Abstract
Background
Postoperative cholangitis is a common but severe complication after Kasai portoenterostomy for biliary atresia (BA). This study aimed to identify its prognostic factors.
Methods
Two sets of liver paraffin-embedded tissue samples were collected from BA patients who received Kasai portoenterostomy (n = 25 and n = 31, respectively). Patients were divided into non-cholangitis and cholangitis groups. The infiltration of CD4+, CD8+, CD45RO+, CD68+ cells and expression of Beclin1 were quantitatively evaluated in immunohistochemical analysis.
Results
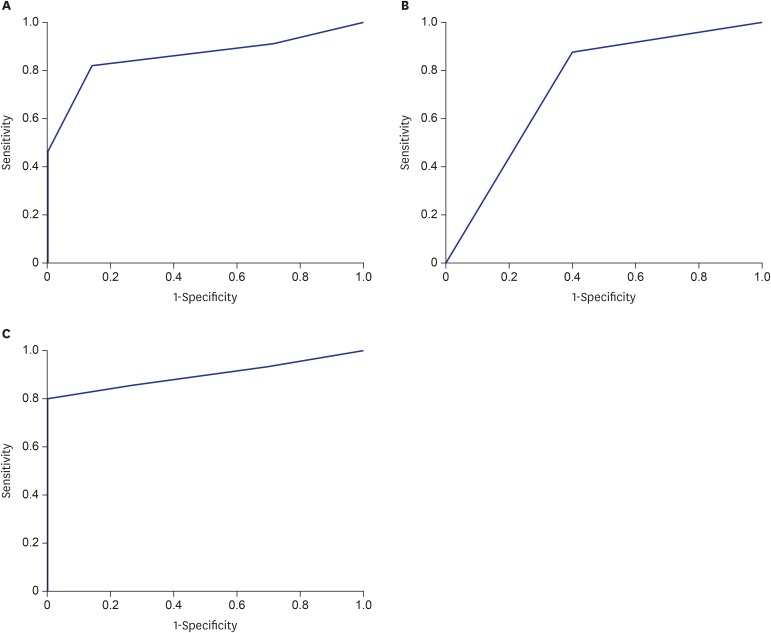
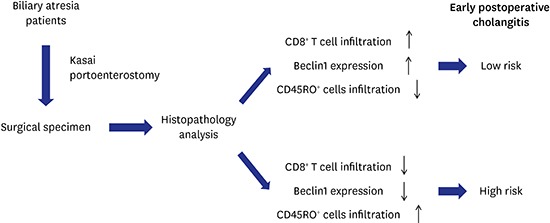
Cholangitis group had a significantly lower CD8+ T cell infiltration but a higher CD45RO+ cell infiltration, and a lower Beclin1 level than non-cholangitis group (all P < 0.01). Multivariate logistic regression analysis indicated that infiltration of CD8+ cells (odds ratio [OR], 0.112; 95% confidence interval [CI], 0.022–0.577) and CD45RO+ cells (OR, 3.88; 95% CI, 1.37–11.03), and Beclin1 level (OR, 0.088; 95% CI, 0.018–0.452) were independent influence factors for early postoperative cholangitis. Receiver operating characteristic (ROC) analysis showed that area under ROC curve (AUROC) values for CD8+ cells, CD45RO+ cells and Beclin1 were 0.857, 0.738 and 0.900, respectively.
Conclusion
Our findings demonstrated the CD8+ cells, CD45RO+ cells and Beclin1 level possessed the prognostic value for early postoperative cholangitis following Kasai operation, which may be helpful to develop new prevention and treatment strategies for postoperative cholangitis.
Keywords: Biliary Atresia, Kasai Portoenterostomy, Postoperative Cholangitis, CD45RO+ T Infiltration, Beclin1
Graphical Abstract
Introduction
Biliary atresia (BA) is a rare but life-threatening neonatal disease characterized by obliteration of one or more bile ducts, which leads to impaired bile flow, cholestasis, rapid progression of liver fibrosis and irreversible cirrhosis, and eventually liver failure. A geographical variation could be observed in the incidence of BA, namely about 1 in 5,000–10,000 live births in East Asia,1,2,3 but about 1 in 15,000–20,000 in Western countries.4,5 The cause of BA can be congenital or acquired, but the precise etiology remains poorly understood.6 At present, the portoenterostomy (the Kasai operation) remains the first-line treatment for BA.7 Kasai operation was first described by the Japanese surgeon Morio Kasai in 1959.8 In this surgical procedure, the porta hepatis is excised to expose the ductules to which a Roux-en-Y jejunal loop is anastomosed, allowing for bile flow into the duodenum.7 A study has demonstrated that Kasai operation can restore bile flow in 30%–80% of BA patients within the first 2–3 months of age.9 However, this surgical management is a palliative but not curative treatment for BA. It has been shown that the disease still progresses in the majority of BA patients with a successful Kasai operation, and the symptoms include progressive inflammation, fibrosis of intrahepatic bile ducts and cirrhosis. As a result, up to 70%–80% of BA patients still require liver transplantation eventually.10 Even though liver transplantation is a curative therapy, however, in China, the cases of liver transplantation for BA patients are still few due to the severe shortage of organ sources and the economic burden of postoperative immunosuppressive therapy. Hence, the Kasai operation remains the main treatment for BA and its therapeutic outcomes directly impact the patients' survival.
