Abstract
OBJECTIVES
This study identified women with unique trajectories of executive function, concentration, and visual working memory before and during adjuvant therapy for breast cancer, and examined phenotypic and genotypic predictors associated with subgroups.
SAMPLE & SETTING
399 postmenopausal women, of whom 288 were women with early-stage breast cancer and 111 were women without breast cancer, matched on age and years of education to the women with breast cancer, and all at an urban cancer center.
METHODS & VARIABLES
A repeated-measures design was used; assessments occurred before adjuvant therapy and every six months post-therapy initiation. Group-based trajectory modeling determined subgroups. Multinomial logistic regression identified phenotypic and genotypic characteristics.
RESULTS
Three executive function and concentration trajectory subgroups were identified: low, moderate, and high; two visual working memory subgroups were identified: low and high.
IMPLICATIONS FOR NURSING
Advancing age, greater pretherapy fatigue, and poorer pretherapy cognitive function are associated with the low subgroups. DNA repair and oxidative stress mechanisms may be involved in the cognitive changes that women experience.
Keywords: breast cancer, cognitive function, aromatase inhibitor, phenotypic, genotypic
Breast cancer is the most common cancer in women in the United States. About 3.6 million female breast cancer survivors were living in the United States in 2016, and 93% of those survivors were aged 50 years or older (Miller et al., 2016). In addition, about 75%–80% of women with breast cancer are postmenopausal at the time of diagnosis (DeSantis et al., 2016). Of those, 75%–79% have hormone receptor–positive disease (Cheang et al., 2015; Clark, Osborne, & McGuire, 1984; Osborne, 1998), and a large portion will receive adjuvant aromatase inhibitor (AI) therapy. Use of endocrine therapy with an AI, such as anastrozole, letrozole, or exemestane, has improved the disease-free survival and overall survival of postmenopausal women with early-stage disease (Schiavon & Smith, 2014); however, negative sequelae associated with AI therapy may include changes in cognitive function.
An estimated 25%–75% of women with breast cancer experience changes in cognitive function with disease and treatment (Wefel et al., 2004). Cognitive decline compromises psychological well-being and interferes with work, decision making, the ability to perform daily activities efficiently, and adherence to cancer therapy (Bender et al., 2014; Bender & Thelen, 2013). Multiple factors likely contribute to changes in cognitive function, including mood, sleep problems, concomitant medications, disease-related factors, and cancer therapy (Bender & Thelen, 2013).
Most research in this area has focused on changes in cognitive function with chemotherapy (Myers, 2012). Deterioration in multiple cognitive domains has been observed in women receiving selective estrogen receptor modulators (SERMs), such as tamoxifen (Castellon et al., 2004; Chen et al., 2017; Ganz, Petersen, Bower, & Crespi, 2016; Palmer, Trotter, Joy, & Carlson, 2008; Schilder et al., 2009, 2010). Less is known about the changes in cognitive function associated with AI therapy because few studies have focused on cognitive function with these agents. In addition, methodologic issues reduce the ability to compare results across studies (Wu & Amidi, 2017). Samples in some studies were heterogeneous, combining premenopausal with postmenopausal women (Ganz et al., 2014; Hermelink et al., 2008; Jenkins et al., 2006; Palmer et al., 2008) and women who received AIs with women who received SERMs (Collins, Mackenzie, Stewart, Bielajew, & Verma, 2009; Ganz et al., 2014; Mar Fan et al., 2007; Root, Andreotti, Tsu, Ellmore, & Ahles, 2016; Schilder et al., 2009). Several studies had small sample sizes, relied solely on self-report of cognitive function, or lacked control groups, which hindered the ability to make comparisons and determine the influence of practice effects (Ganz et al., 2014; Hermelink et al., 2008; Jenkins et al., 2006; Paganini-Hill & Clark, 2000). Several studies also lacked pretherapy assessments, precluding the ability to determine changes in cognitive function with treatment.
In addition, most studies of cognitive function in patients with breast cancer have a priori grouped women according to the type of therapy they receive (Ahles et al., 2010; Bender et al., 2015; Wefel, Saleeba, Buzdar, & Meyers, 2010). For example, the authors previously reported the results of a study in which postmenopausal women with breast cancer were grouped according to whether they received an AI alone or following chemotherapy (Bender et al., 2015). Although this approach is useful for uncovering the role of cancer therapy in changes in cognitive function, it precludes identification of subgroups of women who may be more vulnerable to poorer cognitive function regardless of therapy. Identification of distinct subgroups, their trajectories of change in cognitive function, and the characteristics that are associated with subgroup membership are critical for developing and targeting interventions to improve cognitive function in women with breast cancer. Knowledge of the behavioral and biological factors that are associated with subgroup membership can help to identify characteristics of women at greatest risk for changes in cognitive function as well as characteristics that may be protective. This approach is also useful in clarifying the mechanisms underlying changes in cognitive function, which remain unclear.
One useful guide for understanding the mechanisms of the problem is the theory of accelerated aging (Lisanti et al., 2011; Mandelblatt et al., 2014). Cognitive and biologic reserves are needed to compensate for the effects of aging. Cancer and cancer treatment may deplete these reserves and accelerate aging, leading to greater vulnerability to poorer outcomes, including deterioration in cognitive function. Whether cognitive decline is accelerated in individuals with cancer compared to cognitive decline observed in healthy aging adults is unclear. Evidence suggests that individuals with cancer have poorer cognitive function compared to norms and matched controls without cancer (Ahles et al., 2010; Wefel et al., 2010). In the authors’ previous work, deterioration in working memory and concentration was found within the first six months of exposure to AI therapy in postmenopausal women with early-stage breast cancer. Compared to age and education-matched controls without breast cancer, women with breast cancer had poorer executive function at pretherapy and through the first 18 months of therapy (Bender et al., 2015). Others have reported similar results (Ahles et al., 2008; Wefel et al., 2004).
Shared biologic pathways that link cancer, aging, and declines in cognitive function may include oxidative stress and DNA damage with reduced capacity for DNA repair (Ahles & Saykin, 2007; Gorgoulis et al., 2005; Jablonska et al., 2015; Mandelblatt et al., 2013; McAdam, Brem, & Karran, 2016). Cancer and cancer treatment may increase oxidative stress and ensuing DNA damage (Joshi et al., 2005; Lisanti et al., 2011; Wang et al., 2008). Genetic variability in these pathways may result in variable rates of aging and cognitive decline during therapy (Ahles et al., 2010; Mandelblatt et al., 2013). About 81% of breast cancers are diagnosed in women aged 50 years or older (Miller et al., 2016). These women may be at increased risk for accelerated aging with cancer and cancer therapy and more vulnerable to changes in cognitive function. Exploring associations between genetic markers for these biologic pathways and membership in cognitive function subgroups may generate hypotheses for future study of accelerated aging and mechanisms underlying changes in cognitive function in women with breast cancer.
Compared to controls without breast cancer, the authors previously reported poorer function in three of the eight cognitive domains that were examined: executive function, concentration, and visual working memory (Bender et al., 2015). Therefore, group-based trajectory modeling (GBTM) was conducted for these three cognitive domains to identify unique subgroups of women who experience different trajectories of cognitive function before and during adjuvant therapy for breast cancer. Consistent with the theory of accelerated aging, the authors determined whether there are subgroups of women who are vulnerable to poorer cognitive function. The authors also determined demographic, clinical, and pretherapy phenotypic characteristics that were associated with subgroup membership. Controlling for these phenotypic characteristics, the authors then explored associations between subgroup membership and variability in candidate genes involved in oxidative stress and DNA repair.
Methods
The current study used a repeated-measures design. Participants were evaluated before the initiation of chemotherapy, or the AI anastrozole, and at six-month intervals for as many as 18 months after they began anastrozole. Comparable time points were evaluated in the control group. The study protocol was approved by the institutional review board at the University of Pittsburgh, and all participants provided written informed consent.
Sample
The sample included two cohorts of postmenopausal women with early-stage breast cancer: one cohort received chemotherapy followed by anastrozole and the other received anastrozole alone. A third control cohort consisted of postmenopausal women without breast cancer matched on age and education to the cohorts with breast cancer. All participants were postmenopausal, aged 75 years or younger, able to read and speak English, and had at least eight years of education. Women were excluded who had a history of neurologic illness or cancer, or who reported hospitalization for psychiatric illness within the past two years. Women with breast cancer were newly diagnosed with stage I, II, or IIIa disease. Women with stage IIIb or higher breast cancer were excluded because of the potential confounding effects of factors related to the presence of extensive disease, including metabolic changes and greater need for additional medications, such as narcotic analgesics (Silberfarb, 1983).
Women with breast cancer were recruited from the Comprehensive Breast Cancer Program at the University of Pittsburgh Medical Center Hillman Cancer Center. Control participants were recruited from the University Center for Social and Urban Research via random digit dialing, responses to a local advertisement, or social-based referrals (i.e., someone informing a friend about the study). Enrollment began in November 2005 and follow-up assessments concluded in February 2017.
Phenotypic Measures
Cognitive function was assessed at each time point with a battery of objective neuropsychological measures that evaluated multiple cognitive domains. Because of the large number of scores yielded from this battery, a data reduction approach—exploratory factor analysis—was used to minimize type 1 errors. Eight cognitive factors were generated from this approach. Mean z scores for the eight cognitive factors were computed as the average of the z scores of individual cognitive measures. Higher z scores indicate better performance relative to the control group at baseline. A detailed description of the battery and factor analysis was previously reported (Bender et al., 2015). The focus of the current analysis was on the factors for which the authors previously observed significant deterioration with AI therapy (i.e., concentration and visual working memory) or poorer function from pretherapy and through the first 18 months of therapy when compared to control participants (i.e., executive function) (Bender et al., 2015).
