Abstract
All‐trans retinoic acid (ATRA) or mesenchymal stem cells (MSCs) have been shown to promote lung tissue regeneration in animal models of emphysema. However, the reparative effects of the combination of the two and the role of p70S6 kinase‐1 (p70S6k1) activation in the repair process have not been defined. Twenty‐one days after intratracheal instillation of porcine pancreatic elastase (PPE), MSC and/or 10 days of ATRA treatment was initiated. Thirty‐two days later, static lung compliance (Cst), mean linear intercepts (MLIs), and alveolar surface area (S) were measured. After PPE, mice demonstrated increased values of Cst and MLI, and decreased S values. Both ATRA and MSC transfer were individually effective in improving these outcomes while the combination of ATRA and MSCs was even more effective. The combination of p70S6k1−/− MSCs transfer followed by ATRA demonstrated only modest effects, and rapamycin treatment of recipients with wild‐type (WT) MSCs and ATRA failed to show any effect. However, transfer of p70S6k1 over‐expressing‐MSCs together with ATRA resulted in further improvements over those seen following WT MSCs together with ATRA. ATRA activated p70S6k1 in MSCs in vitro, which was completely inhibited by rapamycin. Tracking of transferred MSCs following ATRA revealed enhanced accumulation and extended survival of MSCs in recipient lungs following PPE but not vehicle instillation. These data suggest that in MSCs, p70S6k1 activation plays a critical role in ATRA‐enhanced lung tissue repair, mediated in part by prolonged survival of transferred MSCs. p70S6k1‐activated MSCs may represent a novel therapeutic approach to reverse the lung damage seen in emphysema. stem cells translational medicine 2018;7:551–558
Keywords: Animal models, Cell signaling, Cellular therapy, Mesenchymal stem cells, Tissue regeneration
Significance Statement.
Current therapies are ineffective in repairing emphysema in patients with established disease. All‐trans retinoic acid (ATRA) was reported to promote lung tissue regeneration in animal models of emphysema but had little effect in clinical studies to date. p70S6 kinase‐1 (p70S6k1) is a downstream effector of mammalian target of rapamycin and upregulation of this pathway has been associated with cell growth and differentiation. Mesenchymal stem cells (MSCs) exhibit tissue repair effects mediated through potent regenerative and anti‐inflammatory properties. This study demonstrated the effectiveness of the combination of MSCs and ATRA in the reversal of emphysematous changes in the lung. Activation of the p70S6K1 signaling pathway in MSCs was essential to the reparative activities. Upregulation of this pathway in MSCs may provide therapeutic benefits in the treatment of damaged lung tissue such as emphysema.
Introduction
Emphysema is one phenotype of chronic obstructive pulmonary disease (COPD), a worldwide problem with an estimated 65 million people affected and more than 3 million deaths annually 1. The principal causes of emphysema are cigarette smoking (primarily) and alpha‐1 antitrypsin deficiency (AATD) 2, 3. AATD is a genetic disorder; the number of people with AATD is estimated at 100,000 in the United States, and patients who develop early onset emphysema have a poor prognosis. Treatments for emphysema do not have sustained clinical benefit and do not lead to the repair of underlying pulmonary parenchymal damage. Lung transplantation remains the primary therapeutic option in severe cases and is limited in its utility due to complications, costs, and number of available donors 4.
Mesenchymal stem cells (MSCs) are found in tissues and organs, and their primary role is to maintain and repair damaged tissue, including lung tissue 5. MSCs can be derived from bone marrow and differentiated into a variety of mesenchymal cell types, including fibroblasts, myofibroblasts, osteoblasts, chondroblasts, adipocytes, myoblasts, and also epithelial cells 6, 7. MSCs possess low immunogenicity and can be adoptively transferred into the host, even allogeneically 7, an advantage in the clinical setting. In rodent models of emphysema, transplantation of MSCs has shown significant repair benefits 8, 9, 10. In acute lung injury models, adoptive transfer of MSCs demonstrated immunomodulatory effects and were shown to differentiate into alveolar epithelial cells in a papain‐induced emphysema model in rats 11. However, the role of MSCs in the repair of lung tissue damage has not been well defined. Clinical studies with MSCs in the treatment of COPD patients are underway, but studies are critically needed to identify when and in what type of lung disease treatment with MSCs can be most successfully applied 12.
