At least 15 alpha HPV types are causative agents for 5% of all cancers worldwide, and beta types have been implicated in nonmelanoma skin cancer, whereas others produce benign papillomas, such as genital warts, associated with considerable morbidity and health systems costs. Vaccines targeting the minor capsid protein L2 have the potential to provide broad-spectrum immunity against medically relevant HPVs of divergent genera via the induction of broadly cross-neutralizing serum IgG. Here we examine the mechanisms by which L2-specific serum IgG reaches the viral inoculum in the genital tract to effect protection. Abrasion of the vaginal epithelium allows the virus to access and infect basal keratinocytes, and our findings suggest that this also permits the local exudation of neutralizing IgG and vaccine-induced sterilizing immunity. We also demonstrate the importance of Fc-mediated phagocytosis of L2 antibody-virion complexes for humoral immunity, a protective mechanism that is not detected by current in vitro neutralization assays.
KEYWORDS: human papillomavirus, L2 protein, Fc, FcRn, TRIM21, antibody-mediated protection, humoral immunity, cross-protection, monoclonal antibody, neutralizing antibodies
ABSTRACT
To address how L2-specific antibodies prevent human papillomavirus (HPV) infection of the genital tract, we generated neutralizing monoclonal antibodies (MAbs) WW1, a rat IgG2a that binds L2 residues 17 to 36 (like mouse MAb RG1), and JWW3, a mouse IgG2b derivative of Mab24 specific for L2 residues 58 to 64. By Western blotting, WW1 recognized L2 of 29/34 HPV genotypes tested, compared to only 13/34 for RG1 and 25/34 for JWW3. WW1 IgG and F(ab′)2 bound HPV16 pseudovirions similarly; however, whole IgG provided better protection against HPV vaginal challenge. Passive transfer of WW1 IgG was similarly protective in wild-type and neonatal Fc receptor (FcRn)-deficient mice, suggesting that protection by WW1 IgG is not mediated by FcRn-dependent transcytosis. Rather, local epithelial disruption, required for genital infection and induced by either brushing or nonoxynol-9 treatment, released serum IgG in the genital tract, suggesting Fc-independent exudation. Depletion of neutrophils and macrophages reduced protection of mice upon passive transfer of whole WW1 or JWW3 IgGs. Similarly, IgG-mediated protection by L2 MAbs WW1, JWW3, and RG1 was reduced in Fc receptor knockout compared to wild-type mice. However, levels of in vitro neutralization by WW1 IgG were similar in TRIM21 knockout and wild-type cells, indicating that Fc does not contribute to antibody-dependent intracellular neutralization (ADIN). In conclusion, the Fc domain of L2-specific IgGs is not active for ADIN, but it opsonizes bound extracellular pseudovirions for phagocytes in protecting mice from intravaginal HPV challenge. Systemically administered neutralizing IgG can access the site of infection in an abrasion via exudation without the need for FcRn-mediated transcytosis.
IMPORTANCE At least 15 alpha HPV types are causative agents for 5% of all cancers worldwide, and beta types have been implicated in nonmelanoma skin cancer, whereas others produce benign papillomas, such as genital warts, associated with considerable morbidity and health systems costs. Vaccines targeting the minor capsid protein L2 have the potential to provide broad-spectrum immunity against medically relevant HPVs of divergent genera via the induction of broadly cross-neutralizing serum IgG. Here we examine the mechanisms by which L2-specific serum IgG reaches the viral inoculum in the genital tract to effect protection. Abrasion of the vaginal epithelium allows the virus to access and infect basal keratinocytes, and our findings suggest that this also permits the local exudation of neutralizing IgG and vaccine-induced sterilizing immunity. We also demonstrate the importance of Fc-mediated phagocytosis of L2 antibody-virion complexes for humoral immunity, a protective mechanism that is not detected by current in vitro neutralization assays.
INTRODUCTION
Over 200 types of human papillomavirus (HPV) have been described, and these small DNA tumor viruses are generally classified into either cutaneous or mucosal types based on their epithelial tissue tropism (1). The mucosal types are then further subcategorized into “low-risk” types (lrHPV) associated with genital warts (e.g., HPV6 and HPV11) and 15 “high-risk” types (hrHPV), which have oncogenic potential. Of particular importance among the hrHPV types is HPV16, which accounts for 50% of all cervical cancer cases and approximately 90% of all HPV-associated cancers at other anatomical sites (2, 3).
The licensed HPV vaccines (Gardasil, Gardasil-9, and Cervarix) are based upon highly immunogenic virus-like particles (VLPs) assembled from the major capsid protein L1 (4) and safely confer durable and robust protection against their targeted HPV types (5). They all contain L1 VLPs of HPV16 and HPV18, genotypes that account for approximately 50 and 20% of all cervical cancer cases, respectively, and Gardasil-9 contains L1 VLPs derived from the 5 next most common HPV genotypes detected worldwide in cervical cancer. Both Gardasil vaccines also contain HPV6 and HPV11 L1 VLPs, as these two genotypes cause ∼90% of genital warts, a benign sexually transmitted disease. L1 VLP vaccines elicit a strong type-restricted neutralizing response against the HPV type from which the L1 protein was derived (6–10). Although L1 VLPs can elicit some cross-neutralization and protection against very closely related types (8), their limited breadth of protection and the complexity of producing more multivalent formulations have spurred efforts to produce simpler HPV vaccines that are effective against all oncogenic HPV types and types associated with cutaneous disease.
Another minor component of the nonenveloped viral capsid is the late protein L2, which mediates endosome escape and, in the absence of L1, traffics to the nucleus with the viral genome (reviewed in reference 11). The amino-terminal sequence of L2 possesses several motifs that are both functional in viral infection and conserved, broadly neutralizing epitopes (12–14), including residues 17 to 36 recognized by monoclonal antibody (MAb) RG1 (15) and residues 58 to 81 and residues 108 to 120 mapped with antisera and MAbs (13, 14, 16–18).
Passive-transfer studies demonstrate that neutralizing antibodies specific for either L1 VLPs or L2 are sufficient to mediate protection in animal challenge models. Vaccination with L2 generates lower titers but more broadly neutralizing antibodies than L1 VLPs (19), raising questions about the durability of L2-specific immunity (18, 20). To enhance the titer of the neutralizing serum antibody response, several approaches have been tested, including the use of strong adjuvants and concatenation of L2 epitopes and/or repetitive display on macromolecular scaffolds, such as viral VLP platforms, to increase epitope density (11, 18, 21–23). While these approaches are beneficial, the L2-specific in vitro neutralization titers elicited in serum are still lower than the type-specific responses to L1 VLP vaccines. Nevertheless, active vaccination with L2 or passive transfer of L2 antiserum confers robust protection against viral challenge (24–26).
It is unclear how protective IgG in the systemic circulation reaches the viral inoculum at the epithelial site of infection in the anogenital mucosa or in skin. This may occur via active transcytosis out of the bloodstream mediated by the neonatal Fc receptor (FcRn) and/or possibly exudation associated with epithelial abrasion at the site of infection. Indeed, experimental infection of the murine genital tract requires epithelial brushing (or disruption by pretreatment with nonoxynol-9 [N9]) such that pseudovirions (PsV) can reach and bind to heparin sulfate proteoglycans on the basement membrane (BM). Likewise, cutaneous papillomavirus infection in several animal models requires scarification (27).
Binding of the virus to the BM induces conformational changes in the capsid, which promote their association with the basal keratinocyte and infection. Importantly, this conformational change renders certain amino-terminal L2 epitopes, including residues 17 to 36 recognized by RG1, accessible on the capsid surface to permit neutralization (28). This is not required for HPV L1 antibodies, whereby high concentrations of L1 VLP-specific antibodies block the initial association with the BM, whereas lower antibody levels permit BM association but block the binding of pseudovirions to basal keratinocytes (24), as is also seen for neutralization by L2-specific antibody (for a review, see reference 29). Importantly, antibody-bound pseudovirions on the BM are cleared over 18 h, and this is associated with neutrophil infiltrates. This is suggestive of Fc-mediated opsonization and phagocytosis, but its role in protection was never directly tested.
L2-specific neutralizing antibodies do not prevent virion binding to cultured cell monolayers (28, 30). Day et al. (14) found that following binding, the L2 antibody-virion complex slowly accumulated on the extracellular matrix (ECM) but did not enter cells. However, in another study, uptake was only partially inhibited. Rather, the bulk of L2 antibody-bound pseudovirions accumulated in the perinuclear region in large lamellar bodies and lysosome-like multivesicular bodies, suggesting that neutralization may occur by preventing L2-viral DNA egress from the vesicular compartment (14). Alternatively, L2 antibody-dependent neutralization may occur in the cytoplasm by a mechanism recently described for several viruses as a final defense to counter the “persistent fraction” of virions bound by otherwise nonneutralizing antibodies that escape into the cytoplasm (31, 32). In this mechanism, the Fc of the antibody-virus complex is bound with high affinity by the cytosolic Fc receptor TRIM21. TRIM21 then catalyzes the attachment of K63-linked polyubiquitinated chains, which both commits the antibody-virion complex to proteasomal degradation and activates an innate response, presumably to combat early viral replication (31–34). Since L2 traffics to the nucleus during infection, this TRIM21 Fc receptor-dependent mechanism might contribute to sterilizing immunity provided by L2-specific antibody. Here we examine the potential to induce broadly cross-neutralizing L2-specific antibody by using a polyepitope vaccine containing sequences from diverse HPV types and the mechanisms by which such serum IgGs access the viral inoculum and mediate protection.
