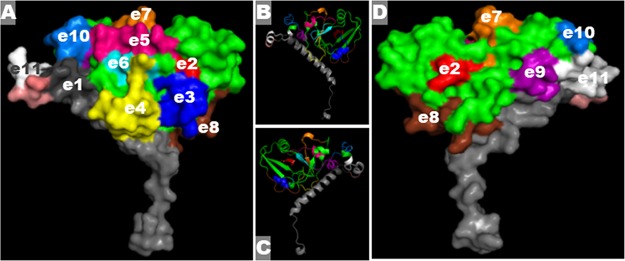
Eleven continuous B-cell epitopes were identified from LTA subunit, ranging from 10 to 13 amino acid residues in length (Table 1). Ten epitopes (e2 to e11) were identified from the enzymatic LT A1 domain (indicated in green in Fig. 1), and one epitope (e1) was located at the LT A2 domain (indicated in gray in Fig. 1). Computation modeling using Phyre2 (36) showed all epitopes were surface exposed (Fig. 1A and D). All predicted epitopes were located at α-helix or β-sheet of protein secondary structure (Fig. 1B and C). This LTA protein model was deposited under PDB accession no. 1LTA at the protein database.
TABLE 1.
LTA subunit continuous B-cell epitopes were identified from epitope prediction programsa
| Epitope | aa sequence | Position (aa) | Length (aa) | CT counterpart epitope sequence |
|---|---|---|---|---|
| e1 | TITGDTCNEETQ | 193–204 | 12 | SSMSTCDEKTQ |
| e2 | NGDKLYRADSR | 1–11 | 11 | NDDKLYRADSR |
| e3 | DSRPPDEIKRSGG | 9–21 | 13 | DSRPPDEIKQSGG |
| e4 | RGHNEYFDRGTQ | 25–36 | 12 | RGQSEYFDRGTQ |
| e5 | YDHARGTQTG | 42–51 | 10 | YDHARGTQTG |
| e6 | RYDDGYVSTS | 54–63 | 10 | RHDDGYVSTS |
| e7 | SPHPYEQEVSA | 105–115 | 11 | SPHPDEQEVSA |
| e8 | HRNREYRDRY | 140–149 | 10 | HRNRGYRDRY |
| e9 | APAEDGYRLAG | 156–166 | 11 | APAADGYGLAG |
| e10 | AGFPPDHQAWREE | 165–177 | 13 | AGFPPEHRAWREE |
| e11 | HHAPQGCGNSSRT | 181–193 | 13 | HHAPPGCGNAPRS |
Epitope amino acid sequences, positions, and lengths are indicated. The epitope prediction programs used are described elsewhere (44, 45). CT counterpart epitopes are also included; heterogeneous residues are indicated by underlining. The sequences of LT and CT were published previously by Holmes et al. (52). aa, amino acids.
FIG 1.
In silico epitope identification and protein computational modeling to show epitopes of the A subunit of LT (LTA). (A and D) The front (A) and back (D) of the LTA protein model show the LTA subunit structure and 11 LTA epitopes (in different colors). Green, LTA1 domain backbone; gray, LTA2 domain. (B and C) Secondary structure of LTA subunit, with identified epitopes in the same colors used in panels A and D.
Epitope-ovalbumin fusion proteins reacted with anti-LT antibodies.
Each LTA epitope was genetically fused to a modified chicken ovalbumin gene (37), by replacing an ovalbumin epitope, for epitope-ovalbumin fusions (Fig. 2A). DNA sequencing confirmed that LTA epitopes were correctly inserted into the modified chicken ovalbumin gene. Computational modeling indicated each LTA epitope was surface exposed, and epitope replacement did not appear to alter the ovalbumin protein overall structure or protein antigenic propensity. All recombinant epitope-ovalbumin fusion proteins expressed in pET28a and E. coli BL21(DE3) were 6×His tag-less, at the expected size of 34 kDa (Fig. 2B).
FIG 2.
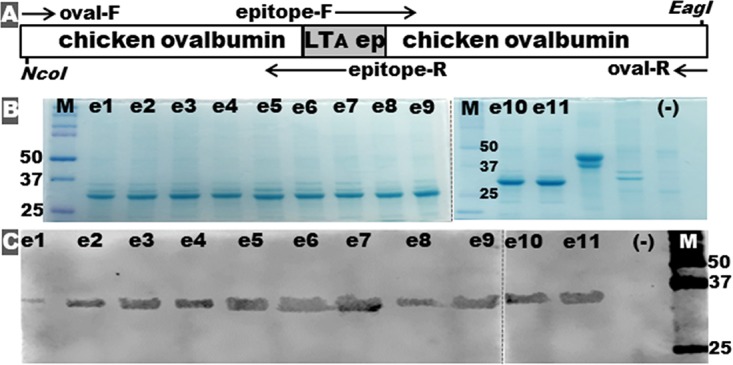
Construction and detection of epitope-ovalbumin fusion proteins. (A) Illustration of the genetic structure of an epitope-ovalbumin fusion gene, with PCR primers used to insert each LTA epitope into the intron-truncating chicken ovalbumin gene. (B) SDS-PAGE Coomassie blue staining shows each extracted epitope-ovalbumin fusion protein (e1 to e11). (C) Western blot with anti-LT antiserum to characterize each epitope-ovalbumin fusion protein (e1 to e11). Note that e1 to e11 represent epitope-ovalbumin fusion proteins carrying the LTA subunit epitope e1, e2, e3, e4, e5, e6, e7, e8, e9, e10, or e11. “(−)” refers to proteins of host strain E. coli BL21-CodonPlus (DE3). M, molecular marker (Bio-Rad Precision Plus Protein Dual Color Standards), with the 25-, 37-, and 50-kDa bands marked.
All 11 epitope-ovalbumin fusion proteins reacted with anti-LT antiserum. Western blot using anti-LT antiserum as the primary antibody showed each epitope-ovalbumin fusion was recognized (Fig. 2C). When they were immobilized on wells of enzyme-linked immunosorbent assay (ELISA) plates, epitope-ovalbumin fusion proteins reacted with anti-LT antiserum at a similar level, indicated by similar optical density (OD) readings. Interestingly, anti-CT (cholera toxin) antiserum reacted with epitope-ovalbumin fusions differently, likely because of the heterogeneity of LT epitopes and CT counterpart epitopes (Table 1). Western blotting showed anti-CT reacted strongly with e3-ovalbumin and e4-ovalbumin fusion proteins (fusion proteins carrying the LTA epitope 3 or 4) and moderately with e7-ovalbumin fusion, but weakly reacted with the rest of the epitope fusions. Anti-CT antiserum reacted with all epitope-ovalbumin fusion proteins immobilized in wells of ELISA plates, with greater reactivity with e3-ovalbumin and e4-ovalbumin fusions.
