Abstract
Background
In patients with suspected native valve infective endocarditis (IE), current guidelines recommend an initial transthoracic echocardiogram (TTE) followed by a transesophageal echocardiogram (TEE) if clinical suspicion remains. The guidelines do not account for quality of the initial TTE or other findings that may alter the study’s diagnostic characteristics. This may lead to unnecessary TEE procedures where the initial TTE was sufficient to rule out vegetation.
Methods
Patients with suspected native valve endocarditis that underwent a TTE followed by a TEE within 7 days between 1/1/2007 and 2/28/2014 were included. A negative TTE for vegetation was defined by either the standard approach (at least possible vegetation seen on TTE), or by applying a set of strict negative criteria incorporating other findings on TTE. Using TEE as the gold standard for presence of vegetation, the diagnostic performance of the two TTE approaches were compared.
Results
In total, 790 TTE/TEE pairs were identified. With the standard approach, 671 of the TTEs were negative, compared to 107 negative studies using the strict negative approach. The sensitivity and negative predictive value (NPV) of TTE for detecting vegetation were substantially improved using the strict negative approach (sensitivity: 98% [95% C.I. 95–99%] vs. 39% [95% C.I. 31%–47%], NPV: 97% [95% C.I. 92–99%] vs. 86% [95% C.I. 83–88%]).
Conclusions
The ability for TTE to exclude vegetation in patients is excellent when strict criteria for a negative study are applied. In low to intermediate risk patients with a strict negative TTE, follow up TEE may be unnecessary.
Keywords: Infective Endocarditis, Echocardiography, Transesophageal Echocardiography, Screening
1. Background
The diagnosis of infective endocarditis (IE) is based on the Modified Duke criteria where presence of endocardial vegetation on echocardiography is a major criterion.1 Guidelines from multiple clinical societies including the American College of Cardiology, the American Heart Association, and the Infectious Disease Society of America recommend transthoracic echocardiography (TTE) as the initial test when evaluating for vegetations in patients with a low or intermediate pretest probability of IE. Transesophageal echocardiography (TEE) is recommended confirm absence of vegetations in patients with a negative TTE if clinical suspicion of IE remains.2,3
These recommendations are based on work from the late 1980s into the 1990s showing that the sensitivity of TTE for detecting valvular vegetations (44% to 75%), is markedly lower than what has been reported for TEE (over 95%).4–7 Most of the prior work reporting the diagnostic characteristics of TTE was performed with older generation echocardiography (echo) technology. Furthermore, these recommendations are based strictly on the presence or absence of a vegetation on TTE, and do not account for image quality or other echo findings which may alter the study’s diagnostic performance characteristics.
The clinical utility of TTE would be markedly improved by increases in sensitivity and negative predictive value, which may allow for a negative TTE to exclude intracardiac vegetations. We hypothesized that when evaluating for IE, using modern echo equipment in combination with a stricter criteria for labeling a TTE as negative would generate a subset of patients where a negative TTE may obviate the need for follow up TEE.
2. Methods
2.1 Study Objective
The objective of this study was to determine whether implementing a strict definition of a negative TTE would improve the performance characteristics of TTE sufficiently to exclude IE.
2.2 Patient Population Derivation
The patient population was derived through the Duke Echocardiography Lab Database DELD). The set up of DELD has been previously described.8 Briefly, the DELD is a prospectively maintained digital archive of all echocardiograms performed at Duke University Hospital and satellite clinics since 1995. This is linked to a corresponding searchable reporting database with clinical information derived from the electronic health record.
2.3 Patient Population
The DELD was searched for adult patients who underwent a TTE followed by a TEE within 7 days between January 1, 2007 and February 28, 2014. Patients who underwent the studies for any of the following indications were included: history of fever, bacteremia, or IE evaluation. The majority of TTEs (over 90%) were performed by the Phillips iE33 platform (Andover, WA), which was fully integrated at our institution by January 1, 2007. The remaining studies were performed with the GE Vivid 7 or E9 platforms (Buckinghamshire, UK). Studies were performed using a 2.5 MHz phased array probe. The TTE IE imaging protocol includes multiple zoomed-in views of each valve, with frequent use of fundamental frequencies to enhance spatial resolution beyond that of harmonic imaging alone. Patients with prior valve repair or replacement, complex congenital heart disease, prior heart transplant or left ventricular assist device were excluded due to the higher incidence of IE, and increased difficulty of imaging in these groups by TTE alone.9–12 TEE was performed using the Phillips iE33 machine and a omniplane 3.5 MHz phased array probe using fundamental frequencies.
