Summary
DNA methylation increases with age. The objective of this study was to investigate whether compound H, a potential activator of DNA demethylases, attenuates aging‐related arterial stiffness and hypertension. Aged mice (24–27 months) and adult mice (12 months) were used. Pulse wave velocity (PWV), a direct measure of arterial stiffness, and blood pressure (BP) were increased significantly in aged mice. Notably, daily treatments with compound H (15 mg/kg, IP) for 2 weeks significantly attenuated the aging‐related increases in PWV and BP. Compound H abolished aging‐associated downregulation of secreted Klotho (SKL) levels in both kidneys and serum likely by enhancing DNA demethylase activity and decreasing DNA methylation. Aging‐related arterial stiffness was associated with accumulation of stiffer collagen and degradation of compliant elastin which are accompanied by increased expression of MMP2, MMP9, TGF‐β1, and TGF‐β3. These changes were effectively attenuated by compound H, suggesting rejuvenation of aged arteries. Compound H also rescued downregulation of Sirt1 deacetylase, AMPKα, and eNOS activities in aortas of aged mice. In cultured smooth muscle cells (SMCc) Klotho‐deficient serum upregulated expression of MMPs and TGFβ which, however, was not affected by compound H. In conclusion, compound H attenuates aging‐associated arterial stiffness and hypertension by activation of DNA demethylase which increases renal SKL expression and consequently circulating SKL levels leading to activation of the Sirt1‐AMPK‐eNOS pathway in aortas of aged mice.
Keywords: aging, AMPK, arterial stiffness, compound H, demethylase, hypertension, secreted Klotho, Sirt1
1. INTRODUCTION
DNA demethylation is an important process that maintains transcriptional activity of genes (Avrahami et al., 2015; Bigot et al., 2015; Wagner, Fernandez‐Rebollo & Frobel, 2016; Zinovkina & Zinovkin, 2015). An increase in methylation in the promoter region of a gene diminishes the promoter activity and gene transcription (Wagner et al., 2016; Zinovkina & Zinovkin, 2015). Numerous studies showed that DNA methylation is increased with age (Alvares, Mayberry, Joyner, Lakowski & Ahmed, 2014; Avrahami et al., 2015; Bigot et al., 2015; Wagner et al., 2016; Zinovkina & Zinovkin, 2015). Coincidently, the prevalence of arterial stiffness and hypertension also increases with age (Sun, 2015). Arterial stiffening is an independent predictor of cardiovascular outcomes, such as hypertension, myocardial infarction, cognitive decline in aging, stroke, and kidney diseases (Hashimoto & Ito, 2011, 2013; Karras et al., 2012; Kitzman et al., 2013; Sun, 2015). However, the relationship of DNA methylation and aging‐related arterial stiffening is unclear. Whether increased methylation led to arterial stiffening has never been determined. Physiologically, an appropriate methylation level is maintained by the balanced methyltransferase and demethylase activity (Wagner et al., 2016). In this study, we assessed if activation of the demethylase affects arterial stiffening and hypertension in aged mice.
The Klotho gene was originally identified as a putative aging‐suppressor gene in mice that extended lifespan when overexpressed and caused multiple premature aging phenotypes when disrupted (Kuro‐o et al., 1997; Kurosu et al., 2005). The Klotho level decreases with age (Xiao, Zhang, Zheng & Gu, 2004), while the prevalence of arterial stiffness and hypertension increases with age (Kotsis & Stabouli, 2011). At age 70 years, the serum level of Klotho is only about one half of what it was at age 40 years (Xiao et al., 2004). Moreover, the serum Klotho level is significantly decreased in patients with arterial stiffness in chronic kidney diseases (Karras et al., 2012). Our recent study showed that haplodeficiency of Klotho gene caused arterial stiffness (Chen, Zhou & Sun, 2015). We found, in the cultured renal tubule cells, that a small compound (compound H) may be a potential inducer of Klotho gene expression (Jung, Xu & Sun, 2017). Whether compound H promotes Klotho expression and release in vivo has never been determined. In this study, we investigated whether compound H increases Klotho levels and attenuates aging‐associated arterial stiffening and hypertension.
Our results demonstrated that aging‐related arterial stiffening and hypertension are attributed, at least in part, to the increased DNA methylation. Compound H activates demethylases and attenuates arterial stiffening and hypertension in aged mice likely via increasing the Klotho levels.
2. METHODS
A detailed method section is available in the Data S1.
2.1. Animal study protocols
Six adult mice (12 months) and 20 old mice (24–27 months) were used in this study (129/sv). The old mice were randomly divided into three subgroups and each group had equal number of male mice. Three doses of compound H were tested, and 10 mg/kg bw was chosen as an optimal dose for treating animals. No obvious toxic effect of compound H was observed at this dose. One subgroup received compound H (10 mg kg−1 day−1, IP, Enamine LLC, Monmouth Jct., NJ) and one group received an equal volume of dimethyl sulfoxide (5%) and served as a control. The structure of compound H was provided in Figure S1. The third group received no treatment. Blood pressure (BP) was measured before and after treatment with compound H at 1 and 2 weeks. Pulse wave velocity (PWV) was measured after 2 weeks treatment with compound H. All animals were euthanized (ketamine/xylazine, 90/10 mg, IP) and perfused transcardially with PBS. The aortas were then quickly removed, washed, and cut into pieces for subsequent analyses.
2.2. Histological and immunohistochemical (IHC) examination
The histological and IHC analysis was performed as described in our recent studies (Chen, Lin & Sun, 2016; Gao et al., 2016; Lin, Chen & Sun, 2016; Lin & Sun, 2015a,b; Varshney, Ali, Wu & Sun, 2016). For details, see Data S1.
2.3. Western blot analysis
For details, see Data S1.
