Abstract
Background:
The significance of the gut microbiome for the pathogenesis of multiple sclerosis (MS) has been established, although the underlying signaling mechanisms of this interaction have not been sufficiently explored.
Objectives:
We address this point and use serotonin (5-hydroxytryptamine (5-HT))—a microbial-modulated neurotransmitter (NT) as a showcase to demonstrate that NTs regulated by the gut microbiome are potent candidates for mediators of the gut–brain axis in demyelinating disorders.
Methods, Results, and Conclusion:
Our comprehensive overview of literature provides evidence that 5-HT levels in the gut are controlled by the microbiome, both via secretion and through regulation of metabolites. In addition, we demonstrate that the gut microbiome can influence the formation of the serotonergic system (SS) in the brain. We also show that SS alterations have been related to MS directly—altered expression of 5-HT transporters in central nervous system (CNS) and indirectly—beneficial effects of 5-HT modulating drugs on the course of the disease and higher prevalence of depression in patients with MS. Finally, we discuss briefly the role of other microbiome-modulated NTs such as γ-aminobutyric acid and dopamine in MS to highlight a new direction for future research aiming to relate microbiome-regulated NTs to demyelinating disorders.
Keywords: Multiple sclerosis, gut microbiome, gut–brain axis, serotonin, 5-HT, altered SS (SS), neuroinflammation, neurotransmitters
Introduction
Over the past years, several studies demonstrated that individual microbial species affect the course of experimental autoimmune encephalomyelitis (EAE)1 and showed that altered enteral microbiota is related to multiple sclerosis (MS).2,3 Notwithstanding the evidence for the role of the gut–brain axis in MS, the underlying mechanism remains unknown. To address this question, we use serotonin (5-hydroxytryptamine (5-HT)) as a showcase of a link between microbial-regulated neurotransmitters (NTs) and MS pathogenesis. We base our novel idea on a comprehensive overview of literature, providing circumstantial evidence for an important role of microbiome-induced 5-HT alterations in MS pathology. Although our focus is on 5-HT, the role of other microbial-regulated NTs such as γ-aminobutyric acid (GABA) and dopamine is also discussed to highlight a new area of future research.
NTs: the link between gut and brain?
NTs are neuronal signaling mediators controlling physiological functions ranging from motor control and hunger to immunity and cognition. Altered NT levels have been related to a variety of diseases such as neuroinflammatory diseases.
Depending on their chemical characteristics, NTs are divided into four main groups: amino acids (glycine, glutamic acid, GABA, aspartic acid); peptides (vasopressin, somatostatin, neurotensin); biogenic amines (norepinephrine, 5-HT, dopamine); and acetyl choline.4
NTs are synthesized in neurosecretory or neuroendocrine cells and function in the central or peripheral nervous system. Intestinal bacterial communities alter the production of NTs in the central nervous system (CNS) influencing the levels of host metabolites necessary for their production.5 Moreover, the gut microbiome secretes many NTs such as 5-HT, dopamine, and GABA thus regulating also their peripheral levels.6,7 Finally, the NTs exert an effect on the immune system through respective immune cell receptors.8,9 The link between NTs and neuroinflammatory diseases, their function in the nervous and immune system, and the microbial regulation of their levels makes them potent candidates for mediators of the gut–brain interactions in MS, of which 5-HT may be a dominant player.
5-HT and the gut microbiome
About 90% of the human 5-HT is synthesized by the gut enterochromaffin cells through a process modulated by the intestinal microflora.10 In the gut, 5-HT controls peristalsis, secretion, vasodilation, nociperception, and nausea via 5-HT receptors (5-HTR) expressed on afferent nerves in the lamina propria of the intestinal epithelium.11 Comparison of the fecal metabolome and 5-HT serum levels between germ-free (GF) mice and mice with humanized microbiome reveals that the gut microbiome accounts for 64% of the gut 5-HT concentrations and 49% of its serum levels.10 Other experiments exploiting the same animal model demonstrate that microbial metabolites such as short-chain fatty acids promote transcription of tryptophan hydroxylase 1 which is crucial for 5-HT biosynthesis.5 Combined, these data show a major effect of gut bacteria on 5-HT synthesis. However, there is a need for better understanding how this modulation affects host’s health and disease.