Mice immunized with epitope-ovalbumin fusion proteins developed anti-LT antibodies.
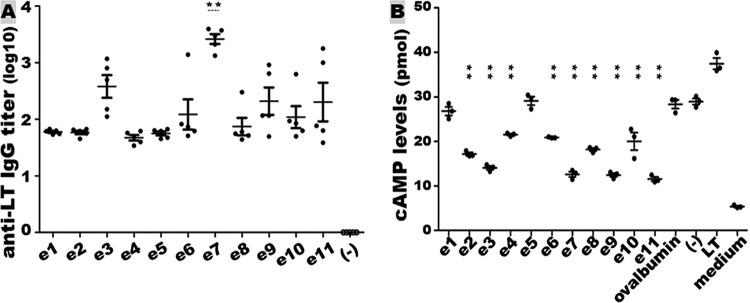
Mice subcutaneously immunized with each epitope-ovalbumin fusion protein developed antibody response to LT (Fig. 3A). The anti-LT IgG titers (in log10) in the groups immunized with the fusion proteins carrying epitope e1, e2, e3, e4, e5, e6, e7, e8, e9, e10, or e11 were 1.78 ± 0.04, 1.77 ± 0.07, 2.58 ± 0.45, 1.67 ± 0.11, 1.75 ± 0.08, 2.08 ± 0.60, 3.42 ± 0.20, 2.13 ± 0.56, 2.24 ± 0.62, 2.04 ± 0.44, or 2.30 ± 0.76, respectively. The group immunized with the fusion carrying epitope 7 (e7) developed serum anti-LT IgG titers significantly greater than the other immunization groups (P ≤ 0.01). Serum samples from the mice immunized with ovalbumin protein or phosphate-buffered saline (PBS) had no anti-LT antibody response detected.
FIG 3.
Mouse serum anti-LT IgG antibody titration and neutralization. (A) Anti-LT IgG titers (log10) from the group subcutaneously immunized with the epitope-ovalbumin fusion protein (e1 to e11) or the control group immunized with protein carrier ovalbumin (−). Bars indicate the mean titer in each group. e1 to e11 represent the groups of mice immunized with fusion proteins carrying different LTA1 epitopes. Each solid dot refers to an IgG titer from each mouse immunized with the epitope-ovalbumin fusion protein, and empty cycles for the titer of the control mice. **, P < 0.01 compared to all other immunized groups. (B) Mouse serum antibody in vitro neutralization activity against LT enterotoxicity. Mouse serum samples from the group immunized with each epitope-ovalbumin fusion protein (e1 to e11) and from two control groups immunized with ovalbumin protein (ovalbumin) or PBS (−) mixed with 20 ng of LT were incubated with T-84 cells. T-84 cell intracellular cAMP levels were measured with an EIA cAMP kit (Enzo Life Science). Portions (20 ng) of LT directly were added to T-84 cells to show enterotoxicity in the stimulation of cAMP levels in T-84 cells. Inclusion of T-84 cells in cell culture medium (without LT or serum) was to show baseline cAMP levels. “**” (in the vertical scale) indicates P < 0.01, when the cAMP levels in T-84 cells incubated with LT exposed to the immunized mouse sera were compared to the cAMP levels in T-84 cells incubated with LT exposed to the serum of the group immunized with ovalbumin or PBS.
Mouse serum samples from the groups immunized with epitope-ovalbumin fusions except the fusions carrying e1 (e1 located at the nonenzymatic LT A2 domain) or e5 significantly prevented LT from stimulating intracellular cyclic AMP (cAMP) in T-84 cells (Fig. 3B), indicating antibody neutralization activities against LT enterotoxicity. The cAMP levels in T-84 cells incubated with 20 ng of LT exposed to the serum sample of mice immunized with e1-ovalbumin or e5-ovalbumin fusion were 27.6 ± 1.7 or 28.5 ± 2.2 (pmol/ml), respectively. These cAMP levels were not significantly different compared to the levels in T-84 cells incubated with LT exposed to the sera of mice immunized with PBS (29.8 ± 1.2 pmol/ml; P = 0.10, P = 0.58) or ovalbumin protein (29.3 ± 1.1 pmol/ml; P = 0.17, P = 0.73). The cAMP levels in T-84 cells incubated with LT exposed to the sera of mice immunized with e2-ovalbumin (17.3 ± 1.7 pmol/ml), e3-ovalbumin (13.7 ± 1.1 pmol/ml), e4-ovalbumin (21.7 ± 0.7 pmol/ml), e6-ovalbumin (20.9 ± 1.4 pmol/ml), e7-ovalbumin (12.2 ± 0.9 pmol/ml), e8-ovalbumin (18 ± 0.7 pmol/ml), e9-ovalbumin (12.2 ± 0.7 pmol/ml), e10-ovalbumin (21.9 ± 1.1 pmol/ml), or e11-ovalbumin (11.1 ± 0.7 pmol/ml) were significantly lower than the cAMP in the cells treated with LT exposed to the serum from mice immunized with PBS (P < 0.01) or ovalbumin (P < 0.01).
LT mutants with each LTA1 epitope substituted by a foreign epitope retained AB5 holotoxin structure and GM1-binding ability.
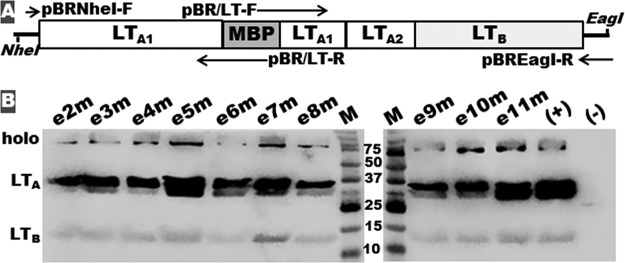
LT mutants were produced by replacing nucleotides coding each LTA1 epitope of native eltAB genes with nucleotides coding maltose-binding protein (MBP) epitope KDAQTNSSS (Fig. 4A). All 10 LT mutants formed AB5 holotoxin structure. Western blotting with anti-LT antiserum detected LTB subunit, LTA subunit, and holotoxin mutant proteins from each mutant strain and control CT, but not host strain DH5α (Fig. 4B).