Cholangitis is a common postoperative complication of Kasai operation. Postoperative recurrent cholangitis accelerated the development of liver fibrosis and other severe complications, such as ascites, gastrointestinal bleeding, and growth retardation. It has been suggested that disease progression after Kasai operation most likely occurs in the patient with recurrent cholangitis,11 suggesting that occurrence of postoperative cholangitis indicates a poor prognosis. Cholangitis is one of the most crucial factors for long-term survival after a successful portoenterostomy.12 Early postoperative cholangitis has a serious impact on patient's quality of life, physical and mental development, as well as causes a heavy burden on the patients and their parents. Prevention and treatment of early postoperative cholangitis after Kasai operation is crucial for BA treatment. Therefore, to reduce the incidence of postoperative cholangitis, a variety of modifications of original Kasai operation have been developed.13,14,15,16,17 Nevertheless, the benefits of these anti-cholangitis modifications could not be approved. In clinical practice, even a strict anti-infective treatment is adopted for BA patients after Kasai operation, but the incidence of early postoperative cholangitis is still high to 60%–100%.18 In addition, once postoperative cholangitis occurs, 90% of patients will suffer from repeated attacks of cholangitis.11
Pathological studies on the hepatic tissue of BA patients showed that the pathological basis of cholangitis includes atrophy, disappearance and necrosis in the bile duct. Infiltration of immune cells around the ductules and necrosis of cholangiocytes lead to partial bile duct obstruction and eventually cholangitis. However, the precise mechanism underlying postoperative cholangitis after Kasai operation remains not fully understood. Immune systems have been shown to involve in the pathogenesis of BA. T lymphocytes infiltration around the intrahepatic bile ducts is a pathological characteristic of BA, and CD4+ and CD8+ T cells are the predominant types of the infiltrated cells.9 Kotb et al.19 have reported patients with a higher CD4+/CD8+ ratio have a favorable survival outcome, suggesting a prognostic value of infiltrated CD4+ and CD8+ T cells in BA patients. Autophagy is a form of programmed cell death by which cells undergo partial autodigestion to obtain nutrients and prolong survival for a short time under starvation, hypoxia and high-temperature conditions.20 Beclin1 is a protein associated with BCL-2 or PI3k class III protein, and play a crucial role in the regulation of both autophagy and apoptosis.21 Thus far, it is still unknown whether Beclin1 is implicated in the pathogenesis of BA.
When confirming the diagnosis of postoperative cholangitis, the liver of BA patients has already begun to be damaged, which may eventually induce irreversible disease progress. If a preventive therapy could be conducted prior to the onset of symptoms, it may reduce the incidence of postoperative cholangitis and improve the therapeutic outcomes of Kasai operation for BA patients. In this context, identifying the prognostic markers for early postoperative cholangitis may be useful to conduct preventive therapies for patients with a high risk of early postoperative cholangitis. Therefore, the purpose of this study was to investigate the immune cells infiltration and the expression of Beclin1 in the hepatic tissue of BA patients, and to address if these molecules possess the prognostic value. By assessment of these molecules in the hepatic tissue excised from BA patients, three prognostic markers for early postoperative cholangitis were identified in BA patients after Kasai operation.
Methods
Patients and tissue samples collection
In the current study, we performed two independent sample collections. The Study 1 aimed to investigate the infiltration of CD4+ and CD8+ T cells in the hepatic tissue of BA patients and its prognostic value for early postoperative cholangitis. Based on the findings of the study, we subsequently designed Study 2 to further investigate the subtypes of inflammatory cells and if the autophagy is involved in the hepatic tissue of BA patients. Due to the small size of paraffin specimens used in Study 1 (about 2 cm × 1 cm), some paraffin-embedded tissue blocks were insufficient for the Study 2 after serially sectioned into 5–8 sections in the Study 1. Therefore, some new specimens were supplemented in the Study 2. A total of 31 BA patients receiving surgical treatment at our center from January 2007 to January 2009 were included in Study 2. There were 11 patients overlapping between Study 1 and Study 2. The inclusion criteria were: 1) patients received exploratory laparotomy at our center, and had a confirmed diagnosis of type III BA by intraoperative extrahepatic cholangiography or methylene blue injection. The patients then underwent Kasai operation; 2) intraoperative wedge biopsy specimens were collected from the anterior right lobe of the liver.