2.4 Definition of Vegetation
Vegetation was defined as an independently mobile or oscillating, echogenic target or mass in a heart chamber or valve on an echocardiographic study done for the above indication. To find studies with at least possible vegetation, a string search was performed in the DELD on the ‘Final Interpretation’ free text field and valve comments fields for “vegetation”, “target”, or “mass”. The comments for the resulting studies were then manually reviewed. Studies with mobile target(s) seen on a valve, without a more likely alternative etiology (e.g. senile degeneration, benign neoplasm, or annular calcification), were considered suggestive of vegetation.3 The result of the follow up TEE was considered the gold standard for presence of vegetation.
2.5 Strict Criteria for Negative TTE
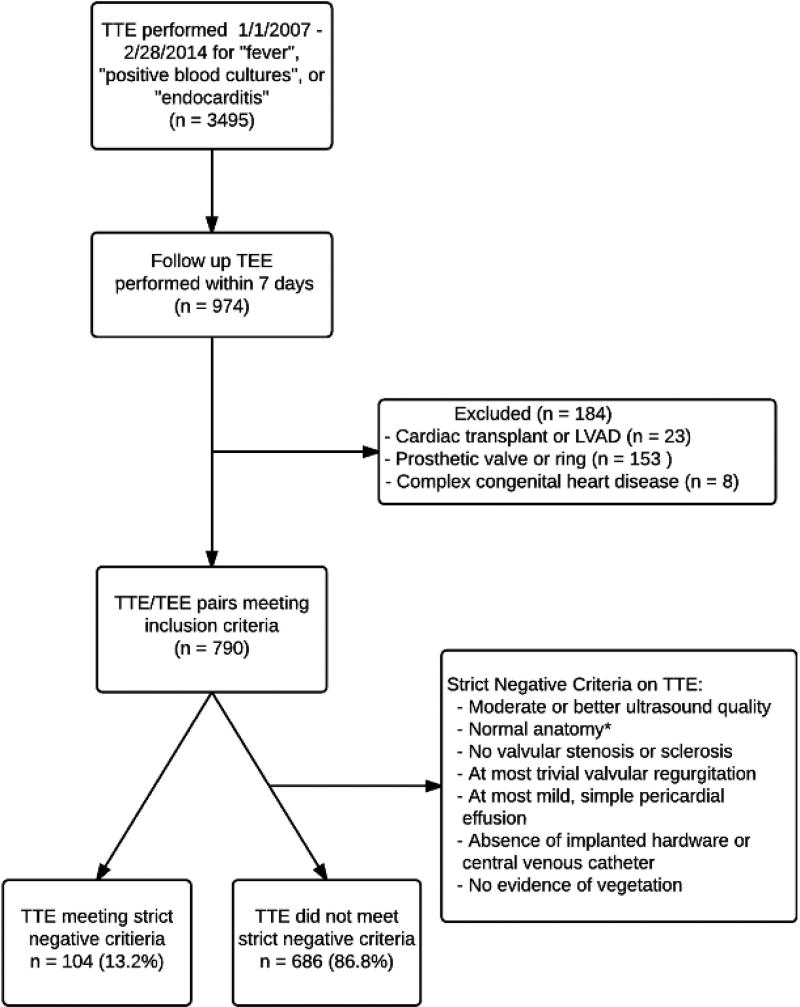
Two separate analyses were performed to evaluate the diagnostic characteristics of TTE in the evaluation of IE. In the first analysis, the standard definition of a positive or negative TTE was used, based solely on the presence or absence of possible vegetation. For the second analysis, a strict definition of a negative TTE was developed, and studies not meeting these criteria were considered “positive or indeterminate”. The criteria for a “strict negative” TTE are shown in figure 1.
Figure 1.
Study cohort derivation and strict negative criteria
This figure outlines the derivation of the study cohort and shows the list of strict negative criteria
Abbreviations: TTE, Transthoracic Echocardiogram; TEE, Transesophageal Echocardiogram; LVAD, Left Ventricular Assist Device;
*Normal anatomy defined as tricuspid aortic, pulmonic, and tricuspid valves, mitral valve without mitral annular calcification, no mitral valve prolapse, no atrial septal defect or ventricular septal defect, repaired or unrepaired.
The strict negative criteria were designed to identify TTEs that provide strong evidence against vegetation. Valvular regurgitation is characteristic of IE and has been associated with increased morbidity and mortality in patients diagnosed with IE.1,13,14 Thus, studies with mild or greater regurgitation of any valve were labeled indeterminate. Because serial echocardiographic data may not be available for all patients presenting with suspected IE, stability of regurgitant lesions was not included in the criteria.
Findings that suggest increased risk of IE were also exclusions in the strict negative criteria. These include valvular stenosis or sclerosis, intracardiac foreign bodies, and anatomic abnormalities such as ventricular septal defects and structural valvular disease, which have all been shown to increase the risk of IE.10,15–22 Large or complex pericardial effusions were also excluded, as these have been associated with both increased rates of IE, and worse outcomes when IE is present.23,24
Finally, all studies were required to have adequate technical quality to detect all components of the strict negative criteria, with moderate or better sound quality defined as adequate visualization of anatomic structures, chamber morphology, endocardial borders, and cardiac function from the standard acoustic windows without the need for contrast.25 Thus, studies without adequate image quality to detect the presence of valvular regurgitation, valvular sclerosis, or presence of intracardiac hardware would be considered ‘poor’ and not meeting the strict negative criteria.