The microbiome also affects the central serotonergic system (SS).12,13 This system originates from the raphe nucleus where the bodies of 5-HT–producing neurons are located. Almost all brain regions express 5-HTR and are innervated by serotonergic neurons, but the main target of these afferent pathways is the limbic system, including the hippocampus. In the CNS, 5-HT has a wide spectrum of functions ranging from locomotor activation and nociperception to control over reproductive and addictive behavior.14 The microbiome modulates the central SS affecting both 5-HT levels and 5-HTR expression. For instance, elevated levels of Bifidobacterium in the gut disable the increase in 5-HT receptor type 2A (5-HTR2A) in the frontal cortex of mice in response to treatment with lipopolysaccharide (LPS).12 This effect is combined with steady levels of peripheral 5-HT indicating a mechanism of central SS modulation, which bypasses the 5-HT synthesis in the gut. Furthermore, studies on gnotobiotic mice demonstrate that lack of microbiota at an early age increases 5-HT and its main metabolite (5-hydroxyindoleacetic acid) in the hippocampus.13 This event is accompanied by elevated levels of the 5-HT precursor tryptophan in the circulation, pointing to a microbial control of the central SS via modulation of peripheral 5-HT.
The described research is mostly performed in animals and needs to be translated into the clinic. Nevertheless, it provides evidence that the gut microbiome is an essential regulator of the peripheral and central SS. It is thus reasonable to assume that the gut microbiome might play a role in SS alterations.
Altered SS in MS
Evidence for an altered SS in MS comes from positron emission tomography studies on a cohort of 23 MS patients and 22 healthy controls,15 which revealed lower expression of 5-HT transporters (serotonin transporter (SERT)) in the limbic and paralimbic regions in patients. The analysis reveals a reduction in SERT expression in the hippocampal/parahippocampal regions of patients with relapsing–remitting multiple sclerosis (RRMS) and an increase in the orbitofrontal cortex of patients with primary-progressive multiple sclerosis (PPMS). The data suggest differential 5-HT signaling in RRMS and PPMS and underline the necessity of a deeper understanding of the role of the SS in MS pathology. It is speculated that altered SERT expression might reflect the homeostatic function of the CNS in response to changed 5-HT availability, although the underlying mechanisms remain unknown.15
Interestingly, treatment of mice with monoamine oxidase (MAO) inhibitors, which increase 5-HT availability in the CNS, improved 5-HT innervation of the spinal cord and reduced EAE severity.16 MAO inhibitors affect also the CNS levels of GABA and norepinephrine. Therefore, it can be assumed that observed effects are the result of the combined modulation of the three NTs and are not solely dependent on 5-HT.16 Similarly, treatment with arylpiperazine ligands, which bind to 5-HTR1A and dopamine receptor 2, demonstrates that combined serotonergic and dopaminergic stimulation has a neuroprotective effect and reduces CNS immune infiltration in EAE mice.17 Clinical investigations have also explored the effects of fluoxetine – a selective 5-HT re-uptake inhibitor, which increases the concentration of 5-HT in the CNS of MS patients.18,19 Fluoxetine – a candidate for drug repurposing18 is now clinically tested in patients with secondary-progressive MS as a neuroprotective compound.19 These clinical studies are the next step in the verification of the role of 5-HT in MS, and the confirmation of this hypothesis would be a starting point for future research to elucidate the effects of serotonergic signaling on the disease pathogenesis.
In addition, evidence for an altered SS in MS comes from studies on depression. Depression, generally accepted to be caused by low 5-HT levels, is a frequent MS comorbidity. The prevalence of depression is higher in individuals suffering from the demyelinating disease, compared to healthy individuals.20 Although depression could manifest as a result of being diagnosed with MS, a current study suggests that depression in MS has a different pathophysiology and a different cause.20 A 4-year questionnaire-based follow-up on a cohort of over thousand MS patients indicates that depression in MS has a chronic character, lasts longer, and is not related to the course of MS.20 The authors of this study argue that the cause of depression during MS is a result of alterations in the CNS and not a reaction to being diagnosed with an incurable disease. This study and the investigations on the beneficial effects of antidepressants on MS highlight a possible shared SS alteration in the pathogenesis of MS and depression.