Diagnostic criteria of early postoperative cholangitis were within one month after surgery, patients with: 1) high fever or remittent, bile discharge reduced or completely stopped; 2) abdominal distension, vomiting and reduced liver function; 3) worsening jaundice in a short period, elevated levels of serum bilirubin and alanine aminotransferase; 4) pale or clay-colored stools, dark yellow-colored urine; 5) level of white blood cells elevated significantly, especially the neutrophils.
Surgery and treatment
BA Patients were treated with hilar jejunum anastomosis surgical treatment. Postoperative adjuvant treatments for all patients included: 1) fasting and intravenous nutrition support for 3 or 7 days after the Kasai operation; 2) intravenous dexamethasone (0.4 mg/kg) for one week at the 2nd week postoperation; 3) for anti-infective treatment, intravenous or oral third-generation cephalosporins at least 1 month postoperation. For patients with repeated fever, the fourth-generation cephalosporins would be used: 4) intravenous infusion of Yinzhihuang injection (proprietary Chinese medicine) and polyene phosphatidylcholine for liver protection; 5) after discharge, long-term oral administration of ursodeoxycholic acid, Yinzhihuang decoction, glucurolactone to protect gallbladder and liver.
Hematoxylin and eosin (H & E) staining and IHC analysis
Paraffin-embedded tissue blocks were pre-cooled at −20°C for 30 minutes, and were then serially sectioned in 3 µm–4 µm thick sections, transferred to slides. Sections were incubated at 40°C overnight, then de-paraffinized in dimethylbenzene and rehydrated in graded ethanol solutions. For H & E staining, slides were stained in hematoxylin solution for 5 minutes, and immersed in 0.5% hydrochloric alcohol, 0.5% ammonia and 0.5% eosin 3 minutes, slides were extensively washed between each immersion step. The slides were then dehydrated in graded ethanol solutions and dimethylbenzene, followed by sealed with neutral resin. For immunohistochemical (IHC) staining, slides were treated with 10 mM sodium citrate buffer (pH 6.0) at 96°C for 10 minutes for antigen retrieval. The slides were then incubated with blocking solution (5%–10% goat serum) at room temperature for 20 minutes, and subsequently primary antibodies (20 × dilution) at 37°C for 1 hour and secondary antibodies at 37°C for 30 minutes. After another PBS wash, the peroxidase reaction was developed using a DAB detection kit (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) according to manufacturer's protocol. The slides were stained with hematoxylin for 2 minutes, differentiated in 0.1% hydrochloric acid alcohol for 5 seconds, treated with bluing in running water for 10 minutes, dehydrated by gradient ethanol treatments. The sections were then sealed with neutral resin. The slides were visualized and photographed using a phase contrast microscope (CX41; Olympus, Tokyo, Japan). Primary antibodies (mouse anti-human) specific for CD4 (Novocastra, London, UK), CD8 (Novocastra), CD68 (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), CD45PRO (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), PCNA (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd)., Beclin1 (Abcam, Cambridge, MA, USA), TGF-β1 (Santa Cruz, Dallas, TX, USA) and secondary antibody goat anti-mouse IgG/HRP (DAKO, Carpenteria, CA, USA) were all used in the IHC analyses.
IHC scoring assessment
Each IHC slide was examined by two pathologists (JZ and MS) blinded to the subject's clinical history. Slide of lymph node specimen from children without biliary disease was used as a positive control. A negative control slide was prepared by substituting saline buffer for the primary antibodies. Five representative high power fields were selected for each slide, and the total number of cells should be over 1,000. Semi-quantitative analysis was performed for the results based on the proportion of positive cells in all the cells and the intensity of antibody staining in positive cells.22 The staining intensity was scored as 0 (no staining), 1 (light yellow), 2 (yellow), 3 (brown) or 4 (dark brown). The proportion of positive cells was scored as 0 (< 5%), 1 (5% to 25%), 2 (26% to 50%), 3 (51% to 75%) or 4 (> 75%). The scoring results from two pathologists were averaged, and the average score from staining intensity and proportion of positive cells were summed up as the total score. Total scores were ranked into 4 grades: rank 1 (0–2 points), rank 2 (3–4 points), 3 (5–6 points), 4 (7–8 points).
Statistical analysis
Continuous data were presented as the mean ± standard deviation (SD) and tested by Mann-Whitney U test. Categorical data were demonstrated by number (N) and percentage in cross-tables and tested by χ2 test. Rank data including CD4, CD8, CD68, CD45RO, and Beclin1 were analyzed as both continuous and categorical variables. Univariate and multivariate logistic regression were utilized to investigate the independent variables which were associated with early cholangitis. Significant independent variables would be further analyzed by receiver operating characteristic (ROC) curve to observe their area under ROC curve (AUROC) and potential capacity in diagnosis. A rough guide for assessing the utility of a biomarker based on its AUC is as follows: 0.9–1.0 = excellent; 0.8–0.9 = good; 0.7–0.8 = fair; 0.6–0.7 = poor; 0.5–0.6 = fail.23 All statistical analyses were performed using IBM SPSS Version 20 (SPSS Statistics V20; IBM Corporation, Somers, NY, USA). The significance level was set at P < 0.05, two-tailed.