2.6 Baseline Characteristics and Outcome Data
DEDUCE (Duke Enterprise Data Unified Content Explorer) is a web-based query tool used to extract data generated as a byproduct of the care of patients in the Duke University Health System.26 The cohort of patients generated from the initial query of the DELD was uploaded to DEDUCE, and clinical data including prior diagnoses, blood culture results, and outcomes data was extracted. The available outcomes data applicable to this study were mortality and surgical intervention.
2.7 Statistical Analysis Approach
The sensitivity (True positives/Condition positive), specificity (True Negative/Condition Negative), negative predictive value (True negative/Test Outcome Negative), and positive predictive value (True Positive/Test Outcome Positive) were determined for both the standard and strict negative criteria approaches using the standard 2×2 table approach. From this, negative and positive likelihood ratios (False Negative Rate/True Negative Rate, and True Positive Rate/False Positive Rate respectively) and the resulting relationships for each approach between pre-test and post-test odds using Bayes’ Theorem were determined. A test indication curve was then generated to compare the effect of the negative likelihood ratio on post-test probability using the two TTE interpretation approaches.27 Differences in baseline characteristics between the strict negative sub group and the positive or indeterminate sub group were determined by using Fisher’s exact test or unpaired Student’s t test. Statistical calculations were performed using GraphPad (San Diego, CA), and p-values <0.05 were considered statistically significant. Results are presented as mean ± standard deviation or value with 95% confidence intervals (calculated via Wilson score interval with continuity correction).
2.8 Ethics
This study was performed using only information gathered as part of the routine clinical evaluation of patients at our institution, and was carried out under approval of the Duke University School of Medicine Institutional Review Board.
3. Results
Between January 1, 2007 and February 28, 2014, 3,495 TTEs were performed to evaluate for IE. Of these, 790 TTEs met inclusion criteria and were followed by a TEE within 7 days. Among these 790 TTE/TEE paired examinations, 107 of the TTEs met the strict criteria for a negative TTE (13.5% of all included TTEs, Figure 1). For the overall cohort, 143 TEEs were performed in the outpatient setting (18.1%), and among patients meeting strict negative criteria, 23 TEEs were outpatient (21.5%).
Baseline characteristics and blood culture data of the overall cohort, as well as the subgroups classified by the strict negative criteria are shown in table 1. The strict negative group was significantly younger with lower rates of congestive heart failure, coronary artery disease, and chronic kidney disease, and hypertension. The proportion of patients with positive blood cultures was similar in each group, as was the distribution of infectious organisms.
Table 1.
Baseline characteristics and blood culture data
| Overall (n = 790) |
Strict Negative (n = 107) |
Indeterminate/ Positive(n = 683) |
P Value |
|
|---|---|---|---|---|
| Baseline Characteristics | ||||
|
|
||||
| Age (years, mean ± SD) | 57.3 ± 15.5 | 45.7 ± 13.5 | 59.2 ± 15.0 | <0.001 |
| Female (%) | 335 (42%) | 39 (36%) | 296 (43%) | 0.21 |
| Hypertension (%) | 599 (76%) | 66 (62%) | 533 (78%) | <0.001 |
| Diabetes Mellitus (%) | 366 (46%) | 41 (38%) | 325 (48%) | 0.08 |
| Congestive Heart Failure (%) | 310 (39%) | 9 (8%) | 301 (44%) | <0.001 |
| Coronary Artery Disease (%) | 293 (37%) | 9 (8%) | 284 (42%) | <0.001 |
| Chronic Kidney Disease (%) | 372 (47%) | 33 (31%) | 339 (50%) | <0.001 |
| Renal Dialysis Status (%) | 169 (21%) | 18 (17%) | 151 (22%) | 0.25 |
| COPD (%) | 51 (6%) | 5 (5%) | 46 (7%) | 0.53 |
| Cerebrovascular Disease (%) | 183 (23%) | 12 (11%) | 171 (25%) | 0.001 |
| HIV Positive (%) | 22 (3%) | 8 (7%) | 14 (2%) | 0.005 |
| History Of Illicit Drug Abuse (%) | 42 (5%) | 12 (11%) | 30 (4%) | 0.008 |
| Metastatic Cancer (%) | 31 (4%) | 4 (4%) | 27 (4%) | 1.0 |
|
|
||||
| Blood Culture Data | ||||
|
|
||||
| Positive Blood Cultures (%) | 595 (75%) | 88 (82%) | 507 (74%) | 0.09 |
| Species (% of Positive Cultures): | ||||
| Staphylococcus aureus | 157 (26%) | 27 (31%) | 130 (19%) | 0.15 |
| Streptococcus Species | 28 (5%) | 2 (2%) | 26 (4%) | 0.41 |
| Coagulase-Negative | 61 (10%) | 7 (8%) | 54 (8%) | 0.85 |
| Staphylococcus | ||||
| Enterococcus | 28 (5%) | 1 (1%) | 27 (4%) | 0.16 |
| Polymicrobial including SA | 209 (35%) | 38 (43%) | 171 (25%) | 0.03 |
| Polymicrobial excluding SA | 76 (13%) | 5 (6%) | 71 (10%) | 0.08 |
| Other | 36 (6%) | 8 (9%) | 28 (4%) | 0.13 |
SA = Staphylococcus aureus, COPD = Chronic Obstructive Pulmonary Disease, HIV = Human Immunodeficiency Virus. Note that statistical comparisons are between outlined columns.