Altogether, these studies demonstrate a role of decreased SS functionality in MS, which may be due to altered cellular immunity.
5-HT and cellular immunity
5-HT has an effect on immune regulation via 5-HTRs expressed on cells of the innate and adaptive immune systems,21 and changes in the communication between the SS and leukocytes are related to diseases with an inflammatory pathological component (rheumatoid arthritis, psoriasis, asthma). 5-HT regulates the function of macrophages, dendritic cells (DCs), and T cells21 which are major immunological components of MS.
Macrophages in MS lesions exhibit an intermediate phenotype expressing both pro-inflammatory (M1) and growth-promoting (M2) markers depending on their polarization.22 It is thus not clear whether the macrophage phenotype determines lesion progression or limitation and repair.23 5-HT influences macrophage polarization. In vitro experiments utilizing cells from healthy donors and MS patients demonstrate that through 5-HTR2B and 5-HTR7 on monocytes, 5-HT skews monocyte polarization to M2 macrophages via suppression of LPS-induced pro-inflammatory cytokines and reciprocal regulation of M2 and M1 polarization-related genes—a potentially beneficial effect for lesion recovery in MS.23,24
DCs in MS patients have a pro-inflammatory phenotype resulting in augmented T-cell activation.25 5-HT regulates maturation of DCs in the bone marrow affecting their migration capacity and shaping their cytokine repertoire and subsequent secretion and T-cell activation.21 Finally, DCs can take up and store 5-HT from the local environment, which is shuttled to T cells to regulate their function.21
5-HT directly regulates the differentiation, proliferation, and effector functions of naïve and activated T cells.21 Naïve T cells express 5-HTR7, which signals to the activation of extracellular signal–regulated kinase (ERK) and nuclear factor κB (NF-κB) pathways, resulting in expression of 5-HTR1B and 5-HTR2B. 5-HTR1B and 5-HTR2B modulate chemotaxis and other effector functions of activated T cells in a multidirectional manner depending on the T-cell environment and activation state.21
Platelets control inflammation and tissue repair through interaction with leukocytes and secretion of soluble immune mediators including 5-HT.26 Platelets take up 5-HT from the gut and transport it to the peripheral blood, where they are the only source of the NT. This transportation provides a link between microbial modulation of gut 5-HT levels and its effects on immune cells at other locations. 5-HT secreted from platelets stimulates differentiation of CD4 T cells to Th1 cells. Platelets from MS patients fail to secrete 5-HT and this is linked to disease activation. Depletion of platelets during a peak of EAE exacerbates inflammation and increases immune cell infiltration of the CNS, illustrating their importance in MS.26
Other potential gut–brain axis mediators
Besides 5-HT other NTs are also regulated by the gut microbiome,6 function in the immune system,9 and have altered levels in MS patients.27,28 For instance, GABA, the main inhibitory mediator in CNS, downregulates pro-inflammatory mediators which target T-cell dampening and thus T-cell–mediated immunity.29 In addition, GABA production is regulated by the gut microbiome with Lactobacillus brevis being the most efficient GABA producer from the enteral flora.30 Moreover, treatment of mice with Lactobacillus rhamnosus shows altered GABA receptor expression in CNS accompanied by related changes in the mice behavior31 indicating that bacteria regulate GABA signaling both in the CNS and in the periphery. Finally, altered GABAergic pathways are related to MS.32 Essential regulators of GABA signaling in the brain are GABA transporters (GATs). GATs are expressed on macrophages and lymphocytes and are upregulated during immune activation to deplete extracellular GABA.31 Decreased GAT expression is detected in the white brain matter and brain macrophages of MS patients. Treatment with ganaxolone (a positive allosteric modulator of GABA receptor) suppresses GAT in macrophages and dampens neuroinflammation, highlighting the role of GABAergic alterations in MS.32
Another microbial-modulated NT is dopamine. Dopamine controls locomotion, cognition, endocrine regulation and has been related to neurodegenerative diseases.8 In vitro, human gut bacteria produce dopamine in very high concentrations compared to its physiological levels.6 Moreover, experiments with GF rats demonstrate lower dopaminergic turnover rate in the CNS accompanied by enhanced anxiety-like behavior and neuroendocrine response, highlighting the role of the microbiome in dopaminergic system regulation.33 Finally, dopamine also plays a role in immune modulation and its decreased levels are linked to MS relapse.28 A study on a cohort of 43 patients with RRMS reveals lowered serum levels of dopamine during relapse related to higher T helper 17 (Th-17) and interleukin 17 (IL-17) serum levels. Dopamine suppresses IL-17 production of peripheral blood mononuclear cells (PBMCs) from MS patients suggesting an anti-inflammatory effect on MS.28
The discussed NTs do not exhaust the arsenal of microbiome-modulated compounds with effects on neuroinflammation but point to a new direction of future investigation to relate other microbiome-regulated NTs to demyelinating disorders.
