ABSTRACT
Cytosolic DNA arising from intracellular pathogens is sensed by cyclic GMP-AMP synthase (cGAS) and triggers a powerful innate immune response. However, herpes simplex virus 1 (HSV-1), a double-stranded DNA virus, has developed multiple mechanisms to attenuate host antiviral machinery and facilitate viral infection and replication. In the present study, we found that HSV-1 tegument protein VP22 acts as an inhibitor of cGAS/stimulator of interferon genes (cGAS/STING)-mediated production of interferon (IFN) and its downstream antiviral genes. Our results showed that ectopic expression of VP22 decreased cGAS/STING-mediated IFN-β promoter activation and IFN-β production. Infection with wild-type (WT) HSV-1, but not VP22-deficient virus (ΔVP22), inhibited immunostimulatory DNA (ISD)-induced activation of the IFN signaling pathway. Further study showed that VP22 interacted with cGAS and inhibited the enzymatic activity of cGAS. In addition, stable knockdown of cGAS facilitated the replication of ΔVP22 virus but not the WT. In summary, our findings indicate that HSV-1 VP22 acts as an antagonist of IFN signaling to persistently evade host innate antiviral responses.
IMPORTANCE cGAS is very important for host defense against viral infection, and many viruses have evolved ways to target cGAS and successfully evade the attack by the immune system of their susceptible host. This study demonstrated that HSV-1 tegument protein VP22 counteracts the cGAS/STING-mediated DNA-sensing antiviral innate immunity signaling pathway by inhibiting the enzymatic activity of cGAS. The findings in this study will expand our understanding of the interaction between HSV-1 replication and the host DNA-sensing signaling pathway.
KEYWORDS: HSV-1, VP22, cGAS, DNA sensing
INTRODUCTION
The innate immune system is the first line of host defense against invading pathogens, which depends on various pathogen recognition receptors (PRRs). The PRRs detect structurally conserved pathogen-associated molecular patterns (PAMPs) generated during the pathogen life cycle and trigger the production of type I interferon (IFN-I) and other antiviral factors (1–3). Besides Toll-like receptors, nucleotide-binding oligomerization domain, leucine-rich repeat-containing receptors, RIG-I-like receptors, C-type lectin receptors, and AIM-2-like receptors (4, 5), a number of cytosolic DNA sensors have been identified and characterized, such as DNA-dependent activator of IFN regulatory factors, interferon gamma inducible protein 16, RNA polymerase III, DEAD box helicase 41, and cyclic GMP-AMP synthase (cGAS) (6–12). Among these DNA sensors, cGAS, which is a nucleotidyltransferase, is an important cytoplasmic sensor that recognizes various DNA ligands in different cell types (13–16). Upon binding to cytosolic double-stranded DNA, cGAS will be activated and utilize ATP and GTP to produce cyclic GMP-AMP (cGAMP) through its enzymatic activity (17–19). The cGAMP produced by cGAS activates stimulator of interferon genes (STING), which then recruits TANK-binding kinase 1 (TBK1), leading to the activation of interferon regulatory factor 3 (IRF3) and inducing the production of IFN-β (13, 14, 20).
Herpes simplex virus 1 (HSV-1) belongs to the alphaherpesvirus subfamily, with a large, linear double-stranded DNA that encodes over 80 proteins. HSV-1 causes an acute lytic infection and then establishes a lifelong latent infection in the trigeminal ganglia. HSV-1 is extremely successful in establishing an effective infection due to its capacity to counteract the host innate antiviral response (21, 22). During HSV-1 replication, viral DNA that leaks into the cytosol can be sensed by cGAS. Although the cytosolic DNA-sensing pathway is activated, HSV-1 has developed multiple mechanisms to attenuate host antiviral machinery and facilitate virus infection and replication. Our previous studies showed that HSV-1 UL41 could degrade cGAS mRNA (23). A recent study showed that in infection with HSV-1 d109, the level of cGAMP is below the limit of detection in infected cells, indicating that there might be at least one viral component capable of inhibiting cGAS enzymatic activity (24).
VP22, encoded by the UL49 gene, is a highly abundant tegument protein in HSV-1, and its amino acid sequence is conserved in the subfamily Alphaherpesvirinae. In infected cells, VP22 is phosphorylated, possibly by CKII and a viral protein kinase encoded by the UL13 gene. The phosphorylation of VP22 is involved in the regulation of incorporation into virions and in its dissociation from the tegument upon entry of the virus into the cell (25). Without VP22, the UL49-null virus reduces plaque size and causes a nearly global shutoff protein synthesis at late times in infection (26). These findings prove that VP22 plays a critical role in HSV-1 replication and pathogenesis. However, whether VP22 affects the host DNA-sensing pathway is unknown. In the present study, we found that VP22 acted as an important inhibitor of cGAS/STING-mediated production of IFN-β and downstream antiviral genes. Moreover, compared with VP22-deficient (ΔVP22) HSV-1 infection, wild-type (WT) HSV-1 infection could inhibit immunostimulatory DNA (ISD)-induced activation of the IFN signaling pathway. Further study showed that VP22 interacted with cGAS and inhibited the enzymatic activity of cGAS. In addition, stable knockdown of cGAS facilitated the replication of ΔVP22 virus but not the WT. In summary, these findings expand our understanding of evasion of host antiviral innate immune responses by HSV-1.