FIG 4.
Illustration of the construction and detection of LT mutants e2m to e11m and LT with LTA1 epitope e2, e3, e4, e5, e6, e7, e8, e9, e10, or e11 substituted by the MBP epitope KDAQTNSSS. (A) LT mutant genetic structure and chimeric gene construction. Primers pBR/LT-F and pBR/LT-R were specific for the substitution of each LTA1 epitope with the MBP epitope. (B) Western blot with anti-LT antiserum to detect LTB subunit, mutated LTA subunit, and AB5 holotoxin-structured LT mutants. Lanes: (+), LT from recombinant LT strain 8460; (−), proteins from host strain E. coli DH5α. M, molecular marker, in kilodaltons.
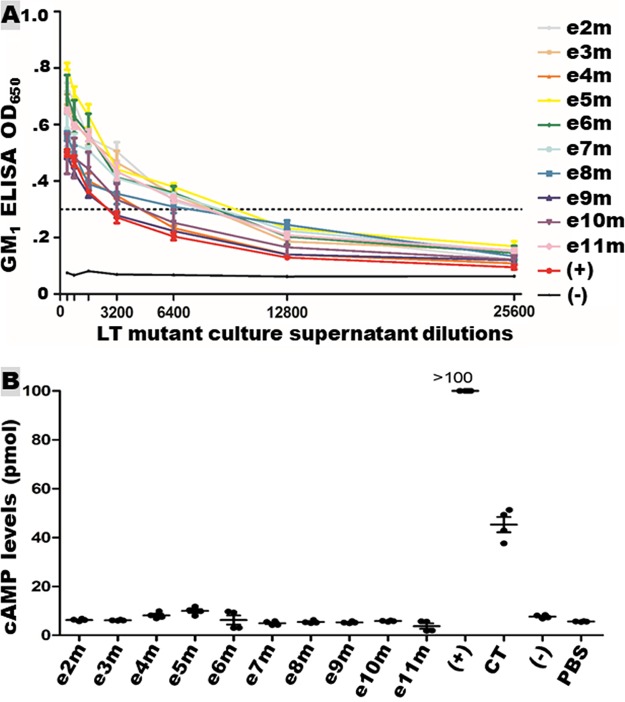
Holotoxin-structured LT mutants were secreted across outer membrane. LT mutant proteins recovered from bacterial culture filtrates bound GM1 (Fig. 5A). GM1 ELISA using antibodies specific to LTA subunit detected holotoxin-structured GM1-binding LT proteins from each mutant strain. The ELISA OD values were similar to the OD detected from the culture filtrate of LT recombinant strain 8460. GM1-binding activity was not detected from proteins of host strain DH5α.
FIG 5.
Holotoxin-structured LT mutant protein secretion and enterotoxicity. (A) GM1 ELISA to show LT mutant protein across outer membrane secretion and GM1-binding activity. Overnight culture filtrates of LT mutants e2m to e11m (LT toxin with epitope e2, e3, e4, e5, e6, e7, e8, e9, e10 or e11 substituted with MBP epitope KDAQTNSSS), positive strain 8460, and negative-control strain E. coli DH5α were used in GM1 ELISA. Mouse antiserum specific to LTA detected only the LTA peptide linked to LTB pentamer (the A subunit of AB5 LT mutants). (B) LT mutant protein enterotoxicity detected in T-84 cells using an EIA cAMP kit (Enzo Life Sciences). Overnight growth filtrates (75 μl) from each LT mutant (across outer membrane secreted LT mutant proteins), positive-control strain 8460, or negative-control strain E. coli DH5α were added to T-84 cells for stimulation of intracellular cAMP levels (pmol). Portions (10 ng) of CT and PBS were added to T-84 cells to show CT enterotoxicity in elevating cAMP and baseline cAMP levels, respectively.
LT mutants with an LTA1 epitope substituted showed no LT enterotoxicity.
LT showed no enterotoxicity in vitro after each individual LTA1 epitope substituted with the MBP epitope. T-84 cells incubated with overnight growth filtrates of each LT mutant strain showed no elevation of intracellular cAMP levels (Fig. 5B). The overall cAMP levels in T-84 cells treated with 10 LT mutant filtrates were 6.2 ± 2.2 pmol/ml, which were not different than the baseline cAMP levels in cells treated with PBS, which were 5.6 ± 0.1 pmol/ml (P = 0.10). In contrast, a significant elevation of cAMP levels was detected in T-84 cells incubated with 10 ng of CT (P < 0.001) or the filtrates of LT-producing E. coli strain 8460 (P < 0.001) (Fig. 5B).
DISCUSSION
Epitopes from LTA subunit particularly the A1 domain were investigated for the significance in LT immunogenicity and enterotoxicity in this study. While LT A2 peptide associates LTA subunit to the LTB pentamer to form LT AB5 holotoxin and LTB subunits (pentamer) bind GM1 receptor, LT A1 peptide is the enzymatic domain responsible for LT enterotoxicity. Consequently, while antibodies specific to LT A2 peptide and LTB subunit may disrupt the formation of LT holotoxin or block LT binding to host receptor GM1, it is antibodies derived from the A1 peptide that are expected to directly neutralize LT enterotoxicity. Data from the present study showed that while all in silico-identified epitopes from the LT A1 peptide (when inserted in chicken ovalbumin protein) reacted with anti-LT antiserum and induced anti-LT antibodies in subcutaneously immunized mice, epitopes 3, 7, 9, and 11 induced greater anti-LT IgG antibody responses. Among them, epitope 7 was significantly effective at inducing anti-LT IgG antibody. In vitro antibody neutralization study has indicated that while antibodies derived from all epitopes except e1 and e5 showed neutralizing activity, antibodies derived from epitopes 3, 7, 9, and 11 were more effective in neutralizing LT enterotoxicity. Antibodies derived from epitope 1 (e1) did not show any neutralizing activity against LT enterotoxicity, confirming that A2 peptide plays no role in LT enzymatic activity and that antibodies derived from A2 do not neutralize LT enterotoxicity. It remained unclear why antibodies induced by immunodominant epitope 5 showed no neutralizing activity. This may suggest that immunodominant epitopes are not necessarily neutralizing epitopes. It needs to be pointed out that the present study used the same volume of mouse serum from each group in an LT enterotoxicity neutralization assay; thus, the greater neutralization activity against LT from antibodies derived from fusions with 3, 7, 9, and 11 epitopes may result from higher anti-LT antibody titers. Future studies using standardized anti-LT antibodies or the same amount of mouse serum anti-LT antibodies (e.g., μg) from each group or in vivo studies may measure better anti-LT neutralizing activities.