Conclusion and perspectives
Gut microbiota alterations are linked to MS and some pathological mechanisms have been proposed for a few individual species. These mechanisms include production of neuroactive toxins, immunogenicity of lipid membranes, production of inflammatory metabolites, and others (summarized in the works by Mirza and Mao-Draayer34). The gut microbiome varies between people, depending on their genetic makeup, lifestyle, and environment. Due to this heterogeneity, it is difficult to translate the effects of separate microbial species on MS between different patients. In this Future Perspectives paper, we look at the microbiome as a system and provide a framework linking whole microbiome alterations to MS pathogenesis through effects on the metabolic pathway of 5-HT. Using this approach, it would therefore be interesting to investigate whether the variabilities in the microbiome of different patient cohorts (such as between the Harvard2 and Mayo clinic3 reports from 2016) which are likely due to sequencing methods and patients’ population differences are, however, not related to common global effects on 5-HT signaling.
We have combined data demonstrating that the enteral flora influences the peripheral and central SS5–7,10,12,13 with clinical studies which provide evidence for lowered SS functionality in MS patients.15–20 In our opinion, these data strongly suggest a role of the gut microbiome in the alterations of the SS in MS. To further strengthen our hypothesis, we looked into studies on the effects of 5-HT on macrophages, DCs, and T cells21–25 – key players in MS. Although there is a need for greater understanding of the effects of 5-HT on cellular immunity and neuroinflammation specifically, the discussed studies provide a plausible theoretical framework linking decreased levels of 5-HT to inflammation in MS.
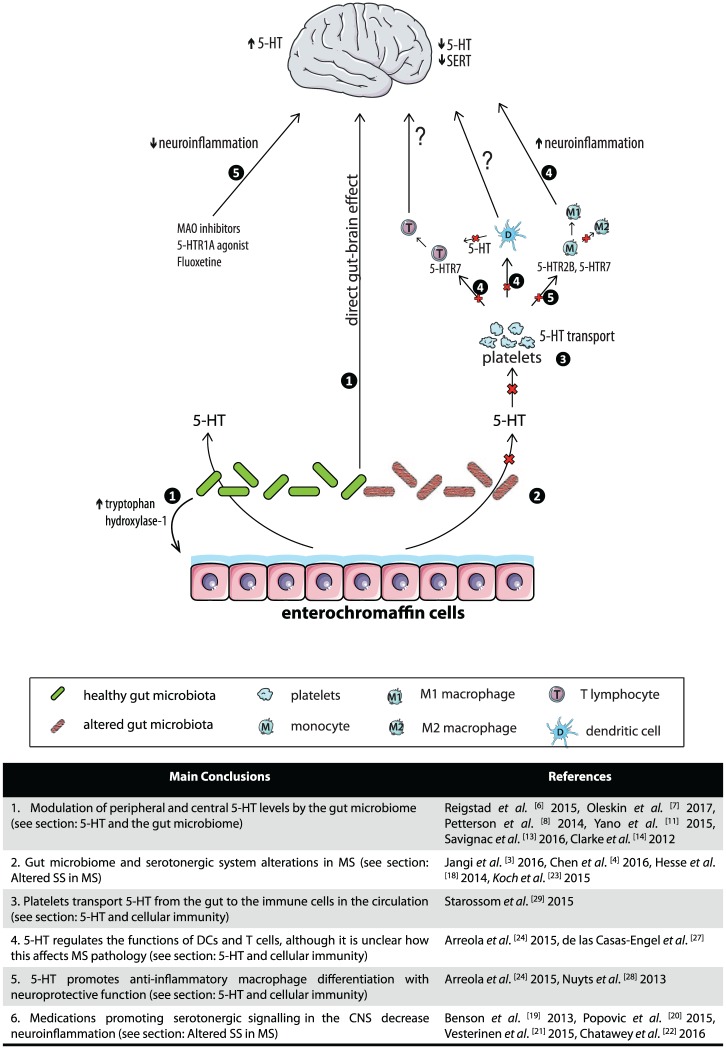
We propose that a decrease in SS function is a factor contributing to MS pathology (Figure 1). Since the microbiome is a major regulator of the SS, we suggest that the root cause of this alteration may lie in an unbalanced microflora during early life, when the SS in the periphery and in the CNS is shaped, or later through modulation of peripheral 5-HT levels. The reduced 5-HT levels affect neuroinflammation in the CNS directly or through skewing macrophages and T cells to an autoinflammatory phenotype.