RESULTS
HSV-1 VP22 inhibits IFN-β activation induced by cGAS/STING.
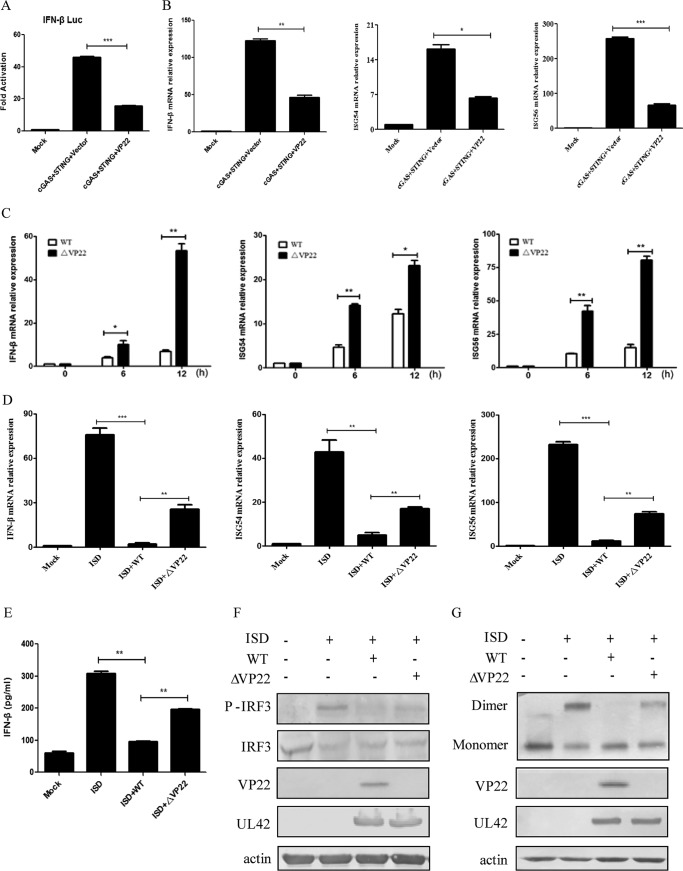
Previous studies from our laboratory and others showed that exogenous expression of a minimal amount of either cGAS or STING alone is unable to induce the activation of IFN-β in HEK293T cells and that the IFN-β promoter can be activated only when a minimal amount of cGAS and a trace amount of STING are cotransfected (13, 23, 27–29). To investigate the role of VP22 in the cGAS/STING-mediated IFN-β signaling pathway, HEK293T cells were cotransfected with cGAS-Flag, STING-hemagglutinin (STING-HA), pRL-TK, and IFN-β promoter construct along with empty vector or a Flag-tagged VP22 expression plasmid and subjected to dual-luciferase reporter (DLR) assays to detect IFN-β promoter activity. As shown in Fig. 1A, ectopic expression of VP22 could significantly inhibit the activation of the IFN-β promoter. Next, we validated the DLR result by measuring IFN-β mRNA levels through quantitative reverse transcription-PCR (qRT-PCR). As indicated in Fig. 1B, the expression of cGAS and STING activated the IFN-β by 45-fold. However, coexpression of VP22 could significantly inhibit this induction. Likewise, the VP22 protein suppressed the production of the ISG54 and ISG56 induced by cGAS/STING (Fig. 1B).
FIG 1.
HSV-1 VP22 inhibits IFN-β activation induced by cGAS/STING. (A) HEK293T cells were cotransfected with IFN-β promoter plasmid (IFN-β-Luc, 200 ng), Renilla luciferase (pRL-TK, 50 ng) reporter plasmid, cGAS-Flag (15 ng), and STING-HA (2.5 ng), along with empty vector (200 ng) or VP22-Flag plasmid (200 ng). Luciferase activity was measured 24 h posttransfection in the cell lysates. (B) HEK293T cells were cotransfected with cGAS-Flag and STING-HA, along with empty vector or VP22-Flag plasmid. At 24 h posttransfection, cells were harvested and subjected to qRT-PCR analysis. (C) Effects of VP22 deficiency on transcription of downstream antiviral genes. HFF cells were infected with WT (MOI = 1) or ΔVP22 (MOI = 1) virus for the indicated times before qRT-PCR analysis. (D and E) HFF cells were infected with WT or ΔVP22 virus at an MOI of 5 for 2 h, cell medium was then replaced by DMEM containing 10% FBS, and ISD (2 μg/ml) was transfected using Lipofectamine LTX according to the manufacturer's recommendations. Cells were harvested and subjected to qRT-PCR analysis at 7 h posttransfection (D) or ELISA analysis at 18 h posttransfection (E). (F and G) HFF cells were infected and transfected as described for panel D, and then cells were harvested at 7 h posttransfection and subjected to WB analysis to detect IRF3 phosphorylation (F) or native PAGE to detect IRF3 dimerization (G). The data represent results from one of triplicate experiments. Error bars represent the standard deviation of results of three independent experiments. Statistical analysis was performed using Student's t test with the GraphPad Prism 5.0 software. (*, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, P < 0.001).