Concentration is defined as sustained attention or vigilance. Working memory is the capacity to temporarily retain information for processing. Closely linked to concentration and working memory is executive function, which includes the ability to organize, reason, and problem solve (Lezak, Howieson, Loring, Bigler, & Tranel, 2004). Potential covariates of cognitive function, including depressive symptoms (Beck Depression Inventory II) (Beck, Steer, & Brown, 1996), anxiety (Profile of Mood States [POMS] Tension/Anxiety subscale), fatigue (POMS Fatigue/Inertia subscale) (McNair, Lorr, & Droppleman, 1992), age, and estimated verbal intelligence (IQ) (National Adult Reading Test–Revised [NART-R]) (Nelson, 1982) were also assessed. Data collection occurred either at participants’ homes or in a private conference room by a nurse trained in the proper administration of the study battery.
Genotypic Measures
Genotyping was conducted in a subgroup of participants (n = 190) who provided whole blood or saliva samples. Genotypic data was available from a subgroup of the study participants because funding to support collection and banking of genomic samples was secured for an ancillary study subsequent to the initiation of the main study.
As described in Koleck et al. (2016), 39 functional and tagging single nucleotide polymorphisms (SNPs) for four DNA repair genes (ERCC2, ERCC3, ERCC5, and PARP1) and five oxidative stress genes (CAT, GPX1, SEPP1, SOD1, and SOD2) were selected. SNP selection was based on a literature review and database review (Koleck et al., 2016). When a functional SNP was not identified from the literature, tagging SNPs were selected using the Phase III HapMap database to more fully represent the variability in each candidate gene based on the following criteria: R2 ≥ 0.8, minor allele frequency ≥ 20%, and selected for Caucasian or African ancestry. Genotypes were also determined for the two functional SNPs (rs429358 and rs7412) that comprise the e2, e3, and e4 alleles of the APOE gene, which performs antioxidant activities throughout the body (Koleck et al., 2014). A more detailed description of candidate gene selection and the processes for extraction and quality control is provided elsewhere (Koleck et al., 2014, 2016).
Statistical Analysis
GBTM was performed with SAS, version 9.4, using PROC TRAJ to determine subgroups of women with distinct trajectories of executive function, concentration, and visual working memory (Nagin, 2005). An advantage of this analytic approach is that it does not apply a priori assignment rules, such as therapy types to trajectories, which is commonly used in linear mixed modeling. The authors previously conducted linear mixed modeling and identified changes in cognitive function based on disease (breast cancer) and treatment type (AI with and without chemotherapy) (Bender et al., 2015). However, this analytic approach precluded the authors’ ability to determine whether subgroups of women were more vulnerable to poorer cognitive function, regardless of treatment, and which factors were associated with membership in those subgroups. GBTM allows for identification of distinct subgroups of individual trajectories and permits the identification of key factors that are associated with the trajectories of those groups (Nagin, 2014; Nagin & Tremblay, 2001). Therefore, the authors were able to identify subgroups of women who experienced distinct trajectories of cognitive function and the phenotypic and genotypic factors associated with their temporal patterns of cognitive performance. It is important to examine the trajectories of both subgroups for women who experience poorer cognitive function and women who experience better cognitive function. Knowledge of factors associated with subgroup membership aids in the identification of characteristics of women at greatest risk for poor cognitive function as well as factors that may be protective.
GBTMs were determined using a two-stage approach. First, the optimal number of subgroups for each cognitive domain mean z score was identified. Second, the shape of each subgroup’s trajectory was determined, yielding intercept (b0), linear (b1), or quadratic (b2) regression coefficients. The Bayesian Information Criterion (BIC), based on the number of participants and the number of repeated measures from competing group-based trajectory models, were compared using the BIC log Bayes factor approximation for the selection of models. Participants were assigned to trajectory subgroups having the largest posterior probability. All group-based trajectory models were deemed adequate because the average predicted posterior probabilities of assignment were at least 0.7 for all subgroups, odds of correct classification were at least 5 for all subgroups, and good correspondence was found between the estimated subgroup probabilities and the proportion assigned to the subgroup. Finally, the authors used the chi-square test of independence to examine associations between predicted trajectory groupings for each pairing of the three cognitive domains.
Multinomial logistic regression using STATA 14 identified significant (p < 0.05) baseline phenotypic factors associated with subgroup membership for each of the three cognitive domains. Univariate analyses were preformed initially to identify potential variables to include in the multivariable models. Any variables that were p < 0.1 uncorrected were considered as candidates for the multivariable models. Correlations among the phenotypic and genotypic factors were examined separately to assess for possible multicollinearity and confounding. In each of the two sets of multivariable models, the authors included in the final models only variables that had an overall significance of p < 0.05. Then, using the Bonferroni correction for post-hoc pairwise contrasts between the subgroups, the authors interpreted p < 0.05/j as significant and p < 0.1/j as a trend, where j represents the number of subgroups. Therefore, for executive function, where there were three subgroups, the authors interpreted p < 0.0167 as significant and p < 0.0333 as a trend.
For the exploratory genotypic models, a similar process was followed. Differences among subgroups in minor allele distributions identified in the bivariate analysis were evaluated, controlling for variables from each of the phenotypic models. SNPs with overall p < 0.05 were retained in the final models. A complete summary of the bivariate associations between SNPs and trajectory cognitive function subgroups is available upon request from the corresponding author. Each final genotypic model was fit to determine covariate-adjusted odds ratios and 95% confidence intervals for association between genotype and subgroups in pairwise comparisons. The same criteria for Bonferroni correction were used for subgroup comparisons.
Finally, the authors examined the association between predicted trajectory groupings among pairs of the three cognitive domains examined: executive function, concentration, and visual working memory. For these analyses, the authors used chi-square tests of independence statistics.
Results
The sample size for the GBTM analyses was 399 for the executive function and visual working memory domains and 397 for the concentration domain. This discrepancy was caused by missing concentration measure scores for two women. The sample size for the multiple logistic regression analysis performed to examine the phenotypic characteristics associated with subgroup membership was 367 because of missing data for some phenotypic measures; 261 were women with breast cancer and 106 were controls. Finally, the sample size for the genotypic factors was 190, as described earlier.
Women were, on average, aged 60.2 years, had completed 14.8 years of education, and scored 109.6 on the NART-R (see Table 1). Most women were White (95%) and married/partnered (63%). Most women with breast cancer had lumpectomy (80%) and radiation therapy (71%), and 28% received chemotherapy before anastrozole. The women who provided genomic samples did not differ from the women who did not provide genomic samples in any demographic or clinical characteristics. A greater proportion of controls (62%) than women with breast cancer receiving anastrozole alone (45%) (p = 0.007) provided a genomic sample.
TABLE 1.
Characteristics of the Overall Sample at Enrollment (N = 367)
| Characteristic |
|
SD | |
|---|---|---|---|
| Age (years) | 60.2 | 6.15 | |
| Education (years) | 14.8 | 2.71 | |
| NART-R score | 109.6 | 8.78 | |
|
| |||
| Characteristic | n | % | |
|
| |||
| Married or partnered | |||
|
| |||
| Yes | 232 | 63 | |
|
| |||
| Race | |||
|
| |||
| White | 348 | 95 | |
|
| |||
| Breast-conserving surgerya | |||
|
| |||
| Yes | 209 | 80 | |
|
| |||
| Radiation therapya | |||
|
| |||
| Yes | 185 | 71 | |
|
| |||
| Systemic therapy | |||
|
| |||
| Controls (no systemic therapy) | 106 | 29 | |
| AI only | 157 | 43 | |
| Chemotherapy plus AI | 104 | 28 | |
|
| |||
| Stage of diseasea | |||
|
| |||
| I | 177 | 68 | |
| IIa | 55 | 21 | |
| IIb | 18 | 7 | |
| IIIa | 11 | 4 | |
Breast cancer cohort only (n = 261)
AI—aromatase inhibitor; NART-R—National Adult Reading Test–Revised
Executive Function
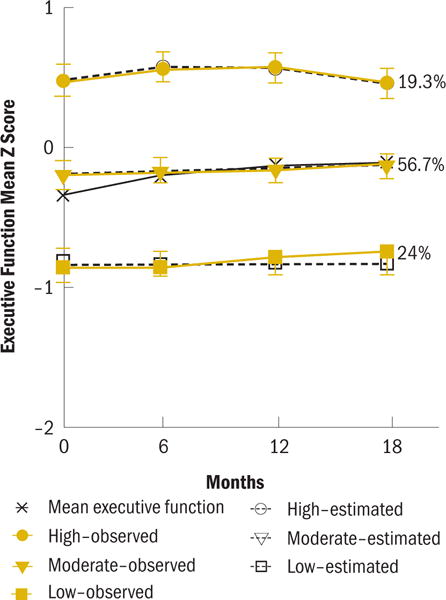
Using GBTM, three distinct trajectory subgroups were identified for executive function: low performance at pretherapy that remained low (low) (b0 = −0.83); performance slightly below the norm based on the age and education-matched control group at pretherapy that improved linearly (moderate) (b0 = −0.17, b1 = 0.05), and high performance that improved and then declined (high) (b0 = 0.56, b1 = 0.31, and b2 = −0.1) (see Figure 1).
FIGURE 1.