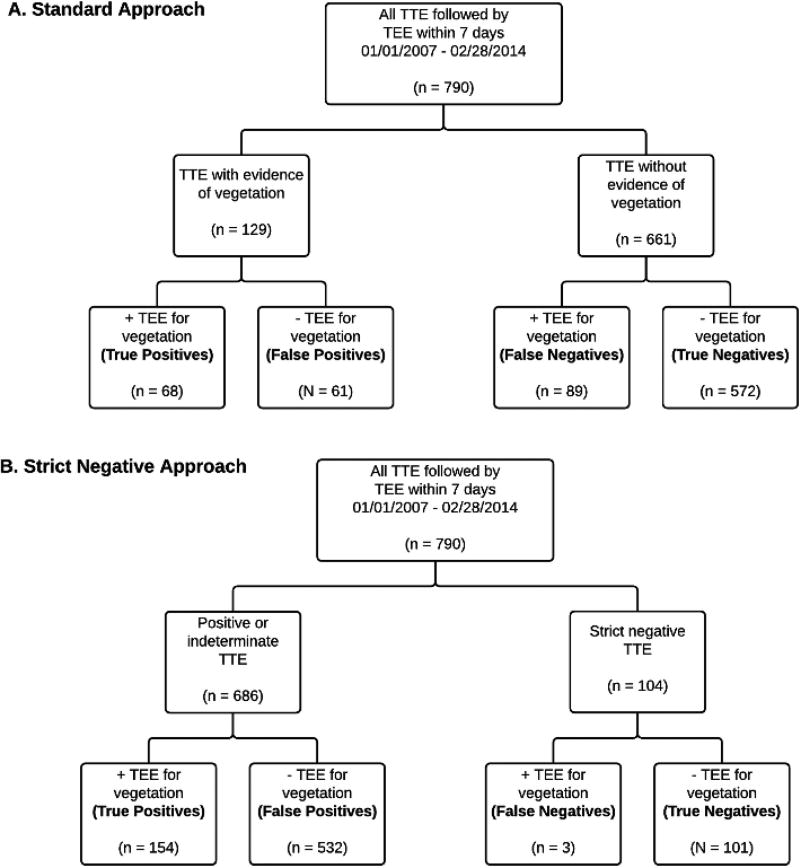
Using the standard approach, 671 out of 790 TTEs were read as negative for vegetation, and 119 were read as at least possible for vegetation (figure 2A). Of the 671 negative TTEs, 95 had vegetation on subsequent TEE. The resulting sensitivity of TTE using the standard approach was (60/155) = 39% (95% CI 31%–47%), with a NPV of (576/671) = 86% (95% CI 83–88%, Table 2).
Figure 2.
A. Flow diagram showing the results of using the standard approach.
B. Flow diagram showing the results of using the strict negative approach.
Abbreviations: TTE, Transthoracic Echocardiogram; TEE, Transesophageal Echocardiogram
Table 2.
Diagnostic characteristics of the standard approach and the strict negative approach
| Standard Approach (95% CI) | Strict Negative Approach (95% CI) |
|
|---|---|---|
| Sensitivity (%) | 38.7 (31.4 – 46.6) | 98.1 (94.5 – 99.3) |
| Specificity (%) | 90.7 (88.2 – 92.7) | 16.3 (13.7 – 19.4) |
| PPV (%) | 50.4 (41.6 – 59.2) | 22.2 (19.2 – 25.5) |
| NPV (%) | 85.8 (83.0 – 88.3) | 97.2 (92.1 – 99.0) |
| LR+ | 4.166 (3.045 – 5.700) | 1.17 (1.13 – 1.22) |
| LR− | 0.676 (0.595 – 0.768) | 0.119 (0.038 – 0.369) |
PPV = Positive Predictive Value, NPV = Negative Predictive Value, LR+ = Positive Likelihood Ratio, LR− = Negative Likelihood Ratio, CI = Confidence Interval
The strict criteria, along with the number of TTEs not meeting each criterion are listed in table 3. Of the 107 patients meeting strict negative criteria, 3 had evidence of vegetation on subsequent TEE (Figure 2B). The resulting sensitivity of TTE using the strict negative approach was (152/155) = 98% (95% CI 95–99%), with a NPV of (104/107) = 97% (95% CI 92–99%, Table 2).