2.4. Reverse transcription–PCR (RT–PCR)
The RT–PCR procedure was performed as described recently (Fan & Sun, 2016; Lin & Sun, 2015b). For details, see Data S1.
2.5. Methylation analyses of Klotho gene
For details, see Data S1.
2.6. Measurement of DNA demethylase and DNA methyltransferase activity
For details, see Data S1.
2.7. Measurement of MMPs activity
For details, see Data S1.
2.8. Cell culture and treatment
For details, see Data S1.
2.9. Statistical analysis
Quantitative data were presented as the means ± SE. Differences between experimental groups were examined by one‐way analysis of variance (ANOVA) followed by the Bonferroni post‐test using Prism software (GraphPad, La Jolla, CA). For all analysis, p < .05 were considered statistically significant.
3. RESULTS
3.1. Compound H attenuated arterial stiffness and hypertension in aged mice
Pulse wave velocity, a direct measure of arterial stiffness, was increased significantly in aged mice (Figure 1a). The widening of pulse pressure is another direct surrogate of arterial stiffness. Pulse pressure of aged mice was significantly increased compared to that of adult mice (Figure 1b). Thus, aged mice developed arterial stiffness. Daily treatment with compound H for 2 weeks remarkably decreased PWV and pulse pressure in aged mice (Figure 1a,b). Compound H also significantly decreased systolic BP (Figure 1c), diastolic BP (Figure 1d), and mean BP (Figure 1e) in aged mice. Therefore, compound H effectively reduced aging‐related arterial stiffness and hypertension.
Figure 1.
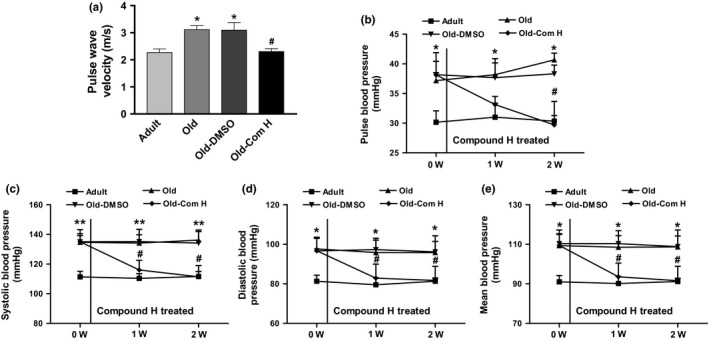
Compound H attenuated arterial stiffness and hypertension in aged mice. (a) PWV was measured by 10‐MHz Doppler probes. (b–e) BP was measured weekly by the volume–pressure recording (VPR) tail‐cuff method using a CODA 6 BP monitoring system during treatment with compound H. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. n = 6–7. *p < .05, **p < .01 vs. adult mice; # p < .05, vs. old mice
3.2. Compound H increased secreted Klotho (SKL) expression in aged mice
To investigate the potential mechanism contributing to the beneficial effects of compound H on aging‐related arterial stiffening, we measured Klotho expression levels in kidneys, a major source of circulating Klotho (Lindberg et al., 2014; Xu & Sun, 2015b). We found that protein and mRNA expression of both transmembrane (full‐length) and secreted Klotho (SKL) were remarkably decreased in kidneys of aged mice (Figure 2a). Compound H rescued the aging‐related downregulation of SKL expression but did not affect full‐length Klotho expression (Figure 2a). Notably, serum level of SKL was also downregulated in aged mice which was largely rescued by compound H (Figure 2b).
Figure 2.
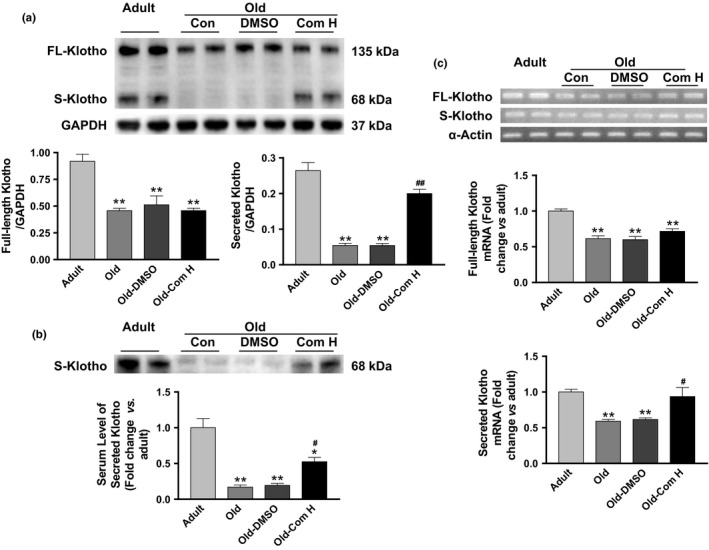
Compound H increased secreted Klotho levels in the kidney and serum of aged mice. (a) Western blot analysis of Klotho expression in kidneys. n = 4. (b) Western blot analysis of Klotho in serum (fold change vs. adult). n = 4. (c) mRNA expression of Klotho in kidneys (fold change vs. adult). n = 4. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. *p < .05, **p < .01 vs. adult mice; # p < .05, ## p < .01 vs. old mice
Compound H rescued aging‐associated downregulation of SKL mRNA expression but did not affect full‐length Klotho mRNA in kidneys (Figure 2c).
3.3. Compound H attenuated DNA hypermethylation of Klotho gene in aged mice
To further explore the potential mechanism of induction of Klotho expression by compound H, we measured the DNA demethylase activity and DNA methyltransferase activity in kidneys. The DNA demethylase activity was decreased while the DNA methyltransferase activity was increased in aged mice (24–27 months old) in relative to those of adult mice (12 months old) (Figure 3a,b). Compound H rescued the downregulation of DNA demethylase activity but did not affect DNA methyltransferase activity in aged mice (Figure 3a,b). We then measured methylation of the Klotho gene. The methylation of CpG islands of the Klotho gene was significantly increased in aged mice compared to that of adult mice (Figure 3c). Compound H effectively attenuated the aging‐related methylation of the Klotho gene (Figure 3c), which was associated with increased expression of SKL in aged mice (Figure 2a). The molecular mechanism of compound H‐induced SKL expression warrants additional investigation.