Ethics statement
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-Sen University on January 9, 2009, and written informed consent was obtained from all patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
Patient demographics
A total of 25 and 31 BA patients receiving Kasai operation were included in Study 1 and Study 2, respectively. In both studies, patients were divided into non-cholangitis and cholangitis groups depending on whether they had early postoperative cholangitis or not. At one month postoperation, there were 14 and 16 patients diagnosed with early postoperative cholangitis according to the clinical manifestations and laboratory tests in the Study 1 and Study 2, respectively. The demographic and clinical baseline characteristics of the patients were summarized in Table 1. There was no significant difference in the demographic and clinical baseline characteristics, including pathological diagnosis, jaundice duration, levels of total bilirubin, direct bilirubin, indirect bilirubin, white blood cell red blood cell, neutrophil and hemoglobin between non-cholangitis and cholangitis groups in both studies (all P > 0.05, Table 1), indicating the two groups are comparable.
Table 1. Patient demographic and baseline clinical characteristics.
| Parameters | Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|---|
| Non-cholangitis (n = 11) | Cholangitis (n = 14) | P | Non-cholangitis (n = 15) | Cholangitis (n = 16) | P | ||
| Sex | 0.697 | 0.376 | |||||
| Male | 6 (55) | 9 (64) | 8 (53) | 6 (38) | |||
| Female | 5 (45) | 5 (36) | 7 (47) | 10 (63) | |||
| Age, day | 67.09 ± 13.49 | 62.29 ± 13.05 | 0.661 | 52.93 ± 20.36 | 58.88 ± 14.22 | 0.477 | |
| Pathological diagnosis | 1.000 | 1.000 | |||||
| Neonatal hepatitis syndrome | 11 (100) | 13 (93) | 12 (80) | 13 (81) | |||
| Biliary atresia | 0 | 1 (7) | 3 (20) | 3 (19) | |||
| Jaundice, day | 5.55 ± 5.24 | 11.79 ± 13.13 | 0.348 | 13.07 ± 13.10 | 11.88 ± 12.29 | 0.736 | |
| TBIL, µmol/L | 171.02 ± 54.32 | 207.64 ± 66.58 | 0.239 | 159.38 ± 49.70 | 184.72 ± 60.89 | 0.252 | |
| DBIL, µmol/L | 107.23 ± 31.66 | 124.26 ± 53.19 | 0.381 | 108.63 ± 33.45 | 125.12 ± 34.26 | 0.453 | |
| IBIL, µmol/L | 63.79 ± 50.10 | 83.38 ± 51.13 | 0.208 | 50.75 ± 33.17 | 62.07 ± 49.27 | 0.540 | |
| WBC, × 109/L | 11.50 ± 3.33 | 19.43 ± 16.77 | 0.055 | 10.56 ± 2.91 | 13.32 ± 4.57 | 0.102 | |
| Neutrophil, % | 3.65 ± 2.79 | 4.07 ± 4.12 | 0.951 | 2.48 ± 1.26 | 3.30 ± 2.77 | 0.662 | |
| RBC, × 1012/L | 3.72 ± 0.48 | 3.69 ± 0.59 | 1.000 | 3.52 ± 0.46 | 3.41 ± 0.39 | 0.582 | |
| Hb, g/L | 112.50 ± 15.21 | 109.31 ± 16.31 | 0.663 | 102.29 ± 15.61 | 103.07 ± 10.61 | 0.913 | |
Data are presented as means ± standard deviation (range) or number (%).
TBIL = total bilirubin, DBIL = direct bilirubin, IBIL = indirect bilirubin, WBC = white blood cell, RBC = red blood cell, Hb = hemoglobin.
Infiltration of CD4+ and CD8+ T cells in BA patients
Tissue samples from Study 1 were used to investigate the infiltration degree of CD4+ and CD8+ T cells. IHC analysis showed that varying degrees of infiltration of CD4+ (Supplementary Fig. 1A) and CD8+ T (Supplementary Fig. 1B) cells were observed in the hepatic tissue of all the BA patients. The infiltrated CD4+ and CD8+ T cells mainly distributed in the portal area and some cells infiltrated to the liver sinusoid. To compare degrees of infiltration between the two groups, the IHC data was quantitated. As shown in Table 2, there is no significant difference in CD4+ T infiltration between non-cholangitis and cholangitis groups (both P > 0.05), while the level or rank of CD8+ T infiltration was significantly higher in the non-cholangitis group than in cholangitis group (both P < 0.01). This data suggested that patients with early postoperative cholangitis had a lower infiltration degree of CD8+ T cells.