Previously, it has been shown that human foreskin fibroblast (HFF) cells can express downstream antiviral genes in response to HSV-1 infection (24, 30). HFF cells contain the complete GAS/STING pathway, which is the major pathway activated by DNA transfection or DNA virus infection (30, 31). Our results indicated that IFN-β induction was significantly inhibited in cGAS stable knockdown HFF cells (HFF-shcGAS cells) under ISD transfection compared with that in HFF-shNC (negative control) cells (data not shown). To further confirm the role of VP22 in immune evasion of HSV-1, we generated ΔVP22 virus by CRISPR-Cas9 gene knockout technology. We examined the expression of downstream antiviral genes in HFF cells infected with WT or ΔVP22 virus, and qRT-PCR was performed to measure IFN-β, ISG54, and ISG56 mRNA accumulation. As shown in Fig. 1C, the mRNA levels of IFN-β, ISG54, and ISG56 induced by ΔVP22 virus were significantly higher than those induced by the WT in HFF cells from 6 to 12 h. These data indicate that VP22 downregulated the activation of the IFN pathway mediated by cGAS/STING.
ISD is a double-stranded DNA 60-mer oligonucleotide derived from the HSV-1 genome, and transfection of ISD into HFF cells can induce a robust production of IFN-β (10). To determine whether VP22 could affect the production of IFN-β induced by ISD, HFF cells were infected with WT or ΔVP22 virus for 2 h before ISD transfection. Then cells were harvested and subjected to qRT-PCR to analyze IFN-β, ISG54, and ISG56 mRNA. As shown in Fig. 1D, infection of HFF cells with WT but not ΔVP22 virus significantly inhibited ISD-induced accumulation of IFN-β, ISG54, and ISG56 mRNA, suggesting that VP22 inhibited the production of IFN-β induced by ISD. To further confirm this assertion, enzyme-linked immunosorbent assays (ELISAs) were performed to measure the secretion of IFN-β in the supernatant of HFF cells infected by WT or ΔVP22 virus at a multiplicity of infection (MOI) of 5, followed by transfection with ISD. We found that IFN-β was significantly decreased in cells infected with the WT, while ΔVP22 infection recovered IFN-β to a certain extent (Fig. 1E). Collectively, these results indicated that VP22 was capable of inhibiting the cGAS/STING-dependent DNA-sensing signaling pathway.
IRF3 is a crucial transcription factor in the IFN-β signaling pathway, and phosphorylation and dimerization of IRF3 are a hallmark of IFN-mediated early antiviral responses. We next examined the effect of VP22 on ISD-mediated activation of IRF3. HFF cells were infected with WT or ΔVP22 virus for 2 h before ISD transfection, and robust phosphorylation and dimerization of IRF3 were observed following ISD stimulation. We found that infection with WT virus abrogated ISD-induced phosphorylation of IRF3, while infection with ΔVP22 virus did not (Fig. 1F). Similarly, ISD-triggered IRF3 dimerization was markedly reduced under WT but not ΔVP22 infection (Fig. 1G). Taken together, those results indicate that VP22 inhibited IFN-β activation by the cGAS/STING pathway.
VP22 inhibits IFN-β signaling pathway at the level upstream of STING.
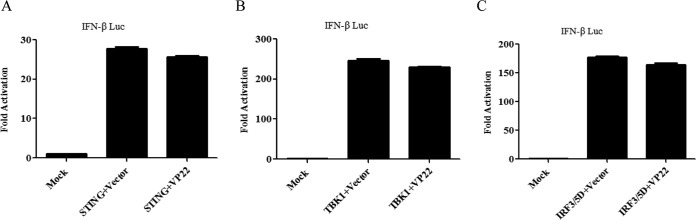
To determine at what level in the pathway VP22 blocks IFN-β activation, we cotransfected empty vector or VP22-expressing plasmids along with the IFN-β-Luc reporter and expression plasmids of cGAS/STING signaling pathway components, including STING, TBK1 kinase, and the active form of IRF3 (IRF3/5D) into HEK293T cells. Although ectopic expression of a minimal amount of STING alone failed to activate the IFN-β promoter, expression of a large amount of STING could induce IFN-β promoter activation (29, 32). All expression constructs resulted in a 27- to 251-fold induction of IFN-β-Luc reporter activity (Fig. 2A to C). We found that ectopic expression of VP22 did not affect the activation of the IFN-β promoter driven by STING, TBK1, and IRF3/5D (Fig. 2A to C). Collectively, those results suggested that VP22 inhibited the IFN signaling pathway at the level upstream of STING.
FIG 2.
VP22 inhibits the IFN signaling pathway at the level upstream of STING. (A to C) HEK293T cells were cotransfected with IFN-β-Luc reporter, pRL-TK, and STING (A), TBK1 (B), or IRF3/5D (C), along with empty vector or VP22-Flag plasmid. Cells were harvested 24 h after transfection and subjected to a DLR assay. The data represent results from one of triplicate experiments. Error bars represent the standard deviation of results of three independent experiments. Statistical analysis was performed using Student's t test with the GraphPad Prism 5.0 software.
VP22 interacts with cGAS and inhibits the enzymatic activity of cGAS.