Figure 3.
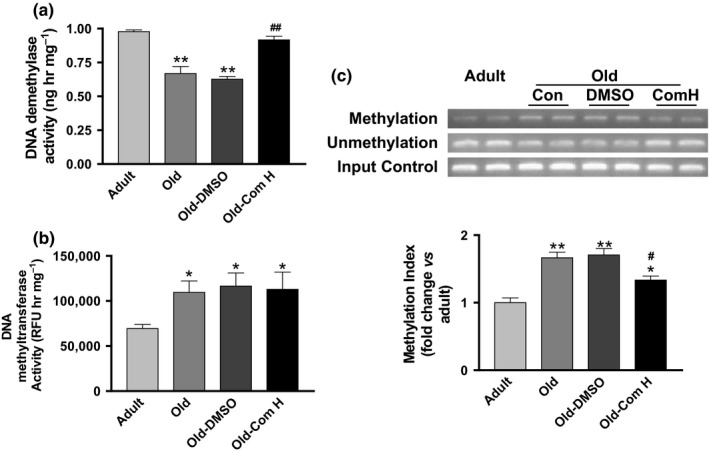
Compound H decreased DNA hypermethylation of Klotho gene in kidneys of aged mice. (a) DNA demethylase activity. n = 6. (b) DNA methyltransferase activity. n = 6. (c) DNA methylation of Klotho gene (fold change vs. adult). n = 4. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. *p < .05, **p < .01 vs. adult mice; # p < .05, ## p < .01 vs. old mice
3.4. Compound H attenuated accumulation of stiffer collagen and degeneration of compliant elastin fibers in aortas of aged mice
To investigate the molecular basis of arterial stiffening, we measured arterial collagen and elastin levels. The immunostaining assay showed that aortic collagen levels were increased significantly in aged mice (Figure 4a). The aging‐related collagen deposition (blue) was mainly found in the medial and adventitial layer of the aorta. On the other hand, aortic elastin levels (brown) were decreased significantly in aged mice (Figure 4a). Western blot analysis further confirmed that aging upregulated collagen I levels but downregulated elastin levels in aortas (Figure 4b). The ratio of elastin to collagen in aortas was markedly decreased in aged mice (Figure 4b), indicating that aging causes aortic remodeling. Treatments with compound H abolished upregulation of collagen and downregulation of elastin in aortas leading to attenuation of arterial remodeling in aged mice (Figure 4a,b). No significant differences were found between the Old‐Com H groups and the Adult groups (Figure 4a,b).
Figure 4.
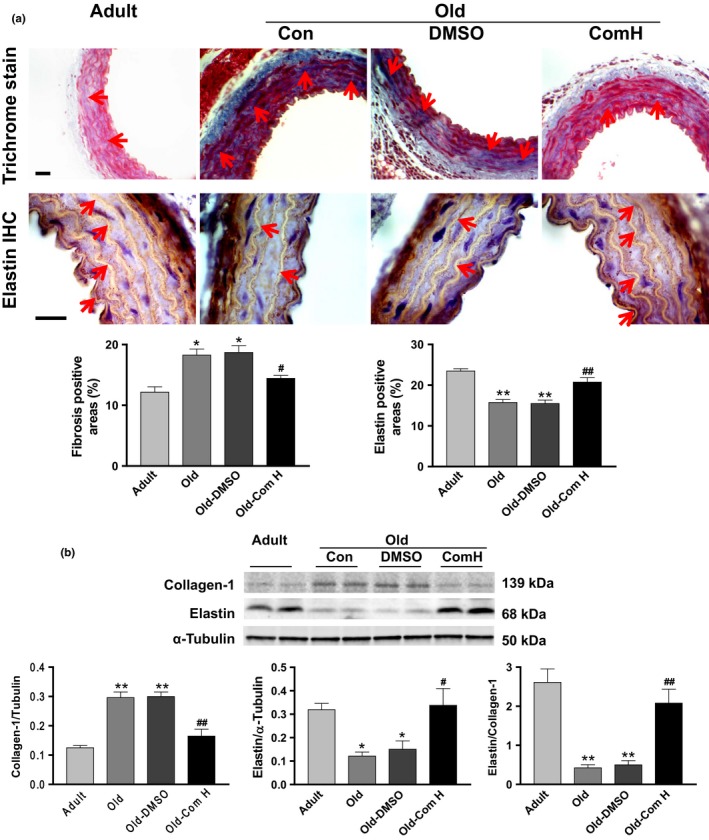
Compound H attenuated the accumulation of collagen and degeneration of elastin in the aorta of aged mice. (a) Histological staining of collagen and immunohistochemical staining of elastin. n = 5. (b) Western blot analysis of collagen‐1 and elastin. n = 4. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. *p < .05, **p < .01 vs. adult mice; # p < .05, ## p < .01 vs. old mice
3.5. Compound H abolished the aging‐associated increases in arterial MMP activity and expression
MMPs are a family of proteases that play important roles in extracellular matrix (ECM) remodeling and degradation. Increased MMP activity could contribute to ECM remodeling and fibrosis. MMPs activity was increased significantly in aortas of aged mice (Figure 5a). An increase in circulating levels of Klotho by compound H remarkably decreased MMPs activity in aged mice (Figure 5a). We also measured MMP protein expression levels by Western blot. MMP2 and MMP9 protein expressions were increased significantly in aortas of aged mice which were effectively attenuated by compound H (Figure 5b). No significant differences in MMPs activity and protein expressions were found between the Old‐Com H groups and the Adult groups (Figure 5a,b).
