Abstract
Aim: Acute myeloid leukemia (AML) is an aggressive hematopoietic clonal disorder characterized by the increased blasts and poor survival outcome, which is mainly driven by cytogenetic and molecular abnormalities. Here, we investigated the prognostic impact of other demographic parameters on the survival outcomes in AML patients.
Method: We reviewed the Surveillance, Epidemiology, and End Result (SEER) database to collect demographic information, including age, diagnosis, gender, race, and geographic region in patients with non-acute promyelocytic leukemia AML, between 2004–2008. The primary end-point of our study was 3-year overall survival (OS), which was estimated by the Kaplan–Meier method and Cox regression model.
Results: A total of 13,282 patients were included in our analyses. Increasing age (HR 1.2, p < .0001), male gender (HR 1.05, p = .01), and geographic region of Midwest (HR 1.07, p = .002) were associated with inferior 3-year OS in univariate analysis, and these parameters remained independent prognostic factors in multivariate analyses.
Conclusions: AML is a heterogeneous myeloid neoplasm with patient outcomes largely dictated by the cytogenetics and somatic mutations. In our study, additional demographic factors, including advanced age, male gender, and geographic region of AML diagnosis were associated with OS outcome in non-APL AML patients.
Keywords: AML, acute leukemia, leukemia, overall survival, gender, region, race
Introduction
Many pre-treatment factors, including cytogenetic/molecular abnormalities and age have been associated with survival in acute myeloid leukemia (AML) [1]. Here, we used data from the Surveillance, Epidemiology, and End Result (SEER) Program to study the relation between age, gender, race, and region of diagnosis and survival in AML.
Methods
The SEER database (version 8.1.5) was reviewed for patients with histologically confirmed non-acute promyelocytic (APL) AML (ICD-O-3), between 2004 and 2008. We collected information on: date of, and age at, diagnosis, gender, ethnicity, geographic region of diagnosis, reported follow-up intervals, and date of death. Primary outcome of interest was 3-year overall survival (OS), defined as the time from AML diagnosis to death from any cause [2], with censoring if the patient was still alive after 3 years of follow-up. Data from all SEER registries were included. OS probabilities were estimated using the Kaplan–Meier method. Hazard ratios (HR) for OS were calculated using univariate and multivariate Cox proportional hazard regressions.
Results
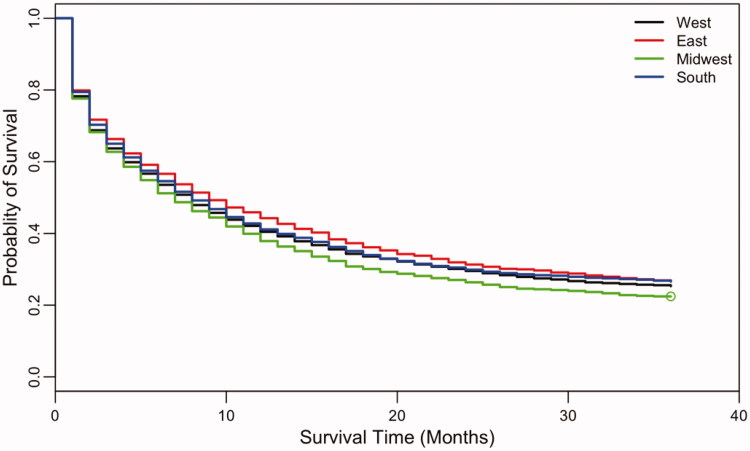
Thirteen thousand two hundred and eighty-two patients with non-APL AML were included between 2004 and 2008. The median age was 65 years (range 15–99). The Caucasian to non-Caucasian ratio was 5:1 and male:female ratio 7:6. In univariate analysis, 3-year OS was worse with increasing age (HR 1.2, p < .0001), male gender (HR 1.05, p = .01), residence in the Midwest (MI, IW, KY, HR 1.07, p = .002) and Caucasian ethnicity (HR 1.10, p = .0005). Multivariate analysis found that each of the parameter, age, sex, and region, were independently associated with 3-year OS, while the ethnicity was not (Table 1). Additionally, the hazard ratio independently increased by 22% with every 5-year increment in age, despite adjustments for the other covariates. OS was also associated with region of diagnosis in both univariate and multivariate analysis, based on limited available regional data (Table 1). Patients in the Eastern (NJ, CT) and Southern regions (GA, NM, and LA) had better 3-year overall survival (26.8% and 26.6% respectively) when compared with patients in the Western region (25.3%; CA, WA, HI, AK, and UT). Patients in the Midwest (MI, IW, and KY) had inferior 3-year OS compared to the Western region (22.4%; Figure 1).
Table 1.
Summary of a Multivariate Cox regression model between overall survival and covariates age, sex, ethnicity and region.
| Category | N/ Median (Range) | 3-years survival (%) | HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age | 65 (15, 99) | 25.3% | 1.219 | <.0001 | |
| Sex | Female (Ref) | 6113 | 27.0% | .0288 | |
| Male | 7169 | 23.8% | 1.047 | ||
| Ethnicity | Non-Caucasian (Ref) | 2282 | 27.2% | .1354 | |
| Caucasian | 11,000 | 24.9% | 0.958 | ||
| Region | West (Ref) | 6792 | 25.3% | .0041 | |
| East | 2147 | 26.8% | 0.900 | ||
| Midwest | 2090 | 22.4% | 1.000 | ||
| South | 2253 | 26.6% | 0.973 |
Figure 1.