Results from this study suggested that LT, if serving as a carrier protein, can have any of the LT A1 epitopes potentially substituted with foreign epitopes. However, if LT is also used as an antigen for ETEC vaccine development, epitopes 7, 9, 11, and/or 3 need to be retained to induce LTA1-specific antibodies to neutralize LT enterotoxicity. Data from this study showed that a replacement of any one of the LTA1 epitopes with a foreign epitope resulted in a complete loss of LT enterotoxicity in vitro. It was reported that substitution of a single amino acid residue at certain regions of the LT A1 peptide can significantly affect LT enterotoxicity (38). Indeed, LT mutated at R7K, V53D(E), S61F, S63K, A72R, V97K(Y), Y104K(D/S), E110D, E112K, S114K(E), or R192G showed abolition or reduction of toxicity (38–42). Replacing an epitope (10 to 13 amino acid residues) can alter LTA subunit binding to NAD, thus abolishing LT ribosylating and enterotoxic activities, particularly for epitopes 2, 6, 7, and 11 that are at or near the NAD binding site. Future in vivo studies will help to confirm or better characterize the enterotoxicity of these LT epitope mutants.
Data also indicated that LT mutants, after having an individual epitope substituted with a foreign epitope, still formed an AB5 holotoxin structure. It was reported that certain individual amino acid residues have a significant effect on LT holotoxin structure formation or stability (38, 43). While LT mutants R7K, V53D, S63K, V97K, and Y104K formed a stable holotoxin structure, with only a reduction in holotoxin yield (43), mutants with a mutation at other residues, for example, L41F, A45Y or A45E, V60G, and H70P, showed a poor assembly of AB5 structure (38). Data from the present study showed that all epitope mutants formed an AB5 holotoxin structure. However, this study did not quantitatively measure mutant AB5 holotoxin stability. Western blotting examining periplasm proteins suggested variations in the yield of LTA subunit and holotoxin structured LT mutant proteins (Fig. 4B). GM1 ELISA to analyze proteins across the outer membrane indicated that all epitope mutants secreted AB5 LT proteins at a similar level.
GM1 ELISA to examine LT proteins across the outer membrane suggested that LT with an individual epitope substituted remained bound to the GM1 receptor. Since the eltA gene coding the LTA subunit and the eltB gene coding the LTB subunit have individual promoters and ribosome-binding sites, the two elt genes transcribe and translate independently. In addition, the B subunits assembled into the LTB pentamer and noncovalently associated with the LTA subunit to form LT holotoxin in bacterial periplasm. Without altering the eltB gene structure, all LT epitope mutants were expected to produce LTB subunits and LTB pentamer, just like native LT. GM1 ELISA using antibodies specific to the LTA subunit in the present study detected only the LT proteins which were assembled in the AB5 structure, secreted across the outer membrane, and bound to the GM1 receptor.
A nontoxic, GM1-binding LT molecule secreted across the outer membrane can be an ideal protein carrier and an antigen delivery vehicle for epitopes from heterogeneous virulence factors to target host mucosal immunity. Such a molecule can serve a versatile MEFA (multiepitope fusion antigen) platform for the development of multivalent mucosal vaccines against enteric or other pathogens. MEFA is a structure-based vaccine technology to carry and to present multiple heterogeneous epitopes to induce broad antibody responses. Currently, this MEFA technology is mainly applied to produce chimeric proteins for development of multivalent parenteral vaccines. With outer membrane secretion and GM1-binding ability, this LT-platform MEFA can be used for mucosal vaccine development in general. The LT epitope mutants examined in this study had a single epitope substituted with a foreign epitope; future studies using multiple epitopes simultaneously replaced by different foreign epitopes are needed to confirm a stable AB5 structure and GM1-binding activities. MEFA technology using protein modeling and molecule dynamics simulation can optimize epitope substitution for stable protein structure (35). Indeed, a tripartite LT mutant, which had LT segments replaced by porcine ETEC adhesin K88 and F18 subunit domains, not only formed the AB5 holotoxin structure and bound GM1 but also induced antigen-specific protective mucosal immunity (29). A recent study showed that replacing LTA1 epitopes 2, 4, 5, 6, 8, 9, and 10 with seven epitopes from pig ETEC fimbrial adhesins K88 and F18, STb, Stx2e, and STa toxoid (with epitope 3 and 7 as LT antigens to induce antibodies neutralizing LT enterotoxicity) did not affect holotoxin formation, protein secretion, or binding to GM1 (data not shown). Replacing these LTA1 epitopes with epitopes of human ETEC CFA adhesins (CFA/I, CS1-CS6) and an STa toxoid, we can develop a multivalent ETEC vaccine to target small intestinal mucosal immunity. Similarly, by replacing LT A1 epitopes with neutralizing epitopes from the virulence factors of other enteric pathogens, effective multivalent vaccines can be developed against different enteric diseases.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 2. Recombinant strain 9511 which carries intron-truncated chicken ovalbumin gene (37) was used as the template to construct epitope-ovalbumin fusion genes. Vector pET28a (Novagen, Madison, WI) was used to clone epitope-ovalbumin fusion genes; E. coli strain BL21-CodonPlus (DE3) (Agilent Technologies, Santa Clara, CA) was used to express recombinant epitope-ovalbumin fusion proteins. Recombinant E. coli strains were cultured in Luria broth (LB) medium supplemented with kanamycin (30 μg/ml).