Figure 5.
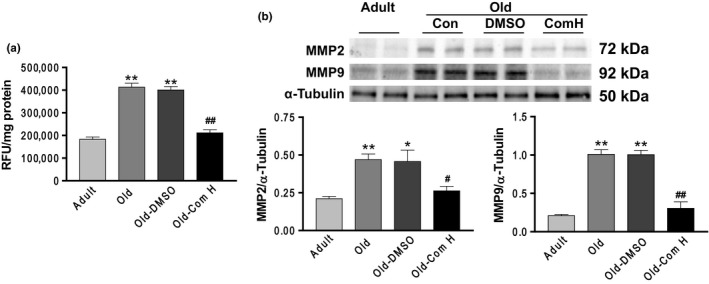
Compound H abolished the aging‐associated increases in arterial MMP activity and expression. (a) MMPs activity analysis. (b) Western blot analysis of MMP2 and MMP9 protein expressions. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. n = 4. *p < .05, **p < .01 vs. adult mice; # p < .05, ## p < .01 vs. old mice
3.6. Aging increased arterial TGF‐β1, TGF‐β3, RUNX2, and ALP expressions which can be abolished by compound H
TGFβ promotes matrix protein synthesis and decreases matrix protein degradation, contributing to tissue fibrosis. Runt‐related transcription factor 2 (RUNX2) and alkaline phosphatase (ALP) are markers of fibrosis and arterial stiffening (Capelli, Lusuardi, Cerutti & Donner, 1997; Lin, Chen, Leaf, Speer & Giachelli, 2015). Western blot analysis showed that TGFβ1, TGF‐β3, RUNX2, and ALP expressions were increased significantly in aortas of aged mice (Figure 6a–e). An increase in circulating levels of Klotho by compound H abolished the aging‐related increase in expression of TGFβ1, TGF‐β3, RUNX2, and ALP (Figure 6a–e), suggesting that aging‐associated upregulation of these factors may be due to downregulation of circulating Klotho levels.
Figure 6.
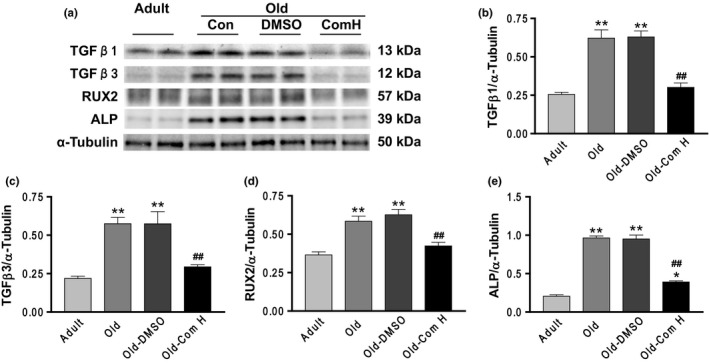
Aging increased arterial TGF‐β1, TGF‐β3, RUNX2, and ALP expressions which can be abolished by compound H. (a) Western blots analysis of TGF‐β1, TGF‐β3, RUNX2, and ALP. (b) Quantification of TGF‐β1 protein levels. (c) Quantification of TGF‐β3 protein levels. (d) Quantification of RUX2 protein levels. (e) Quantification of ALP protein levels. Data are expressed as mean ± SE and analyzed by one‐way ANOVA. n = 4. *p < .05, **p < .01 vs. adult mice; ## p < .01 vs. old mice
3.7. Compound H abolished aging‐related downregulation of the SIRT1‐AMPK‐eNOS pathway
Silent information regulator T1 (SIRT1) plays an important role in the regulation of aging and longevity in mammals (Alcendor et al., 2007; Satoh et al., 2013; Wang, Chen, Lv & Liu, 2013). There exist cross‐talks between SIRT1‐ and AMP‐activated protein kinase (AMPK)/eNOS, which are involved in the control of the senescence program (Wang, Liang & Vanhoutte, 2011). Therefore, we assessed the effect of compound H on the SIRT1‐AMPK‐eNOS cascade in aged mice. SIRT1 expression and activity in aortas of aged mice were decreased significantly in relative to the adult mice. Although it did not affect SIRT1 expression, compound H increased SIRT1 activity significantly, as evidenced by an increase in deacetylation of p53 tumor suppressor protein in aged mice (Figure S2A). Consistently, the aging‐related increase in histone acetylation was rescued by compound H (Figure S2A). The phospho‐AMPK and phospho‐eNOS expressions were decreased in the aortas of aged mice (Figure S2B,C), indicating that AMPK and eNOS activities were downregulated with aging. Compound H, an inducer of klotho, rescued the aging‐related downregulation of AMPK and eNOS activities in aortas (Figure S2B,C). Therefore, the aortic SIRT1‐AMPK‐eNOS pathway was suppressed by aging, which can be activated by compound H.
3.8. Compound H did not affect MMP2, MMP9, TGFβ1, and TGFβ3 expressions in mouse aortic smooth muscle cells (MOVAS)
The in vivo study showed that compound H increased circulating levels of Klotho and attenuated arterial stiffening and hypertension in aged mice. So we investigated whether compound H and SKL have direct effects on remodeling factors in cultured SMCs. Mouse aortic smooth muscle cells (MOVAS) which do not express endogenous Klotho(Lindberg et al., 2013) were treated with compound H for 16 hr and then harvested for Western blot analysis. In regular medium which has constant levels of Klotho, compound H treatment did not change MMP2, MMP9, TGFβ1, and TGFβ3 expressions (Figure S3), suggesting that compound H did not affect expression of these remodeling factors in SMCs.