Table 2. Infiltration degrees of CD4+ and CD8+ T cells in patients of Study 1.
| Parameters | Non-cholangitis (n = 11) | Cholangitis (n = 14) | P | |
|---|---|---|---|---|
| CD4 | 0.82 ± 0.60 | 0.57 ± 0.51 | 0.308 | |
| Rank 0 | 3 (27) | 6 (43) | 0.348 | |
| Rank 1 | 7 (64) | 8 (57) | ||
| Rank 2 | 1 (9) | 0 (0) | ||
| Rank 3 | 0 (0) | 0 (0) | ||
| Rank 4 | 0 (0) | 0 (0) | ||
| CD8 | 3.18 ± 0.98 | 1.86 ± 0.66 | 0.002 | |
| Rank 0 | 0 (0) | 0 (0) | 0.002 | |
| Rank 1 | 1 (9) | 4 (29) | ||
| Rank 2 | 1 (9) | 8 (57) | ||
| Rank 3 | 4 (36) | 2 (14) | ||
| Rank 4 | 5 (45) | 0 (0) | ||
Data are presented as means ± standard deviation (range) or number (%).
Levels of CD68+ cells, CD45RO+ T cells and Beclin1 in BA patients
In Study 2, we compared the infiltration degrees of CD68+ cells and CD45RO+ T cells and the expression levels of Beclin. IHC data showed that CD68+ cells mainly distributed among the hepatocytes, especially within the liver sinusoid, and around the central venous, but less in the portal area (Supplementary Fig. 2). In contrast, CD45RO+ T cells were more abundant in the portal area, particularly in areas with other infiltrated inflammatory cells, but less among the hepatocytes (Supplementary Fig. 3). Beclin1 expressed in the cytoplasm and cell membrane (Supplementary Fig. 4). The IHC data was quantitated to compare levels of these markers between two groups. As shown in Table 3, the cholangitis group had a higher level or rank of CD45RO+ T cells infiltration and a significantly lower level or rank of Beclin1 protein as compared with the non-cholangitis group. No significant difference was found in the level of CD68+ cells between the two groups. This data suggested that patients with early postoperative cholangitis had a higher level of CD45RO+ T infiltration and a lower level of Beclin1 protein.
Table 3. Levels of CD68+, CD45RO+ T cells and Beclin1 in patients of Study 2.
| Parameters | Non-cholangitis (n = 15) | Cholangitis (n = 16) | P | |
|---|---|---|---|---|
| CD68 | 2.40 ± 0.91 | 2.44 ± 0.89 | 0.904 | |
| Rank 0 | 0 (0) | 0 (0) | 0.992 | |
| Rank 1 | 4 (27) | 4 (25) | ||
| Rank 2 | 1 (7) | 1 (6) | ||
| Rank 3 | 10 (67) | 11 (69) | ||
| CD45RO | 1.80 ± 1.01 | 2.75 ± 0.68 | 0.007 | |
| Rank 0 | 0 (0) | 0 (0) | 0.009 | |
| Rank 1 | 9 (60) | 2 (13) | ||
| Rank 2 | 0 (0) | 0 (0) | ||
| Rank 3 | 6 (40) | 14 (88) | ||
| Beclin1 | 2.60 ± 0.91 | 1.00 ± 0.82 | < 0.001 | |
| Rank 0 | 1 (7) | 5 (31) | < 0.001 | |
| Rank 1 | 1 (7) | 6 (38) | ||
| Rank 2 | 1 (7) | 5 (31) | ||
| Rank 3 | 12 (80) | 0 (0) | ||
Data are presented as means ± standard deviation (range) or number (%).
Independent influence factors associated with early postoperative cholangitis
Since differential levels of Beclin1 and infiltration of immune cells were observed between non-cholangitis and cholangitis groups, we attempted to identify the influence factors associated with early postoperative cholangitis. In Study 1, logistic regression showed that CD8+ cells level was a significant influence factor both in the univariate and multivariate analyses (both P < 0.05). The estimated odds ratio (OR) of CD8+ T cells after adjusting gender and age was 0.140 (95% confidence interval [CI], 0.027–0.721). Even including CD4+ cells level in the multivariate model, CD8+ T cells still reached significant (OR, 0.112; 95% CI, 0.022–0.577; P = 0.009). These results indicated that the low infiltration of CD8+ T cells was a risk factor for early postoperative cholangitis.
In Study 2, CD45RO+ T cells and Beclin1 were found steady significant in both univariate and multivariate results (all P < 0.05). In the multivariate model with adjustment for gender and age, the estimated ORs of CD45RO+ T cells and Beclin1 were 3.88 (95% CI, 1.37–11.03) and 0.088 (95% CI, 0.018–0.452). These data indicated that high infiltration of CD45RO+ T cells and low Beclin1 were risks factors for early postoperative cholangitis.