All‐trans retinoic acid (ATRA), a retinoid, is structurally related to Vitamin A and has multiple roles in tissues, including modulation of the structure and function of a variety of inflammatory, immune, and structural cells, and the development of organs, including lungs 13. ATRA has been shown to reverse lung tissue damage in an elastase‐induced rat emphysema model, and retinoids have been shown to play a role in lung development 14, 15. Despite these findings, similar studies in mouse models of elastase‐induced emphysema and in clinical studies, demonstrated that ATRA treatment alone failed to show these effects 16, 17, 18. Retinoic acid has also been shown to play a critical role in differentiating stem cells through ligation of retinoic acid receptors followed by homodimerization with retinoid X receptors which activate transcription factors 19. However, the signaling pathways and functional alteration of MSCs following retinoid stimulation in the repair of lung tissue have not been fully defined.
S6 kinase (S6k) is a key downstream substrate of the mammalian target of rapamycin (mTOR). The mTOR/S6k signaling pathway contributes to several pathological conditions, including diabetes, cancer, obesity, and emphysema 20, 21, 22 The pivotal role of mTOR in the functional differentiation of MSCs has been also demonstrated 23, 24. The mTOR/S6k axis stimulates protein synthesis, cell growth, and survival 25. To date, two highly homologous S6ks have been reported: S6k1 and S6k2 26. S6k1 and S6k2 are phosphorylated by a specific hetelotrimetric complex including mTOR (mTORC1 and mTORC2, respectively). The mTORC1‐S6k1 axis has been shown to control fundamental cellular processes, including transcription, translation, protein and lipid synthesis, cell growth/size, cell metabolism including induction of wound healing, gastric epithelial cell migration, and promotion of differentiation and regeneration of neurons 25, 26, 27, 28. To date, three isoforms of S6k1 have been reported: p70S6k1, p85S6k1, and p31S6k1. Among these isoforms, p70S6k1 has been reported as predominate and is the only isoform regulated by a growth factor 25, 26, 27, 28. Previously, we reported the outcomes of p70S6k1 deficiency at the cellular level and showed that rapamycin blocked protein synthesis through inhibition of the S6k1 signaling pathway 29. Despite these functions of p70S6k1, p70S6k1‐deficient mice appeared to be relatively normal except for a slightly smaller body size 30. Since the mTORC1‐S6k1 signaling pathway plays a critical role in lung development and mTOR plays a preventive role in cigarette smoking‐induced emphysema 21, the role of p70S6k1 in the repair of damaged lung tissue was determined in this study.
In a mouse model of elastase‐induced emphysema, emphysematous changes progressed gradually for up to 10 months following a single administration of elastase 31. Thus, the elastase‐induced emphysema model may reproduce some of the pathogenic events seen with progressive emphysema in smoking‐ or AATD‐associated COPD. Excessive neutrophil elastase activity has been associated with the progression of COPD 32. In light of the potential for beneficial interactions between MSCs, ATRA, and p70S6k1 in the repair of damaged lung tissue, we investigated the effects of combining treatment with MSC transfer and ATRA administration on elastase‐induced emphysema in settings where p70S6k1 activity was enhanced by overexpression or reduced by genetic or pharmacologic manipulation. Although advanced methodologies for isolation of bone marrow MSCs have been developed and different characteristics have been described for isolated and cultured MSCs, conventional MSC isolation and expansion in culture should remain a valid approach for studying potential clinical applications 33. In this setting, we hypothesized that ATRA would enhance the repair activity of MSCs in emphysema through the activation of p70S6k1.
Materials and Methods
Animals
Female C57Bl/6 wild‐type (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used at 8–10 weeks of age. This age and gender were chosen as body sizes and lung volume, which affect lung function values, are less variable compared to male or older mice. p70S6k1‐deficient (S6k1−/−) mice, created previously by homologous recombination, were genotyped and maintained on an inbred C57Bl/6 background as previously described 30. Transgenic mice expressing tdTomato B6.CgGt(ROSA)26Sortm14(CAGtdTomato)Hze/J (tdTomato mice) were provided by Dr. Jordan Jacobelli at National Jewish Health, Denver, CO. All of the mice were housed under specific pathogen‐free conditions. Experiments were conducted under a protocol approved by Institutional Animal Care and Use Committee of National Jewish Health.
Reagent
ATRA (Cayman Chemical, Ann Arbor, MI) was dissolved in cottonseed oil and administered to mice at a dose of 500 µg/kg by i.p. injection. ATRA treatment was initiated 21 days after elastase instillation and continued for 10 consecutive days. For in vitro experiments, 10 μM of ATRA was used. Rapamycin (LC Laboratories, Woburn, MA) was administered by i.p. injection at a dose of 1.5 mg/kg/day in 200 μl of vehicle (5.2% Tween‐80 and 5.2% polyethylene glycol in water) for 10 consecutive days 34. All of the reagent solutions and vehicles were sterilized by 0.2 µm filtration prior to use.