RESULTS
Generation of L2-specific rat MAbs WW1 and S10.
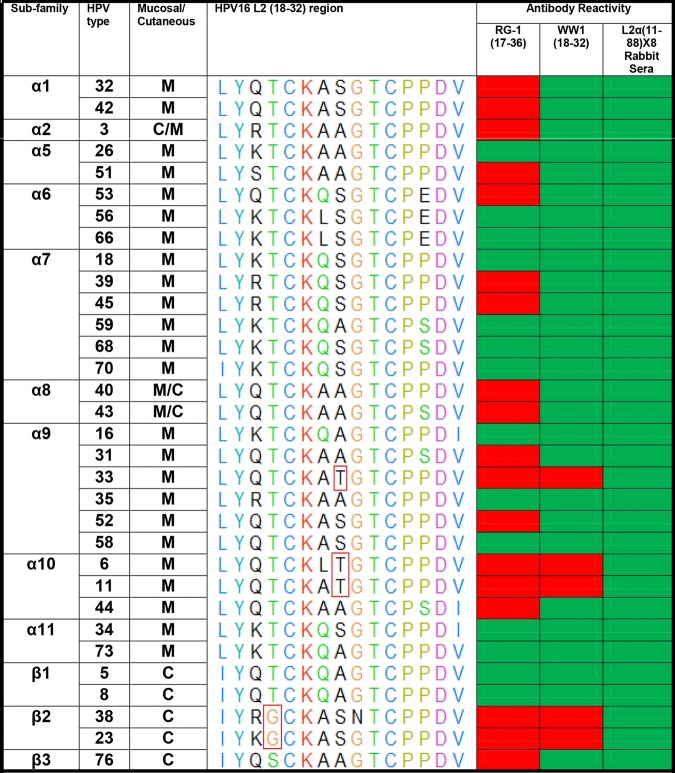
Previous studies showed that vaccination of mice with a fusion protein comprising residues 11 to 88 of HPV types 6, 16, 18, 31, 39, 51, 56, and 73 (L2 α11–88×8) provided robust protection against vaginal challenge with 11 clinically relevant genital HPV genotypes (35). Rabbit antisera to L2α11-88×8 also neutralized 34 clinically relevant HPV types from the alpha and beta families in vitro (36) and was protective via passive transfer (35). Therefore, in an effort to generate new, more broadly neutralizing L2-specific MAbs and identify their epitopes in a new species, we vaccinated Lewis rats with the same L2 α11-88×8 antigen. L2 α11–88×8 enzyme-linked immunosorbent assay (ELISA) screening of hybridoma supernatants identified two rat IgG2a MAbs, named WW1 and S10. A second round of ELISA screening using full-length L2 from HPV6, HPV11, HPV16, HPV18, or HPV31 as the coating antigen showed that WW1 reacted with HPV16/18/31 L2 but not HPV6 or HPV11 L2, whereas S10 reacted with the HPV6/11 L2 protein but not with HPV16, -18, or -31 L2 (data not shown). These specificities were further confirmed by Western blotting using the same L2 proteins (data not shown). To further test the breadth of WW1 and S10 reactivity, pseudovirion preparations of 34 clinically relevant HPV genotypes from the alpha and beta families were probed by Western blotting, first with MAb WW1, S10, or RG1 and then, after stripping, with rabbit antiserum to L2 α11-88×8 as a positive control to confirm the presence of L2 in all of the pseudovirion preparations (Fig. 1). S10 bound only to L2 proteins of HPV6, -11, and -44, members of the α10 subfamily, as well as HPV58 L2 from the α9 subfamily. In contrast, WW1 reacted with L2 proteins of 29 out of the 34 HPV genotypes tested, whereas RG1 recognized L2 in 13 of these 34 pseudovirion preparations (summarized in Fig. 1).
FIG 1.
Summary of RG1 and WW1 reactivity with diverse HPV genotypes. Red boxes indicate no MAb binding detectable by Western blotting, and green boxes indicate detectable reactivity. The WW1 epitope on HPV16 L218–32 and the corresponding regions of other HPV types were aligned using UCSF chimera software. Red boxes in the multiple-sequence alignment indicate putative key amino acid mutations from HPV16L218–32 that resulted in WW1 nonreactivity.
WW1 recognizes a linear epitope within HPV16 L2 residues 18 to 32.
A 15-mer peptide array of L2 peptides within the regions spanning residues 11 to 88 of 15 different HPV types, each overlapping by 5 residues, was utilized to map the MAb epitopes. WW1 recognized only two overlapping L2 peptides, comprising residues 13 to 32 and 18 to 37 derived from HPV16 L2, as well as the equivalent L2 peptides from HPV types 18, 31, 35, 39, 45, 51, 52, 58, 59, and 73. WW1 did not bind to HPV16 L2 peptide amino acids (aa) 23 to 42, suggesting that its epitope likely resides within residues 18 to 32 of HPV16 L2 (LYKTCKQAGTCPPDI). To further map the epitope, we analyzed WW1 binding via an ELISA using HPV16 PsV containing either wild-type L2 or several point mutants within this region (Fig. 2). Mutation to alanine of either of the conserved cysteines at HPV16 L2 amino acid 22 or 28, or proline 29, eliminated WW1 binding, as previously described for MAb RG1 (37). However, while RG1 binding to HPV16 L2 was also previously reported to be affected if lysine 20 was changed to alanine, WW1 binding was not affected by this mutation.
FIG 2.
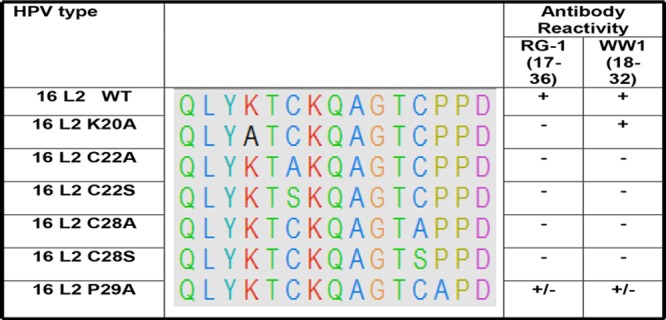
Conserved cysteines in L2 are required for WW1 binding. RG1 and WW1 binding with either wild-type (WT) HPV16 PsV or HPV16 point mutant PsV at the HPV16L218–32 site was assessed by Western blotting. The WW1 epitope on HPV16 and the corresponding L2 point mutant regions on other mutants were aligned using UCSF chimera software. “+” indicates strong binding, “+/−” indicates weak binding, and “−” indicates no binding.
S10 binds L2 residues 63 to 89 of HPV types 6, 11, 44, and 58.
To determine whether S10 bound L2 on the surface of pseudovirions, we further performed ELISAs with HPV6, -11, -44, and -58 and HPV16 pseudovirions. S10 bound to pseudovirions with a type specificity consistent with the Western blot data (data not shown). However, S10 failed to bind to any of the overlapping 15-mer HPV6 L2 peptides in the array by an ELISA (data not shown). Since S10 binds within residues 12 to 88 of HPV6 L2 but not to the overlapping peptide array, an alternative strategy was devised to map its epitope, specifically, Western blotting of three previously described L2 fusion proteins containing HPV6 L2 residues 12 to 88 (within L2 α11-88×8) (20) and HPV6 L2 residues 12 to 46 and 63 to 88 (within L2α11-88×8ΔTM) (21) and residues 12 to 46 (within 13-47×15) (38). S10 bound to α11-88×8 and α11-88×8ΔTM but not to the 13-47×15 fusion protein (data not shown). This indicates that the L2 region spanning residues 63 to 89 is required for S10 recognition of L2.
In vitro neutralization of HPV PsV by WW1 and S10.
The in vitro neutralizing activities of WW1 and S10 (serially titrated from 1,666 μM) were compared against HPV6/16/31/35/45/58 PsV using the standard L1 pseudovirion-based neutralization assay (PBNA). MAb RG1 and an irrelevant rat IgG were also included as positive and negative controls, respectively (Table 1). Consistent with previous findings (39), RG1 could neutralize only HPV16 and HPV18 (<13 nM) (15, 39), whereas WW1 neutralized HPV16 (<13 nM), HPV18 (26 nM), and HPV45 (69 nM). WW1, however, did not neutralize HPV31 or HPV58 PsV even at 1,666 nM. S10 also failed to consistently show any neutralization activity, even against HPV6 PsV (Table 1).