ROC curve analysis
To further evaluate the potential prognostic value of the independent influence factors for early postoperative cholangitis (CD8+, CD45RO+ T cells, and Beclin1), ROC curve analysis was utilized. As shown in Fig. 1, ROC analysis showed that all the three factors reached statistical significance (all P < 0.05) and have a good AUROC. The AUROC values were 0.857, 0.738 and 0.900 for CD8+, CD45RO+ cells and Beclin1, respectively. These data suggested that CD8+, CD45RO+ cells and Beclin1 possessed the prognostic value for early postoperative cholangitis in BA patients undergoing Kasai operation.
Fig. 1. The ROC curve of significant independent variables associated with early cholangitis, including (A) CD8, AUROC = 0.857, P = 0.003; (B) CD45RO, AUROC = 0.738, P = 0.024 and (C) Beclin1, AUROC = 0.900, P < 0.001.
ROC = receiver operating characteristic, AUROC = area under receiver operating characteristic.
Discussion
In this study, we investigated the immune cells infiltration and the expression of Beclin1 in the hepatic tissue of BA patients, and evaluated the prognostic value of these molecules for early postoperative cholangitis. The results showed the infiltrated CD4+ and CD8+ T cells mainly distributed in the portal area. CD68+ cells mainly distributed within the liver sinusoid, and CD45RO+ T cells were more abundant in the portal area. The cholangitis group had a significantly lower level of CD8+ T cells infiltration, a higher level of CD45RO+ T cells infiltration and a lower level of Beclin1 protein as compared with the non-cholangitis group. In addition, multivariate logistic regression analysis indicated that low infiltration of CD8+ T cells, high infiltration of CD45RO+ T cells and low Beclin1 level were risks factors for early postoperative cholangitis. Furthermore, ROC analysis suggested that CD8+, CD45RO+ T cells and Beclin1 possessed the prognostic value for early postoperative cholangitis in BA patients undergoing Kasai operation. Our findings may be helpful to better understand the mechanism underlying early postoperative cholangitis in BA patients, and to provide a theoretical basis for developing new prevention and treatment strategies for postoperative cholangitis in BA patients.
The cause of cholangitis is still unclear. The surgical approach of bile duct dilatation is similar with Kasai operation, but the incidence of postoperative cholangitis is much lower and is easier to control as compared with those in BA patients. Clinically, postoperative cholangitis does not occur in surgery for obstructive jaundice in patients with infant hepatitis syndrome. It has been suggested that BA is an immune-mediated inflammatory process.24 Our data showed that before Kasai operation, there have been already varying degrees of immune cells infiltrations in the hepatic tissue in all the BA patients, which is in line with previous studies.25,26,27 H & E staining showed that infiltrated CD4+ and CD8+ T cells mainly distributed in the portal area. These results suggested that CD4+ and CD8+ T cells involved in the immune injury in the pathogenesis of BA. The data also suggested that the surgical anastomosed site has the potential immune injury before Kasai operation, which may contribute to the occurrence of postoperative cholangitis. Comparison in the infiltration degree between the non-cholangitis and cholangitis group showed that the non-cholangitis group had a significantly higher infiltration degree of CD8+ T cells as compared with the cholangitis group. Consistently, Kotb et al.19 also found that BA patients with a lower CD8+ T cells infiltration have a worse prognosis. In the present study, multivariate logistic regression analysis showed that high CD8+ T cells infiltration was a protective factor for postoperative cholangitis (OR, 0.112). ROC curve analysis also confirmed that CD8+ T cells infiltration in hepatic tissue of BA patients has a good prognostic value for early postoperative cholangitis in BA patients undergoing Kasai operation (AUROC = 0.857).
CD8+ T cells may exert a cytotoxic and immunomodulatory effect. To determine if the infiltrated CD8+ T cells were cytotoxic cells, we conducted IHC staining for three CD8 cytotoxic-relative proteins, including granzyme B, perforin and Fas receptors. The results showed that all the three protein staining data was negative in hepatic tissues of BA patients (data not shown). Ahmed et al.28 have also demonstrated that granzyme B, perforin were negative in BA patients, which is consistent with our observation. These results indicated that the cytotoxic effect of CD8+ T cells may not involve in the immune injury of BA, and the infiltrated CD8+ T cells might play an immunomodulatory role rather than a cytotoxic effect in the injured region. The non-cholangitis group had a higher level of CD8+ infiltration, suggesting that these cells may have the protective function to prevent the early postoperative cholangitis. Further study is necessary to determine if the infiltrated CD8+ cells have a protective effect, and its detailed molecular mechanism.