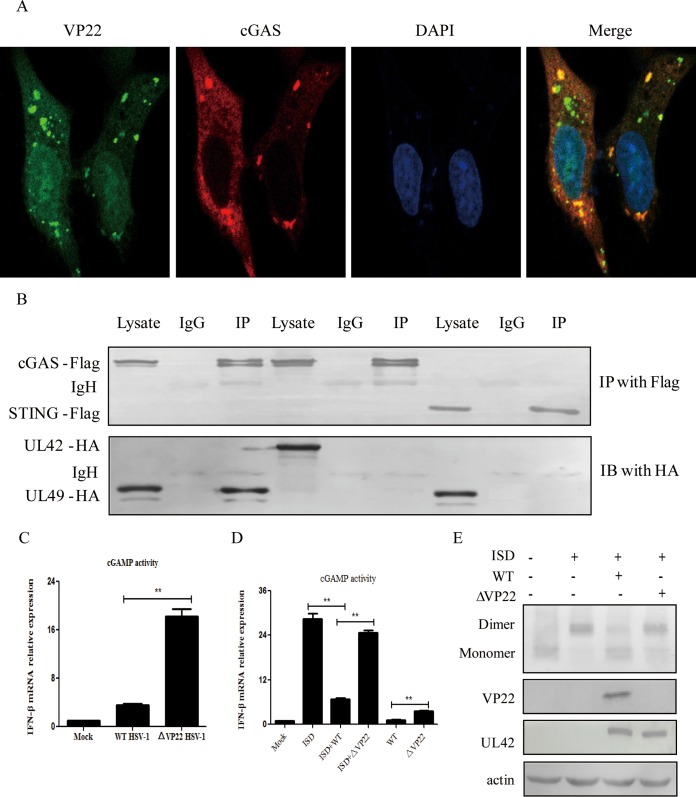
The aforementioned data led us to hypothesize that VP22 might act directly on cGAS. To explore this hypothesis, an indirect immunofluorescence assay was performed. HeLa cells were transfected with VP22-enhanced yellow fluorescent protein (VP22-EYFP) and cGAS-Flag expression plasmids, and localization of VP22 and cGAS was assessed by confocal microscopy. As shown in Fig. 3A, VP22 was colocalized with cGAS. Next, we analyzed the potential interaction between VP22 and cGAS. HEK293T cells were transfected with VP22-HA along with cGAS-Flag or STING-Flag, and coimmunoprecipitation (co-IP)-Western blot (WB) analysis was performed with anti-Flag and anti-HA antibodies. HSV-1 tegument protein UL42 was applied as a negative control to obviate nonspecific interactions. We found that the VP22 protein was immunoprecipitated by cGAS but not STING (Fig. 3B). These results demonstrated that VP22 interacted with cGAS.
FIG 3.
VP22 interacts with cGAS and inhibits the enzymatic activity of cGAS. (A) Colocalization of VP22 with cGAS. HeLa cells were transfected with VP22-EYFP and cGAS-Flag for 20 h, as indicated. Cells were stained with mouse anti-Flag MAb, and TRITC (tetramethyl rhodamine isothiocyanate)-conjugated goat anti-mouse antibody was used as the secondary antibody before confocal microscopy. DAPI, 4′,6-diamidino-2-phenylindole. (B) VP22 interacts with cGAS but not STING. HEK293T cells were transfected with VP22-HA along with cGAS-Flag or STING-Flag for 24 h. HEK293T cells were transfected with UL42-HA and cGAS-Flag as a negative control. The cells were lysed, and the extracts were subjected to IP with anti-Flag MAb or control IgG. Precipitates were analyzed by WB. IB, immunoblotting. (C and D) HFF cells were infected with WT or ΔVP22 virus at an MOI of 1 for 24 h (C) or were infected with WT or ΔVP22 virus at an MOI of 5 for 2 h before ISD transfection (D). Extracts from cells were prepared, DNase treated, heat treated, and incubated with permeabilized HFF cells for 6 h. Cells were then harvested and subjected to qRT-PCR analysis. (E) VP22 inhibits the dimerization of STING. HFF cells were infected with WT or ΔVP22 virus at an MOI of 5 for 2 h, cell medium was then replaced with DMEM containing 10% FBS, and ISD (2 μg/ml) was transfected using Lipofectamine LTX according to the manufacturer's recommendations; cells were then harvested at 7 h posttransfection and subjected to native PAGE to detect STING dimerization. The data represent results from one of triplicate experiments. Error bars represent the standard deviation of results of three independent experiments. Statistical analysis was performed using Student's t test with GraphPad Prism 5.0 software. (**, 0.001 < P < 0.01).
To define the interaction of cGAS and VP22 during innate sensing, we examined the production of cGAMP in HFF cells upon infection with WT or ΔVP22 virus for 24 h. cGAMP activity was measured in cell extracts by using a modified bioassay based on the original method of Orzalli et al. (24) in which the induction of IFN-β transcripts, as a marker of cGAMP activity, was analyzed in permeabilized secondary reporter cells (HFF cells). As shown in Fig. 3C, an increase in cGAMP activity was observed in HSV-1-infected HFF cells. However, the lack of VP22 in cells infected with ΔVP22 virus resulted in more robust cGAMP activity than in cells infected with the WT, indicating that the interaction of VP22 with cGAS reduced cGAMP production.
Next, to determine whether VP22 could affect the cGAMP induced by ISD, HFF cells were infected with WT or ΔVP22 virus for 2 h prior to ISD transfection. At 6 h, the induction of IFN-β transcripts in permeabilized secondary reporter cells was measured. As expected, transfection with ISD resulted in robust cGAMP activity. However, infection with WT but not ΔVP22 virus resulted in reduced cGAMP activity (Fig. 3D).