Table 3.
The strict negative criteria, and number of TTEs in overall cohort not satisfying each criterion
| Criteria | N not meeting each criterion (%) |
|---|---|
| Moderate or better sound quality | 117 (15%) |
| Normal anatomy* | 282 (36%) |
| No valvular stenosis or sclerosis | 168 (21%) |
| Less than mild valvular regurgitation | 478 (61%) |
| Less than moderate, simple pericardial effusion | 6 (0.7%) |
| Absence of pacemaker/defibrillator leads, central venous catheter | 164 (21%) |
| No evidence of vegetation | 119 (15%) |
Normal anatomy defined as tricuspid aortic, pulmonic, and tricuspid valves, mitral valve without mitral annular calcification. No mitral valve prolapse. No atrial septal defect or ventricular septal defect - repaired or unrepaired.
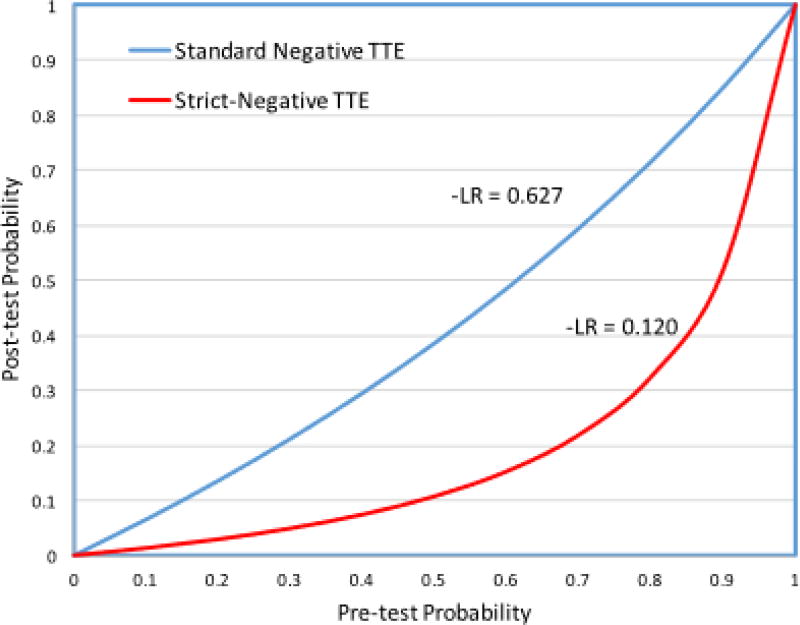
By applying the calculated negative likelihood ratios listed in table 2, the effect of a negative TTE on posttest probability was estimated using each approach, and is displayed as a test indication curve in figure 3.
Figure 3.
Test indication curve comparing the negative likelihood ratios for the standard and strict negative approach.
This figure uses the negative likelihood ratio to display the relationship between pre and post test probability for each approach. In order to focus on the improved negative predictive value when using the strict negative criteria, the positive likelihood ratio curves were not included.
Abbreviations: TTE, Transthoracic Echocardiogram;
Among all 790 patients with TTE/TEE pairs, there was an 8.6% 30-day mortality rate and a 5.6% incidence of valve surgery within 180 days. The mortality and morbidity in the strict negative cohort was much less, with a 1.9% 30-day mortality rate, and no patients undergoing valve surgery.
The clinical scenarios for the 3 patients with false-negative strict criteria TTEs were reviewed. Patient A had a remote history of L4/L5 fusion, and was found to have Streptococcus mitus bacteremia and osteomyelitis of his lumbar spine. TEE showed vegetation on the right coronary cusp of the aortic valve. Patient B was on chronic intravenous total-parenteral nutrition, and was found to have subclavian thrombophlebitis and Candida albicans bacteremia. He had no central line at time of TTE (removed due to candidemia), and thus met our criteria for strict negative TTE. TEE showed a small mobile target on aortic side of right coronary cusp. Patient C had a history of intravenous drug abuse, and was found to have pulmonary septic emboli and Staphylococcus aureus bacteremia. TEE showed a vegetation on the tricuspid valve. There was no evidence of abscess, fistula, or leaflet perforation on TEE, and no IE related mortality or surgery for these three patients.
4. Discussion
This large observational study demonstrates that by using strict criteria to define a negative TTE for vegetation, the sensitivity and NPV of TTE for presence of vegetation is greatly improved compared to the standard approach.