Preparation and Transfer of MSC
Bone marrow‐derived WT MSCs were provided by the Texas A&M Health Science Center College of Medicine (TAMHSC). Additionally, MSCs were generated from bone marrow cells of naive WT, S6k1−/−, or tdTomato mice. Briefly, bone marrow cells were obtained from femurs and tibias of mice and placed in culture medium (MesenCult MSC Basal Medium with MesenCult Stem Cell Stimulatory Supplements; STEMCELL Technologies, Vancouver, Canada) containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 µg/ml amphotericin B (Mediatech, Manassas, VA) until second passage. After second passage, MSCs were cultured in Iscove's Modified Dulbecco's Medium with L‐Glutamine and HEPES (Hyclone, Logan, UT), containing 10% heat‐inactivated fetal calf serum (Flowery Branch, GA), 10% horse serum (Hyclone), 50 µM 2‐ME (Sigma‐Aldrich, St. Louis, MO), and antibiotics/antimycotics as above. Between four and eight passages of MSCs were used in the experiments. Purity of MSCs was approximately 80% for MSCs from TAMHSC and 97% for in‐house generated MSCs. Purity was determined by flow cytometry analysis using anti‐mouse Sca‐1, CD11b, CD29, CD34, CD45, CD90.2, and CD105. All antibodies were purchased from eBioscience (San Diego, CA). MSCs were suspended in phosphate buffered saline (PBS) and 0.2 × 106 cells in 200 µl injected i.v. into recipient mice 21 days after porcine pancreatic elastase (PPE) instillation. In cell tracking experiments, 0.33 × 106 of MSCs were injected i.v. into recipient mice three times at least 30 minutes apart (providing a total of 1.0 × 106 labeled MSCs).
Development and Evaluation of Elastase‐Induced Emphysema
Mice were anesthetized with isoflurane, and 120 U/kg of PPE (Elastin Products Company, Owensville, MO) dissolved in 40 μl of physiological saline solution was instilled via the trachea without causing any adverse health events. Control mice received saline alone. Thirty‐two days after elastase administration, lung function was measured. Mice were euthanized using pentobarbital and the inserted tracheal cannula was attached to a FlexiVent system (Scireq, Montreal, Canada) to ventilate and analyze lung function. Pressure‐volume curves were generated and static compliance (Cst) and total lung capacity (TLC) were calculated using FlexiVent software.
After lung function measurements, lung tissues were collected for further morphometric analysis to determine the degree of emphysematous changes. Slides of left lung tissue were stained by hematoxylin‐eosin and analyzed for mean linear intercept (MLI) according to the guidelines of the American Thoracic Society/European Respiratory Society 35. The slide images of peripheral lung tissues along visceral pleura were captured using a microscope (BX40; Olympus America, Melville, NY) equipped with a digital camera (Q‐color 3; Olympus America, Center Valley, PA). Mean‐free distance was quantified as MLI in the captured images by randomly set test lines using NIH Image J (available at http://imagej.nih.gov/ij/download.html). Values of alveolar surface area (S) were also calculated with MLI and TLC as described previously 36.
Lentiviral Transduction of p70S6k1 in MSC
The p70S6k1 open‐reading frame expression clone was purchased from Genecopoeia (Rockville, MD) and used along with the Lenti‐Pac HIV Expression Packaging Kit (Genecopoeia) according to the manufacturer's instructions to produce recombinant lentivirus. The 293Ta packaging cell line was transfected overnight with the p70S6k1 expression clone and packaging plasmids. After 16 hours, fresh medium was added to the cells and incubated overnight. Pseudoviral supernatants were then collected at 24 and 48 hours post‐transfection and used for transduction. MSCs were plated in 6 well plates (1 × 106 cells per well) and viral supernatants (4 ml) containing 10 mM HEPES pH 7.4 (Life Technologies, Carlsbad, CA) and 7 μg/ml polybrene (Sigma‐Aldrich) were added to the cells. The plates were centrifuged at room temperature for 1 hour at 2,000 rpm (spinfection). The viral supernatants were removed and replaced with fresh medium. The cells were incubated overnight at 37°C. This centrifuge/incubation step was repeated two additional times. After the third spinfection, the cells were allowed to expand for 2 days and positively selected using 100 μg/ml Geneticin (Life Technologies).