The cGAMP produced by cGAS activates STING, which then forms a dimer. So we analyzed the dimerization of STING as a marker of cGAMP activity. HFF cells were infected with WT or ΔVP22 virus for 2 h before ISD transfection, and robust dimerization of STING was observed following ISD stimulation. We found that infection with the WT abrogated ISD-induced dimerization of STING, while infection with ΔVP22 virus did not (Fig. 3E).
cGAS mediates the defense against the replication of ΔVP22 virus.
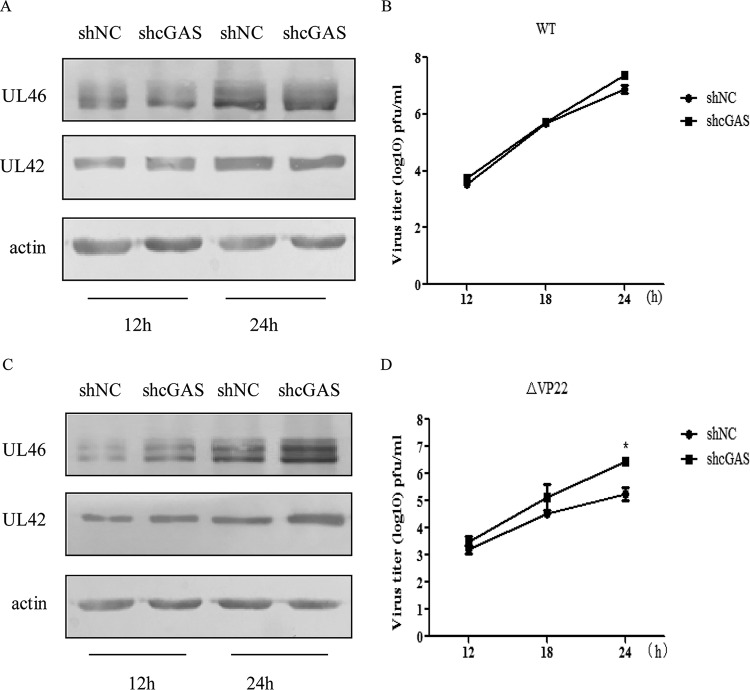
Previous studies have revealed that the activation of cGAS elicits a potent antiviral response, while the aforementioned data suggest that HSV-1 infection inhibits cGAS/STING-mediated activation of the IFN pathway by inhibiting cGAS enzymatic activity via VP22 (33, 34). Therefore, to better delineate the antiviral role of cGAS in HSV-1 replication, the stably transfected HFF-shNC and HFF-shcGAS cells were infected with WT or ΔVP22 virus and the virus was harvested at the indicated time points (see Fig. 4) for WB analysis and viral plaque assay. With WT infection, the expression levels of UL46 in HFF-shNC and HFF-shcGAS cells were similar, while the protein level of UL46 was increased in HFF-shcGAS cells compared to HFF-shNC cells upon ΔVP22 infection (Fig. 4A and C). Similarly, a viral plaque assay also demonstrated that knockdown of cGAS did not affect the replication of WT virus but facilitated the replication of ΔVP22 virus (Fig. 4B and D). Collectively, these data indicate that cGAS mediates the defense against the replication of ΔVP22 virus. The antiviral innate immunity mediated by cGAS against the WT is counteracted by VP22.
FIG 4.
cGAS mediates the defense against replication of ΔVP22 virus. (A to D) The stably transfected HFF-shNC and HFF-shcGAS cells were infected with WT or ΔVP22 virus at an MOI of 2, and then the virus was harvested at the indicated time points postinfection and subjected to WB analysis (A and C) or viral plaque assay on Vero cells (B and D). The data represent results from one of the triplicate experiments. Statistical analysis was performed using Student's t test with GraphPad Prism 5.0 software. (* 0.01 < P < 0.05).
DISCUSSION
HSV-1 has developed multiple mechanisms to evade host antiviral machinery and facilitate viral infection and replication. For example, US3, ICP0, VP16, UL42, UL24, and UL36USP have previously been proven to inhibit IFN production and NF-κB activation by targeting IRF3, p65, or IκBα (28, 35–39). UL46 and ICP27 inhibited IRF3 activation and IFN-I production by interacting with STING and TBK1 (40, 41). And UL41 counteracts the antiviral activity of viperin, ZAP, IFIT3, Tetherin, and Ch25h by targeting its mRNA for degradation (42–46). All the protein of HSV-1 may function cooperatively during HSV-1 infection. VP22, a highly abundant tegument protein in HSV-1, plays a critical role in HSV-1 replication and pathogenesis. However, the role of VP22 in the innate immune response of the host is still largely unknown.