These findings lend support to previous studies which have suggested that in the setting of Staphylococcus aureus bacteremia, vegetation may be sufficiently excluded by a negative TTE in patients with low risk features, such as absence of: intracardiac devices, embolic phenomena, prolonged bacteremia, prosthetic valves, hemodialysis, and secondary foci of infection.28–34 The present study suggests that simple criteria based on TTE findings can be applied across a variety of infectious organisms. In contrast to prior investigators, we chose to examine the diagnostic characteristics of TTE in patients with native valves only.35,36 In light of known sound transmission limitations and echo artifacts associated with prosthetic valves, this approach is consistent with current guidelines for IE where an initial TEE in patients with prosthetic valves is recommended.2,3
The overall cohort of patients in this study had an intermediate pretest probability for IE. The prevalence of cardiovascular and renal disease was high in this group, as was the number of patients with pacemaker/defibrillator leads or central venous catheters. Furthermore, all patients were felt to warrant a TEE by their care providers at the time of hospitalization, signifying greater clinical suspicion. The strict negative group had a lower prevalence of cardiovascular and renal disease, but had a similar infectious organism distribution to the overall cohort, with Staphylococcus aureus bacteremia representing the majority. Of note, 25% of the overall cohort did not have positive blood cultures recorded at our health system, which is likely due in large part to the high volume of referrals to our health center from outside institutions, where cultures may have already been performed and antibiotics started.
The findings of this study suggest that in a patient with a low to intermediate pre-test risk of endocarditis, a TTE meeting the proposed strict-criteria can effectively rule-out intracardiac vegetation. Based on the negative likelihood ratio of a strict-negative TTE, patients with pre-test probability of up to 50% have a post-test probability of less than 10% (low-risk) after a strict-negative TTE. Compare this to the standard approach, where patients with a pre-test probability of 50% and a negative TTE still have a 40% post-test probability (Figure 3). Although TEE is a safe and generally well tolerated procedure, as an invasive procedure it is not without risk and cost.37 Application of the proposed strict criteria for a negative TTE can identify a group of patients in whom vegetation is effectively excluded by TTE and who likely do not need the de-facto follow-up TEE standard in many practices.
It should be noted that this study looked at the ability of TTE to rule out vegetation associated with IE as compared to TEE – not the clinical diagnosis of IE. Not only is this approach consistent with the methodology of prior work,36,38 the goal of this analysis was to identify patients in whom a follow-up TEE may not add clinically relevant information. While only 13.5% of all TTEs met the strict negative criteria, these patients potentially represent a sizeable population in whom the risk and costs of TEE can be avoided. Future work should evaluate the impact of each strict negative criterion on the sensitivity and NPV of a negative TTE, as the ideal criteria would be maximally inclusive while maintaining a strong sensitivity and NPV.
4.1 Limitations
This large single-center observational study has several limitations. All echo data was based on the clinical report stored in the DELD, without reviewing stored images. As described in the methods section, our lab has a specific TTE image acquisition protocol for IE studies. We are unable to tell how well the protocol was followed for each TTE in this study, which may have implications for the diagnostic characteristics of TTE. In the 7 years covered by this study, there were on average 24 accredited sonographers, and 10 COCATS level 3 readers per year, so while variability in image acquisition and interpretation was unavoidable, this study tested the strict negative criteria in a real-world setting with expert personnel.
Two of the three patients with TTEs meeting strict negative criteria, but who went onto have positive TEEs (false-negatives), had high-risk features not accounted for by our study methods (Patients B and C). Excluding these patients based on a thorough chart review would have increased both the sensitivity and NPV of a strict-negative TTE to 99%. These cases also highlight that while echocardiography can provide valuable information to support the diagnosis of IE, it does not override clinical experience and judgment.
Our ability to assess pre-test probability of IE for the cohort was limited by the charted diagnoses available for each patient, and follow up was limited to events occurring in our hospital system. Future studies testing strict negative criteria should be performed prospectively with accurate assessment of pre-test probability, and a standardized imaging and interpretation protocol for the study.
5. Conclusion
In patients undergoing evaluation for suspected IE, TTE has a greatly improved sensitivity and NPV for vegetation when using strict criteria for a negative study. In low to intermediate risk patients with a strict negative TTE, follow up TEE may not be necessary to exclude vegetation.
Acknowledgments
No sponsor or funding to report
Abbreviations
- IE
Infective Endocarditis
- TTE
Transthoracic Echocardiogram
- TEE
Transesophageal Echocardiogram
- Echo
Echocardiogram
- DELD
Duke Echocardiography Lab Database
- DEDUCE
Duke Enterprise Data Unified Content Explorer
- NPV
Negative Predictive Value
- SA
Staphylococcus aureus
Footnotes
Author Disclosures
JA Sivak: Dr. Sivak has no relevant disclosures to report.
AN Vora: Dr. Vora has no relevant disclosures to report.
AM Navar: Dr. Navar has no relevant disclosures to report.
PJ Schulte: Dr. Schulte has no relevant disclosures to report.