TABLE 1.
Neutralization and protection by MAbs against different HPV genotypesa
| Genotype | Monoclonal antibody | L1-PBNA IC50 (nM) | FC-PBNA IC50 (nM) | Mean % protection by passive transfer (5 mice) |
|---|---|---|---|---|
| HPV6 (luciferase reporter) | RG1 | >1,666 | >1,666 | None |
| WW1 | >1,666 | >1,666 | None | |
| S10 | >1,666 | 8.44 | 87.6 | |
| Rat IgG control | >1,666 | >1,666 | None | |
| HPV16 (luciferase reporter) | RG1 | <13 | 0.26 | 100 |
| WW1 | <13 | 0.52 | 98.9 | |
| S10 | >1,666 | >1,666 | None | |
| Rat IgG control | >1,666 | >1,666 | None | |
| HPV18 (luciferase reporter) | RG1 | <13 | 0.28 | ND |
| WW1 | 26 | 0.42 | ND | |
| S10 | >1,666 | >1,666 | ND | |
| Rat IgG control | >1,666 | >1,666 | ND | |
| HPV31 (luciferase reporter) | RG1 | >1,666 | >1,666 | None |
| WW1 | >1,666 | >1,666 | None | |
| S10 | >1,666 | >1,666 | None | |
| Rat IgG control | >1,666 | >1,666 | None | |
| HPV45 (luciferase reporter) | RG1 | >1,666 | >1,666 | None |
| WW1 | 69 | 4.15 | 97.2 | |
| S10 | >1,666 | >1,666 | None | |
| Rat IgG control | >1,666 | >1,666 | None | |
| HPV58 (luciferase reporter) | RG1 | >1,666 | >1,666 | None |
| WW1 | >1,666 | 2.08 | 93.7 | |
| S10 | >1,666 | 4.15 | 67.8 | |
| Rat IgG control | >1,666 | >1,666 | None |
Shown is a summary of neutralization IC50s (nanomolar) against HPV6/16/18/31/45 and HPV58 for MAb RG1, WW1, and S10 determined by 2-fold antibody titration from 1,666 nM and measured using the L1-PBNA or FC-PBNA. Also shown is the percent protection obtained by passive transfer of 50 μg (166 nM) MAb prior to challenge with HPV6/16/18/31/45 and HPV58. ND, not done; None, no significant protection.
We then performed passive-transfer studies of WW1 and S10 followed by vaginal challenge of mice with pseudovirions of different HPV types, as it is a more sensitive and biologically relevant test of protective capacity than in vitro neutralization (25, 40). The rat MAbs were administered intraperitoneally (i.p.) to each naive mouse (n = 5) in a single dose of 50 μg. Given a 2-ml estimated plasma volume for a mouse, this corresponds to a concentration of ∼166 nM assuming equal distribution and no degradation or excretion. These mice were then subjected to vaginal challenge with the same PsV types used in the L1-PBNA. Passive transfer of 50 μg WW1 conferred significant protection against vaginal challenge with HPV16 (98.9%), HPV45 (97.2%), or HPV58 (93.7%) but not HPV6 or HPV31. Consistent with its in vitro neutralization data, passive transfer of 50 μg RG1 protected mice against vaginal challenge with HPV16 PsV (100%) but not the other HPV types 31, 45, and 58. Mice that received 50 μg of S10 also exhibited a reduction of luciferase activity (87.6%) after challenge with HPV6 PsV, and some protection was observed against HPV58 PsV (67.8%), although no significant protection was seen for S10 against HPV types 16, 31, and 45 (Table 1).
The inconsistency of in vitro and in vivo data assessing inhibition of HPV58 infectivity by WW1 and of HPV6 and HPV58 infectivity by S10 suggests that the L1-PBNA may be insufficiently sensitive for L2-neutralizing antibody analysis. However, the use of the furin-cleaved PBNA (FC-PBNA) to reanalyze WW1 and S10 provided in vitro neutralization data more consistent with the findings by passive transfer: WW1 neutralized, in the nanomolar range, all HPV types tested, including HPV58 (2 nM), except HPV6 and HPV31 (Table 1), whereas RG1 neutralized only HPV16 and HPV18. Importantly, using the FC-PBNA, we found that the 50% inhibitory concentrations (IC50s) were ∼10-fold lower than those measured using the L1-PBNA. Furthermore, S10 could also neutralize HPV6 (8.44 nM) and HPV58 (4.15 nM) in the FC-PBNA, but this was not detected by the L1-PBNA.
Affinity of MAbs for HPV L2.
Despite WW1 recognizing epitopes within the same L217–36 region of several HPV types as RG1, there were observed differences between Western blot and ELISA reactivities as well as neutralization toward different HPV types. For example, WW1 is reactive to HPV31 via Western blotting or an ELISA; however, WW1 is nonneutralizing to HPV31. To further investigate and understand if this is related to binding kinetics for each specific virus type, we performed quantitative measurement of antibody affinity using the BLItz label-free assay system (FortéBio Inc.), using streptavidin biosensors loaded with biotinylated HPV L217–36 peptides from HPV6/16/31/45 and -58, respectively. Following loading, the L2 sensors were exposed to different concentrations of RG1 and WW1 IgG to measure the association rate constant (Ka), and dissociation rate constant (Kd) to calculate the equilibrium dissociation constant (KD) (Table 2). The KD of RG1 toward the HPV16 L2 peptide was 8 nM, while the KD values were 10- to 100-fold higher for HPV6/31/45/58. These findings corresponded with the IC50s determined by the L1-PBNA, <13 nM for HPV16 and >1,666 nM for the remaining types tested. Likewise, RG1 gave robust protection against vaginal challenge with HPV16 upon passive transfer at an estimated concentration of 166 nM, but it was not protective against challenge with HPV6/31/45/58.
TABLE 2.
Measurement of MAb affinity for L2 proteins of different HPV types by interferometrya
| HPV L2 aa 17–36 | RG1 |
WW1 |
||||
|---|---|---|---|---|---|---|
| KD (M) | Ka (M−1 s−1) | Kd (s−1) | KD (M) | Ka (M−1 s−1) | Kd (s−1) | |
| HPV6 | 3.82E−07 | 1.94E+04 | 7.42E−03 | 1.79E−06 | 4.88E+03 | 8.71E−03 |
| HPV16 | 8.34E−09 | 7.92E+04 | 6.60E−04 | 3.70E−08 | 8.87E+03 | 3.28E−04 |
| HPV31 | 2.79E−07 | 2.50E+04 | 6.97E−03 | 2.61E−07 | 7.96E+03 | 2.08E−03 |
| HPV45 | 3.54E−07 | 2.48E+04 | 8.77E−03 | 2.77E−07 | 2.77E+03 | 7.68E−04 |
| HPV58 | 1.80E−07 | 3.69E+04 | 6.64E−03 | 4.09E−07 | 6.54E+03 | 2.67E−03 |
Measurements of the dissociation constants for MAb RG1 and WW1 binding to peptides corresponding to L2 residues 17 to 36 of several HPV genotypes were determined by interferometry using the BLItz system. S10 was unreactive to all peptides.
In contrast, the KD values for WW1 were generally 1 log higher than those for RG1. For example, the WW1 KD value for HPV16 was 37 nM, consistent with the in vitro neutralization IC50 observed in the FC-PBNA for WW1 against HPV16. The KD values for WW1 against HPV31/45/58 were 261 nM, 277 nM, and 409 nM, respectively (Table 2), and there was a less clear correlation between WW1 affinity and in vitro neutralization. Thus, WW1 has a lower affinity for this peptide than does RG1, but this could also account for its broader Western blot and ELISA reactivities. Given the broader reactivity of WW1 than of S10 and RG1, WW1 was selected for all subsequent studies.
FcRn is not required for protection via passive transfer of WW1.
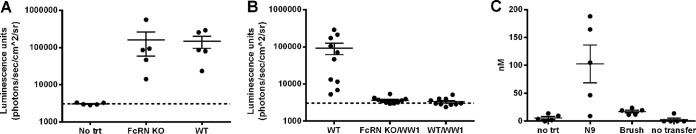
It is not well understood how neutralizing IgG in serum protects against a viral inoculum infecting the vaginal mucosa, since virions do not cross the basement membrane. Studies indicate that IgG concentrations in vaginal fluid exceed those of IgA, reflecting the transcytosis of IgG from the plasma into the genital tract via Fc interaction with the neonatal Fc receptor (FcRn) (41). FcRn-mediated intracellular neutralization has been described in polarized epithelial cells (42). Therefore, to assess if FcRn is required to transport protective antibodies into the genital tract or keratinocytes to prevent HPV infection, we performed passive transfer of WW1 IgG followed by HPV16 vaginal challenge in both wild-type and FcRn-deficient C57BL/6 mice. Both groups were equally susceptible to infection after preincubation with nonoxynol-9 (N9) and vaginal challenge with HPV16 PsV (Fig. 3A). Upon systemic administration of WW1 to groups of 10 mice followed by vaginal challenge with HPV16 PsV, similar levels of protection were observed for wild-type and FcRn-deficient mice (Fig. 3B), suggesting that transcytosis is not required or possibly that there is another undefined transcytosis mechanism independent of FcRn.