Executive Function Trajectory
At pretherapy, the low and moderate subgroups were older (p < 0.001) and had less education (p < 0.001) than the high subgroup (see Table 2). IQ differed among the subgroups, with the low subgroup having the lowest NART-R scores (p < 0.001). The low subgroup was more likely to have received adjuvant therapy (AI with or without chemotherapy) (p < 0.001) and reported greater pain severity than the other subgroups (p = 0.007). The low subgroup also reported greater fatigue (p = 0.035) than the moderate subgroup (see Table 3). The pretherapy neuropsychological performance of the low subgroup was poorer on every cognitive domain except mental flexibility (p < 0.001), and the performance of the moderate subgroup was poorer than the high subgroup on verbal and visual memory, psychomo-tor speed, and executive function.
TABLE 2.
Executive Function Subgroup Characteristics at Enrollment
| Characteristic | Low (1) (N = 88)
|
Moderate (2) (N = 208)
|
High (3) (N = 71)
|
Test Statistic | p | Post-Hoc Comparis ons | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
SD |
|
SD |
|
SD | ||||
| Age (years) | 61.8 | 5.85 | 60.6 | 6.48 | 56.8 | 4 | F(2,185) = 25.9 | < 0.001 | 1, 2 > 3 |
| Education (years)a | 14.2 | 2.26 | 14.7 | 2.67 | 15.9 | 3.02 | F(2,157) = 7.9 | 0.001 | 1, 2 < 3 |
| NART-R score | 106.4 | 8.51 | 109.6 | 8.5 | 113.5 | 8.49 | F(2,364) = 13.5 | < 0.001 | 1 < 2 < 3 |
|
| |||||||||
| Characteristic | n | % | n | % | n | % | Test Statistic | p | Post-Hoc Comparisons |
|
| |||||||||
| Married or partnered | χ2 = 3 | 0.224 | – | ||||||
|
| |||||||||
| Yes | 49 | 56 | 135 | 65 | 48 | 68 | |||
|
| |||||||||
| Race | FE | 0.193 | – | ||||||
|
| |||||||||
| White | 81 | 92 | 197 | 95 | 70 | 99 | |||
|
| |||||||||
| Mastectomy | χ2 = 2.2 | 0.331 | – | ||||||
|
| |||||||||
| Yes (versus BCS) | 16 | 18 | 17 | 8 | 6 | 8 | |||
|
| |||||||||
| Radiation therapy | FE | 0.593 | – | ||||||
|
| |||||||||
| Yes | 58 | 66 | 102 | 49 | 25 | 35 | |||
|
| |||||||||
| Systemic therapy | χ2 = 49.2 | < 0.001 | – | ||||||
|
| |||||||||
| None (controls) | 4 | 5 | 64 | 31 | 38 | 54 | |||
| AI only | 53 | 60 | 89 | 43 | 15 | 21 | |||
| Chemotherapy plus AI | 31 | 35 | 55 | 26 | 18 | 25 | |||
|
| |||||||||
| Stage of diseasea | FE | 0.969 | – | ||||||
|
| |||||||||
| I | 59 | 70 | 94 | 65 | 24 | 73 | |||
| IIa | 17 | 20 | 31 | 22 | 7 | 21 | |||
| IIb | 5 | 6 | 12 | 8 | 1 | 3 | |||
| IIIa | 3 | 4 | 7 | 5 | 1 | 3 | |||
Breast cancer cohort only (N = 261; low, n = 84; moderate, n = 144; high, n = 33)
AI—aromatase inhibitor; BCS—breast-conserving surgery; FE—Fisher’s exact test; NART-R—National Adult Reading Test–Revised
Note. Although actual means and SDs are reported for each subgroup, statistical tests were performed on the square-root transformed variable.
TABLE 3.
Significant Differences in Self-Reported Symptom Scores and Cognitive Domain Z Scores Among the Predicted Trajectory Subgroups at Enrollment (N = 367)
| Item | Low (1)
|
Moderate (2)
|
High (3)
|
Test Statistic | p | Post-Hoc Comparisons | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
SD |
|
SD |
|
SD | ||||
| Executive functiona
| |||||||||
| Self-reported symptom score | |||||||||
|
| |||||||||
| POMS fatigue/inertia | 6.9 | 6.68 | 5.1 | 5.65 | 5.1 | 4.21 | F(2,170) = 3.4 | 0.035 | 1 > 2 |
| BPI average pain | 2.5 | 2.49 | 1.8 | 2.13 | 1.5 | 2.16 | F(2,363) = 5.1 | 0.007 | 1 > 2, 3 |
|
| |||||||||
| Cognitive domain z score | |||||||||
|
| |||||||||
| Verbal memory | −0.48 | 0.645 | −0.18 | 0.642 | 0.07 | 0.753 | F(2,364) = 13.5 | < 0.001 | 1 < 2 < 3 |
| Psychomotor speed | −0.43 | 0.896 | −0.12 | 0.846 | 0.38 | 0.555 | F(2,178) = 29.1 | < 0.001 | 1 < 2 < 3 |
| Attention | −0.48 | 1.062 | −0.1 | 0.872 | 0.19 | 0.939 | F(2,359) = 10.4 | < 0.001 | 1 < 2, 3 |
| Visual memory | −0.25 | 0.865 | 0.06 | 0.708 | 0.28 | 0.468 | F(2,174) = 12.9 | < 0.001 | 1 < 2 < 3 |
| Executive function | −0.92 | 0.434 | −0.27 | 0.502 | 0.35 | 0.65 | F(2,153) = 118 | < 0.001 | 1 < 2 < 3 |
| Visual working memory | −0.37 | 0.914 | −0.01 | 0.78 | 0.26 | 0.693 | F(2,364) = 12.5 | < 0.001 | 1 < 2, 3 |
| Concentration | −0.38 | 0.734 | −0.03 | 0.584 | 0.1 | 0.526 | F(2,156) = 11.7 | < 0.001 | 1 < 2, 3 |
|
| |||||||||
| Concentrationb
| |||||||||
| Self-reported symptom score | |||||||||
|
| |||||||||
| POMS fatigue/inertia | 4.3 | 5.04 | 6.6 | 6.43 | 4.9 | 5.12 | F(2,364) = 3.5 | 0.031 | 2 > 3 |
|
| |||||||||
| Cognitive domain z score | |||||||||
|
| |||||||||
| Verbal memory | −0.62 | 0.618 | −0.24 | 0.669 | −0.09 | 0.685 | F(2,364) = 9.7 | < 0.001 | 1 < 2, 3 |
| Mental flexibility | −0.39 | 0.842 | −0.02 | 0.736 | 0.22 | 0.7 | F(2,364) = 12.4 | < 0.001 | 1 < 2 < 3 |
| Psychomotor speed | −0.93 | 0.999 | −0.32 | 0.868 | 0.23 | 0.621 | F(2,89) = 37.5 | < 0.001 | 1 < 2 < 3 |
| Attention | −0.64 | 0.924 | −0.31 | 0.972 | 0.09 | 0.889 | F(2,359) = 13.3 | < 0.001 | 1, 2 < 3 |
| Visual memory | −0.39 | 1.003 | −0.06 | 0.782 | 0.17 | 0.579 | F(2,88) = 8.3 | < 0.001 | 1, 2 < 3 |
| Executive function | −0.69 | 0.542 | −0.38 | 0.675 | −0.17 | 0.646 | F(2,364) = 11.6 | < 0.001 | 1 < 2 < 3 |
| Visual working memory | −0.57 | 0.972 | −0.12 | 0.835 | 0.12 | 0.734 | F(2,92) = 9.8 | < 0.001 | 1 < 2 < 3 |
| Concentration | −1.14 | 0.565 | −0.42 | 0.416 | 0.36 | 0.316 | F(2,88) = 257.5 | < 0.001 | 1 < 2 < 3 |
|
| |||||||||
| Visual working memoryc
| |||||||||
| Cognitive domain z score | |||||||||
|
| |||||||||
| Verbal memory | −0.51 | 0.679 | – | – | −0.05 | 0.64 | t = 6.4 | < 0.001 | – |
| Mental flexibility | −0.07 | 0.771 | – | – | 0.13 | 0.734 | t = 2.4 | 0.016 | – |
| Psychomotor speed | −0.42 | 1.015 | – | – | 0.06 | 0.712 | t = 4.7 | < 0.001 | – |
| Attention | −0.36 | 1.046 | – | – | −0.02 | 0.892 | t = 3.2 | 0.001 | – |
| Visual memory | −0.34 | 0.928 | – | – | 0.2 | 0.534 | t = 5.9 | < 0.001 | – |
| Executive function | −0.62 | 0.592 | – | – | −0.15 | 0.649 | t = 6.6 | < 0.001 | – |
| Visual working memory | −0.65 | 0.856 | – | – | 0.25 | 0.622 | t = 10.3 | < 0.001 | – |
| Concentration | −0.23 | 0.669 | – | – | −0.02 | 0.608 | t = 3 | 0.003 | – |
For the executive function subgroup, participants were classified as low (n = 88), moderate (n = 208), or high (n = 71).
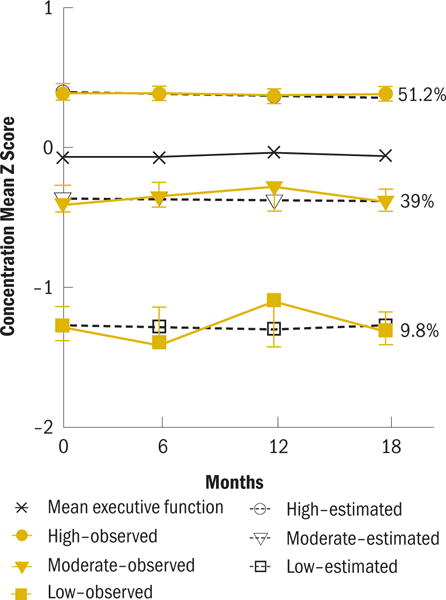
For the concentration subgroup, participants were classified as low (n = 36), moderate (n = 143), or high (n = 188).