Kaplan–Meier curves on overall survival by region of diagnosis. Available regional data included the following regions/states: West (California, Washington, Hawaii, Alaska, Utah); East (New Jersey, Connecticut); Midwest (Michigan, Iowa, Kentucky); South (Georgia, New Mexico, Louisiana).
Discussion
AML is a heterogeneous entity with many clinical and biologic factors influencing treatment response and survival. In our study, gender, age, and region of diagnosis were independently associated with overall survival in AML. This study suggests that female gender may serve as a favorable risk factor in AML, which is consistent with a publication reporting on younger patients with AML [3]. While the exact mechanism accounting for this disparity is unclear, it certainly alludes to the potential role of hormonal variations in altering disease biology and social influences.
Additionally, this study further confirms that advancing age confers inferior survival in AML, with a hazard ratio increase of 22% with every 5-year increment in age. This is in keeping with prior studies and is likely to be a result of poorer performance status, higher frequency of multidrug resistance, and more complex disease biology, which accompany the disease with increasing age [4,5].
Lastly, our study demonstrates that regional disparities may account for outcome differences in this population with survival favoring the Eastern and Southern states. However, it is notable that the proportion of the various regions covered by the SEER database is limited and does not include many populous states.
Analysis of a large cohort of AML patients from the National Cancer Database analyzed over 60,000 patients and demonstrated that short and long-term overall survival favored patients who received initial treatment at an academic medical center versus non-academic centers [6]. However, patients receiving care at academic medical centers were also noted to be younger, having fewer comorbidities, and more often received multimodal therapy including transplant which are known prognosticators influencing improved survival in AML. Data on the patients with greater access to National Cancer Institute and National Comprehensive Cancer Center Network –designated cancer centers exhibited improved survival outcomes in other disease states including multiple myeloma [7], prostate cancer [8], and adolescent and young adult acute lymphoblastic leukemia [9]. Thus, variations in concentration and access to academic medical centers may have plausibly contributed to the regional outcome disparities as evidenced in our studied AML population.
Survival may also be influenced by the case volume of treating facility, as shown in a study examining volume-outcome relationships which favored survival among AML patients treated at “high volume” centers [10]. This phenomenon may be due to increased provider experience, rate of adherence to standardized guidelines, robust ancillary services, and greater resources which may impact survival in disease treatment [10]. It is quite possible that regional disparities in access to high volume sub-specialty AML centers could also have impacted outcome differences as reported in our patient population.
It is further unknown, whether environmental stressors or variations in socioeconomic class influenced the differences in survival outcomes when comparing the regional differences. Additional limitations of the study include lacking information on patient performance status, type of treatment, cytogenetic and molecular data, and treatment setting, which are known to impact survival in AML. Therefore, it is quite possible that outcome disparities noted in our study could have been influenced by the variables mentioned above and requires further exploration. Nevertheless, based on all the available metrics, this study suggests that female gender may serve as a favorable risk factor in AML, further confirms that advancing age confers inferior survival, and that regional disparities may account for some outcome differences in this population.
Transparency
Declaration of funding
There is no funding to report for this study.
Declaration of financial/other relationships
All authors declare that there are no relevant conflict of interests pertaining to this manuscript. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–1152. [DOI] [PubMed] [Google Scholar]
- 2.Cheson BD, Bennett JM, Kopecky KJ, et al. . Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. JCO. 2003;21:4642–4649. [DOI] [PubMed] [Google Scholar]
- 3.Hossain MJ, Xie L. Sex disparity in childhood and young adult acute myeloid leukemia (AML) survival: evidence from us population data. Cancer Epidemiol. 2015;39:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. . Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leith CP, Kopecky KJ, Godwin J, et al. . Acute Myeloid Leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest oncology group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 6.Bhatt VR, Shostrom V, Giri S, et al. . Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol. 2017;92:764–771. [DOI] [PubMed] [Google Scholar]
- 7.Ailawadhi S, Advani P, Yang D, et al. . Impact of access to NCI- and NCCN-designated cancer centers on outcomes for multiple myeloma patients: A SEER registry analysis. Cancer. 2016;122:618–625. [DOI] [PubMed] [Google Scholar]
- 8.Ghaffary C, Dafashy T, Kosarek CD, et al. . Impact of proximity to NCI- and NCCN- designated cancer centers on outcomes for patients with prostate cancer undergoing radical prostatectomy. Journal of Clinical Oncology. 2017;35:14–14.27918724 [Google Scholar]
- 9.Muffly L, Alvarez E, Lichtensztajn D, et al. . Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giri S, Pathak R, Aryal MR, et al. . Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood. 2015;21125:3359–3360. [DOI] [PubMed] [Google Scholar]