FIG 3.
FcRn-independent release of WW1 into the vagina and protection. (A) HPV16 PsV encoding a luciferase reporter plasmid infects FcRn knockout (KO) and wild-type (WT) C57BL/6 mice equivalently after vaginal challenge. (B) Passive transfer of WW1 (50 μg) protects both FcRn KO and WT mice against vaginal challenge with HPV16 PsV. (C) Higher concentrations of WW1 IgG are present in vaginal lavage samples obtained from WT and FcRn KO mice 12 h after N9 treatment than with cytobrush-induced disruption of the murine cervicovaginal epithelium.
Alternatively, plasma IgG might escape the circulation to reach the virus via exudation since HPV infection in the genital tract of mice requires epithelial abrasion/disruption (25). Epithelial trauma is elicited in the mouse model by brushing or the administration of nonoxynol-9 in the vagina, presumably to allow the pseudovirions to reach the basement membrane and access basal keratinocytes. To measure their impact on exudation, 6 h after systemic administration of WW1 IgG, three groups (n = 5) of BALB/c mice were administered N9 (50 μl; 4%, vol/vol), cytobrush treatment in the vaginal lumen, or no additional treatment. Twelve hours later, vaginal washes with saline were then collected and tested for WW1 IgG. The level of WW1 detected in the vaginal lavage fluid was dramatically increased by N9 treatment (105 ± 35 nM) compared to the no-treatment control (5.9 ± 1.5 nM) (Fig. 3C). Surprisingly, the cytobrush method exhibited a less dramatic increase in the level of WW1; it was ∼20% of that released by N9 treatment (mean = 17.2 nM) although significantly higher than that for untreated mice (P = 0.03) (Fig. 3C). Together, these results suggest that FcRn is not required for the transport of WW1 into the mouse genital tract, since epithelial trauma required for HPV infection permits the exudation of plasma IgG.
The WW1 F(ab′)2 fragment is less protective against HPV16 challenge than whole IgG.
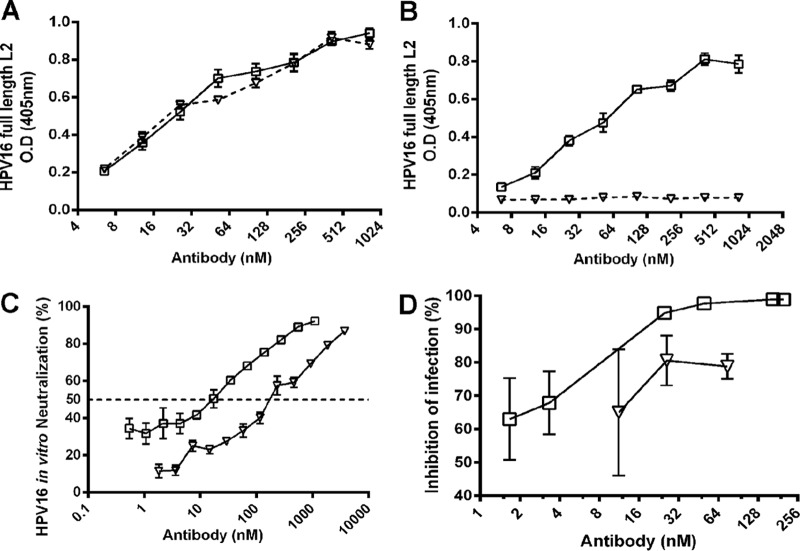
To further dissect how L2 antibody neutralization occurs, we examined the importance of the Fc portion of L2 antibodies in mediating protection. WW1 F(ab′)2 fragments were prepared by digestion on pepsin-Sepharose beads, and undigested Fc was removed by passage through protein G-Sepharose. The completeness of digestion and purity of the fragments were assessed by reducing and nonreducing SDS-PAGE and Coomassie staining (not shown). To determine whether the F(ab′)2 fragments retained epitope binding, their recognition of either HPV16 PsV or a synthetic HPV16 L2 peptide spanning residues 17 to 36 was first examined by an ELISA using a peroxidase-linked mouse MAb specific for the rat kappa light chain as the secondary antibody. Both WW1 IgG and WW1 F(ab′)2 fragments were able to bind to both the HPV16 L2 peptide and HPV16 PsV, while S10, as expected, did not (Fig. 4A). When peroxidase-linked mouse anti-rat IgG2a Fc was used as the secondary antibody, no reactivity was detected with the F(ab′)2 preparation, suggesting that the F(ab′)2 preparation was free of whole WW1 IgG2a. Conversely, whole WW1 IgG2a was detected with mouse anti-rat IgG2a Fc (Fig. 4B). Taken together, these results show that WW1 binding to HPV16 L2 was not impacted by the removal of the Fc domain. However, when examining the in vitro neutralizing activity, the F(ab′)2 fragment of WW1 was not as effective as IgG (Fig. 4C), suggesting that the antibody is not as stable in culture or that the Fc domain contributes to in vitro neutralization.
FIG 4.
WW1 IgG and F(ab′)2 bind L2 equivalently, but IgG is more neutralizing and protective. (A) WW1 whole IgG and WW1 F(ab′)2 bind comparably to the HPV16 L2 protein, as determined by an ELISA using a Fab-specific secondary antibody. (B) The WW1 F(ab′)2 preparation that lacked a detectable signal compared to whole WW1 IgG toward anti-IgG2a secondary antibody failed to detectably react in an HPV16 L2 ELISA. (C) Whole WW1 IgG2a can neutralize HPV16 better in vitro in the FC-PBNA than WW1 F(ab′)2. (D) Whole WW1 IgG2a better protects naive mice from vaginal challenge with HPV16 upon passive transfer than WW1 F(ab′)2. Open squares indicate WW1 whole IgG, and open triangles indicate WW1 F(ab′)2.
To address the relevance of Fc to protection, naive mice were passively transferred with titrations of WW1 whole IgG or its F(ab′)2 fragment and then challenged 6 h later with HPV16 PsV. Whole WW1 IgG provided robust protection (94%) at 7.5 μg (25 nM), while the administration of WW1 F(ab′)2 failed to achieve >90% protection even at 15 μg (75 nM) (Fig. 4D). This difference might reflect a lower stability of F(ab′)2 in vivo than IgG and/or the loss of Fc-mediated protective functions.
Contribution of phagocytes to WW1-mediated protection.
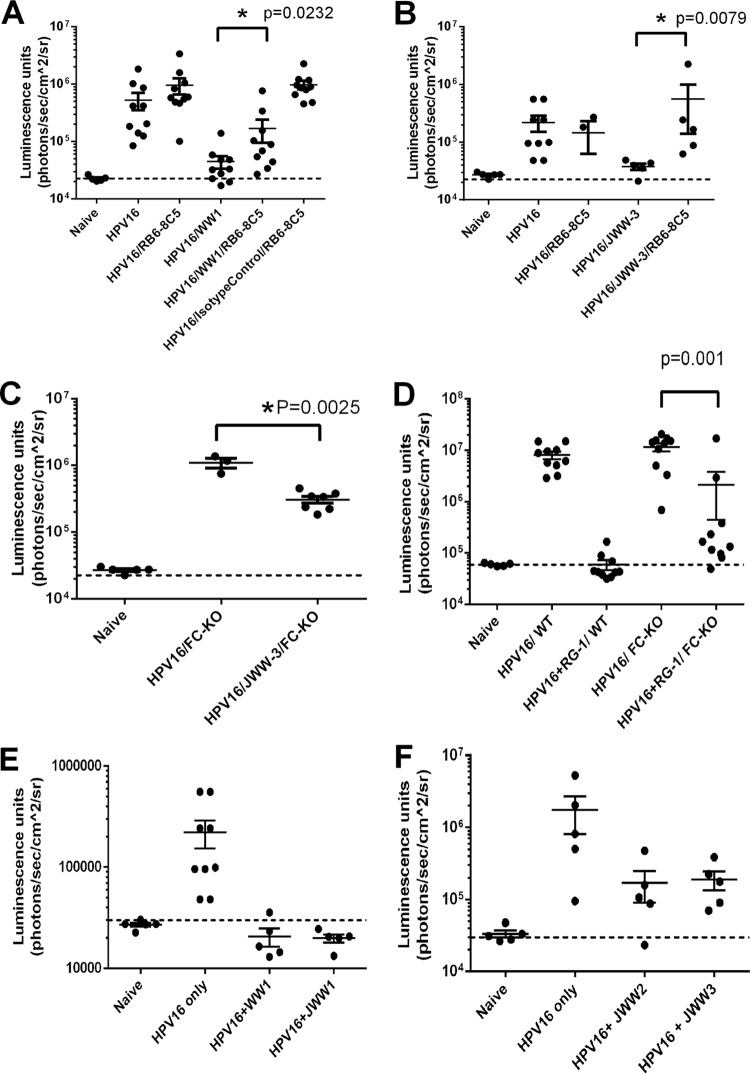
Opsonization via the recognition of the Fc of IgG bound to extracellular pathogens promotes their engulfment and clearance by phagocytes. Since WW1 F(ab′)2 fragments lack the Fc portion, it is possible that their lower protective capacity in vivo reflects an inability to opsonize extracellular HPV16 pseudovirions. Indeed, it was also previously described that neutrophils accumulated at the site of HPV16 PsV challenge in mice treated with L2 antibody, which was associated with the disappearance of pseudovirions over 18 h (24). However, the role of phagocytes in L2 antibody-mediated protection was not directly tested. Therefore, we compared the abilities of the passive transfer of WW1 IgG to protect against vaginal challenge with HPV16 in normal mice or those depleted of neutrophils and Gr1+ macrophages (data not shown). While the depletion of neutrophils and Gr1+ macrophages had no impact on HPV16 PsV infection in the genital tract (Fig. 5A), passive transfer of WW1 IgG (25 μg/mouse; ∼83 nM) was significantly less protective (P = 0.02) against HPV16 in mice depleted of neutrophils and Gr1+ macrophages (inhibition of 82% ± 7.9%) than in control mice (inhibition of 95% ± 0.9%) (Fig. 5A). This implies that the Fc portion of WW1 IgG contributes to protection by promoting phagocytosis of extracellular virus, as suggested by previous immunofluorescent staining studies (24).