Figure 1.
Schematic representation of the links between the gut microbiome, 5-HT production, and neuroinflammation in CNS. 5-HT: 5-hydroxytryptamine, serotonin; 5-HTR1A: serotonin receptor 1A; MAO: monoamine oxidase; 5-HTR7: serotonin receptor 7; 5-HTR5: serotonin receptor 5; and SERT: serotonin transporter. The left side illustrates serotonergic pathways related to maintenance of 5-HT homeostasis and inhibition of neuroinflammation. These pathways include 5-HT production by healthy gut microbiota (depicted green with smooth texture) and 5-HT increase in CNS by 5-HT modulating medications. The right side represents neuroinflammatory pathways triggered by lowered 5-HT production in the gut as a result of altered gut microbiota (depicted in red with striated texture). The red crosses indicate decreased gut 5-HT synthesis, lowered levels of 5-HT uptake in platelets, and less 5-HT available to promote macrophage differentiation to anti-inflammatory M2 phenotype. The numbers circled in black indicate the main conclusions related to the specific branch of the pathway made throughout the paper. These conclusions are summarized in the accompanying table along with the relevant references. Images are adapted from Servier Medical Art by Servier and modified by the authors under the following terms: Creative Commons Attribution 3.0 Unported (CC BY 3.0) (https://creativecommons.org/licenses/by/3.0/).
Summarizing the available data on the role of the gut microbiome in SS regulation and on the role of SS in MS pathology, we demonstrate that 5-HT is a critical mediator of the gut–brain axis in MS. This is a novel insight, which opens up a broad horizon for future investigation to relate the microbiome modulation of a wide range of NTs to demyelinating disorders.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Tsveta S Malinova, Department of Biochemistry, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Christine D Dijkstra, Department of Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, The Netherlands.
Helga E de Vries, Department of Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, The Netherlands.
References
- 1. Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol 2015; 17(4): 344. [DOI] [PubMed] [Google Scholar]
- 2. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016; 7: 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016; 6(1): 28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humphries P, Pretorius E, Naudé H. Direct and indirect cellular effects of aspartame on the brain. Eur J Clin Nutr 2008; 62(4): 451–462. [DOI] [PubMed] [Google Scholar]
- 5. Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015; 29(4): 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oleskin AV, Shenderov BA, Rogovsky VS. Role of neurochemicals in the interaction between the microbiota and the immune and the nervous system of the host organism. Probiotics Antimicrob Proteins 2017; 9: 215–234. [DOI] [PubMed] [Google Scholar]
- 7. Patterson E, Cryan JF, Fitzgerald GF, et al. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc 2014; 73: 477–489. [DOI] [PubMed] [Google Scholar]
- 8. Idova GV, Alperina EL, Cheido MA. Contribution of brain dopamine, serotonin and opioid receptors in the mechanisms of neuroimmunomodulation: Evidence from pharmacological analysis. Int Immunopharmacol 2012; 12(4): 618–625. [DOI] [PubMed] [Google Scholar]
- 9. Devoino L, Idova G, Alperina E, et al. Brain neuromediator systems in the immune response control: Pharmacological analysis of pre- and postsynaptic mechanisms. Brain Res 1994; 633(1–2): 267–274. [DOI] [PubMed] [Google Scholar]
- 10. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015; 161(2): 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mawe GM, Hoffman JM. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013; 10(8): 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savignac HM, Couch Y, Stratford M, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav Immun 2016; 52: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2012; 18(10): 666–673. [DOI] [PubMed] [Google Scholar]