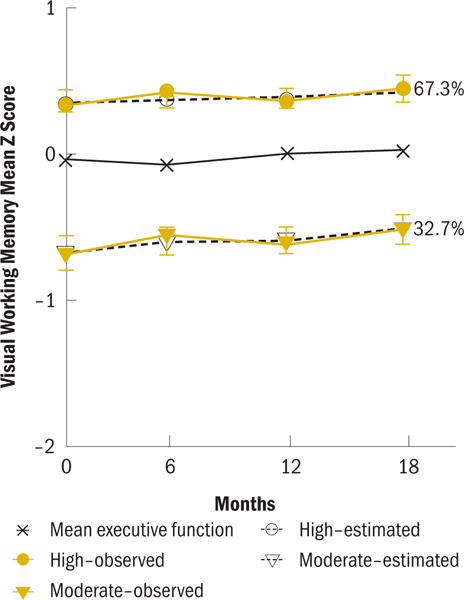
For the visual working memory subgroup, participants were classified as low (n = 120) or high (n = 247).
Note. Although actual means and SDs are reported for each subgroup, statistical tests were performed on the square-root transformed variable.
In the multivariable phenotypic model, age (p = 0.024), IQ score (p = 0.003), treatment group (p < 0.001), and pretherapy psychomotor speed (p < 0.001) and concentration (p = 0.002) z scores were associated with executive function subgroup membership. In pairwise subgroup comparisons (see Table 4), the authors found that relative to the high subgroup, membership in the low executive function subgroup was associated with lower estimated IQ, receipt of anastrozole therapy with or without chemotherapy, and poorer pretherapy psychomotor speed.
TABLE 4.
Multinomial Logistic Regression Models for Pretherapy Phenotypic Predictors of Subgroup Membership
| Phenotypic Predictor | Adjusted OR | SE | 95% CI | z | p |
|---|---|---|---|---|---|
| Executive functiona
| |||||
|
High (RG) versus low
| |||||
| Age | 1.44 | 0.262 | [1.007, 2.054] | 2 | 0.046 |
| Estimated verbal intelligence score | 0.66 | 0.08 | [0.524, 0.84] | −3.41 | 0.001 |
| Receipt of chemotherapy plus anastrozole | 20.68 | 13.637 | [5.676, 75.313] | 4.59 | < 0.001 |
| Receipt of anastrozole alone | 34.95 | 22.61 | [9.832, 124.203] | 5.49 | < 0.001 |
| Psychomotor speed z score | 0.28 | 0.092 | [0.148, 0.534] | −3.88 | < 0.001 |
| Concentration z score | 0.47 | 0.163 | [0.236, 0.926] | −2.18 | 0.029 |
|
| |||||
|
Moderate (RG) versus low
| |||||
| Age | 0.95 | 0.121 | [0.742, 1.219] | −0.39 | 0.694 |
| Estimated verbal intelligence score | 0.85 | 0.072 | [0.721, 1.004] | −1.91 | 0.056 |
| Receipt of chemotherapy plus anastrozole | 10.14 | 5.941 | [3.213, 31.973] | 3.95 | < 0.001 |
| Receipt of anastrozole alone | 10.35 | 5.848 | [3.417, 31.326] | 4.13 | < 0.001 |
| Psychomotor speed z score | 0.76 | 0.141 | [0.526, 1.09] | −1.5 | 0.134 |
| Concentration z score | 0.45 | 0.102 | [0.287, 0.7] | 3.53 | < 0.001 |
|
| |||||
|
Moderate (RG) versus high
| |||||
| Age | 0.66 | 0.1 | [0.492, 0.89] | −2.73 | 0.006 |
| Estimated verbal intelligence score | 1.28 | 0.125 | [1.06, 1.553] | 2.55 | 0.011 |
| Receipt of chemotherapy plus anastrozole | 0.49 | 0.189 | [0.23, 1.044] | −1.85 | 0.064 |
| Receipt of anastrozole alone | 0.3 | 0.112 | [0.141, 0.623] | −3.21 | 0.001 |
| Psychomotor speed z score | 2.69 | 0.778 | [1.529, 4.744] | 3.43 | 0.001 |
| Concentration z score | 0.96 | 0.292 | [0.527, 1.741] | −0.14 | 0.887 |
|
| |||||
| Concentrationb
| |||||
|
High (RG) versus low
| |||||
| Estimated verbal intelligence score | 0.95 | 0.116 | [0.748, 1.206] | −0.43 | 0.67 |
| POMS fatigue/inertia | 0.96 | 0.04 | [0.886, 1.043] | −0.95 | 0.344 |
| Psychomotor speed z score | 0.18 | 0.047 | [0.104, 0.298] | −6.46 | < 0.001 |
| Attention z score | 0.57 | 0.13 | [0.368, 0.894] | −2.45 | 0.014 |
|
| |||||
|
Moderate (RG) versus low
| |||||
| Estimated verbal intelligence score | 0.83 | 0.098 | [0.654, 1.042] | −1.61 | 0.107 |
| POMS fatigue/inertia | 0.92 | 0.037 | [0.855, 1.001] | −1.95 | 0.052 |
| Psychomotor speed z score | 0.49 | 0.106 | [0.318, 0.746] | −3.31 | 0.001 |
| Attention z score | 0.84 | 0.179 | [0.557, 1.277] | −0.81 | 0.42 |
|
| |||||
|
Moderate (RG) versus high
| |||||
| Estimated verbal intelligence score | 0.87 | 0.064 | [0.752, 1.004] | −1.9 | 0.057 |
| POMS fatigue/inertia | 0.96 | 0.02 | [0.923, 1.002] | −1.85 | 0.064 |
| Psychomotor speed z score | 2.77 | 0.552 | [1.877, 4.098] | 5.12 | < 0.001 |
| Attention z score | 1.47 | 0.21 | [1.111, 1.944] | 2.7 | 0.007 |
|
| |||||
| Visual working memoryc
| |||||
|
High (RG) versus low
| |||||
| Verbal memory z score | 0.51 | 0.103 | [0.339, 0.756] | −3.33 | 0.001 |
| Visual memory z score | 0.52 | 0.107 | [0.349, 0.78] | −3.17 | 0.002 |
| Executive function z score | 0.42 | 0.093 | [0.274, 0.652] | −3.9 | < 0.001 |
Overall model fit (n = 366): χ2 = 136.94, p < 0.001, pseudo R2 = 0.191
Overall model fit (n = 362): χ2 = 97.91, p < 0.001, pseudo R2 = 0.145
Overall model fit (n = 367): χ2 = 76.63, p < 0.001, pseudo R2 = 0.165
CI—confidence interval; GBTM—group-based trajectory model; OR—odds ratio; POMS—Profile of Mood States; RG—reference group; SE—standard error
Note. Unadjusted p values prior to Bonferroni correction: p < 0.0167 are significant; p > 0.0167 and p < 0.0333 are trends.
Note. Age is reported in five-year increments; estimated verbal intelligence score is reported in five-point increments.
Relative to the moderate subgroup, membership in the low executive function subgroup was associated with receipt of anastrozole with or without chemotherapy and poorer pretherapy concentration. Finally, relative to the moderate subgroup, membership in the high executive functioning subgroup was associated with younger age, higher IQ, no receipt of anastrozole, and better pretherapy psychomotor speed.
The authors explored whether there were associations between variability in candidate genes involved in oxidative stress and DNA repair and executive function subgroup membership. After controlling for the variability in these participant characteristics and self-reported race and ethnicity, genetic variation in three SNPs in DNA repair genes (PARP1 rs2271347 [p = 0.01], ERCC3 rs4150402 [p = 0.024], and ERCC5 rs751402 [p = 0.034]) imparted differential risk for subgroup membership. Carrying at least one copy of the PARP1 rs2271347 minor A allele conferred about four times greater odds of belonging to the low versus moderate subgroup (p = 0.005). In addition, carrying at least one copy of the ERCC3 rs4150402 minor A allele was marginally associated with about three times increased odds of belonging to the low versus moderate subgroup (p = 0.025). Finally, carrying at least one copy of the ERCC5 rs751402 minor T allele conferred about 81% reduced odds of belonging to the low versus high subgroup (p = 0.011), and was marginally associated with 71% reduced odds of belonging to the low versus moderate subgroup (p = 0.02).
Concentration
Three distinct trajectory subgroups were found for concentration; low and stable (low; b0 = −1.24), below norm and stable (moderate; b0 = −0.32) and high and stable (high; b0 = 0.43). At pretherapy (see Figure 2), the subgroups differed on age (p < 0.001), race (p = 0.014) and fatigue (p = 0.031) (see Table 5). The low concentration subgroup was the oldest subgroup, the high subgroup was the youngest. The moderate subgroup was less likely to report being White and had greater fatigue than the high subgroup. At pretherapy, the low concentration subgroup had poorer performance on every cognitive function factor compared to the other subgroups. Relative to the high subgroup; the performance of the moderate concentration subgroup was poorer on every cognitive factor except verbal memory.
FIGURE 2.
Concentration Trajectory
TABLE 5.