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| BL21-CodonPlus (DE3) | F− ompT hsdS(rB− mB−) gal dcm | Agilent Technologies |
| 9511 | 3×STa-chicken ovalbumin fusion + pET28α in DH5α Kan+ | 37 |
| 9529 | e1-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9546 | e2-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9550 | e3-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9547 | e4-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9551 | e5-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9552 | e6-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9548 | e7-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9543 | e8-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9553 | e9-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9544 | e10-ovalbumin + pET28α in BL21 Kan+ | This study |
| 9549 | e11-ovalbumin + pET28α in BL21 Kan+ | This study |
| 8460 | eltAB genes + pBR322 in TOP10, LT recombinant strain | 25 |
| 9646 | LT mutant with e2 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9647 | LT mutant with e3 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9648 | LT mutant with e4 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9649 | LT mutant with e5 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9650 | LT mutant with e6 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9651 | LT mutant with e7 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9652 | LT mutant with e8 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9653 | LT mutant with e9 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9654 | LT mutant with e10 substituted with MBP epitope, in DH5α Amp+ | This study |
| 9655 | LT mutant with e11 substituted with MBP epitope, in DH5α Amp+ | This study |
| Plasmids | ||
| pBR322 | Promega | |
| pMAL-c5X | NEB | |
| pET28a | Novagen |
LT mutants were constructed by using LT recombinant strain 8460 (25), which had the native eltAB genes cloned in pBR322 (Promega, Madison, WI) to express holotoxin-structured GM1-binding LT toxin, as the template. Mutant LT genes with nucleotides coding each LTA1 epitope substituted with MBP epitope KDAQTNSSS were cloned in pBR322 vector and expressed in E. coli strain DH5α (Promega).
LTA epitope in silico identification and protein computational modeling.
LTA subunit B-cell continuous epitopes were predicted using B-cell epitope prediction software (44, 45). Peptides that have the highest antigenicity scores were identified as epitopes. Protein modeling was carried out by using Phyre2 online server (36) based on amino acid sequences; the secondary structure stability of epitope-ovalbumin fusion proteins was assessed using CHARMM (46), as previously described (35).
Epitope-ovalbumin fusion gene construction.
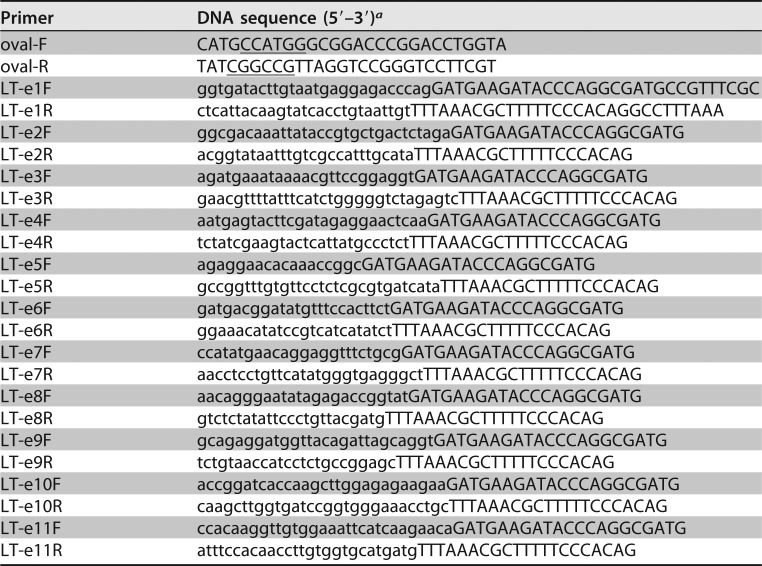
Nucleotide fragments coding each in silico identified LTA epitope were genetically fused to a modified chicken ovalbumin gene which had introns truncated (37), by using splicing overlap extension (SOE) PCR and specific primers (Table 3). Two PCR products amplified with the primers oval-F/epitope-R and epitope-F/oval-R (primers epitope-F and epitope-R were specific to each LTA epitope) were overlapped. Each overlapped fragment was digested with restriction enzymes EagI and NcoI (New England BioLabs, Ipswich, MA). Digested epitope-ovalbumin products were ligated into expression vector pET28α and expressed as tag-less proteins in E. coli strain BL21-CodonPlus (DE3).
TABLE 3.
PCR primers used to construct epitope-ovalbumin fusions in this study
aUnderlined nucleotides indicate NcoI and EagI restriction sites; nucleotides in lowercase are from each LTA epitope.
Epitope-ovalbumin fusion protein expression and characterization.
Epitope-ovalbumin fusion proteins that carried each LTA epitope (e1 to e11) were expressed and purified as described previously (33, 34, 37). Briefly, a single colony from each epitope-ovalbumin fusion recombinant strain was cultured in 5 ml of LB medium supplemented with kanamycin (30 μg/ml) at 37°C overnight. Next, 2 ml of overnight bacterial culture was added to 200 ml of 2× yeast extract tryptone (Fisher Scientific, Waltham, MA) medium supplemented with kanamycin (30 μg/ml) and cultured at 37°C. After the culture OD reached 0.5 to 0.7, bacteria were induced with 30 μM IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma, St. Louis, MO) and cultured for four more hours. Bacteria pellets collected from centrifugation (12,000 rpm for 15 min) were suspended in 10 ml of bacterial protein extraction reagent (B-PER; Thermo Fisher Scientific, Rochester, NY) to collect inclusion body proteins according to the manufacturer's protocol.
Extracted inclusion body proteins after three to five washes with PBS were solubilized and refolded by following a protein refolding protocol (Novagen). Briefly, each extracted epitope-ovalbumin fusion protein was mixed with 1× IB solubilization buffer {50 mM CAPS [3-(cyclohexylamino)propanesulfonic acid] [pH 11.0]} supplemented with 0.3% N-lauroylsarcosine and 1 mM dithiothreitol (DTT), followed by incubation at room temperature for 40 min. The suspension was centrifuged at 12,000 rpm for 20 min at room temperature to collect solubilized proteins (in supernatant). Proteins in supernatant were transferred to molecular porous membrane tubing (Spectrum Laboratories, Inc., Rancho Dominguez, CA), refolded using dialysis buffer (20 mM Tris-HCl [pH 8.5]) supplemented with 0.1 mM DTT at 4°C for 3 to 4 h, dialyzed in dialysis buffer without DTT with two to three buffer changes, and collected and stored at −80°C.