- 14. Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Reports 2009; 61(5): 761–777. [DOI] [PubMed] [Google Scholar]
- 15. Hesse S, Moeller F, Petroff D, et al. Altered serotonin transporter availability in patients with multiple sclerosis. Eur J Nucl Med Mol Imaging 2014; 41(5): 827–835. [DOI] [PubMed] [Google Scholar]
- 16. Benson CA, Wong G, Tenorio G, et al. The MAO inhibitor phenelzine can improve functional outcomes in mice with established clinical signs in experimental autoimmune encephalomyelitis (EAE). Behav Brain Res 2013; 252: 302–311. [DOI] [PubMed] [Google Scholar]
- 17. Popovic M, Stanojevic Z, Tosic J, et al. Neuroprotective arylpiperazine dopaminergic/serotonergic ligands suppress experimental autoimmune encephalomyelitis in rats. J Neurochem 2015; 135(1): 125–138. [DOI] [PubMed] [Google Scholar]
- 18. Vesterinen HM, Connick P, Irvine CMJ, et al. Drug repurposing: A systematic approach to evaluate candidate oral neuroprotective interventions for secondary progressive multiple sclerosis. PLoS ONE 2015; 10(4): e0117705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chataway J, Chandran S, Miller D, et al. The MS-SMART trial in secondary progressive MS—Current update. J Neurol Neurosurg Psychiatry 2016; 87(12): e1. [Google Scholar]
- 20. Koch MW, Patten S, Berzins S, et al. Depression in multiple sclerosis: A long-term longitudinal study. Mult Scler J 2015; 21(1): 76–82. [DOI] [PubMed] [Google Scholar]
- 21. Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, et al. Immunomodulatory effects mediated by serotonin. J Immunol Res 2015; 2015: 354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogel DY, Glim JE, Stavenuiter AW, et al. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014; 219(9): 695–703. [DOI] [PubMed] [Google Scholar]
- 23. Vogel DY, Vereyken EJ, Glim JE, et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 2013; 10(1): 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol 2013; 190(5): 2301–2310. [DOI] [PubMed] [Google Scholar]
- 25. Nuyts A, Lee W, Bashir-Dar R, et al. Dendritic cells in multiple sclerosis: Key players in the immunopathogenesis, key players for new cellular immunotherapies? Mult Scler J 2013; 19(8): 995–1002. [DOI] [PubMed] [Google Scholar]
- 26. Starossom SC, Veremeyko T, Yung AW, et al. Platelets play differential role during the initiation and progression of autoimmune neuroinflammation. Circ Res 2015; 117(9): 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cawley N, Solanky BS, Muhlert N, et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 2015; 138(9): 2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melnikov M, Belousova O, Murugin V, et al. The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. J Neuroimmunol 2016; 292: 97–101. [DOI] [PubMed] [Google Scholar]
- 29. Wu C, Qin X, Du H, et al. The immunological function of GABAergic system. Front Biosci 2017; 22: 1162–1172, http://www.ncbi.nlm.nih.gov/pubmed/28199198 (accessed 22 March 2017). [DOI] [PubMed] [Google Scholar]
- 30. Barrett E, Ross RP, O’Toole PW, et al. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 2012; 113(2): 411–417. [DOI] [PubMed] [Google Scholar]
- 31. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011; 108(38): 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul AM, Branton WG, Walsh JG, et al. GABA transport and neuroinflammation are coupled in multiple sclerosis: Regulation of the GABA transporter-2 by ganaxolone. Neuroscience 2014; 273: 24–38. [DOI] [PubMed] [Google Scholar]
- 33. Neufeld KM, Kang N, Bienenstock J, et al. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2011; 23(3): 255–264. [DOI] [PubMed] [Google Scholar]
- 34. Mirza A, Mao-Draayer Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin Immunol. Epub ahead of print 9 March 2017. DOI: 10.1016/j.clim.2017.03.001. [DOI] [PubMed] [Google Scholar]