In Klotho‐free medium (KL(‐))‐treated cells, MMP2, MMP9, TGFβ1, and TGFβ3 expressions were increased significantly compared to those of regular medium‐treated cells (Figure S3), suggesting that Klotho deficiency directly upregulates these remodeling factors. Notably, SKL protein treatment effectively rescued Klotho deficiency‐induced upregulation of MMP2, MMP9, TGFβ1, and TGFβ3 expressions (Figure S3), suggesting that Klotho directly regulates these factors in SMCs. By contrast, compound H treatment did not affect Klotho deficiency‐induced upregulation of MMP2, MMP9, TGFβ1, and TGFβ3 expressions in SMCs (Figure S3).
These results suggest that the in vivo suppressing effect of compound H on aging‐related upregulation of MMP2, MMP9, TGFβ1, and TGFβ3 expression is mediated by induction of klotho expression and release, which further inhibit arterial remodeling. Therefore, compound H attenuates arterial stiffening and hypertension through increasing circulating levels of Klotho.
4. DISCUSSION
SKL may function as a hormone (Xu & Sun, 2015). The circulating SKL may have direct action in vascular endothelial cells or SMCs which do not express SKL. Klotho is an aging‐suppressor gene (Xu & Sun, 2015). Klotho gene deficiency led to arterial stiffening (Chen et al., 2015). This study provides the first evidence that an increase in serum SKL attenuated arterial stiffening.
Aging is associated with increased cardiovascular complications, such as arterial stiffness and hypertension (Sun, 2015). Arterial stiffness is an independent risk factor for myocardial infarction, cognitive decline in aging, stroke, and kidney diseases (Boutouyrie et al., 2002; Matsuoka et al., 2005; Waldstein et al., 2008; Zoungas & Asmar, 2007). Unfortunately, the current antihypertensive drugs were mainly designed to reduce peripheral resistance and are not specific to alter the pathological process of vascular remodeling or stiffening (Dao, Essalihi, Bouvet & Moreau, 2005). Our results showed that PWV and BP were increased in mice of 24–27 months which is equivalent of 70–80 years in humans (Flurkey, Currer & Harrison, 2007), indicating the development of aging‐related arterial stiffness. Importantly, this study demonstrates that activation of demethylases by compound H reversed arterial stiffening and hypertension in aged mice (Figure 1), suggesting that increased methylation plays a critical role in aging‐related arterial stiffening and hypertension. This finding suggests that compound H may be an effective therapeutic agent for arterial stiffness and hypertension.
DNA methylation is one of several epigenetic mechanisms that control gene expression. During development, the pattern of DNA methylation in the genome changes as a result of a dynamic process involving both DNA methylation and demethylation (Moore, Le & Fan, 2013). We measured the methylation state of the promoter region of the Klotho gene to assess whether expression of Klotho, an aging‐suppressor gene, is regulated by DNA methylation in aged mice. We found that aging led to downregulation of DNA demethylase activity and upregulation of DNA methyltransferase activity leading to upregulation of DNA methylation of Klotho gene in aortas of aged mice (Figure 3). Interestingly, compound H rescued the aging‐related downregulation of DNA demethylase activity but did not affect the DNA methyltransferase activity. Compound H restored SKL gene transcription and expression in aged mice (Figure 2) which is likely through attenuating hypermethylation of Klotho gene (Figure 3). Our data suggest that compound H increased DNA demethylase activity in kidneys and increased renal Klotho expression and circulating klotho levels which largely explain the beneficial effect of compound H on arterial stiffening and hypertension. In a recent study, we showed that compound H directly stimulates DNA demethylase activity, decreases DNA methylation, and enhances Klotho expression in the cultured kidney cells (Jung, Xu & Sun, 2017). Our recent study showed that haplodeficiency of Klotho gene caused arterial stiffness and hypertension (Chen et al., 2015). Therefore, we believe that the mechanism of the antihypertensive effect of compound H includes enhanced circulating Klotho levels. The beneficial effect of compound H is mediated, at least partially, by Klotho because compound H does not affect MMPs and TGFβ in vascular SMCs which do not express Klotho (Figure S3). Nevertheless, an additional study is required to determine to what degree the beneficial effect of Compound H is mediated by Klotho.
This study provides the first evidence that stimulation of Klotho expression attenuates aging‐associated arterial stiffening and hypertension. It is intriguing that a small chemical compound effectively induced SKL expression and increases circulating levels of SKL. It was reported that at age of 70 years, the serum level of Klotho is only about one half of what it was at age of 40 years (Xiao et al., 2004), while the prevalence of arterial stiffness and hypertension is increased in the aged population (Kotsis & Stabouli, 2011). Thus, Klotho deficiency may be a pathological factor for aging‐associated arterial stiffness and hypertension. It is interesting that a kidney‐derived protein (SKL) plays a critical role in the maintenance of normal vascular structure and function.
We further investigated whether compound H and SKL affect remodeling factors in MOVAS which do not express endogenous Klotho. Compound H had no direct effect on expression of MMP2, MMP9, TGFβ1, and TGFβ3 in cultured MOVAS (Figure S3). By contrast, SKL‐deficient medium upregulated MMP2, MMP9, TGFβ1, and TGFβ3 expressions in MOVAS. Interestingly, Klotho deficiency‐induced upregulation of these factors was abolished by adding SKL but was not affected by compound H. Compound H may not affect demethylase or methyltransferase activities in vascular cells because it did not alter expression of MMPs and TGFβ in cultured SMCs (Figure S3). This hypothesis needs to be validated.
It is noticed that compound H reversed aging‐associated arterial stiffening and remodeling, suggesting that compound H via induction of SKL expression and release rejuvenates aged arteries. The beneficial effect of compound H may be partially attributed to reversal of upregulation of collagen and downregulation of elastin (Figure 4). This process may include collagen degradation and elastin synthesis which may be likely due to upregulation of MMP and TGFβ induced by increased SKL levels. Although aging led to a decrease in autophagy, the beneficial effect of compound H may not involve autophagy which was not affected by compound H (Figure S4).