In this study, IHC data showed that CD45RO+ T cells were more abundant in the portal area, particularly in areas with other infiltrated immune cells. In contrast, CD68+ cells mainly distributed within the liver sinusoid, and around the central venous, but less in the portal area. CD68 is a cell marker of the macrophage/monocyte. Liver macrophages are also known as Kupffer cells which are mainly located in the liver sinus cavity.29 CD45RO+ cells are memory T cells, which often accumulate in the inflammatory area or the region exposure to antigen.30 Studies have shown that CD45RO+ T cells are activated at the early stage of inflammation, and express lymphocyte-associated antigen to promote the memory of T cells homing, leading to a variety of lymphocytes infiltrating into the lesion region.31,32 Our IHC data showed that the cholangitis group had a significantly higher infiltration of CD45RO+ T cells than the non-cholangitis group. Multivariate logistic regression analysis showed that high CD45RO+ cells infiltration was a risk factor for postoperative cholangitis. The incidence of cholangitis in patients with high CD45RO+ T cells infiltration was 3.88 times higher than that of patients with low CD45RO+ T cells infiltration. ROC curve analysis showed that CD45RO+ cells infiltration had a moderate prognostic value for early postoperative cholangitis in BA patients undergoing Kasai operation (AUROC = 0.738). A variable with an AUROC > 0.8 is typically recognized as having good diagnostic value.23 Although slightly lower than 0.8, the AUROC level of CD45RO still reached a fair rank.23 Taken together, these results indicated that CD45RO+ T cells may account for the chronic inflammation in the liver of BA patients. However, the role of infiltrated CD45RO+ cells in the hepatic tissues of BA patients and the mechanism for postoperative cholangitis remains to be further investigated.
We also assessed the Beclin1 expression level in the hepatic tissues of BA patients. IHC data showed that the non-cholangitis group had a significantly higher level of Beclin1 than the cholangitis group. In addition, 80% of patients in the non-cholangitis group were strongly positive for Beclin1 protein (rank 3), whereas 31% of patients in the cholangitis group exhibited negative for Beclin1 (rank 0). Multivariate logistic regression showed that high level of Beclin1 was a protective factor for postoperative cholangitis (OR, 0.088), suggesting that the incidence of postoperative cholangitis was 11.36 times higher in patients with a low Beclin1 than in patients with a high level of Beclin1. Furthermore, ROC curve analysis showed that Beclin1 level had an excellent prognostic value for early postoperative cholangitis in BA patients undergoing Kasai operation (AUROC = 0.900). To our best knowledge, this is the first study reporting the expression level of Beclin1 in hepatic tissue of BA patients and revealing its prognostic value for postoperative cholangitis. Since Beclin1 is a crucial regulator for apoptosis and phagocytosis, its role in BA is worthy to be further investigated.
There are still some limitations in the current study. Firstly, we only investigated the hepatic histology but not other potential risk factors for cholangitis, such as blood culture results or surgical adverse events. In addition, two different sets of paraffin-embedded specimens were used in this study, and the sample sizes of two sets of paraffin-embedded specimens were both small. A well-designed prospective study with a large sample size is necessary to further validate the findings of this study. If the findings of this study could be validated, some prophylaxis treatments, such as enhancing immunotherapy, high-level antibiotics, prolonged antibiotic treatment or active hormone therapy, could be considered for patients with high risk of early postoperative cholangitis. Meanwhile, these patients need to be closely followed-up, and their parents could be educated about the symptoms of early postoperative cholangitis. Once the patient develops early postoperative cholangitis, the disease should be timely controlled to avoid adverse consequences and improve the prognosis. Furthermore, even though we identified that infiltration of CD8+ and CD45RO+ T cells and Beclin1 level were the independent influence factors, we did not further investigate their roles in the pathogenesis of postoperative cholangitis in BA patients. All these limitations should be addressed in the following study.
In summary, our data showed that low infiltration of CD8+ T cells, high infiltration of CD45RO+ T cells and low Beclin1 level were risks factors for early postoperative cholangitis, and these three markers possessed the prognostic value for early postoperative cholangitis in BA patients. Our findings may be helpful to develop new strategies for prevention and treatment of postoperative cholangitis in BA patients.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Liu J, Zeng J. Data curation: Chen H, Zhong Z, Zhang Z. Formal analysis: Zhong Z, She J. Methodology: Gao P, Shu M. Writing - original draft: Jiang H. Writing - review & editing: Liu J.
SUPPLEMENTARY MATERIALS
Infiltration of CD4+ T cells (red arrows, A) and CD8+ T cells (red arrows, B) mainly distributed in the portal area. Representative images were shown at 400 × magnification.
CD68+ cells mainly distributed among the hepatocytes, but less in the portal area (A, B). CD68+ cells mainly distributed within the liver sinusoid (C), and around the central venous (D).
Infiltration of CD45RO+ T cells mainly distributed in the portal area.
Representative IHC images of Beclin1 protein of non-cholangitis group and cholangitis group were shown at 200 × magnification.