Concentration Subgroup Characteristics at Enrollment
| Characteristic | Low (1) (N = 36)
|
Moderate (2) (N = 143)
|
High (3) (N = 188)
|
Test Statistic | p | Post-Hoc Comparisons | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
SD |
|
SD |
|
SD | ||||
| Age (years) | 64.2 | 6.5 | 60.7 | 5.95 | 59 | 5.89 | F(2,364) = 12.2 | < 0.001 | 1 > 2 > 3 |
| Education (years) | 14.2 | 2.88 | 14.8 | 2.46 | 14.9 | 2.85 | KW = 1.5 | 0.463 | – |
| NART-R score | 106.4 | 8.74 | 110 | 8.78 | 109.9 | 8.71 | F(2,364) = 2.7 | 0.067 | – |
|
| |||||||||
| Characteristic | n | % | n | % | n | % | Test Statistic | p | Post-Hoc Comparisons |
|
| |||||||||
| Married or partnered | χ2 = 3.2 | 0.201 | – | ||||||
|
| |||||||||
| Yes | 19 | 53 | 87 | 61 | 126 | 67 | |||
|
| |||||||||
| Race | FE | 0.014 | 2 < 3 | ||||||
|
| |||||||||
| White | 34 | 94 | 130 | 91 | 184 | 98 | |||
|
| |||||||||
| Mastectomy | FE | 0.284 | – | ||||||
|
| |||||||||
| Yes (versus BCS) | 7 | 19 | 14 | 10 | 18 | 10 | |||
|
| |||||||||
| Radiation therapy | FE | 0.7 | – | ||||||
|
| |||||||||
| Yes | 23 | 64 | 71 | 50 | 91 | 48 | |||
|
| |||||||||
| Systemic therapy | χ2 = 7.3 | 0.12 | – | ||||||
|
| |||||||||
| None (controls) | 6 | 17 | 46 | 32 | 54 | 29 | |||
| AI only | 17 | 47 | 66 | 46 | 74 | 39 | |||
| Chemotherapy plus AI | 13 | 36 | 31 | 22 | 60 | 32 | |||
|
| |||||||||
| Stage of diseasea | FE | 0.619 | – | ||||||
|
| |||||||||
| I | 20 | 67 | 70 | 72 | 87 | 65 | |||
| IIa | 5 | 16 | 18 | 19 | 32 | 24 | |||
| IIb | 3 | 10 | 7 | 7 | 8 | 6 | |||
| IIIa | 2 | 7 | 2 | 2 | 7 | 5 | |||
Breast cancer cohort only (N = 261; low, n = 30; moderate, n = 97; high, n = 134)
AI—aromatase inhibitor; BCS—breast-conserving surgery; FE—Fisher’s exact test; KW—Kruskal-Wallis test; NART-R—National Adult Reading Test–Revised
Note. Although actual means and SDs are reported for each subgroup, statistical tests were performed on the square-root transformed variable.
In the multivariable phenotypic model, pretherapy fatigue, psychomotor speed (p < 0.001), and attention (p = 0.009) were associated with concentration subgroup membership. IQ was marginally associated with concentration subgroup membership (p = 0.085). In pairwise subgroup comparisons, relative to both the high and moderate concentration subgroups, membership in the low concentration subgroup was associated with poorer pretherapy psychomotor speed. Membership in the low concentration subgroup versus the high subgroup was also predicted by poorer pretherapy attention. Better pretherapy performance on measures of psychomotor speed and attention predicted membership in the high subgroup compared to the moderate subgroup.
Controlling for variability in these participant characteristics, variation in one SNP in an oxidative stress gene (GPX1 rs1050450) (p = 0.048) was associated with subgroup membership (see Table 6). Carrying at least one copy of the GPX1 rs1050450 minor A allele was associated with about six times increased odds of belonging to the high versus moderate concentration subgroup (p = 0.014). In addition, variation in one SNP in a DNA repair gene (ERCC3 rs4150407) (p = 0.043) was associated with subgroup membership. Carrying at least one copy of the minor G allele was marginally associated with more than eight times increased odds of belonging to the low versus moderate concentration subgroup (p = 0.023).
TABLE 6.
Multinomial Logistic Regression Models for Pretherapy Genotypic Predictors of Subgroup Membership
| Gene Family/Genotypic Predictor | OR | SE | 95% CI | z | p |
|---|---|---|---|---|---|
| Executive function
| |||||
|
Moderate (RG) versus lowa
| |||||
| DNA repair/PARP1 rs2271347 | 3.87 | 1.875 | [1.494, 10.003] | 2.79 | 0.005 |
| Age | 1.23 | 0.255 | [0.819, 1.847] | 1 | 0.318 |
| Estimated verbal intelligence score | 0.81 | 0.106 | [0.624, 1.045] | −1.62 | 0.105 |
| Chemotherapy plus anastrozole | 15.87 | 14.644 | [2.603, 96.811] | 3 | 0.003 |
| Anastrozole alone | 14.95 | 13.757 | [2.463, 90.754] | 2.94 | 0.003 |
| Psychomotor speed z score | 0.91 | 0.24 | [0.547, 1.527] | −0.34 | 0.731 |
| Concentration z score | 0.36 | 0.132 | [0.179, 0.741] | −2.79 | 0.005 |
|
| |||||
|
Moderate (RG) versus lowb
| |||||
| DNA repair/ERCC3 rs4150402 | 2.75 | 1.238 | [1.136, 6.643] | 2.24 | 0.025 |
| Age | 1.13 | 0.228 | [0.766, 1.682] | 0.63 | 0.529 |
| Estimated verbal intelligence score | 0.86 | 0.113 | [0.664, 1.111] | −1.16 | 0.247 |
| Chemotherapy plus anastrozole | 14.58 | 13.445 | [2.39, 88.881] | 2.9 | 0.004 |
| Anastrozole alone | 16.55 | 15.203 | [2.733, 100.186] | 3.05 | 0.002 |
| Psychomotor speed z score | 0.95 | 0.246 | [0.569, 1.574] | −0.21 | 0.831 |
| Concentration z score | 0.36 | 0.132 | [0.175, 0.739] | −2.78 | 0.005 |
|
| |||||
|
High (RG) versus low
| |||||
| DNA repair/ERCC5 rs751402 | 0.19 | 0.125 | [0.053, 0.688] | −2.53 | 0.011 |
| Age | 2.12 | 0.604 | [1.216, 3.708] | 2.65 | 0.008 |
| Estimated verbal intelligence score | 0.63 | 0.111 | [0.443, 0.886] | −2.65 | 0.008 |
| Chemotherapy plus anastrozole | 25.37 | 25.24 | [3.609, 178.323] | 3.25 | 0.001 |
| Anastrozole alone | 35.64 | 35.814 | [4.973, 255.434] | 3.56 | < 0.001 |
| Psychomotor speed z score | 0.21 | 0.1 | [0.082, 0.536] | −3.27 | 0.001 |
| Concentration z score | 0.62 | 0.284 | [0.25, 1.519] | −1.05 | 0.293 |
|
| |||||
|
Moderate (RG) versus lowc
| |||||
| DNA repair/ERCC5 rs751402 | 0.29 | 0.154 | [0.099, 0.823] | −2.32 | 0.02 |
| Age | 1.22 | 0.247 | [0.82, 1.813] | 0.98 | 0.327 |
| Estimated verbal intelligence score | 0.86 | 0.109 | [0.675, 1.107] | −1.16 | 0.248 |
| Chemotherapy plus anastrozole | 13.62 | 12.367 | [2.295, 80.761] | 2.87 | 0.004 |
| Anastrozole alone | 11.62 | 10.443 | [1.996, 67.64] | 2.73 | 0.006 |
| Psychomotor speed z score | 0.77 | 0.21 | [0.448, 1.309] | −0.98 | 0.329 |
| Concentration z score | 0.4 | 0.145 | [0.196, 0.813] | −2.53 | 0.011 |
|
| |||||
| Concentration
| |||||
|
Moderate (RG) versus lowd
| |||||
| DNA repair/ERCC3 rs4150407 | 8.26 | 7.669 | [1.337, 50.984] | 2.27 | 0.023 |
| Estimated verbal intelligence score | 0.9 | 0.176 | [0.613, 1.318] | −0.55 | 0.585 |
| POMS fatigue/inertia score | 0.93 | 0.053 | [0.834, 1.042] | −1.24 | 0.214 |
| Psychomotor speed z score | 0.38 | 0.118 | [0.205, 0.698] | −3.11 | 0.002 |
| Attention z score | 0.82 | 0.278 | [0.423, 1.596] | −0.58 | 0.562 |
|
| |||||
|
Moderate (RG) versus highe
| |||||
| Oxidative stress/GPX1 rs1050450 | 5.83 | 4.172 | [1.434, 23.706] | 2.46 | 0.014 |
| Estimated verbal intelligence score | 0.93 | 0.1 | [0.754, 1.149] | −0.67 | 0.503 |
| POMS fatigue/inertia score | 1 | 0.033 | [0.939, 1.068] | 0.04 | 0.965 |
| Psychomotor speed z score | 2.7 | 0.779 | [1.538, 4.755] | 3.45 | 0.001 |
| Attention z score | 1.84 | 0.403 | [1.194, 2.822] | 2.77 | 0.006 |
|
| |||||
| Visual working memory
| |||||
|
High (RG) versus lowf
| |||||
| DNA repair/ERCC5 rs873601 | 3.84 | 2.342 | [1.162, 12.691] | 2.21 | 0.027 |
| Verbal memory z score | 0.28 | 0.096 | [0.145, 0.549] | −3.72 | < 0.001 |
| Visual memory z score | 0.46 | 0.166 | [0.229, 0.936] | −2.14 | 0.032 |
| Executive function z score | 0.5 | 0.169 | [0.254, 0.968] | −2.05 | 0.04 |
Overall model fit (n = 187): χ2 = 94.65, p < 0.001, pseudo R2 = 0.258
Overall model fit (n = 188): χ2 = 92.08, p < 0.001, pseudo R2 = 0.25
Overall model fit (n = 187): χ2 = 92.78, p < 0.001, pseudo R2 = 0.253
Overall model fit (n = 189): χ2 = 67.6, p < 0.001, pseudo R2 = 0.192
Overall model fit (n = 179): χ2 = 74.43, p < 0.001, pseudo R2 = 0.224
Overall model fit (n = 189): χ2 = 66.08, p < 0.001, pseudo R2 = 0.275
CI—confidence interval; OR—odds ratio; POMS—Profile of Mood States; RG—reference group; SE—standard error
Note. For executive function, genotypic predictors evaluated in the models were PARP1 rs2271347 (GA plus AA versus GG), ERCC3 rs4150402 (GA plus AA versus GG), and ERCC5 rs751402 (CT plus TT versus CC). Self-reported race/ethnicity was retained in the models to adjust for potential confounding because of population stratification (data not shown).