cGAS, a recently discovered predominant cytosolic DNA sensor, recognizes cytosolic DNA and subsequently produces cGAMP, a second messenger that activates STING, and eventually, the series of events leads to IFN-β production (47). cGAS/STING-mediated activation of IFN pathway is very important in host antiviral responses. Several DNA viruses induce IFN-I in a cGAS/STING-dependent manner, including vaccinia virus, adenovirus, cytomegalovirus, Kaposi sarcoma-associated herpesvirus (KSHV), and hepatitis B virus (29, 48–50). cGAS is also a key sensor for retroviruses, including HIV-1 and HIV-2 (51, 52). Viral infection induces mitochondrial DNA (mtDNA) stress, which leads to the activation of antiviral innate immune responses through the cGAS/STING pathway (53, 54). Since cGAS is so important in the host's defense against viral infection, many viruses have evolved ways to target cGAS to successfully evade the attack by the immune system of their susceptible host. For example, the tegument protein ORF52 of KSHV directly interacts with and inhibits cGAS enzymatic activity (49). Human cytomegalovirus tegument protein pUL83 dampens IFN-I production by inactivating cGAS, but the molecular mechanisms have not been delineated (55). HSV-1 UL41 may partly degrade cGAS mRNA (23). A recent study showed that in HSV-1 d109-infected cells, the level of cGAMP is below the limit of detection, indicating that there might be at least one viral component capable of inhibiting cGAS enzymatic activity (24). In the present study, we demonstrated that HSV-1 tegument protein VP22 inhibited the cGAS/STING-mediated IFN-I signaling pathway by inhibiting the enzymatic activity of cGAS.
Several evidences suggest that VP22 inhibits IFN-β activation by the cGAS/STING pathway. First, ectopic expression of VP22 could significantly inhibit the activation of the IFN-β promoter. VP22 protein suppressed induction of the IFN-β, ISG54, and ISG56 mRNA induced by cGAS/STING. Second, the mRNA levels of IFN-β, ISG54, and ISG56 induced by ΔVP22 virus were significantly higher than those induced by the WT in HFF cells from 6 to 12 h. Third, WT HSV-1 infection could inhibit IFN-β transcription induced by ISD, in comparison with ΔVP22 infection. Similarly, ISD-triggered IRF3 phosphorylation and dimerization were markedly reduced under WT but not ΔVP22 infection.
In our study, we found that ectopic expression of VP22 did not significantly affect STING-, TBK1-, and IRF3/5D-mediated activation of the IFN-β promoter. Moreover, our data demonstrate that the HSV-1 tegument protein VP22 directly binds and inhibits cGAS enzymatic activity, leading to downregulation of IFN-β production. The functional relevance of the inhibitory activity of viral VP22 against cGAS can be inferred by the observation that infection with ΔVP22 virus results in a significant increase in cGAS activity, accompanied by a significant increase in IFN-β production.
In summary, we have shown here for the first time that HSV-1 tegument protein VP22 counteracts the cGAS/STING-mediated DNA-sensing pathway by inhibiting the enzymatic activity of cGAS. Infection with WT HSV-1, but not ΔVP22 HSV-1, could inhibit ISD-induced activation of the IFN signaling pathway. Moreover, stable knockdown of cGAS significantly facilitated replication of ΔVP22 virus but not the WT. Our findings in this study are important for understanding the interaction between HSV-1 replication and the host DNA-sensing signaling pathway.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
HEK293T, HFF, and Vero cell lines were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml of penicillin and streptomycin. The wild-type (WT) HSV-1 F strain was propagated in Vero cells and titrated as described previously (56). The protease inhibitor mixture cocktail was purchased from Thermo Fisher Scientific (MA, USA). Radioimmunoprecipitation assay (RIPA) lysis buffer was purchased from Beyotime (Shanghai, China). Mouse anti-HA and anti-Flag monoclonal antibodies (MAbs) were purchased from Abmart (Shanghai, China). Goat anti-cGAS polyclonal antibody (PAb) and mouse anti-β-actin MAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-IRF3, anti-STING, anti-TBK1, anti-UL46, and anti-VP22 PAbs were made by GL Biochem Ltd. (Shanghai, China). Phospho-IRF-3 (Ser396) rabbit MAb was purchased from Cell Signaling Technology (Danvers, MA, USA).
Plasmid construction.
All enzymes used for cloning procedures were purchased from Vazyme (Nanjing, China). Commercial reporter plasmid pRL-TK (RL stands for Renilla luciferase, and TK stands for thymidine kinase) was purchased from Promega Corporation (Madison, WI, USA). Other gift plasmids used include the following: cGAS-Flag (13), pcDNA3.1-Flag-TBK1 (57), IRF3/5D (58), and IFN-β promoter reporter plasmid (59).
CRISPR/Cas9-mediated genome editing of HSV-1.
Potential guide RNAs (gRNAs) targeting the VP22 gene were analyzed using the CRISPR design tool. Double-stranded oligonucleotides were cloned into the PX330-U6-chimeric-BB-CBh-hSpCas9 vector, cotransfected into HEK293T cells, and then infected with WT HSV-1 for 20 h. The VP22-deficient virus was purified by plaque assay in Vero cells. The VP22 gRNA target sequence is 5′-ACTCGTTGGCGCGCTGAAGC-3′.
Co-IP assay and WB analysis.
The coimmunoprecipitation (co-IP) assay and Western blot (WB) analysis were performed as previously described (36).
RNA isolation and qRT-PCR.
Total RNA was extracted using TRIzol (Invitrogen, California) according to the manufacturer's manual. Samples were digested with DNase I and subjected to reverse transcription as previously described. The cDNA was used as the template for qRT-PCR to test the levels of IFN-β, and 18S rRNA was used as an internal reference as previously described (42).
Transfection and dual-luciferase reporter (DLR) assays.
HFF cells were transfected with Lipofectamine LTX (Invitrogen, CA, USA) according to the manufacturer's recommendations. HEK293T cells were cotransfected with reporter plasmids, such as IFN-β-Luc and internal control plasmid pRL-TK, with or without expression plasmids, as indicated, by standard calcium phosphate precipitation (60). At 24 h posttransfection, luciferase assays were performed with a dual-specific luciferase assay kit (Promega, Madison, WI) as described in our previous studies (56, 61).