AL Crowley: Dr. Crowley has no relevant disclosures to report.
J Kisslo: Dr. Kisslo reports consulting and educational activities or lectures for Phillips.
GR Corey: Dr. Corey has no relevant disclosures to report.
L Liao: Dr. Liao has no relevant disclosures to report.
A Wang: Dr. Wang has no relevant disclosures to report.
EJ Velazquez: Dr. Velazquez has no relevant disclosures to report.
Z Samad: Dr. Samad has no relevant disclosures to report.
References
- 1.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin Infect Dis. 2000 Apr 1;30(4):633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 10;129(23):2440–92. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 3.Baddour LM. Infective Endocarditis: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Statement for Healthcare Professionals From the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation. 2005 Jun 6;111(23):e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 4.Shively BK, Gurule FT, Roldan CA, Leggett JH, Schiller NB. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J Am Coll Cardiol. 1991 Aug;18(2):391–7. doi: 10.1016/0735-1097(91)90591-v. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro SM. Transesophageal echocardiography in diagnosis of infective endocarditis. CHEST J. 1994 Feb 1;105(2):377. doi: 10.1378/chest.105.2.377. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Li J, Corey GR, Boley J, Marr KA, Gopal AK, et al. Role of Echocardiography in Evaluation of Patients With Staphylococcus aureus Bacteremia: Experience in 103 Patients. J Am Coll Cardiol. 1997 Oct;30(4):1072–8. doi: 10.1016/s0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 7.Erbel R, Rohmann S, Drexler M, Mohr-Kahaly S, Gerharz CD, Iversen S, et al. Improved diagnostic value of echocardiography in patients with infective endocarditis by transoesophageal approach. A prospective study. Eur Heart J. 1988 Jan;9(1):43–53. [PubMed] [Google Scholar]
- 8.Ersboll M, Schulte PJ, Al Enezi F, Shaw L, Køber L, Kisslo J, et al. Predictors and Progression of Aortic Stenosis in Patients With Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2015 Jan 1;115(1):86–92. doi: 10.1016/j.amjcard.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Athan E, Pappas PA, Fowler VG, Olaison L, Paré C, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007 Mar 28;297(12):1354–61. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 10.Rushani D, Kaufman JS, Ionescu-Ittu R, Mackie AS, Pilote L, Therrien J, et al. Infective Endocarditis in Children With Congenital Heart Disease Cumulative Incidence and Predictors. Circulation. 2013 Sep 24;128(13):1412–9. doi: 10.1161/CIRCULATIONAHA.113.001827. [DOI] [PubMed] [Google Scholar]
- 11.Nienaber JJC, Kusne S, Riaz T, Walker RC, Baddour LM, Wright AJ, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013 Nov;57(10):1438–48. doi: 10.1093/cid/cit536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman-Weber S, Axelrod P, Suh B, Rubin S, Beltramo D, Manacchio J, et al. Infective endocarditis following orthotopic heart transplantation: 10 cases and a review of the literature. Transpl Infect Dis Off J Transplant Soc. 2004 Dec;6(4):165–70. doi: 10.1111/j.1399-3062.2004.00074.x. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe WM, Morgan DE, Pearlman AS, Otto CM. Infective endocarditis, 1983–1988: Echocardiographic findings and factors influencing morbidity and mortality. J Am Coll Cardiol. 1990 May 1;15(6):1227–33. doi: 10.1016/s0735-1097(10)80005-1. [DOI] [PubMed] [Google Scholar]
- 14.Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981 Apr;94(4 pt 1):505–18. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- 15.Gersony WM, Hayes CJ. Bacterial endocarditis in patients with pulmonary stenosis, aortic stenosis, or ventricular septal defect. Circulation. 1977 Aug;56(1 Suppl):I84–7. [PubMed] [Google Scholar]
- 16.Gersony WM, Hayes CJ, Driscoll DJ, Keane JF, Kidd L, O’Fallon WM, et al. Bacterial endocarditis in patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation. 1993 Feb;87(2 Suppl):I121–6. [PubMed] [Google Scholar]
- 17.Corone P, Doyon F, Gaudeau S, Guérin F, Vernant P, Ducam H, et al. Natural history of ventricular septal defect. A study involving 790 cases. Circulation. 1977 Jun 1;55(6):908–15. doi: 10.1161/01.cir.55.6.908. [DOI] [PubMed] [Google Scholar]
- 18.Duval X, Selton-Suty C, Alla F, Salvador-Mazenq M, Bernard Y, Weber M, et al. Endocarditis in patients with a permanent pacemaker: a 1-year epidemiological survey on infective endocarditis due to valvular and/or pacemaker infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004 Jul 1;39(1):68–74. doi: 10.1086/421493. [DOI] [PubMed] [Google Scholar]