Note. For concentration, genotypic predictors evaluated in the models were ERCC3 rs4150407 (AG plus GG versus AA) and GPX1 rs1050450 (GA plus AA versus GG). Self-reported race/ethnicity was retained in the models to adjust for potential confounding because of population stratification (data not shown).
Note. For visual working memory, genotypic predictor evaluated in the model was ERCC5 rs873601 (GG versus AA plus AG). Self-reported race/ethnicity was retained in the models to adjust for potential confounding because of population stratification (data not shown).
Note. Unadjusted p values prior to Bonferroni correction: p < 0.0167 are significant; p > 0.0167 and p < 0.0333 are trends.
Note. Age is reported in five-year increments; estimated verbal intelligence score is reported in five-point increments.
Visual Working Memory
For visual working memory, a two-group model was identified: low and improving (low; b0 = −0.67, b1 = 0.11) and above the norm and improving (high; b0 = 0.32, b1 = 0.07) (see Figure 3). The low working memory group was older (p = 0.009), had less education (p = 0.005) and lower IQ (p = 0.001), and was less likely to report being White (p < 0.001) than the high visual working memory subgroup (see Table 7). Compared to the high verbal working memory subgroup, the low verbal working memory subgroup had poorer performance on every cognitive function factor at pretherapy (p < 0.05).
FIGURE 3.
Visual Working Memory Trajectory
TABLE 7.
Visual Working Memory Subgroup Characteristics at Enrollment
| Characteristic | Low (N = 120)
|
High (N = 247)
|
Test Statistic | p | ||
|---|---|---|---|---|---|---|
|
|
SD |
|
SD | |||
| Age (years) | 61.4 | 6.38 | 59.6 | 5.96 | t = 2.6 | 0.009 |
| Education (years)a | 14.3 | 2.4 | 15.1 | 2.82 | t = 2.8 | 0.005 |
| NART-R score | 107.5 | 9.39 | 110.6 | 8.3 | t = 3.3 | 0.001 |
|
| ||||||
| Characteristic | n | % | n | % | Test Statistic | p |
|
| ||||||
| Married or partnered | χ2 = 1.8 | 0.176 | ||||
|
| ||||||
| Yes | 70 | 58 | 162 | 66 | ||
|
| ||||||
| Race | χ2 = 15.3 | < 0.001 | ||||
|
| ||||||
| White | 106 | 88 | 242 | 98 | ||
|
| ||||||
| Mastectomy | χ2 = 1.2 | 0.275 | ||||
|
| ||||||
| Yes (versus BCS) | 17 | 14 | 22 | 9 | ||
|
| ||||||
| Radiation therapy | χ2 = 2.9 | 0.089 | ||||
|
| ||||||
| Yes | 62 | 52 | 123 | 50 | ||
|
| ||||||
| Systemic therapy | χ2 = 5.9 | 0.052 | ||||
|
| ||||||
| None (controls) | 28 | 23 | 78 | 32 | ||
| AI only | 62 | 52 | 95 | 38 | ||
| Chemotherapy plus AI | 30 | 25 | 74 | 30 | ||
|
| ||||||
| Stage of diseasea | FE | 0.488 | ||||
|
| ||||||
| I | 63 | 69 | 114 | 68 | ||
| IIa | 17 | 18 | 38 | 22 | ||
| IIb | 9 | 10 | 9 | 5 | ||
| IIIa | 3 | 3 | 8 | 5 | ||
Breast cancer cohort only (N = 261; low, n = 92; high, n = 169)
AI—aromatase inhibitor; BCS—breast-conserving surgery; FE—Fisher’s exact test; NART-R—National Adult Reading Test–Revised
Note. Although actual means and SDs are reported for each subgroup, statistical tests were performed on the square-root transformed variable.
In the multivariable phenotypic model, pretherapy verbal memory (p < 0.001), visual memory (p = 0.002), and executive function (p < 0.001) were associated with visual working memory subgroup membership. In pairwise subgroup comparisons, controlling for phenotypic predictors, membership in the low visual working memory subgroup was associated with poorer pretherapy verbal and visual memory and poorer executive function relative to the high subgroup. Controlling for variability in participant characteristics, genetic variation in one SNP in a DNA repair gene (ERCC5 rs873601 [p = 0.027]) was associated with subgroup membership. Carrying two copies of the minor G allele conferred almost four times increased odds of belonging to the low versus high visual working memory subgroup (p = 0.027).
The authors examined the association between trajectory grouping among pairs of the three cognitive domains (executive function, concentration, and visual working memory) using chi-square analyses. Highly significant associations (p < 0.0001) were observed for all pairwise associations. In particular, membership in high subgroups was correlated, indicating consistent membership in the high subgroups across the three cognitive domains. For the most part, membership in the low performing subgroups was also consistent across the cognitive domains.
Discussion
Ample evidence across multiple types of cancer suggests that, compared to healthy individuals, women with cancer have poorer cognitive function before they begin adjuvant therapy (Ahles et al., 2008; Hermelink et al., 2015). In addition, studies suggest that deterioration in cognitive function is associated with adjuvant therapy in individuals with cancer (Wefel et al., 2010). In the current article, the authors report the results of one of the first applications of subgroup trajectory modeling to examine objectively measured cognitive function in postmenopausal women with early-stage breast cancer. The authors focused on three cognitive domains in this analysis: executive function, concentration, and visual working memory. By performing GBTM, the authors identified subgroups of women with unique trajectories of cognitive function and examined phenotypic factors that are related to subgroup membership. The authors also explored relationships between specified genotypic characteristics and subgroup membership.
Subgroups and Their Trajectories
The high subgroups for concentration and visual working memory comprised the largest portion of the sample for these domains, 51% and 67%, respectively. The performance of women in the high visual working memory subgroup improved across all time points, suggesting practice effects, which are improvements in performance with repeated cognitive assessments (Lezak et al., 2004). The influence of practice effects was observed in the high and low visual working memory subgroups and the high and moderate executive function subgroups. Practice effects are not evident in any of the three concentration subgroups. Although practice effects are an expected phenomenon with retesting using cognitive function measures, they are more likely to occur with executive function and learning and memory domains than other cognitive domains, such as concentration (Bartels, Wegrzyn, Wiedl, Ackermann, & Ehrenreich, 2010; Basso, Bornstein, & Lang, 1999; Basso, Carona, Lowery, & Axelrod, 2002). In addition, the influence of practice effects does tend to be more prominent with initial retesting but then plateaus with later retesting (Bartels et al., 2010). This fact may partially explain the initial improvements in executive function in the high subgroup followed by the decline in performance. Cognitive reserve theory may also help to explain the waning performance at the final assessment in the high subgroup. According to reserve theory, individuals with greater cognitive reserve are better able to compensate for insults related to disease or treatment (Ahles et al., 2010; Stern, 2009), but the influence of these compensations may fade over time. Therefore, although some individuals may be more vulnerable to accelerated aging with the effects of cancer and cancer treatment, individuals with greater reserve may be less vulnerable. Interestingly, the performance of the low executive function subgroup did not improve with retesting, suggesting that this group had less cognitive reserve.
Twenty-four percent of the sample comprised the low executive function subgroup. The performance of this subgroup was stable across the trajectory, at nearly 1 standard deviation below the mean. The performance of the low concentration subgroup was nearly 1.5 standard deviations below the mean. Although this group was only 10% of the sample, these results indicate that some women may be particularly vulnerable to poor concentration that is present at pretherapy and persists through the first 18 months of AI therapy. These results are clinically meaningful, but it is unclear whether the performance in these subgroups constitutes impaired cognitive function. Debate continues regarding the criteria for impaired cognitive function (Shilling, Jenkins, & Trapala, 2006). Some contend that, to be considered impaired, performance must be at or greater than 1.5 standard deviations below the mean on multiple cognitive domains. Others suggest that impairment is not an appropriate label; rather, they propose the term lower than expected performance, determined by scores falling at or below 1.5 standard deviations in multiple cognitive domains (Ahles et al., 2008; Wefel, Vardy, Ahles, & Schagen, 2011). The authors’ analysis of the association between predicted trajectory groupings indicates that membership in the low subgroups was largely consistent across the three cognitive domains. Conversely, membership in the high performing subgroups was highly consistent across domains. These results indicate that women tend to be vulnerable to poorer performance across multiple cognitive domains. Additional research is needed to confirm these results in a larger sample and to determine whether poor performance across multiple cognitive domains persists through the remainder of therapy. Understanding the phenotypic and genotypic factors that are associated with membership in these subgroups helps to identify underlying mechanisms and women who are at risk for poorer cognitive function.