ELISA.
Concentrations of the IFN-β in cell culture supernatants were determined by using the VeriKine human interferon beta ELISA kit from PBL Assay Science (Piscataway, NJ, USA) according to the manufacturer's instructions.
Native PAGE.
Native polyacrylamide gel electrophoresis (PAGE) was performed using ReadyGels (7.5%; Bio-Rad) as described in our previous study (35). In brief, gels were prerun with 25 mM Tris and 192 mM glycine, pH 8.4, with 1% deoxycholate (DOC) in the cathode chamber for 30 min at 40 mA. Samples in native sample buffer (10 μg protein, 62.5 mM Tris-Cl [pH 6.8], 15% glycerol, and 1% DOC) were size fractionated by electrophoresis at 25 mA and transferred to nitrocellulose membranes for WB analysis.
cGAMP activity assay.
The cGAMP activity assay was performed as previously described (24, 55). Infected or transfected cells were washed with phosphate-buffered saline (PBS) and lysed in hypotonic buffer. Cells extracts were incubated with 1 kU/ml Benzonase for 30 min at 37°C. Cells extracts were then heated at 95°C for 5 min and centrifuged for 5 min at maximum speed (16,000 × g). HFF cells were used as reporter cells to measure cGAMP production. HFF cells were permeabilized as described previously (62), with modifications. Briefly, medium was aspirated from the HFF cells, and digitonin permeabilization solution was added to treated cell extracts. HFF reporter cells were incubated with extracts for 30 min at 37°C and then replaced with supplemented medium. RNA was harvested 6 h after the initial addition of extracts and qRT-PCR for IFN-β, as a marker of cGAMP activity, and 18S rRNA, as a housekeeping gene.
Statistical analysis.
Data are represented as the mean ± standard deviation (SD) where indicated, and Student's t test was used for all statistical analyses with GraphPad Prism 5.0 software. Differences between groups were considered significant when the P value was <0.05.
ACKNOWLEDGMENTS
We thank Zhijian J. Chen for the cGAS-Flag plasmid, Rongtuan Lin for the STING-HA plasmid, and Takashi Fujita for IFN-β-Luc. We also thank Yuhui Huang for providing the lab benches.
Work in the Zheng laboratory relevant to this article was supported by the National Natural Science Foundation of China (grants 81571974 and 81772182).
ADDENDUM IN PROOF
When our manuscript was under review, Maruzuru et al. (63) showed that VP22 had little effect on cGAS/STING-mediated antiviral innate immunity. The discrepancy could be due to differences in the amounts of cGAS/STING and in the choice of cell lines used.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Cunha A. 2012. Innate immunity: pathogen and xenobiotic sensing—back to basics. Nat Rev Immunol 12:400. doi: 10.1038/nri3237. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Hornung V. 2014. SnapShot: nucleic acid immune sensors, part 1. Immunity 41:868.e1. doi: 10.1016/j.immuni.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Unterholzner L. 2013. The interferon response to intracellular DNA: why so many receptors? Immunobiology 218:1312–1321. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Chen ZJ. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 9.Chiu YH, Macmillan JB, Chen ZJ. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. 2011. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X, Chiu YH, Chen ZJ. 2014. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, Patel DJ. 2013. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. 2013. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. 2013. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa H, Barber GN. 2011. The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell Mol Life Sci 68:1157–1165. doi: 10.1007/s00018-010-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C. 2018. Evasion of cytosolic DNA-stimulated innate immune responses by herpes simplex virus 1. J Virol 92:e00099-17. doi: 10.1128/JVI.00099-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su C, Zhan G, Zheng C. 2016. Evasion of host antiviral innate immunity by HSV-1, an update. Virol J 13:38. doi: 10.1186/s12985-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su C, Zheng C. 2017. Herpes simplex virus 1 abrogates the cGAS/STING-mediated cytosolic DNA-sensing pathway via its virion host shutoff protein, UL41. J Virol 91:e02414-16. doi: 10.1128/JVI.02414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orzalli MH, Broekema NM, Diner BA, Hancks DC, Elde NC, Cristea IM, Knipe DM. 2015. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc Natl Acad Sci U S A 112:E1773–E1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison EE, Wang YF, Meredith DM. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol 72:7108–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy C, Mbong EF, Baines JD. 2009. VP22 of herpes simplex virus 1 promotes protein synthesis at late times in infection and accumulation of a subset of viral mRNAs at early times in infection. J Virol 83:1009–1017. doi: 10.1128/JVI.02245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Su C, Zheng C. 2016. Herpes simplex virus 1 serine protease VP24 blocks the DNA-sensing signal pathway by abrogating activation of interferon regulatory factor 3. J Virol 90:5824–5829. doi: 10.1128/JVI.00186-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Su C, Pearson A, Mody CH, Zheng C. 2017. Herpes simplex virus 1 UL24 abrogates the DNA sensing signal pathway by inhibiting NF-kappaB activation. J Virol 91:e00025-. doi: 10.1128/JVI.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Z, Jacobs SR, West JA, Stopford C, Zhang Z, Davis Z, Barber GN, Glaunsinger BA, Dittmer DP, Damania B. 2015. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau L, Gray EE, Brunette RL, Stetson DB. 2015. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 350:568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 31.Fu YZ, Su S, Gao YQ, Wang PP, Huang ZF, Hu MM, Luo WW, Li S, Luo MH, Wang YY, Shu HB. 2017. Human cytomegalovirus tegument protein UL82 inhibits STING-mediated signaling to evade antiviral immunity. Cell Host Microbe 21:231–243. doi: 10.1016/j.chom.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, Garcia-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, Zuo X, Kao CC, Herr AB, Li P. 2013. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39:1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J Virol 87:12814–12827. doi: 10.1128/JVI.02355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Wang K, Wang S, Zheng C. 2013. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. J Virol 87:12935–12948. doi: 10.1128/JVI.01952-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-kappaB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Wang S, Wang K, Zheng C. 2013. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. Med Microbiol Immunol 202:313–325. doi: 10.1007/s00430-013-0295-0. [DOI] [PubMed] [Google Scholar]
- 39.Ye R, Su C, Xu H, Zheng C. 2017. Herpes simplex virus 1 ubiquitin-specific protease UL36 abrogates NF-kappaB activation in DNA sensing signal pathway. J Virol 91:e02417-. doi: 10.1128/JVI.02417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deschamps T, Kalamvoki M. 2017. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus 1. J Virol 91:e00535-. doi: 10.1128/JVI.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen MH, Jensen SB, Miettinen JJ, Luecke S, Prabakaran T, Reinert LS, Mettenleiter T, Chen ZJ, Knipe DM, Sandri-Goldin RM, Enquist LW, Hartmann R, Mogensen TH, Rice SA, Nyman TA, Matikainen S, Paludan SR. 2016. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J 35:1385–1399. doi: 10.15252/embj.201593458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen G, Wang K, Wang S, Cai M, Li ML, Zheng C. 2014. Herpes simplex virus 1 counteracts viperin via its virion host shutoff protein UL41. J Virol 88:12163–12166. doi: 10.1128/JVI.01380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su C, Zhang J, Zheng C. 2015. Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA. Virol J 12:203. doi: 10.1186/s12985-015-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J Virol 87:13115–13123. doi: 10.1128/JVI.02167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Z, Su C, Zheng C. 2016. Herpes simplex virus 1 tegument protein UL41 counteracts IFIT3 antiviral innate immunity. J Virol 90:11056–11061. doi: 10.1128/JVI.01672-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You H, Yuan H, Fu W, Su C, Wang W, Cheng T, Zheng C. 2017. Herpes simplex virus type 1 abrogates the antiviral activity of Ch25h via its virion host shutoff protein. Antiviral Res 143:69–73. doi: 10.1016/j.antiviral.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Sun L, Chen ZJ. 2016. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 48.Paijo J, Doring M, Spanier J, Grabski E, Nooruzzaman M, Schmidt T, Witte G, Messerle M, Hornung V, Kaever V, Kalinke U. 2016. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells. PLoS Pathog 12:e1005546. doi: 10.1371/journal.ppat.1005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu JJ, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, Ma S, Liu X, Miley W, Konrad A, Neipel F, Sturzl M, Whitby D, Li H, Zhu F. 2015. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dansako H, Ueda Y, Okumura N, Satoh S, Sugiyama M, Mizokami M, Ikeda M, Kato N. 2016. The cyclic GMP-AMP synthetase-STING signaling pathway is required for both the innate immune response against HBV and the suppression of HBV assembly. FEBS J 283:144–156. doi: 10.1111/febs.13563. [DOI] [PubMed] [Google Scholar]
- 51.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. 2013. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 53.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. 2015. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang C, Wei X, Wei Y. 2016. Mitochondrial DNA in the regulation of innate immune responses. Protein Cell 7:11–16. doi: 10.1007/s13238-015-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biolatti M, Dell'Oste V, Pautasso S, Gugliesi F, von Einem J, Krapp C, Jakobsen MR, Borgogna C, Gariglio M, De Andrea M, Landolfo S. 2018. The human cytomegalovirus tegument protein pp65 (pUL83) dampens type I interferon production by inactivating the DNA sensor cGAS without affecting STING. J Virol 92:e01774-17. doi: 10.1128/JVI.01774-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xing J, Wang S, Lin R, Mossman KL, Zheng C. 2012. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5. J Virol 86:3528–3540. doi: 10.1128/JVI.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paz S, Vilasco M, Arguello M, Sun Q, Lacoste J, Nguyen TL, Zhao T, Shestakova EA, Zaari S, Bibeau-Poirier A, Servant MJ, Lin R, Meurs EF, Hiscott J. 2009. Ubiquitin-regulated recruitment of IkappaB kinase epsilon to the MAVS interferon signaling adapter. Mol Cell Biol 29:3401–3412. doi: 10.1128/MCB.00880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang TH, Liao CL, Lin YL. 2006. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect 8:157–171. doi: 10.1016/j.micinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Lin R, Lacoste J, Nakhaei P, Sun Q, Yang L, Paz S, Wilkinson P, Julkunen I, Vitour D, Meurs E, Hiscott J. 2006. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKepsilon molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol 80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jordan M, Schallhorn A, Wurm FM. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J Virol 85:11079–11089. doi: 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A, Kawaguchi Y. 2018. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe 23:254–265.e7. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]