Portions (10 μg) of each refolded epitope-ovalbumin fusion protein were examined by using sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis. Electrophoresed proteins were visualized with Coomassie blue straining and characterized in a Western blot using anti-LT mouse serum or anti-CT rabbit polyclonal antiserum (Sigma) and IRDye-labeled goat anti-mouse or anti-rabbit IgG secondary antibodies (1:10,000 dilution; LI-COR, Lincoln, NE), as described previously (31, 47, 48).
The immunodominance of each recombinant epitope-ovalbumin fusion protein was examined by ELISA with anti-LT or anti-CT antiserum. Each fusion protein (500 ng per well) was used to coat wells of Immulon 2HB plates (Thermo Fisher Scientific) at 37°C for 1 h, followed by 4°C overnight. Wells were incubated with 10% skim milk (in PBS) at 37°C for 1 h, followed by three washes with PBS–0.05% Tween 20 (PBST). Wells were incubated with 2-fold-diluted anti-LT mouse serum or anti-CT rabbit serum (1:200 to 1:12,800) at 37°C for 1 h. After three washes with PBST, 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse or goat anti-rabbit IgG (Sigma; 1:3,000) was added to each well. Wells were incubated at 37°C for 1 h, washed with PBST, and combined with 100 μl of TMB (3,3′,5,5′-tetramethylbenzidine) Microwell peroxidase substrate (KPL, Gaithersburg, MD) at room temperature for 30 min. The OD650 was recorded to measure the epitope reactivity with anti-LT or anti-CT antiserum.
Epitope-ovalbumin fusion protein mouse immunization.
Eight-week-old female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA), five per group, were subcutaneously immunized with each epitope-ovalbumin fusion protein. Portions (40 μg) of fusion protein (in 25 μl of PBS) mixed with 25 μl of Montanide ISA 51 VG (Seppic, Fairfield, NJ) were injected into each mouse. Two control groups were included: one immunized with the modified chicken ovalbumin protein (40 μg in 25 μl) and ISA adjuvant (25 μl) and the other immunized with sterile PBS (50 μl). Immunized mice received two boosters at the same dose of the primary in an interval of 2 weeks. Mice were euthanized 2 weeks after the second booster. The mouse immunization study complied with the Animal Welfare Act by following the 1996 National Research Council guidance and was approved by the Kansas State University Institutional Animal Care and Use Committee (IACUC 3800).
Epitope-ovalbumin fusion-induced anti-LT IgG antibody titration.
The anti-LT IgG antibody response was examined from each mouse serum sample as described previously (32, 47, 48). Briefly, LT (a gift from John Clements, Tulane University; 200 ng per well) was coated in wells of Immulon 2HB 96-well plates (Thermo Fisher Scientific). Wells washed with PBST were incubated with 10% skim milk at 37°C for 1 h to block uncoated sites, followed by three washes with PBST and incubation with 2-fold-diluted mouse serum samples (1:400 to 1:25,600). Wells were washed with PBST, incubated with HRP-conjugated goat anti-mouse IgG secondary antibodies (1:3,000; Sigma), and then washed with PBST and incubated with TMB Microwell peroxidase substrate (KPL). OD650 readings were used to calculate anti-LT antibody titers, as previously described (32, 33, 47–49).
Epitope-ovalbumin fusion-induced anti-LT antibody neutralization assay.
Serum samples pooled from five mice in each group were used for an antibody neutralization assay with T-84 cells (American Type Culture Collection [ATCC], Fairfax, VA) and an EIA cAMP kit (Enzo Life Sciences, Inc., Farmingdale, NY) as described previously (24, 25, 32, 47). Briefly, 30 μl of serum sample pooled from each immunization group or the control group (in duplicate) was incubated with 20 ng of LT for 30 min at room temperature. Each serum-toxin mixture was transferred to T-84 cells (105 cells per well) cultured in Dulbecco's modified Eagle medium-F12 (DMEM-F12) medium (ATCC) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) in a 24-well microplate (Corning, Inc., Corning, NY). After incubation for 3 h in a CO2 incubator, the T-84 cells were lysed. Collected cell lysates were measured for intracellular cAMP levels (pmol or pmol/ml) with an EIA cAMP kit according to the manufacturer's protocol (Enzo Life Sciences). LT alone (without serum) was used as a positive control to show the LT enterotoxicity in stimulation of cAMP levels in T-84 cells, and culture medium only (without toxin or serum) was used as a control to show the T-84 cell baseline intracellular cAMP levels.
LT mutant construction and expression.
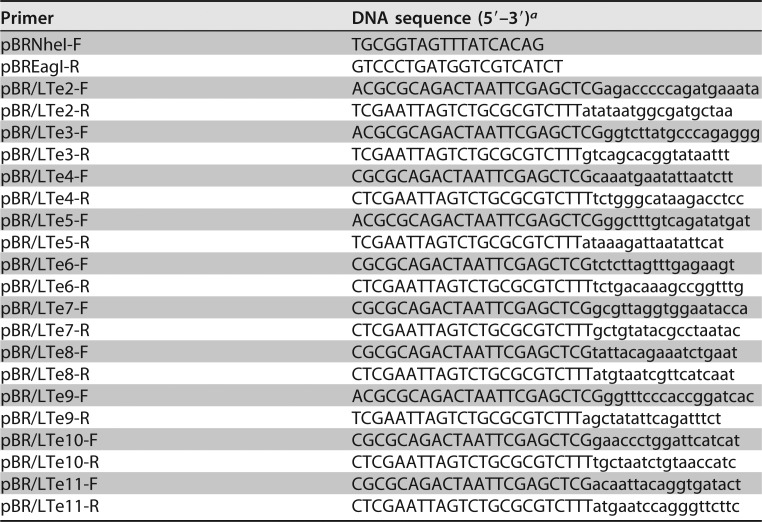
Native eltAB genes cloned in pBR322 from recombinant strain 8460 (Table 2) and specifically designed primers (Table 4) were used in PCRs to replace each LTA1 epitope with MBP epitope KDAQTNSSS for LT mutants. SOE PCR to overlap two fragments, one amplified with the primers pBRNheI-F and pBR/LT-R and the other amplified with the primers pBR/LT-F and pBREagI-R, generated mutated LT genes (Fig. 4). Primers pBR/LT-R and pBR/LT-F were specific for the replacement of each epitope (Table 4). Overlapped PCR products were digested with NheI and EagI restriction enzymes; digested products were ligated into pBR322 with T4 DNA ligase (New England BioLabs).