Phenotypic Factors
The results of this study related to the phenotypic factors associated with subgroup membership provides compelling evidence in support of accelerated aging in women with breast cancer and suggest that the presence of cognitive reserve in some women may mitigate the effects of cancer and cancer therapy on cognitive function.
Consistent with the theory of accelerated aging, receipt of adjuvant therapy was associated with membership in the low executive function subgroup relative to the high and moderate subgroups. The presence of greater concurrent fatigue at pretherapy was also associated with membership in the low executive function subgroup. The phenotype for aging is frailty, and among the criteria for frailty is fatigue (Fried et al., 2001). Aging may be accelerated with cancer and cancer therapy, and a consequence of this acceleration may be deterioration in cognitive function (Hurria, Jones, & Muss, 2016; Mandelblatt et al., 2013). Accelerated aging may be particularly relevant in women who have lower cognitive reserve and, therefore, greater vulnerability to insult related to cancer and cancer therapy.
Older age and lower estimated IQ scores (both proxies for cognitive reserve) were associated with membership in the low executive function subgroup; lower IQ score was also associated with the low visual working memory subgroup. In addition, poorer pretherapy cognitive function was consistently related to the low subgroups across the three domains examined. Taken together, these results suggest that older age, lower IQ, and poorer pretherapy cognitive function, which are all indicative of less cognitive reserve, place women at greater vulnerability to poorer cognitive function with breast cancer and its therapy. In addition, the results indicate that receipt of adjuvant therapy accelerates cognitive aging in women with breast cancer.
Genotypic Factors
Higher levels of oxidative stress and DNA damage with reduced capacity for repair of DNA damage occur with aging. Cancer and cancer therapy increase oxidative stress and ensuing DNA damage (Joshi et al., 2005; Lisanti et al., 2011; Wang et al., 2008), and may result in an accelerated aging and cognitive decline (Ahles et al., 2010; Mandelblatt et al., 2013). The authors explored whether polymorphisms in genes involved in DNA repair and oxidative stress were associated with changes in cognitive function and found preliminary evidence that variability in DNA repair gene polymorphisms imparted differential risk for subgroup membership. This was particularly evident with respect to the executive function domain, where polymorphisms associated with two DNA repair genes (PARP1 and ERCC3) were related to membership in the low subgroup relative to the moderate subgroup. In addition, carrying at least one copy of the ERCC5 rs751402 T minor allele was protective for executive function. Less evidence of these relationships was found for concentration and visual working memory. A SNP related to one DNA repair gene (ERCC3 rs4150407) was associated with membership in the low concentration subgroup relative to the moderate subgroup. Similarly, a SNP related to DNA repair gene (ERCC5 rs873601) was associated with membership in the low visual working memory subgroup relative to the high subgroup.
Mechanisms to repair the inevitable DNA damage associated with exposure to endogenous and exogenous agents are critical to the development and function of the nervous system as well as to protection against development of diseases, such as cancer (Cha & Yim, 2013; Curtin, 2012; Narciso et al., 2016; Smetana et al., 2016). Cancer and cancer treatment can initiate DNA damage and reduction in the effectiveness of some DNA repair mechanisms (Cha & Yim, 2013). In addition, there is reduced capacity of DNA repair mechanisms with advancing age, resulting in increased risk for cancer (Smetana et al., 2016). Mandelblatt et al. (2013) propose a confluence of these factors, suggesting that cancer and its treatment may accelerate the effects of aging on neurons and oxidative stress and DNA repair pathways, which is reflected in accelerated cognitive aging in individuals with the disease. The results of the exploratory analysis of candidate gene polymorphisms related to DNA repair and oxidative stress suggest that this may be true. However, additional research is needed to confirm these findings in a fully powered study.
The mechanisms for the associations found between oxidative stress and DNA repair gene polymorphisms and subgroup membership could be increased levels of oxidative stress and weakened DNA repair. However, the functions of these polymorphisms have not been substantiated, and future studies are needed to clarify their functional roles.
Limitations
The results related to the associations between genotypic factors and trajectory subgroups must be interpreted with caution. Genetic samples were available from a subgroup of the full sample of this study, and the genotypic analysis was exploratory in nature and intended to generate hypotheses for testing in future studies. In addition, the sample lacked racial and ethnic diversity, limiting generalizability.
Implications for Practice and Future Research
The results of this study indicate that nurses who care for postmenopausal women with breast cancer can recognize women who may be at greater risk for poorer cognitive function with disease and treatment. Women who are older at diagnosis may have less cognitive reserve, placing them at greater risk for poorer cognitive function with cancer and cancer therapy. In addition, women who report greater levels of fatigue and poorer cognitive function at diagnosis may be at greater risk for poorer cognitive function with cancer therapy. Although self-report of cognitive problems is generally not related to objectively measured cognitive function (Bender et al., 2008), cognitive problems are commonly related to self-reports of fatigue and, therefore, may be an indirect indication of poorer cognitive function (Downie, Mar Fan, Houédé-Tchen, & Tannock, 2006; Merriman et al., 2017). Assessing women for other factors that may increase their risk for poorer cognitive function is important; these include previous cancer and cancer therapy, certain comorbidities, such as a history of substance abuse, and neurologic disease or neurotrauma and serious psychiatric illness, such as clinical depression and concomitant medications (Jansen, 2013). Nurses should also assess the functional and emotional implications of changes in cognitive function. Poor cognitive function can have a deleterious effect on women’s ability to maintain their functions at home and at work (Nugent et al., 2016). It can also be a source of distress for women and their families who may misattribute changes in cognitive function to failure to respond to therapy and worsening disease.
Unfortunately, strong evidence supporting the efficacy of interventions to prevent or manage changes in cognitive function with cancer and cancer therapy is lacking (Oncology Nursing Society, 2016; Von Ah, Storey, Jansen, & Allen, 2013). Nurses may be able to support women who experience this problem by managing their co-occurring symptoms. For example, interventions to reduce fatigue, pain, and sleep problems and improve mood may reduce cognitive problems. Although most women who experience changes in cognitive function with cancer and cancer therapy do not have impaired cognitive function, women who do have more severe or persistent deterioration in cognitive function should have a comprehensive neuropsychological assessment. In addition, referral for cognitive rehabilitation to help women regain their functional ability at home and at work may be needed.
More research is needed to understand this problem. The long-term effects of cancer and cancer therapy on cognitive function are unclear. It is important to continue research that will help to develop a characteristic profile of patients at risk by uncovering the behavioral and biologic mechanisms underlying cognitive changes. The research in this current article suggests that factors related to oxidative stress and weakened DNA repair should be considered in future mechanistic studies, and that additional research is needed to more fully explicate these and other mechanisms of cognitive changes. It will be important to identify approaches for assessment of cognitive function that provide meaningful data for clinical and research settings. Exhaustive neuropsychological batteries are not practical for use in clinical settings, and valid, reliable assessment tools appropriate for clinical assessment of cognitive function are not available. Finally, research is needed to test interventions to prevent and manage changes in cognitive function with cancer and cancer therapy.
Conclusion
The results of this study suggest that subgroups of postmenopausal women with breast cancer have poorer executive function, concentration, and visual working memory that preexists the initiation of systemic adjuvant therapy and persists through the first 18 months of therapy. Importantly, the results indicate characteristics of women at greater risk for poorer cognitive function with disease and treatment. Advancing age, greater pretherapy fatigue, and poorer cognitive function prior to the initiation of adjuvant therapy increases the likelihood of having poorer cognitive function with therapy. In addition, cancer and cancer treatment may accelerate the effects of aging on neurons and DNA repair mechanisms, resulting in accelerated cognitive decline in individuals with the disease. These findings give preliminary direction for identification of women with breast cancer who are at risk for poor cognitive function and point to a need to develop interventions that mitigate changes in cognitive function.
KNOWLEDGE TRANSLATION.
■ Subgroups of women with breast cancer are at risk for poorer cognitive function before they begin adjuvant therapy and during the first 18 months of therapy.
■ Women with breast cancer who are older and have more fatigue and poorer cognitive function at pretherapy are at greater risk for poorer cognitive function with adjuvant therapy.
■ Oxidative stress and weakened DNA repair may be related to poorer cognitive function in women with breast cancer.
Acknowledgments
This research was funded by a grant from the National Cancer Institute (R01CA107408, principal investigator: Bender) and an award from the Oncology Nursing Society Foundation (Conley and Bender, co-principal investigators). Merriman was supported by grants from National Institute of Nursing Research (NINR) (K99NR015473 and T32NR009759), and Koleck was also supported by grants from the NINR (F31NR014590 and T32NR009759). Bender was the recipient of the 2017 ONS Congress Distinguished Nurse Researcher Award and presented a lecture at the 42nd annual ONS Congress in Denver, CO.
Footnotes
Bender, Sereika, Rosenzweig, Brufsky, and Conley contributed to the conceptualization and design. Bender, Gentry, Casillo, Koleck, Brufsky, and Conley completed the data collection. Bender, Sereika, Koleck, and Brufsky provided statistical support. Bender, Merriman, Sereika, Brufsky, and Zhu provided the analysis. Bender, Merriman, Sereika, Gentry, Koleck, Rosenzweig, Brufsky, McAuliffe, Zhu, and Conley contributed to the manuscript preparation.