Immunoblot Analysis
Cells were washed with PBS and analyzed for levels of whole protein or phosphorylated p70S6k1 by Western blot. Washed cells were lysed in RIPA buffer containing protease and a phosphatase inhibitor (Halt Protease Inhibitor Cocktail; Thermo Fisher Scientific, Inc., Waltham, MA). After debris was removed by centrifugation, Laemelli buffer (Bio‐Rad, Hercules, CA) was added 1:1 to the cell lysates. Lysates were boiled and then run on a 4%–15% precast gradient gel (Bio‐Rad) at 150 V at room temperature. The proteins were then electrophoretically transferred to a nitrocellulose membrane at 100 V for 1 hour at 4°C. Following transfer, the membrane was washed twice in Tris‐buffered saline with 0.1% Tween 20 (TBST), blocked for 1 hour at room temperature in 2% Bovine Serum Albumin, 0.5% sodium azide in TBST, and then incubated overnight at 4°C with antibody against p70S6k1 (ab9366; AbCam, Cambridge, MA) or phosphorylated p70S6k1 at T389 (ab2571; AbCam). Anti‐β‐actin (Sigma‐Aldrich) was used as a loading control. After incubation with the primary antibody, the membranes were incubated with anti‐rabbit horseradish peroxidase antibody (GE Healthcare Bio‐Sciences, Pittsburgh, PA) for 1 hour at room temperature and then washed extensively with TBST. Detection was performed using Western Lightening ECL (Perkin‐Elmer, Waltham, MA).
Flow Cytometry Analysis
To track transferred MSCs in lung tissue, lung cells were isolated by collagenase digestion following i.v. injection of MSCs derived from tdTomato mice. Numbers of tdTomato+ cells were monitored using the BD LSRFortessa (BD Biosciences, San Jose, CA).
Statistical Analysis
Values for all measurements were expressed as means ± SEM. For comparisons between multiple groups, the Tukey‐Kramer test was used. Nonparametric analyses, using the Mann‐Whitney U test or Kruskal‐Wallis test, were also applied to confirm that statistical differences remained significant, even if the underlying distribution was uncertain. The p value for significance was set at less than .05.
Results
Effects of the Combination of ATRA and MSCs on Elastase‐Induced Emphysema
To investigate the outcomes of the combination of ATRA and MSCs on the reversal of emphysematous tissue damage induced by PPE, MSCs were adoptively transferred into mice 21 days after intratracheal instillation of elastase followed by ATRA administration for 10 consecutive days. As shown in Figure 1A, elastase instillation induced significant peripheral airway destruction which resulted in increased MLI (Fig. 1B) and decreased alveolar surface area (S) (Fig. 1C). ATRA or MSC alone demonstrated modest effects on reduction of peripheral lung tissue damage as seen histologically and by decreases in MLI or increases in S values compared to the vehicle‐treated group. When the results of the combination of MSCs and ATRA were determined, the extent of improvement was significantly greater than either treatment alone, with further decreases in MLI and increases in S values. Cst values paralleled the histopathological findings, MLI, and S results (Fig. 1C). As a measure of lung function, Cst values were increased in the vehicle‐treated group following elastase instillation, MSC, or ATRA treatment alone showed decreased Cst values, and the combination was significantly more effective.
Figure 1.
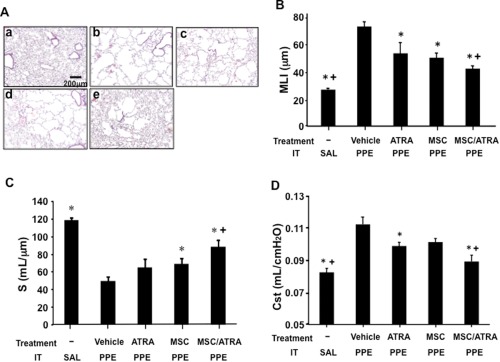
The reparative effects of combination treatment with MSC and ATRA on lung tissue in elastase‐induced emphysema. The treatment groups were: (1) vehicle alone, (2) MSCs, (3) ATRA administration for 10 days, or (4) MSC/ATRA initiated 21 days after elastase (PPE) or saline (SAL) installation (IT). Twelve days after the initiation of the treatment, airway function and lung tissue sampling were performed. (A): Representative lung tissue sections (H&E staining) from mice which received: a. intratracheal vehicle instillation, b. elastase instillation followed by vehicle treatment, c. elastase installation followed by ATRA treatment for 10 days, d. elastase installation followed by MSC transfer, and e. elastase installation followed by MSC/ATRA treatment. Values of MLI (B), alveolar surface area (S) (C) and static compliance (Cst) (D) following MSC or/and ATRA treatments. n = 8 in each group. *, p < .05 versus vehicle‐PPE, +, p < .05 versus ATRA/PPE and MSC/PPE. Abbreviations: ATRA, all‐trans retinoic acid; MLI, mean linear intercept; MSC, mesenchymal stem cell; PPE, porcine pancreatic elastase.