TABLE 4.
PCR primers used to mutate eltAB genes by replacing nucleotides coding each LTA1 epitope with maltose-binding protein epitope KDAQTNSSS in SOE PCRs
aNucleotides in uppercase from the pBT/LT forward or reverse primers are from the MBP epitope; nucleotides in lowercase are from the eltA gene (flanking each LTA1 epitope).
The expression of each mutant LT was verified by SDS-PAGE Western blotting with anti-LT antiserum. Overnight growth culture pellets from each LT mutant strain, positive strain 9460 and negative-control strain DH5α, were used to extract total proteins with B-PER. Portions (20 μl) of the total proteins from each strain were used for SDS-PAGE. Recombinant LT strain 8460 and cholera toxin (CT; Sigma) were included as positive controls. Anti-LT serum (1:3,000; a gift from John Clements at Tulane University) was used as the primary antibody, and IRDye-labeled goat anti-mouse IgG (1:10,000; LI-COR) was used as the secondary antibody.
LT mutant secretion and GM1-binding activity in GM1 ELISA.
GM1 ELISA was used to examine LT mutant protein secretion and GM1-binding activity, as described previously (50, 51). Since GM1 (Sigma) was used as the ELISA coating antigen and antibodies specific to LTA subunit were used as the primary antibodies, only the A subunit of AB5 LT mutants (which bind GM1) could be detected. Briefly, 100 μl of monosialo ganglioside GM1 (4 μg/ml) was used to coat each well of 96-well Maxisorb plates (Nunc, Roskilde, Denmark) overnight at 4°C, followed by incubation with PBST–10% skim milk to block the uncoated sites. After three washes with PBST, wells were incubated with 100 μl of overnight growth filtrates from each LT mutant strain, positive control 8460, or negative-control strain DH5α. After incubation at 37°C for 1 h, the wells were incubated with 2-fold-diluted anti-LTA mouse polyclonal antibodies at 37°C for 1 h. After washes with PBST, each well was incubated with 100 μl of HRP-conjugated goat anti-mouse IgG (1:3,000 dilution; Sigma). Wells were washed with PBST and PBS and incubated with TMB Microwell peroxidase substrate to measure the OD650 readings.
LT mutant enterotoxicity assay.
The enterotoxicity of each LT mutant was examined using a cAMP EIA kit (Enzo Life Sciences) and T-84 cells as described above. Seventy-five microliter overnight growth filtrates from each LT mutant strain, positive strain 8460, or negative strain DH5α (in duplicate) were added to T-84 cells (105) in 24-well plates. Ten nanograms of CT (in 75 μl of PBS) and 75 μl of PBS were included in the assay as a positive control and a negative control, respectively. After 3 h of incubation in a CO2 incubator, the T-84 cells were lysed with 200 μl (per well) of 0.1 M HCl. Cell lysates were measured for intracellular cAMP levels as described above.
Statistical analysis.
Data were analyzed using Prism 5 software (GraphPad, La Jolla, CA). The results are presented as means and standard deviations. Differences between compared groups were calculated with one-way analysis of variance with a confidence interval of 95%. Calculated P values of <0.05 were considered significantly different between groups.
Accession number(s).
The LTA protein model was deposited in the Protein Data Bank under PDB accession no. 1LTA.
ACKNOWLEDGMENTS
We thank John Clements at Tulane University for providing LT, anti-LT, and anti-LTA antisera.
NIH 1R01AI121067 and Kansas State University provided financial support for this study. The funders had no role in study design, data collection, or interpretation.
REFERENCES
- 1.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque AS, Saha D, Sow SO, Sur D, Zaidi AK, Biswas K, Panchalingam S, Clemens JD, Cohen D, Glass RI, Mintz ED, Sommerfelt H, Levine MM. 2012. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study Collaborators. 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang ZD, DuPont HL. 2017. Etiology of travellers' diarrhea. J Travel Med 24:S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 4.Hosangadi D, Smith PG, Kaslow DC, Giersing BK, WHO ETEC and Shigella Vaccine Consultation Expert Group. 2018. WHO consultation on ETEC and Shigella burden of disease, Geneva, 6-7th April 2017: meeting report. Vaccine doi: 10.1016/j.vaccine.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res 133:188–196. [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Sack DA. 2015. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli (ETEC) associated diarrhea. Clin Vaccine Immunol 22:983–991. doi: 10.1128/CVI.00224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss J, Richardson SH. 1978. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest 62:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spangler BD. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev 56:622–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hol WG. 1991. Crystal structure of a cholera toxin-related heat-labile enterotoxin from Escherichia coli. Nature 351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 12.Sixma TK, Kalk KH, van Zanten BA, Dauter Z, Kingma J, Witholt B, Hol WG. 1993. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol 230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 13.Merritt EA, Sixma TK, Kalk KH, van Zanten BA, Hol WG. 1994. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol Microbiol 13:745–753. doi: 10.1111/j.1365-2958.1994.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun 67:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker RI. 2005. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23:3369–3385. doi: 10.1016/j.vaccine.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie R, Bourgeois AL, Frech SA, Flyer DC, Bloom A, Kazempour K, Glenn GM. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684–3691. doi: 10.1016/j.vaccine.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, Clements JD, Nabors GS. 2010. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine 28:1404–1411. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach S, Clements JD, Kaim J, Lundgren A. 2012. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One 7:e51718. doi: 10.1371/journal.pone.0051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated Escherichia coli bacteria overexpressing colonization factors CFA/I, CS3, CS5, and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Sjokvist Ottsjo L, Flach CF, Clements J, Holmgren J, Raghavan S. 2013. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun 81:1532–1540. doi: 10.1128/IAI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Becerra FJ, Chen X, Dickenson NE, Choudhari SP, Harrison K, Clements JD, Picking WD, Van De Verg LL, Walker RI, Picking WL. 2013. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun 81:4470–4477. doi: 10.1128/IAI.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandre RM, Ruan X, Duan Q, Zhang W. 2016. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett 363:1–6. doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect Immun 79:4002–4009. doi: 10.1128/IAI.