Two types of Klotho protein with potentially different functions have been identified: a full‐length transmembrane Klotho and a SKL (Xu & Sun, 2015). The full‐length Klotho is mainly expressed in kidney distal tubule cells and serves as a coreceptor of FGF23 and enhances FGF23 signaling to maintain phosphate homeostasis. The circulating Klotho, that is SKL, may act as a hormone and regulate the functions of tissues or cells that do not express Klotho (e.g., vascular endothelial cells and smooth muscle cells) (Lindberg et al., 2013; Xu & Sun, 2015b). In this study, we found that both full‐length and SKL in kidneys were decreased in aged mice (Figure 2). However, compound H only rescued the downregulation of SKL but did not affect transmembrane Klotho. The molecular mechanism of the selective action of compound H on SKL remains to be explored. SKL and the full‐length klotho are encoded by the same gene. SKL was generated due to alternative splicing (Xu & Sun, 2015). Thus, compound H might demethylase an alternative RNA splicer to rescue the aging‐related downregulation of SKL. This hypothesis warrants further investigation.
SIRT1, known as a class III histone deacetylase, is a nuclear protein implicated in the regulation of many cellular processes, including apoptosis, cellular senescence, endocrine signaling, glucose homeostasis, aging, and longevity. SIRT1 has been reported to deacetylate the lysine residues of a number of nuclear proteins, such as p53 (Yuan et al., 2011), NF‐κB (Salminen & Kaarniranta, 2009), PGC‐1a (Amat et al., 2009), CBP/p300 (Das, Lucia, Hansen & Tyler, 2009), and forkhead family proteins (Brunet et al., 2004). Several recent studies demonstrated that Sirt1 could inhibit TGF‐β signaling and ameliorate fibrosis (Huang et al., 2014; Kume et al., 2007; Zerr et al., 2014). In this study, we found that aging suppressed SIRT1 activity, increased TGFβ and MMP expressions, and induced fibrosis. Compound H rescued aging‐associated downregulation of SIRT1 expression in aortas likely by increasing the circulating SKL levels. Haplodeficiency of Klotho gene led to downregulation of vascular SIRT1 (Gao et al., 2016). Pharmacological activation of SIRT1 attenuated Klotho deficiency‐induced arterial stiffening (Gao et al., 2016). Therefore, downregulation of SIRT1 activity may be an important factor contributing to aging‐induced arterial remodeling and stiffness.
However, the recent studies showed that large‐artery stiffness precedes the development of hypertension (Kaess et al., 2012; Najjar et al., 2008). Although hypertension could also contribute to vascular remodeling and stiffening, it is, however, a slow process. A reduction in BP by 20 mmHg may be insufficient to reverse aging‐related arterial remodeling or arterial stiffening in 2 weeks. Nevertheless, this study does not exclude the possibility that attenuation of BP by compound H may also contribute to the improvement of arterial stiffening in aging mice. The limitation of this study is that it does not elucidate the relationship of arterial stiffening and hypertension (causality). It is noticed that compound H decreased aging‐associated arterial stiffening and hypertension within 2 weeks, which is sooner than expected. This finding suggests that there may be a functional component in arterial stiffening, that is, increased vascular tone. The acute effect of compound H may be partially due to improved endothelial function and decreased vascular resistance. Indeed, treatment with compound H, via stimulation of SKL release, activated the SIRT1‐AMPKα‐eNOS pathway as evidenced by increased activities of SIRT1, AMPKα, and eNOS (Figure S2A–C). eNOS is an important enzyme in the regulation of BP, vascular tone, and angiogenesis. Several protein kinases including AMPK activate eNOS by phosphorylating Ser1177 in response to various stimuli (Dimmeler et al., 1999; Fulton et al., 1999). Therefore, the mechanism of the antihypertensive effect of compound H may include activation of the SIRT1‐AMPK‐eNOS pathway, inhibition of MMP and TGFβ, and attenuation of arterial remodeling and stiffening.
5. PERSPECTIVE
Our study provides the first experimental evidence that aging‐associated arterial stiffening and hypertension are attributed, at least in part, to increased DNA methylation. It is new and interesting that compound H activates DNA demethylase which induces Klotho expression and release leading to attenuation of arterial stiffening and hypertension in aged mice. Our study also provides the first evidence that compound H may be an effective therapeutic agent for aging‐related arterial stiffening and hypertension.
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTION
ZS, design experiment, critical editing manuscript; KC, perform experiment, collect data, and write manuscript.
Supporting information
ACKNOWLEDGMENT
This work was supported by NIH R01 HL118558, DK093403, AG049780, and HL122166.