FIG 5.
Role of neutrophils/macrophages and Fc receptors (FcR) in protection by L2 MAbs. (A) Passive transfer of WW1 and control IgG into control BALB/c mice or mice depleted for neutrophils/macrophages (n = 10) using RB6-8C5 pretreatment followed by HPV16 pseudovirus challenge. (B) Passive transfer of JWW3 IgG into control BALB/c mice or mice depleted for neutrophils/macrophages (n = 7) followed by HPV16 pseudovirus challenge. (C) Passive transfer of JWW3 IgG into Fcγ knockout mice (n = 7) followed by HPV16 pseudovirus challenge. (D) Passive transfer of MAb RG1 IgG into wild-type or Fcγ knockout mice (n = 10) followed by HPV16 pseudovirus challenge. (E) Passive transfer of monoclonal WW1 or JWW1 IgG into BALB/c mice (n = 5) followed by HPV16 pseudovirus challenge. (F) Passive transfer of monoclonal JWW2 or JWW3 IgG into BALB/c mice (n = 5) followed by HPV16 pseudovirus challenge.
The use of a MAb of rat origin in mice might introduce artifacts. Thus, we repeated the above-described experiment with a mouse L2-specific antibody in place of WW1. In addition, since the N terminus of HPV L2 has been shown to contain several neutralizing epitope regions, we also sought to confirm this phenomenon with a mouse MAb to a distinct L2 epitope. To this end, we generated a JWW3 plasmid that expresses a mouse IgG2b constant region fused to the reported VDJ regions of Mab24b (14, 43) that recognizes the region spanning residues 58 to 64 of HPV16 L2 and is broadly cross-neutralizing. Following expression and purification, JWW3 retained in vitro neutralizing activity and reactivity to 25/34 HPV genotypes (Fig. 1). Importantly, the passive transfer of JWW3 IgG (25 μg/mouse; ∼83 nM) was significantly less protective (P = 0.0079) against HPV16 vaginal challenge in mice depleted of neutrophils and Gr1+ macrophages (inhibition of 12% ± 25.4%) than in control mice (inhibition of 95% ± 2.4%) (Fig. 5B).
To further confirm the findings of our neutrophil/macrophage depletion studies, we repeated the same experiment with JWW3 utilizing Fcγ chain knockout mice, which lack the γ chain subunit of their FcgR1, FcgRIII, and FceRI receptors, resulting in impaired Fc receptors on their neutrophils and macrophages. The Fcγ knockout mice demonstrated significant (P = 0.0025) but weak protection (inhibition of 26% ± 3.4%) (Fig. 5C) compared to WW1-treated wild-type BALB/c mice (inhibition of 95% ± 2.4%). Thus, we repeated the experiment with RG1 and observed a significantly (P = 0.001) reduced level of protection in Fcγ knockout mice (inhibition of 82% ± 14.6%) compared to wild-type mice (inhibition of 99% ± 0.2%) (Fig. 5D). Taken together, our results indicate that the Fc portion of L2-specific antibodies contributes to protection by promoting phagocytosis of extracellular virus, as suggested by previous immunofluorescence studies.
To examine the impact of species upon this Fc-mediated protective mechanism, we performed passive-transfer experiments in wild-type BALB/c mice to compare WW1 IgG and JWW1, the latter of which contains the variable region of WW1 fused to the human IgG1 constant region (Fig. 5E). Likewise, we also performed passive-transfer experiments using JWW2 and JWW3 (Fig. 5F), which have human IgG1 and mouse IgG2b constant domains attached to the same variable region of mouse Mab24b that recognizes L2 residues 58 to 64. In both cases, similar levels of protection against HPV16 challenge were observed (Fig. 5E and F).
WW1 does not utilize TRIM21-dependent intracellular neutralization.
The IC50 values for the in vitro neutralization of HPV16 by WW1 IgG and F(ab′)2 were 26 nM and 292 nM, respectively (Fig. 4C), suggesting that WW1 IgG is ∼10-fold more potent than WW1 F(ab′)2. This difference in neutralization is unlikely to reflect Fc-mediated complement activation, since the 293TT cells were cultured in heat-inactivated serum.
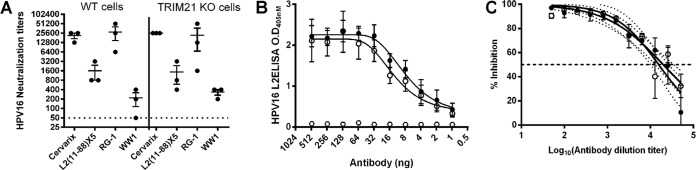
Recently, a mechanism termed antibody-dependent intracellular neutralization (ADIN) was described (34), in which the cytosolic Fc receptor TRIM21 binds to a conserved region (H433, N444, and H435) within the Fc portion of antibody bound to virions that enter the cytosol, shuttling the complex to the proteasome for rapid degradation (8, 26). As L2 may carry the viral DNA to the nucleus via the cytosol, we investigated whether the lower neutralization capabilities of WW1 F(ab′)2 in vitro reflected a loss of TRIM21-dependent ADIN in the cytosol because of Fc removal. First, we compared the capacities of WW1 to neutralize HPV16 infection of wild-type and TRIM21-deficient mouse embryonic fibroblasts. Levels of HPV16 infectivity were similar in both cell lines (data not shown), but there was also no significant difference in neutralization titers when a variety of sera and antibodies were measured in either TRIM21-deficient or wild-type mouse fibroblasts (Fig. 6A).
FIG 6.
Role of TRIM21 in antibody-mediated neutralization. (A) Measurement of in vitro neutralizing antibody titers by the L1-PBNA for mouse antisera to Cervarix or L2α11-88×5 or MAbs WW1 and RG1. The neutralization assays tested the IC50 for HPV16 infection of either wild-type mouse embryo fibroblast (MEF) cells (left) or TRIM21 knockout mouse embryo fibroblast cells (right). (B) Binding of humanized WW1 (closed circles) and humanized WW1 with TRIM21 binding-site mutations (open circles) to HPV16 L2 by an ELISA. (C) In vitro neutralization of HPV16 by humanized WW1 (closed circles) and humanized WW1 with TRIM21 binding-site mutations (open circles).
To further address this question, we used our previously described expression vector for MAb JWW1 in which the variable regions of WW1 are fused onto a human IgG1 Fc. In addition, we created a second construct (JWW1-A3), in which the three critical residues of the TRIM21 binding region of human IgG1 Fc were all mutated to alanine (H433A, N434A, and H435A), a change previously shown to eliminate TRM21 binding (44). Both antibodies were produced by transfection of 293 cells under serum-free conditions and purified from clarified culture supernatants using protein G columns. Subsequently, both the JWW1 and the JWW1-A3 antibodies were tested for binding efficacy against HPV16 L2 (Fig. 6B) and for in vitro neutralization of HPV16 PsV (Fig. 6C). No difference in titers was seen in either assay. These findings suggest that the Fc portion of WW1 does not contribute to HPV16 neutralization in vitro via TRIM21-mediated ADIN. Rather, the lower in vitro neutralization activity of WW1 F(ab′)2 fragments than of WW1 IgG (Fig. 4C) reflects the instability of the disulfide links of the F(ab′)2 fragments in the reducing environment of tissue culture medium produced by cell culture. This is supported by nonreducing SDS-PAGE analysis (not shown) and suggests that Fc contributes to WW1 antibody stability.