References
- 1.Chiu CY, Chen PH, Chan CF, Chang MH, Wu TC, Taiwan Infant Stool Color Card Study Group Biliary atresia in preterm infants in Taiwan: a nationwide survey. J Pediatr. 2013;163(1):100–103.e1. doi: 10.1016/j.jpeds.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 2.Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K, et al. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38(7):997–1000. doi: 10.1016/s0022-3468(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 3.Wada H, Muraji T, Yokoi A, Okamoto T, Sato S, Takamizawa S, et al. Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: a regional survey of over 20 years. J Pediatr Surg. 2007;42(12):2090–2092. doi: 10.1016/j.jpedsurg.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, et al. Biliary atresia: the Canadian experience. J Pediatr. 2007;151(6):659–665. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58(6):1209–1217. doi: 10.1016/j.jhep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374(9702):1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 7.Wildhaber BE. Biliary atresia: 50 years after the first kasai. ISRN Surg. 2012;2012:132089. doi: 10.5402/2012/132089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasai M, Suzuki S. A new operation for non-correctable biliary atresia: hepatic portoenterostomy. Shuiyutsu. 1959;13:733–739. [Google Scholar]
- 9.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57(5 Pt 2):87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 10.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37(1):4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wu ET, Chen HL, Ni YH, Lee PI, Hsu HY, Lai HS, et al. Bacterial cholangitis in patients with biliary atresia: impact on short-term outcome. Pediatr Surg Int. 2001;17(5-6):390–395. doi: 10.1007/s003830000573. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Zheng S. Current concept about postoperative cholangitis in biliary atresia. World J Pediatr. 2008;4(1):14–19. doi: 10.1007/s12519-008-0003-0. [DOI] [PubMed] [Google Scholar]
- 13.Kasai M. Treatment of biliary atresia with special reference to hepatic porto-enterostomy and its modifications. Prog Pediatr Surg. 1974;6:5–52. [PubMed] [Google Scholar]
- 14.Nakajo T, Hashizume K, Saeki M, Tsuchida Y. Intussusception-type antireflux valve in the Roux-en-Y loop to prevent ascending cholangitis after hepatic portojejunostomy. J Pediatr Surg. 1990;25(3):311–314. doi: 10.1016/0022-3468(90)90074-j. [DOI] [PubMed] [Google Scholar]
- 15.Suruga K, Miyano T, Kitahara T, Kojima Y, Fukuda Y. Treatment of biliary atresia: a study of our operative results. J Pediatr Surg. 1981;16(4) Suppl 1:621–626. doi: 10.1016/0022-3468(81)90016-6. [DOI] [PubMed] [Google Scholar]
- 16.Canty TG, Self TW, Collins DL, Bonaldi L. Recent experience with a modified Sawaguchi procedure for biliary atresia. J Pediatr Surg. 1985;20(3):211–216. doi: 10.1016/s0022-3468(85)80104-4. [DOI] [PubMed] [Google Scholar]
- 17.Endo M, Katsumata K, Yokoyama J, Morikawa Y, Ikawa H, Kamagata S, et al. Extended dissection of the portahepatis and creation of an intussuscepted ileocolic conduit for biliary atresia. J Pediatr Surg. 1983;18(6):784–793. doi: 10.1016/s0022-3468(83)80024-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Feng Y. Cholangitis after hepatic portoenterostomy for biliary atresia. J Appl Clin Pediatr. 2007;(23):1769–1772. [Google Scholar]
- 19.Kotb MA, El Henawy A, Talaat S, Aziz M, El Tagy GH, El Barbary MM, et al. Immune-mediated liver injury: prognostic value of CD4+, CD8+, and CD68+ in infants with extrahepatic biliary atresia. J Pediatr Surg. 2005;40(8):1252–1257. doi: 10.1016/j.jpedsurg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 21.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lleo A, Bian Z, Zhang H, Miao Q, Yang F, Peng Y, et al. Quantitation of the rank-rankl axis in primary biliary cholangitis. PLoS One. 2016;11(9):e0159612. doi: 10.1371/journal.pone.0159612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9(2):280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack CL. What causes biliary atresia? Unique aspects of the neonatal immune system provide clues to disease pathogenesis. Cell Mol Gastroenterol Hepatol. 2015;1(3):267–274. doi: 10.1016/j.jcmgh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack CL, Falta MT, Sullivan AK, Karrer F, Sokol RJ, Freed BM, et al. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology. 2007;133(1):278–287. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack CL, Tucker RM, Lu BR, Sokol RJ, Fontenot AP, Ueno Y, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology. 2006;44(5):1231–1239. doi: 10.1002/hep.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115(2):200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed AF, Ohtani H, Nio M, Funaki N, Shimaoka S, Nagura H, et al. CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol. 2001;193(3):383–389. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path793>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33(1):643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10(1):645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 31.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 32.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12(1):39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infiltration of CD4+ T cells (red arrows, A) and CD8+ T cells (red arrows, B) mainly distributed in the portal area. Representative images were shown at 400 × magnification.
CD68+ cells mainly distributed among the hepatocytes, but less in the portal area (A, B). CD68+ cells mainly distributed within the liver sinusoid (C), and around the central venous (D).
Infiltration of CD45RO+ T cells mainly distributed in the portal area.
Representative IHC images of Beclin1 protein of non-cholangitis group and cholangitis group were shown at 200 × magnification.