Chen K, Sun Z. Activation of DNA demethylases attenuates aging‐associated arterial stiffening and hypertension. Aging Cell. 2018;17:e12762 10.1111/acel.12762
REFERENCES
- Alcendor, R. R. , Gao, S. , Zhai, P. , Zablocki, D. , Holle, E. , Yu, X. , … Sadoshima, J. (2007). Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation Research, 100(10), 1512–1521. 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Alvares, S. M. , Mayberry, G. A. , Joyner, E. Y. , Lakowski, B. , & Ahmed, S. (2014). H3K4 demethylase activities repress proliferative and postmitotic aging. Aging Cell, 13(2), 245–253. 10.1111/acel.12166 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat, R. , Planavila, A. , Chen, S. L. , Iglesias, R. , Giralt, M. , & Villarroya, F. (2009). SIRT1 controls the transcription of the peroxisome proliferator‐activated receptor‐gamma Co‐activator‐1alpha (PGC‐1alpha) gene in skeletal muscle through the PGC‐1alpha autoregulatory loop and interaction with MyoD. Journal of Biological Chemistry, 284(33), 21872–21880. 10.1074/jbc.M109.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrahami, D. , Li, C. , Zhang, J. , Schug, J. , Avrahami, R. , Rao, S. , … Kaestner, K. H. (2015). Aging‐dependent demethylation of regulatory elements correlates with chromatin state and improved beta cell function. Cell Metabolism, 22(4), 619–632. 10.1016/j.cmet.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot, A. , Duddy, W. J. , Ouandaogo, Z. G. , Negroni, E. , Mariot, V. , Ghimbovschi, S. , … Duguez, S. (2015). Age‐associated methylation suppresses SPRY1, leading to a failure of re‐quiescence and loss of the reserve stem cell pool in elderly muscle. Cell Reports, 13(6), 1172–1182. 10.1016/j.celrep.2015.09.067. [DOI] [PubMed] [Google Scholar]
- Boutouyrie, P. , Tropeano, A. I. , Asmar, R. , Gautier, I. , Benetos, A. , Lacolley, P. , & Laurent, S. (2002). Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension, 39(1), 10–15. 10.1161/hy0102.099031 [DOI] [PubMed] [Google Scholar]
- Brunet, A. , Sweeney, L. B. , Sturgill, J. F. , Chua, K. F. , Greer, P. L. , Lin, Y. , … Greenberg, M. E. (2004). Stress‐dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science, 303(5666), 2011–2015. 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Capelli, A. , Lusuardi, M. , Cerutti, C. G. , & Donner, C. F. (1997). Lung alkaline phosphatase as a marker of fibrosis in chronic interstitial disorders. American Journal of Respiratory and Critical Care Medicine, 155(1), 249–253. 10.1164/ajrccm.155.1.9001320. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Lin, Y. , & Sun, Z. (2016). Deficiency in the anti‐aging gene Klotho promotes aortic valve fibrosis through AMPKalpha‐mediated activation of RUNX2. Aging Cell, 10.1111/acel.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Zhou, X. , & Sun, Z. (2015). Haplodeficiency of Klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension, 66(5), 1006–1013. 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao, H. H. , Essalihi, R. , Bouvet, C. , & Moreau, P. (2005). Evolution and modulation of age‐related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovascular Research, 66(2), 307–317. 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Das, C. , Lucia, M. S. , Hansen, K. C. , & Tyler, J. K. (2009). CBP/p300‐mediated acetylation of histone H3 on lysine 56. Nature, 459(7243), 113–117. 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler, S. , Fleming, I. , Fisslthaler, B. , Hermann, C. , Busse, R. , & Zeiher, A. M. (1999). Activation of nitric oxide synthase in endothelial cells by Akt‐dependent phosphorylation. Nature, 399(6736), 601–605. 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Fan, J. , & Sun, Z. (2016). The antiaging gene Klotho regulates proliferation and differentiation of adipose‐derived stem cells. Stem Cells, 34(6), 1615–1625. 10.1002/stem.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K., Currer J. M., Harrison D. E. (2007). The mouse in aging research The mouse in biomedical research (2nd ed., pp. 637–672). Burlington, MA: College Laboratory Animal Medicine; 10.1016/B978-012369454-6/50074-1 [DOI] [Google Scholar]
- Fulton, D. , Gratton, J. P. , McCabe, T. J. , Fontana, J. , Fujio, Y. , Walsh, K. , … Sessa, W. C. (1999). Regulation of endothelium‐derived nitric oxide production by the protein kinase Akt. Nature, 399(6736), 597–601. 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, D. , Zuo, Z. , Tian, J. , Ali, Q. , Lin, Y. , Lei, H. , & Sun, Z. (2016). Activation of SIRT1 attenuates Klotho deficiency‐induced arterial stiffness and hypertension by enhancing AMP‐activated protein kinase activity. Hypertension, 68(5), 1191–1199. 10.1161/hypertensionaha.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, J. , & Ito, S. (2011). Central pulse pressure and aortic stiffness determine renal hemodynamics: Pathophysiological implication for microalbuminuria in hypertension. Hypertension, 58(5), 839–846. 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- Hashimoto, J. , & Ito, S. (2013). Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: Potential implication for retrograde embolic stroke in hypertension. Hypertension, 62(3), 542–549. 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- Huang, X. Z. , Wen, D. , Zhang, M. , Xie, Q. , Ma, L. , Guan, Y. , … Hao, C. M. (2014). Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF‐beta/Smad3 pathway. Journal of Cellular Biochemistry, 115(5), 996–1005. 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- Jung, D. , Xu, Y. , & Sun, Z. (2017). Induction of anti‐aging gene klotho with a small chemical compound that demethylates CpG islands. Oncotarget, 8(29), 46745–46755. 10.18632/oncotarget.18608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess, B. M. , Rong, J. , Larson, M. G. , Hamburg, N. M. , Vita, J. A. , Levy, D. , … Mitchell, G. F. (2012). Aortic stiffness, blood pressure progression, and incident hypertension. JAMA, 308(9), 875–881. 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras, A. , Haymann, J. P. , Bozec, E. , Metzger, M. , Jacquot, C. , Maruani, G. , … Nephro Test Study, G (2012). Large artery stiffening and remodeling are independently associated with all‐cause mortality and cardiovascular events in chronic kidney disease. Hypertension, 60(6), 1451–1457. 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- Kitzman, D. W. , Herrington, D. M. , Brubaker, P. H. , Moore, J. B. , Eggebeen, J. , & Haykowsky, M. J. (2013). Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension, 61(1), 112–119. 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsis, V. , & Stabouli, S. (2011). Arterial stiffness, vascular aging, and intracranial large artery disease. American Journal of Hypertension, 24(3), 252 10.1038/ajh.2010.251. [DOI] [PubMed] [Google Scholar]