DISCUSSION
The neutralizing MAb RG1, which was produced by vaccination with full-length HPV16 L2, is cross-reactive to only a small subset of oncogenic HPV types. We hypothesized that a multitype L2 immunogen containing the L2 regions spanning aa 11 to 88 of 8 different HPV genotypes would yield more broadly reactive MAbs. Indeed, this approach yielded the very broadly cross-reactive WW1 MAb as well as the partially cross-reactive S10 antibody. WW1's greater breadth of cross-reactivity than of RG1 (despite both antibodies recognizing epitopes within L2 aa 17 to 36) is consistent with the broader neutralizing antibody response elicited by the multimer than that elicited by a single type L2 immunogen and has been speculated to reflect the superior cross-linking of broadly reactive B cell receptors by the multimer because the chain of constituent subunits is derived from different genotypes.
The cross-reactive natures of WW1 and RG1 reflect their recognition of the only two cysteine residues in L2 (residues 22 and 28), both of which are conserved in all HPV types, as is the conserved proline at residue 29. This L2 epitope appears to be immunodominant not only in mice (15, 18) and some patients (45) but also in rats. ClustalW analysis of the region spanning residues 18 to 32 (Fig. 1) suggests that RG1 requires K at residue 20 for robust binding, whereas WW1 is tolerant to Q/R/K/S at this position and thus has a broader spectrum of binding. Although further direct testing with peptides is required, this ClustalW comparison also suggests that WW1 tolerates A or S at L2 residue 25, but not T, as well as T or S at residue 21, but not G.
It has been puzzling that while L2 vaccination is associated with low neutralization titers in serum, it can provide robust protection from experimental challenge. This in part reflects that the conventional in vitro pseudovirion-based neutralization assay (L1-PBNA) is insensitive for the detection of L2-mediated neutralizing antibodies compared to the furin-cleaved pseudovirion-based neutralization assay (FC-PBNA) (26, 39, 46), as seen again here with L2 MAb. Although the FC-PBNA is more sensitive, it does not assess protection due to opsonization for phagocytosis, which may account for the greater sensitivity of the passive-transfer assay (40).
While WW1 and RG1 are cross-neutralizing (Table 1), IC50s differed substantially by HPV type. For example, the IC50 of WW1 for HPV16 was 0.52 nM but was higher for HPV45 (4.51 nM) or HPV58 (6.71 nM) (FC-PBNA) (Table 1), presumably reflecting the impact of variations in the epitope sequence on binding affinity. The higher binding affinity for the peptide determined by interferometry was associated with the strongest protection in the passive-transfer model. This and the need for multiple L2 immunizations to achieve effective neutralization suggest the importance of affinity maturation.
HPV L1 VLP vaccines elicit high titers of neutralizing serum antibodies and are remarkably effective for prophylaxis, but they lack therapeutic efficacy. This suggests that they provide sterilizing immunity via neutralizing antibody. However, it is still unclear how neutralizing serum IgG reaches the inoculum at the site infection in genital mucosa and skin, since HPV does not cross the basement membrane. Significant quantities of IgG are present in vaginal fluid, and the ratio of IgG to IgA varies across the menstrual cycle (47). In mice, IgG is brought into the genital tract via Fc binding to FcRn (41). After passive transfer into FcRn-deficient mice, there was minimal transfer of WW1 into the vagina in 12 h (Fig. 3C), but physically or chemically induced epithelial trauma, required for effective viral challenge, greatly enhanced its transit (Fig. 3C). Surprisingly, N9 treatment releases more antibody into the vaginal vault than does epithelial abrasion elicited by brushing (Fig. 3C). This suggests that N9 (a mild detergent and widely used spermicide) can cause significant disruption to the epithelial barrier, although the BM remains intact.
There was no difference in protection against HPV16 challenge between wild-type and FcRn-deficient mice after passive transfer of WW1 IgG (Fig. 3B). This suggests that active FcRn-mediated transcytosis of systemically administered WW1 IgG is not required to protect mice from vaginal challenge with HPV, but rather, passive leakage of WW1 IgG at the site of wounding (i.e., exudation) is likely sufficient to mediate protection (Fig. 3C). However, FcRn may contribute other functions, such as extending the antibody half-life while in the systemic circulation (48, 49), which is unlikely to impact our 3-day passive-transfer studies. Our findings suggest that in vaccinated patients, neutralizing serum antibodies may reach the viral inoculum at the genital mucosa as a result of microtrauma to the epithelium during sexual intercourse and local exudation. This notion is consistent with the robust protection observed across all stages of the menstrual cycle despite fluctuating IgG levels in vaginal fluid (50, 51). Nevertheless, a limitation of passive transfer as a model for active vaccination is that it does not account for the possibility that B lymphocytes might be present within the cervicovaginal epithelium and producing neutralizing antibody locally.
Previous studies found that L2-specific neutralization does not prevent HPV interaction with its primary receptors, heparan sulfate proteoglycans (HSPGs), on the basement membrane during infection, but the antibody-bound pseudovirions fail to bind basal keratinocytes and are lost over time (3). The disappearance of the antibody-bound pseudovirions was associated with cellular infiltrates consisting mainly of neutrophils, which is suggestive of opsonization and phagocytosis of L2 antibody-bound virions upon Fc-dependent recognition by neutrophils and/or wound macrophages. To examine whether effector functions mediated by Fc, such as opsonization, contributed to protection, we performed IgG passive-transfer experiments in animals pretreated with monoclonal antibody RB6-8C5, which specifically depletes neutrophils as well as macrophages at the site of wounding (52). While the depletion of these phagocytes does not significantly affect HPV16 PsV infection (Fig. 5A), L2 MAb-mediated protection was reduced ∼10-fold (Fig. 5A), suggesting that a secondary mode of L2 antibody-mediated protection is via phagocytosis by infiltrating Ly6G/C+ neutrophils/macrophages, likely attracted by epithelial trauma. In addition, this supports the phenomenon observed for other viruses whereby antibodies that bind to virions but fail to neutralize in vitro may still contribute to protection in vivo via opsonization. Indeed, such findings have been recently documented for broadly neutralizing influenza virus antibodies that fail to neutralize in vitro but are strongly protective in vivo (53, 54).
In summary, we have created two new broadly reactive L2-specific rat MAbs and shown that L2 antibody mediates protection by both opsonization of extracellular pseudovirions and direct neutralization upon release by exudation at the site of infection. The role of exudation in the access of circulating IgG to the viral inoculum suggests that measurement of serum antibody titers is a relevant correlate. However, the previously unknown role of opsonization in protection may help account for why L2-specific antibodies are more effective at blocking infection in passive-transfer studies than in in vitro neutralization assays.
MATERIALS AND METHODS
Ethics statement.
Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (55) and with the prior approval of the Animal Care and Use Committee of The Johns Hopkins University. Four- to eight-week-old female BALB/c, C57BL/6J, or FcRn knockout (Fcgrttm1Dcr C57BL/6J background) mice were purchased from Jackson Laboratories Inc. For knockout studies, Fc receptor knockout mice [C.129P2(B6)-Fcer1gtm1Rav N12 background] and wild-type control BALB/c mice were purchased from Taconic Inc., and Lewis rats were purchased from Harlan/Envigo.
Cell cultures.
293TT, 293TTF, and LoVoT cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum, 1× penicillin and streptomycin, 1× nonessential amino acids, and 1× sodium pyruvate (Gibco, Life Technologies, Grand Island, NY). Totals of 2 μg/ml and 200 μg/ml of puromycin and hygromycin were added to 293TTF and LoVoT cells, respectively, to maintain the expression of furin/T antigen.
Generation of HPV pseudoviruses.
Pseudoviruses (PsV) and furin-cleaved pseudoviruses (fcPsV) were generated as previously described (see http://home.ccr.cancer.gov/Lco/pseudovirusproduction.htm and reference 39, respectively). The HPV L1/L2 expression plasmids utilized in this study were previously described (36) and are available via http://home.ccr.cancer.gov/LCO/ or http://www.addgene.org/Richard_Roden/.
Recombinant proteins, peptides, monoclonal antibodies, and sera.
The anti-L2 mouse MAb RG1 was previously generated (15). Full-length HPV L2 from HPV6/11/16/18/31 and L2α11-88×8, L2α11-88×8ΔTm, and 13-45×15 antigens were expressed in bacteria and purified as previously described (20). The antisera from rabbits immunized with the indicated type of L1 VLP, PsV, or α11-88×8 L2 antigens were described previously (20).
Generation of rat monoclonal antibodies to L2 and F(ab′)2.