- 19.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008 Jan;83(1):46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- 20.Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S, et al. Rates of and Factors Associated with Infection in 200,909 Medicare Implantable Cardioverter-Defibrillator Implants: Results from the NCDR®. Circulation. 2014 Jul 31; doi: 10.1161/CIRCULATIONAHA.114.009081. CIRCULATIONAHA.114.009081. [DOI] [PubMed] [Google Scholar]
- 21.Athan E, Chu VH, Tattevin P, Selton-Suty C, Jones P, Naber C, et al. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA. 2012 Apr 25;307(16):1727–35. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- 22.Crowley AL, Peterson GE, Benjamin DK, Rimmer SH, Todd C, Cabell CH, et al. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med. 2008 Feb;36(2):385–90. doi: 10.1097/01.CCM.0B013E3181611F914. [DOI] [PubMed] [Google Scholar]
- 23.Regueiro A, Falces C, Cervera C, Del Rio A, Paré JC, Mestres CA, et al. Risk factors for pericardial effusion in native valve infective endocarditis and its influence on outcome. Am J Cardiol. 2013 Nov 15;112(10):1646–51. doi: 10.1016/j.amjcard.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Reid CL, Rahimtoola SH, Chandraratna PAN. Frequency and significance of pericardial effusion detected by two-dimensional echocardiography in infective endocarditis. Am J Cardiol. 1987;60(4):394–5. doi: 10.1016/0002-9149(87)90259-1. [DOI] [PubMed] [Google Scholar]
- 25.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, et al. American Society of Echocardiography Recommendations for Quality Echocardiography Laboratory Operations. J Am Soc Echocardiogr. 2011 Jan;24(1):1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query Tool: Providing Simplified Access to Clinical Data for Research and Quality Improvement. J Biomed Inform. 2011 Apr;44(2):266–76. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein J. Test-indication curves. Med Decis Mak Int J Soc Med Decis Mak. 1997 Mar;17(1):103–6. doi: 10.1177/0272989X9701700112. [DOI] [PubMed] [Google Scholar]
- 28.Joseph JP, Meddows TR, Webster DP, Newton JD, Myerson SG, Prendergast B, et al. Prioritizing echocardiography in Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2013 Feb;68(2):444–9. doi: 10.1093/jac/dks408. [DOI] [PubMed] [Google Scholar]
- 29.Van Hal SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. The role of transthoracic echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect. 2005 Oct;51(3):218–21. doi: 10.1016/j.jinf.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen RV, Høst U, Arpi M, Hassager C, Johansen HK, Korup E, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. 2011 Jun;12(6):414–20. doi: 10.1093/ejechocard/jer023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaasch AJ, Fowler VG, Rieg S, Peyerl-Hoffmann G, Birkholz H, Hellmich M, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011 Jul 1;53(1):1–9. doi: 10.1093/cid/cir320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland TL, Arnold C, Fowler VG., Jr Clinical management of staphylococcus aureus bacteremia: A review. JAMA. 2014 Oct 1;312(13):1330–41. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palraj BR, Baddour LM, Hess EP, Steckelberg JM, Wilson WR, Lahr BD, et al. Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): Scoring System to Guide Use of Echocardiography in the Management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2015 Mar 25;:civ235. doi: 10.1093/cid/civ235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Showler A, Burry L, Bai AD, Steinberg M, Ricciuto DR, Fernandes T, et al. Use of Transthoracic Echocardiography in the Management of Low-Risk Staphylococcus aureus Bacteremia: Results From a Retrospective Multicenter Cohort Study. JACC Cardiovasc Imaging. 2015 Jul 8; doi: 10.1016/j.jcmg.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Lindner JR, Case RA, Dent JM, Abbott RD, Scheld WM, Kaul S. Diagnostic Value of Echocardiography in Suspected Endocarditis An Evaluation Based on the Pretest Probability of Disease. Circulation. 1996 Feb 15;93(4):730–6. doi: 10.1161/01.cir.93.4.730. [DOI] [PubMed] [Google Scholar]
- 36.Barton TL, Mottram PM, Stuart RL, Cameron JD, Moir S. Transthoracic Echocardiography Is Still Useful in the Initial Evaluation of Patients With Suspected Infective Endocarditis: Evaluation of a Large Cohort at a Tertiary Referral Center. Mayo Clin Proc. 2014 Jun;89(6):799–805. doi: 10.1016/j.mayocp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of Transesophageal Echocardiography. J Am Soc Echocardiogr. 2010 Nov;23(11):1115–27. doi: 10.1016/j.echo.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Kini V, Logani S, Ky B, Chirinos JA, Ferrari VA, St John Sutton MG, et al. Transthoracic and Transesophageal Echocardiography for the Indication of Suspected Infective Endocarditis: Vegetations, Blood Cultures and Imaging. J Am Soc Echocardiogr. 2010 Apr;23(4):396–402. doi: 10.1016/j.echo.2009.12.017. [DOI] [PubMed] [Google Scholar]