- Kume, S. , Haneda, M. , Kanasaki, K. , Sugimoto, T. , Araki, S. , Isshiki, K. , … Koya, D. (2007). SIRT1 inhibits transforming growth factor beta‐induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. Journal of Biological Chemistry, 282(1), 151–158. 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- Kuro‐o, M. , Matsumura, Y. , Aizawa, H. , Kawaguchi, H. , Suga, T. , Utsugi, T. , … Nabeshima, Y. I. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51. 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu, H. , Yamamoto, M. , Clark, J. D. , Pastor, J. V. , Nandi, A. , Gurnani, P. , … Kuro‐o, M. (2005). Suppression of aging in mice by the hormone Klotho. Science, 309(5742), 1829–1833. 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. E. , Chen, T. , Leaf, E. M. , Speer, M. Y. , & Giachelli, C. M. (2015). Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. American Journal of Pathology, 185(7), 1958–1969. 10.1016/j.ajpath.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , Chen, J. , & Sun, Z. (2016). Antiaging gene Klotho deficiency promoted high‐fat diet‐induced arterial stiffening via inactivation of AMP‐activated protein kinase. Hypertension, 67(3), 564–573. 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , & Sun, Z. (2015a). Antiaging gene Klotho attenuates pancreatic beta‐cell apoptosis in type 1 diabetes. Diabetes, 64(12), 4298–4311. 10.2337/db15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , & Sun, Z. (2015b). In vivo pancreatic beta‐cell‐specific expression of antiaging gene Klotho: A novel approach for preserving beta‐cells in type 2 diabetes. Diabetes, 64(4), 1444–1458. 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, K. , Amin, R. , Moe, O. W. , Hu, M. C. , Erben, R. G. , Ostman Wernerson, A. , … Larsson, T. E. (2014). The kidney is the principal organ mediating klotho effects. Journal of the American Society of Nephrology, 25(10), 2169–2175. 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, K. , Olauson, H. , Amin, R. , Ponnusamy, A. , Goetz, R. , Taylor, R. F. , … Larsson, T. E. (2013). Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One, 8(4), e60658 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, O. , Otsuka, K. , Murakami, S. , Hotta, N. , Yamanaka, G. , Kubo, Y. , … Ozawa, T. (2005). Arterial stiffness independently predicts cardiovascular events in an elderly community – Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomedicine and Pharmacotherapy, 59(Suppl 1), S40–S44. https://doi.org/S0753-3322(05)80008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. D. , Le, T. , & Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology, 38(1), 23–38. 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar, S. S. , Scuteri, A. , Shetty, V. , Wright, J. G. , Muller, D. C. , Fleg, J. L. , … Lakatta, E. G. (2008). Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology, 51(14), 1377–1383. 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. , & Kaarniranta, K. (2009). NF‐kappaB signaling in the aging process. Journal of Clinical Immunology, 29(4), 397–405. 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- Satoh, A. , Brace, C. S. , Rensing, N. , Cliften, P. , Wozniak, D. F. , Herzog, E. D. , … Imai, S. (2013). Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metabolism, 18(3), 416–430. 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z. (2015). Aging, arterial stiffness, and hypertension. Hypertension, 65(2), 252–256. 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, R. , Ali, Q. , Wu, C. , & Sun, Z. (2016). Monocrotaline‐induced pulmonary hypertension involves downregulation of antiaging protein Klotho and eNOS activity. Hypertension, 68(5), 1255–1263. 10.1161/hypertensionaha.116.08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, W. , Fernandez‐Rebollo, E. , & Frobel, J. (2016). DNA‐methylation changes in replicative senescence and aging: Two sides of the same coin? Epigenomics, 8(1), 1–3. 10.2217/epi.15.100. [DOI] [PubMed] [Google Scholar]
- Waldstein, S. R. , Rice, S. C. , Thayer, J. F. , Najjar, S. S. , Scuteri, A. , & Zonderman, A. B. (2008). Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension, 51(1), 99–104. 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wang F., Chen H. Z., Lv X., Liu D. P. (2013) SIRT1 as a novel potential treatment target for vascular aging and age‐related vascular diseases. Current Molecular Medicine, 13(1), 155–164. http://www.ncbi.nlm.nih.gov/pubmed/22934845 [PubMed] [Google Scholar]
- Wang, Y. , Liang, Y. , & Vanhoutte, P. M. (2011). SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Letters, 585(7), 986–994. 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Xiao, N. M. , Zhang, Y. M. , Zheng, Q. , & Gu, J. (2004). Klotho is a serum factor related to human aging. Chinese Medical Journal, 117(5), 742–747. [PubMed] [Google Scholar]
- Xu, Y. , & Sun, Z. (2015). Molecular basis of klotho: From gene to function in aging. Endocrine Reviews, 36(2), 174–193. 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, F. , Xie, Q. , Wu, J. , Bai, Y. , Mao, B. , Dong, Y. , … Yuan, Z. (2011). MST1 promotes apoptosis through regulating Sirt1‐dependent p53 deacetylation. Journal of Biological Chemistry, 286(9), 6940–6945. 10.1074/jbc.M110.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr, P. , Palumbo‐Zerr, K. , Huang, J. , Tomcik, M. , Sumova, B. , Distler, O. , … Distler, J. H. (2014). Sirt1 regulates canonical TGF‐beta signalling to control fibroblast activation and tissue fibrosis. Annals of the Rheumatic Diseases, 10.1136/annrheumdis-2014-205740. [DOI] [PubMed] [Google Scholar]
- Zinovkina, L. A. , & Zinovkin, R. A. (2015). DNA methylation, mitochondria, and programmed aging. Biochemistry, 80(12), 1571–1577. 10.1134/S0006297915120044. [DOI] [PubMed] [Google Scholar]
- Zoungas, S. , & Asmar, R. P. (2007). Arterial stiffness and cardiovascular outcome. Clinical and Experimental Pharmacology and Physiology, 34(7), 647–651. 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