Two Lewis rats were immunized five times with 50 μg of L2α11-88×8 formulated with TiterMax adjuvant (TiterMax USA Inc.). One week following the final immunization, splenocytes were harvested and fused with SP2/0 myeloma cells using polyethylene glycol (PEG). Supernatants from wells containing viable hybridomas were screened 10 to 14 days after fusion by an ELISA using bacterially expressed 6His-tagged L2, L2α11-88×8, or the full-length L2 peptide derived from HPV type 11, 16, 18, or 31 as a coating antigen. Selected positive clones were recloned three times by limiting dilution, and antibodies secreted were affinity purified using protein G-Sepharose (GE Healthcare). Purified WW1 and S10 rat MAbs were cleaved with pepsin-Sepharose to generate F(ab′)2 fragments using kits according to product instructions (Pierce, ThermoScientific). Coomassie blue staining and L2 ELISAs were performed to assess the purity and concentration of antibody fragments and their binding properties. The rat anti-CD4 antibody (clone GK1.5) was utilized as the nonspecific rat IgG control antibody (BioXCell, West Lebanon, NH).
Generation of JWW3.
The JWW3 monoclonal Ig2b utilized the previously reported variable Fab region sequences of mouse monoclonal antibody Mab24B (17). These variable regions were linked to the constant light and heavy chain regions of a mouse IgG2b sequence by direct synthesis (Biobasic Inc., Ontario, Canada). The heavy and light chains of JWW3 were each cloned into a double expression vector, pVITRO1-neo-mcs (InvivoGen, San Diego, CA), to generate the JWW3 plasmid. Expression and purification were then performed as previously described (43).
Characterization of rat MAbs.
Determination of the Ig subclass of the rat MAbs was performed using a commercial isotyping kit (BD Biosciences, CA). ELISA plates were coated with commercial overlapping 15-mer peptide arrays (Mimotope, Minneapolis, MN) comprising the peptides within the regions spanning residues 11 to 88 of 15 different HPV types for the binding assay. Hybridoma supernatants (1:200) or purified antibodies (1:5,000; from stocks of 1 to 2 mg/ml) were added to the blocked peptide array plates and incubated for 1 h at 37°C. Plates were washed three times with phosphate-buffered saline (PBS) containing 0.01% (vol/vol) Tween 20 (PBST) and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rat IgG for 60 min at 37°C. After washing with PBST, bound antibody was detected with 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt (ABTS; Roche, Basel, Switzerland).
Western blot analysis.
For the detection of L2 in pseudoviruses, samples normalized to the L1 protein (500 ng) were boiled for 5 min in reducing gel sample buffer and analyzed by SDS-PAGE using 4 to 20% precast Tris-HCl gels (Bio-Rad, CA). Proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, CA), and the PVDF membranes were blocked with 5% skim milk in PBST for 1 to 2 h before being incubated with the appropriate MAbs or serum samples at a 1:5,000 dilution. After incubation overnight, blots were washed with PBST three times for 10 min each before the addition of either HRP-conjugated goat anti-rat IgG, sheep anti-mouse IgG (GE Healthcare), or sheep anti-rabbit IgG (GE Healthcare) secondary antibody (1:10,000), as appropriate. The secondary antibody was incubated for 1 to 2 h at room temperature. Membranes were then washed with PBST three times for 10 min each time. A chemiluminescence substrate was added to develop the membranes.
ELISA.
Microtiter plates were coated with 500 ng/well HPV PsV, containing either wild-type L2, alanine mutant L2 (37), or 100 ng/well of L2 antigens, including the synthetic peptide comprising L2 amino acids 17 to 38 derived from various HPV types, in 50 μl PBS per well by incubation at 4°C overnight or at 37°C for 2 h. Coated plates were blocked with PBS supplemented with 1% (wt/vol) bovine serum albumin (BSA) (PBS-BSA) at 37°C for 1 to 2 h and then incubated with 50 μl of monoclonal antibody or serum diluted in PBS-BSA for 1 h at ambient temperature. Plates were then washed three times with PBST, and bound antibodies were detected with HRP-conjugated protein G (Pierce, Rockford, IL) or biotinylated mouse anti-rat IgG2a or Igκ light chain-specific monoclonal antibodies, followed by HRP-streptavidin (1:1,250 dilution of a 0.1-mg/ml stock; KPL, Gaithersburg, MD), before the addition of the ABTS substrate (Roche). The cutoff optical density (ODcutoff) value for the ELISA was set at three times the average absorbance detected from no-primary-antibody control wells. The highest dilution of serum samples that had an OD value equal to or above the ODcutoff was defined as the endpoint ELISA titer and expressed as its reciprocal value or antibody concentration.
HPV in vitro neutralization.
A total of 15,000 293TT or LoVoT cells/well were preplated into a 96-well plate. Twenty-four hours later, on a separate plate, monoclonal antibodies (WW1, RG1, S10, and rat control IgG) were serially diluted 2-fold in culture medium across the plate. Subsequently, an equal volume of HPV pseudovirions containing luciferase reporter genes was added to the plate, and this mixture was incubated at 37°C for 2 h and added to either the preplated 293TT or LoVoT cells. The cells were then incubated for 72 h. To measure neutralization, culture media were removed from each well, and 1× cell culture lysis reagent (Promega, Madison, WI) was added to lyse the cell monolayer for 15 min at room temperature on a rocking platform. The entire lysate from each well was transferred to a black 96-well plate, followed by the addition of 50 μl/well of 1× luciferase substrate (GloMax-Multi detection system; Promega, Madison, WI). The neutralization titer was determined as the reciprocal of the dilution that causes a 50% reduction in luciferase activity.
Passive transfer of antibodies and in vivo PsV vaginal challenge.
Four- to eight-week-old female BALB/c mice were injected subcutaneously with 3 mg of medroxyprogesterone (Depo-Provera; Pfizer) to synchronize their estrus cycles. Three days later, the mice in groups of 5 or 10 were administered i.p. either purified MAbs, control IgGs, or HPV L1 VLP- or L2-specific rabbit antisera as a positive control. Twenty-four hours after passive transfer, each mouse was given a HPV PsV challenge dose of 40 μl comprised of 20 μl PsV mixed with 20 μl of 3% carboxymethyl cellulose (CMC). Half of the challenge dose (20 μl) was injected into the mouse vaginal vault, followed by the insertion of a cytobrush cell collector that was turned both clockwise and counterclockwise 15 times to induce epithelial abrasion without evident release of blood. The dose of HPV PsV was selected by titration to achieve a sufficient signal-to-noise ratio (>20-fold over the background). After removal of the cytobrush, the remaining half of the inoculum was deposited in the vagina while the mice were anesthetized. For experiments using the N9 method, 24 h following passive immunization, mice were pretreated with 4% N9 while being anesthetized, and the entire virus inoculum dosage (40 μl) was delivered into the vaginal vault. Seventy-two hours after HPV PsV challenge, the mice were again anesthetized, and 20 μl of luciferin (7.8 mg/ml in water) was deposited in the vaginal vault. Luciferase signals were acquired for 10 min with a Xenogen IVIS 100 imager, and analysis was performed with Living Image 2.0 software. For wound neutrophil and macrophage depletion, mice were treated with 100 μg/mouse of the rat monoclonal RB6-8C5 antibody (BioXCell, West Lebanon, NH) for 3 consecutive days, followed by the administration of this antibody once a week. Depletion was ensured via flow cytometry prior to passive transfer and HPV16 PsV challenge studies. For knockout studies, 4- to 8-week-old female BALB/c mice or Fc receptor knockout mice [C.129P2(B6)-Fcer1gtm1Rav N12] of the same BALB/c background were utilized.
Vaginal lavage and ex vivo detection of L2-specific MAb.
Rat L2-specific MAb was passively transferred into BALB/c, C57BL/6J, or Fcgrttm1Dcr (C57BL/6J background) mice, and the amount recovered by vaginal lavage was determined with an L2-specific ELISA. Mice were administered 3 mg of medroxyprogesterone (Depo-Provera; Pfizer) 4 days before the passive transfer of 100 μg rat MAbs. Mice were anesthetized, and cytobrush or N9 treatment, as described previously (25), was then performed simultaneously with antibody transfer or 6 h earlier. Lavage fluids were collected 6 or 12 h following the passive transfer of MAb. At the indicated times, the mouse was anesthetized, 500 μl of 1× PBS was deposited twice, and lavage fluids were then collected and kept at −20°C for analysis with an L2-specfic ELISA.
Sequencing of rat antibody genes.
The cDNA encoding WW1 was generated from the hybridoma, cloned, and sequenced by Aldevron (Madison, WI).
Bioinformatic and statistical analyses.
Data were analyzed with either the Mann-Whitney U test or the Kruskal-Wallis test using GraphPad Prism 6.0. Sequences of HPV L2 types were obtained from PaVE (PapillomaVirus Episteme) (http://pave.niaid.nih.gov/#home) and analyzed using the UCSF chimera package (http://www.cgl.ucsf.edu/chimera).
ACKNOWLEDGMENTS
The study was funded by grants from the National Cancer Institute (P50 CA098252 and CA118790) and the V foundation (http://www.jimmyv.org/) to Richard B. S. Roden. Chimera was developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS grant P41-GM103311).