Contributor Information
Catherine M. Bender, Professor and the Nancy Glunt Hoffman Endowed Chair in Oncology Nursing.
John D. Merriman, Postdoctoral associate.
Susan M. Sereika, Professor and director of the Center for Research and Evaluation.
Amanda L. Gentry, Project director.
Frances E. Casillo, Research associate and RN, all in the School of Nursing at the University of Pittsburgh in Pennsylvania.
Theresa A. Koleck, Postdoctoral research fellow in the School of Nursing at Columbia University in New York, NY.
Margaret Q. Rosenzweig, Professor in the School of Nursing at the University of Pittsburgh.
Adam M. Brufsky, Professor of medicine and associate chief of the Division of Hematology/Oncology in the Department of Medicine at the University of Pittsburgh, and co-director of the Comprehensive Breast Cancer Center and associate director of clinical investigation for the University of Pittsburgh Medical Center (UPMC) Cancer Center.
Priscilla McAuliffe, Assistant professor in the Department of Surgery, Division of Surgical Oncology, at the University of Pittsburgh and UPMC Cancer Center.
Yehui Zhu, Doctoral student.
Yvette P. Conley, Professor, both in the School of Nursing at the University of Pittsburgh.
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: A longitudinal study on frequent repetitive cognitive testing. BMC Neuroscience. 2010;11:118. doi: 10.1186/1471-2202-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA, Lang JM. Practice effects on commonly used measures of executive function across twelve months. Clinical Neuropsychologist. 1999;13:283–292. doi: 10.1076/clin.13.3.283.1743. [DOI] [PubMed] [Google Scholar]
- Basso MR, Carona FD, Lowery N, Axelrod BN. Practice effects on the WAIS-III across 3- and 6-month intervals. Clinical Neuropsychologist. 2002;16:57–63. doi: 10.1076/clin.16.1.57.8329. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bender CM, Merriman JD, Gentry AL, Ahrendt GM, Berga SL, Brufsky AM, Sereika SM. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121:2627–2636. doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, Sereika SM. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncology Nursing Forum. 2014;41:274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Pacella ML, Sereika SM, Brufsky AM, Vogel VG, Rastogi P, Ryan CM. What do perceived cognitive problems reflect? Journal of Supportive Oncology. 2008;6:238–242. [PMC free article] [PubMed] [Google Scholar]
- Bender CM, Thelen BD. Cancer and cognitive changes: The complexity of the problem. Seminars in Oncology Nursing. 2013;29:232–237. doi: 10.1016/j.soncn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of Clinical and Experimental Neuropsychology. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Cha HJ, Yim H. The accumulation of DNA repair defects is the molecular origin of carcinogenesis. Tumour Biology. 2013;34:3293–3302. doi: 10.1007/s13277-013-1038-y. [DOI] [PubMed] [Google Scholar]
- Cheang MC, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, Carey LA. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015;20:474–482. doi: 10.1634/theoncologist.2014-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He X, Tao L, Li J, Wu J, Zhu C, Wang K. The working memory and dorsolateral prefrontal-hippocampal functional connectivity changes in long-term survival breast cancer patients treated with tamoxifen. International Journal of Neuropsychopharmacology. 2017;20:374–382. doi: 10.1093/ijnp/pyx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. Journal of Clinical Oncology. 1984;2:1102–1109. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psycho-Oncology. 2009;18:811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nature Reviews Cancer. 2012;12:801–817. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- Downie FP, Mar Fan HG, Houédé-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: Evaluation with patient interview after formal assessment. Psycho-Oncology. 2006;15:921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults. Journals of Gerontology Series A. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: Observational data over 12 months from the mind-body study. Journal of Clinical Oncology. 2016;34:816–824. doi: 10.1200/JCO.2015.64.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Petersen L, Castellon SA, Bower JE, Silverman DH, Cole SW, Belin TR. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: An observational cohort study. Journal of Clinical Oncology. 2014;32:3559–3567. doi: 10.1200/jco.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LF, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer. 2008;113:2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Voigt V, Kaste J, Neufeld F, Wuerstlein R, Bühner M, Harbeck N. Elucidating pretreatment cognitive impairment in breast cancer patients: The impact of cancer-related post-traumatic stress. Journal of the National Cancer Institute. 2015;107:djv099. doi: 10.1093/jnci/djv099. [DOI] [PubMed] [Google Scholar]
- Hurria A, Jones L, Muss HB. Cancer treatment as an accelerated aging process: Assessment, biomarkers, and interventions. American Society of Clinical Oncology Educational Book. 2016;35:E516–E522. doi: 10.14694/edbk_156160. [DOI] [PubMed] [Google Scholar]
- Jablonska E, Gromadzinska J, Peplonska B, Fendler W, Reszka E, Krol MB, Wasowicz W. Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer. 2015;15:657. doi: 10.1186/s12885-015-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CE. Cognitive changes associated with cancer and cancer therapy: Patient assessment and education. Seminars in Oncology Nursing. 2013;29:270–279. doi: 10.1016/j.soncn.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, Butterfield DA. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: Insight into chemobrain. Free Radical Research. 2005;39:1147–1154. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- Koleck TA, Bender CM, Sereika SM, Ahrendt G, Jankowitz RC, McGuire KP, Conley YP. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer [Online exclusive] Oncology Nursing Forum. 2014;41:E313–E325. doi: 10.1188/14.ONF.E313-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleck TA, Bender CM, Sereika SM, Brufsky AM, Lembersky BC, McAuliffe PF, Conley YP. Polymorphisms in DNA repair and oxidative stress genes associated with pre-treatment cognitive function in breast cancer survivors: An exploratory study. SpringerPlus. 2016;5:422. doi: 10.1186/s40064-016-2061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Bigler ED, Tranel D. Neuropsychological assessment. 4th. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Lisanti MP, Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Pestell RG, Howell A, Sotgia F. Accelerated aging in the tumor microenvironment: Connecting aging, inflammation and cancer metabolism with personalized medicine. Cell Cycle. 2011;10:2059–2063. doi: 10.4161/cc.10.13.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, Ahles T. Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Seminars in Oncology. 2013;40:709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, Ahles T. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? Journal of Clinical Oncology. 2014;32:1909–1918. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, Tannock IF. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy. Supportive Care in Cancer. 2007;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- McAdam E, Brem R, Karran P. Oxidative stress-induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Molecular Cancer Research. 2016;14:612–622. doi: 10.1158/1541-7786.MCR-16-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States (POMS)—Revised manual. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- Merriman JD, Sereika SM, Brufsky AM, McAuliffe PF, McGuire KP, Myers JS, Bender CM. Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psycho-Oncology. 2017;26:44–52. doi: 10.1002/pon.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Jemal A. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Myers JS. Chemotherapy-related cognitive impairment: The breast cancer experience [Online exclusive] Oncology Nursing Forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nagin DS. Group-based trajectory modeling: An overview. Annals of Nutrition and Metabolism. 2014;65:205–210. doi: 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Narciso L, Parlanti E, Racaniello M, Simonelli V, Cardinale A, Merlo D, Dogliotti E. The response to oxidative DNA damage in neurons: Mechanisms and disease. Neural Plasticity. 2016;2016:3619274. doi: 10.1155/2016/3619274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): For the assessment of premorbid intelligence in patients with dementia: Test manual. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Nugent BD, Sereika SM, Rosenzweig M, McCue M, Merriman JD, Bender CM. The association between pre-treatment occupational skill level and mood and symptom burden in early-stage, postmenopausal breast cancer survivors during the first year of anastrozole therapy. Supportive Care in Cancer. 2016;24:3401–3409. doi: 10.1007/s00520-016-3161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncology Nursing Society. Cognitive impairment. 2016 Retrieved from http://www.ons.org/practice-resources/pep/cognitive-impairment.
- Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Research and Treatment. 1998;51:227–238. doi: 10.1023/a:1006132427948. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Research and Treatment. 2000;64:165–176. doi: 10.1023/a:1006426132338. [DOI] [PubMed] [Google Scholar]
- Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. Journal of Cancer Survivors. 2008;2:275–282. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- Root JC, Andreotti C, Tsu L, Ellmore TM, Ahles TA. Learning and memory performance in breast cancer survivors 2 to 6 years post-treatment: The role of encoding versus forgetting. Journal of Cancer Survivorship. 2016;10:593–599. doi: 10.1007/s11764-015-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavon G, Smith IE. Status of adjuvant endocrine therapy for breast cancer. Breast Cancer Research. 2014;16:206. doi: 10.1186/bcr3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Schagen SB. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy. Acta Oncologica. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer. Journal of Clinical Oncology. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog—Methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Research and Treatment. 2006;95:125–129. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annual Review of Medicine. 1983;34:35–46. doi: 10.1146/annurev.me.34.020183.000343. [DOI] [PubMed] [Google Scholar]
- Smetana K, Jr, Lacina L, Szabo P, Dvořánková B, Brož P, Šedo A. Ageing as an important risk factor for cancer. Anticancer Research. 2016;36:5009–5017. doi: 10.21873/anticanres.11069. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ah D, Storey S, Jansen CE, Allen DH. Coping strategies and interventions for cognitive changes in patients with cancer. Seminars in Oncology Nursing. 2013;29:288–299. doi: 10.1016/j.soncn.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Wu LM, Amidi A. Cognitive impairment following hormone therapy. Current Opinion in Supportive and Palliative Care. 2017;11:38–45. doi: 10.1097/SPC.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]