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan X, Liu M, Casey TA, Zhang W. 2011. A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection. Clin Vaccine Immunol 18:1593–1599. doi: 10.1128/CVI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Knudsen DE, Liu M, Robertson DC, Zhang W, The STa Toxoid Vaccine Consortium Group. 2013. Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTaA14Q-LTS63K/R192G/L211A in a murine model. PLoS One 8:e77386. doi: 10.1371/journal.pone.0077386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z, Lawson S, Langenhorst R, McCormick KL, Brunick C, Opriessnig T, Baker R, Yoon KJ, Zhang W, Huber VC, Fang Y. 2013. Construction and immunogenicity evaluation of an epitope-based antigen against swine influenza A virus using Escherichia coli heat-labile toxin B subunit as a carrier-adjuvant. Vet Microbiol 164:229–238. doi: 10.1016/j.vetmic.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Ruan X, Zhang W. 2013. Oral immunization of a live attenuated Escherichia coli strain expressing a holotoxin-structured adhesin-toxoid fusion (1FaeG-FedF-LTA2:5LTB) protected young pigs against enterotoxigenic E. coli (ETEC) infection. Vaccine 31:1458–1463. doi: 10.1016/j.vaccine.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Marchioro SB, Fisch A, Gomes CK, Jorge S, Galli V, Haesebrouck F, Maes D, Dellagostin O, Conceicao FR. 2014. Local and systemic immune responses induced by a recombinant chimeric protein containing Mycoplasma hyopneumoniae antigens fused to the B subunit of Escherichia coli heat-labile enterotoxin LTB. Vet Microbiol 173:166–171. doi: 10.1016/j.vetmic.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Ruan X, Knudsen DE, Wollenberg KM, Sack DA, Zhang W. 2014. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin Vaccine Immunol 21:243–249. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan X, Sack DA, Zhang W. 2015. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS One 10:e0121623. doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandre RM, Ruan X, Duan Q, Sack DA, Zhang W. 2016. Antibodies derived from an enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multiepitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21, and EtpA. Vaccine 34:3620–3625. doi: 10.1016/j.vaccine.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rausch D, Ruan X, Nandre RM, Duan Q, Hashish E, Casey TA, Zhang W. 2017. Antibodies derived from a toxoid MEFA (multiepitope fusion antigen) show neutralizing activities against heat-labile toxin (LT), heat-stable toxins (STa, STb), and Shiga toxin 2e (Stx2e) of porcine enterotoxigenic Escherichia coli (ETEC). Vet Microbiol 202:79–89. doi: 10.1016/j.vetmic.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan Q, Lee KH, Nandre RM, Garcia C, Chen J, Zhang W. 2017. MEFA (multiepitope fusion antigen)-novel technology for structural vaccinology, proof from computational and empirical immunogenicity characterization of an enterotoxigenic Escherichia coli (ETEC) adhesin MEFA. J Vaccines Vaccin 8:1000367. doi: 10.4172/2157-7560.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan QD, Zhang W. 2017. Genetic fusion protein 3xSTa-ovalbumin is an effective coating antigen in ELISA to titrate anti-STa antibodies. Microbiol Immunol 61:251–257. doi: 10.1111/1348-0421.12494. [DOI] [PubMed] [Google Scholar]
- 38.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana MR, Giuliani MM, Magagnoli C, Peppoloin S, Manetti R, Rappuoli R. 1994. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol 14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 39.Harford S, Dykes CW, Hobden AN, Read MJ, Halliday IJ. 1989. Inactivation of the Escherichia coli heat-labile enterotoxin by in vitro mutagenesis of the A-subunit gene. Euro J Biochem 183:311–316. doi: 10.1111/j.1432-1033.1989.tb14930.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji T, Inoue T, Miyama A, Okamoto K, Honda T, Miwatani T. 1990. A single amino acid substitution in the A subunit of Escherichia coli enterotoxin results in a loss of its toxic activity. J Biol Chem 265:22520–22525. [PubMed] [Google Scholar]
- 41.Lobet Y, Cluff CW, Cieplak W Jr. 1991. Effect of site-directed mutagenic alterations on ADP-ribosyltransferase activity of the A subunit of Escherichia coli heat-labile enterotoxin. Infect Immun 59:2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickinson BL, Clements JD. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun 63:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magagnoli C, Manetti R, Fontana MR, Giannelli V, Giuliani MM, Rappuoli R, Pizza M. 1996. Mutations in the A subunit affect yield, stability, and protease sensitivity of nontoxic derivatives of heat-labile enterotoxin. Infect Immun 64:5434–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen JE, Lund O, Nielsen M. 2006. Improved method for predicting linear B-cell epitopes. Immunome Res 2:2. doi: 10.1186/1745-7580-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha S, Raghava GPS. 2007. Prediction methods for B-cell epitopes. Methods Mol Biol 409:387–394. doi: 10.1007/978-1-60327-118-9_29. [DOI] [PubMed] [Google Scholar]
- 46.Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch Caflisch SA, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Laxaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M. 2009. CHARMM: the biomolecular simulation program. J Comput Chem 30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, The STa Toxoid Vaccine Consortium Group. 2014. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun 82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan Q, Huang J, Xiao N, Seo H, Zhang W. 2018. Neutralizing anti-STa antibodies derived from enterotoxigenic Escherichia coli (ETEC) toxoid fusions with heat-stable toxin (STa) mutant STaN12S, STaL9A/N12S, or STaN12S/A14T show little cross-reactivity with guanylin or uroguanylin. Appl Environ Microbiol 84:e00550-17. doi: 10.1128/AEM.01737-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nandre RM, Duan Q, Wang Y, Zhang W. 2016. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine 35:552–556. doi: 10.1016/j.vaccine.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berberov EM, Zhou Y, Francis DH, Scott MA, Kachman SD, Moxley RA. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect Immun 72:3914–3924. doi: 10.1128/IAI.72.7.3914-3924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Berberov EM, Freeling J, He D, Moxley RA, Francis DH. 2006. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect Immun 74:3107–3114. doi: 10.1128/IAI.01338-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes RK, Jobling MG, Connell TD. 1995. Cholera toxin and related enterotoxins of Gram-negative bacteria, p 225–255. In Moss J, Iglewski Vangham M, Tu AT (ed), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc, New York, NY. [Google Scholar]