Joshua W. Wang is a founder of PathoVax LLC. Richard B. S. Roden is a cofounder of and has an equity ownership interest in Papivax LLC, and he owns Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.'s scientific advisory board. Richard B. S. Roden is an inventor of L2-related patents licensed by The Johns Hopkins University to PathoVax LLC and BravoVax. Richard B. S. Roden has received research funding from Sanofi Pasteur, Shantha Biotechnic, and GlaxoSmithKline; has an equity ownership interest in PathoVax LLC; and is a member of PathoVax LLC's scientific advisory board. These arrangements have been reviewed and approved by The Johns Hopkins University in accordance with its conflict-of-interest policies.
REFERENCES
- 1.de Villiers EM. 2013. Cross-roads in the classification of papillomaviruses. Virology 445:2–10. doi: 10.1016/j.virol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Cubie HA. 2013. Diseases associated with human papillomavirus infection. Virology 445:21–34. doi: 10.1016/j.virol.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Hausen HZ, Devilliers EM, Gissmann L. 1981. Papillomavirus infections and human genital cancer. Gynecol Oncol 12:S124–S128. doi: 10.1016/0090-8258(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 4.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A 89:12180–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller JT, Castellsague X, Garland SM. 2012. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Future I/II Study Group, Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Lehtinen M, Steben M, Bosch FX, Joura EA, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan JT, Maansson R, Lu S, Vuocolo S, Hesley TM, Barr E, Haupt R. 2010. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversene OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olssonn SE, Tang GWK, Ferris DG, Paavonen J, Lehtinen M, Steben M, Bosch X, Dillner J, Kurmanv RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinaccia LC, Giacoletti KED, Lu S, Vuocolo S, Hesley TM, Haupt RM, Barr E. 2008. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, Chow SN, Kitchener H, Teixeira JC, Hedrick J, Limson G, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, De Carvalho NS, Germar MJ, Peters K, Mindel A, De Sutter P, Bosch FX, David MP, Descamps D, Struyf F, Dubin G, HPV PATRICIA Study Group. 2012. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 9.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, James M, Vuocolo S, Hesley TM, Barr E. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis 199:936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 11.Wang JW, Roden RB. 2013. L2, the minor capsid protein of papillomavirus. Virology 445:175–186. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol 81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Torrimasino M, Muller M, Ottonello S. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949–1956. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 14.Kondo K, Ishii Y, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. 2007. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 358:266–272. doi: 10.1016/j.virol.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawana K, Yasugi T, Kanda T, Kino N, Oda K, Okada S, Kawana Y, Nei T, Takada T, Toyoshima S, Tsuchiya A, Kondo K, Yoshikawa H, Tsutsumi O, Taketani Y. 2003. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine 21:4256–4260. doi: 10.1016/S0264-410X(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 17.Nakao S, Mori S, Kondo K, Matsumoto K, Yoshikawa H, Kanda T. 2012. Monoclonal antibodies recognizing cross-neutralization epitopes in human papillomavirus 16 minor capsid protein L2. Virology 434:110–117. doi: 10.1016/j.virol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Rubio I, Seitz H, Canali E, Sehr P, Bolchi A, Tommasino M, Ottonello S, Muller M. 2011. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology 409:348–359. doi: 10.1016/j.virol.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572–579. doi: 10.1016/0042-6822(91)90890-N. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Yemelyanova AV, Gambhira R, Jagu S, Meyers C, Kirnbauer R, Ronnett BM, Gravitt PE, Roden RB. 2009. Expression pattern and subcellular localization of human papillomavirus minor capsid protein L2. Am J Pathol 174:136–143. doi: 10.2353/ajpath.2009.080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagu S, Kwak K, Karanam B, Huh WK, Damotharan V, Chivukula SV, Roden RB. 2013. Optimization of multimeric human papillomavirus L2 vaccines. PLoS One 8:e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. 2012. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One 7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RBS, Kirnbauer R. 2013. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Investig Dermatol 133:2706–2713. doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. 2010. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 26.Wang JW, Jagu S, Wang C, Kitchener HC, Daayana S, Stern PL, Pang S, Day PM, Huh WK, Roden RB. 2014. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV pseudovirions. PLoS One 9:e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handisurya A, Day PM, Thompson CD, Buck CB, Kwak K, Roden RB, Lowy DR, Schiller JT. 2012. Murine skin and vaginal mucosa are similarly susceptible to infection by pseudovirions of different papillomavirus classifications and species. Virology 433:385–394. doi: 10.1016/j.virol.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. 2008. Mechanisms of human papillomavirus type 16 neutralization by L2 cross-neutralizing and L1 type-specific antibodies. J Virol 82:4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowy DR, Day PM, Thompson CD, Pang YS, Kines RC, Schiller JT. 2013. The mechanisms by which HPV vaccines induce protection against HPV-associated cancers. J Acquir Immune Defic Syndr 62:59. doi: 10.1097/01.qai.0000429256.77899.ee. [DOI] [Google Scholar]
- 30.Ishii Y, Tanaka K, Kondo K, Takeuchi T, Mori S, Kanda T. 2010. Inhibition of nuclear entry of HPV16 pseudovirus-packaged DNA by an anti-HPV16 L2 neutralizing antibody. Virology 406:181–188. doi: 10.1016/j.virol.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. 2013. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol 14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauler F, Mallery DL, McEwan WA, Bidgood SR, James LC. 2012. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc Natl Acad Sci U S A 109:19733–19738. doi: 10.1073/pnas.1210659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keeble AH, Khan Z, Forster A, James LC. 2008. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A 105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, Wang C, Wang HK, Chow LT, Huh WK, Jaganathan KS, Chivukula SV, Roden RB. 2013. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol 87:6127–6136. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak K, Jiang R, Wang JW, Jagu S, Kirnbauer R, Roden RB. 2014. Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta human papillomavirus types. PLoS One 9:e97232. doi: 10.1371/journal.pone.0097232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gambhira R, Jagu S, Karanam B, Day PM, Roden R. 2009. Role of L2 cysteines in papillomavirus infection and neutralization. Virol J 6:176. doi: 10.1186/1743-422X-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jagu S, Malandro N, Kwak K, Yuan H, Schlegel R, Palmer KE, Huh WK, Campo MS, Roden RB. 2011. A multimeric L2 vaccine for prevention of animal papillomavirus infections. Virology 420:43–50. doi: 10.1016/j.virol.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JW, Jagu S, Kwak K, Wang C, Peng S, Kirnbauer R, Roden RB. 2014. Preparation and properties of a papillomavirus infectious intermediate and its utility for neutralization studies. Virology 449:304–316. doi: 10.1016/j.virol.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. 2011. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol 85:13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. 2011. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A 108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai Y, Ye L, Tesar DB, Song H, Zhao D, Bjorkman PJ, Roopenian DC, Zhu X. 2011. Intracellular neutralization of viral infection in polarized epithelial cells by neonatal Fc receptor (FcRn)-mediated IgG transport. Proc Natl Acad Sci U S A 108:18406–18411. doi: 10.1073/pnas.1115348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JW, Jagu S, Wu WH, Viscidi RP, Macgregor-Das A, Fogel JM, Kwak K, Daayana S, Kitchener H, Stern PL, Gravitt PE, Trimble CL, Roden RB. 2015. Seroepidemiology of human papillomavirus 16 (HPV16) L2 and generation of L2-specific human chimeric monoclonal antibodies. Clin Vaccine Immunol 22:806–816. doi: 10.1128/CVI.00799-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEwan WA, Hauler F, Williams CR, Bidgood SR, Mallery DL, Crowther RA, James LC. 2012. Regulation of virus neutralization and the persistent fraction by TRIM21. J Virol 86:8482–8491. doi: 10.1128/JVI.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. 2006. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res 66:11120–11124. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 46.Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. 2012. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol 19:1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell MW, Mestecky J. 2002. Humoral immune responses to microbial infections in the genital tract. Microbes Infect 4:667–677. doi: 10.1016/S1286-4579(02)01585-X. [DOI] [PubMed] [Google Scholar]
- 48.Andersen JT, Dalhus B, Viuff D, Ravn BT, Gunnarsen KS, Plumridge A, Bunting K, Antunes F, Williamson R, Athwal S, Allan E, Evans L, Bjoras M, Kjaerulff S, Sleep D, Sandlie I, Cameron J. 20 March 2014. Extending serum half-life of albumin by engineering FcRn binding. J Biol Chem. doi: 10.1074/jbc.M114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olafsen T. 2012. Fc engineering: serum half-life modulation through FcRn binding. Methods Mol Biol 907:537–556. doi: 10.1007/978-1-61779-974-7_31. [DOI] [PubMed] [Google Scholar]
- 50.Vinzon SE, Braspenning-Wesch I, Muller M, Geissler EK, Nindl I, Grone HJ, Schafer K, Rosl F. 2014. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: a preclinical study using a natural outbred animal model. PLoS Pathog 10:e1003924. doi: 10.1371/journal.ppat.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiller JT, Lowy DR. 2012. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 10:681–692. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 53.DiLillo DJ, Palese P, Wilson PC, Ravetch JV. 2016. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiLillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]