Role of p70S6k1 in MSC‐Induced Lung Tissue Improvement
To determine whether p70S6k1 played a role in the reparative function of MSCs, MSCs derived from the bone marrow of S6k1−/− mice were adoptively transferred into WT mice 21 days after PPE instillation. As a control, MSCs derived from WT mice were similarly transferred. As shown in Figure 2A, recipients of S6k1−/− MSCs followed by ATRA treatment demonstrated limited effects on PPE‐induced tissue damage, as MLI were significantly higher and S values were lower than in the group which received WT MSCs and ATRA treatment (Fig. 2B, 2C). In parallel, Cst values were not significantly decreased by transferred S6k1−/− MSCs followed by ATRA treatment (Fig. 2D).
Figure 2.
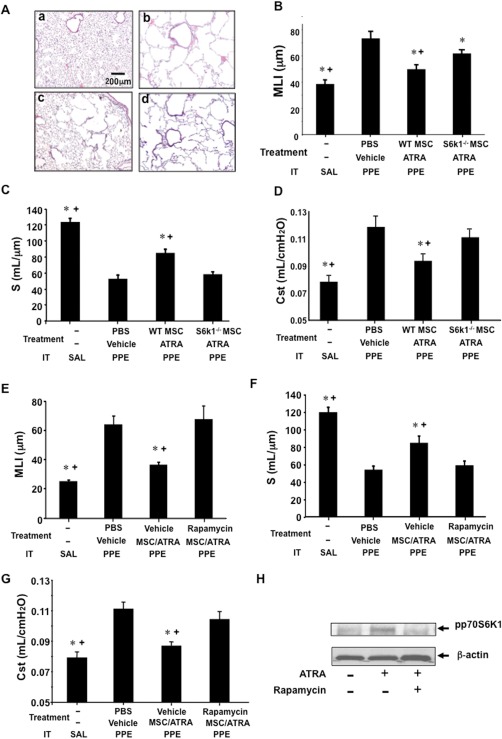
The role of p70S6k1 phosphorylation in MSC/ATRA‐dependent lung tissue repair. Treatment with vehicle alone, transfer of WT‐MSCs, or p70S6k1‐deficient (S6k1−/−) MSCs with ATRA administration for 10 days was initiated 21 days after elastase (PPE) or saline (SAL) installation (IT). Twelve days after the initiation of the treatment, airway function and lung tissue sampling were performed. (A): Representative lung tissue sections (H&E staining) from mice which received: a. intratracheal vehicle instillation, b. elastase instillation followed by vehicle treatment, c. elastase instillation followed by treatment with WT‐MSCs transfer and ATRA administration, and d. elastase instillation followed by transfer of S6k1−/− MSCs and ATRA administration. Values of mean linear intercept (MLI) (B), alveolar surface area (S) (C) and static compliance (Cst) (D) following PBS as vehicle, or WT‐MSCs, or S6k1−/− MSCs with ATRA treatments. MLI (E), alveolar surface area (S) (F) and Cst (G) values from mice treated with rapamycin or vehicle in parallel with WT‐MSC/ATRA treatment following elastase installation. (H): Immunoblot analysis of phosphorylated p70S6k1 levels (ppS6k1) in MSCs, which were cocultured with ATRA or ATRA following rapamycin pretreatment. n = 8 in each group. *, p < .05 versus PBS/vehicle‐PPE, +, p < .05 versus S6k1−/− MSC/ATRA‐PPE or rapamycin/ATRA/WT‐MSC‐PPE. Abbreviations: ATRA, all‐trans retinoic acid; MLI, mean linear intercept; MSC, mesenchymal stem cell; PBS, phosphate buffered saline; PPE, porcine pancreatic elastase; WT, wild‐type.
In light of the known effects of rapamycin on p70S6K1 activation 21, we examined whether rapamycin altered MSC/ATRA‐induced repair by administering the inhibitor following MSC transfer and during the ATRA treatment days. As shown in Figure 2E–2G, both MLI and Cst values in rapamycin‐treated mice remained high, whereas S values remained low compared to the vehicle‐treated group. These results implicated the role of p70S6k1 in the improvement of damaged lung tissue.
To establish that the effects of rapamycin were mediated through inhibition of ATRA stimulation of p70S6k1 in MSCs, protein levels of phosphorylated p70S6k1 were examined in MSCs cocultured with ATRA. As shown in Figure 2H, phosphorylated p70S6k1 was increased compared to vehicle‐treated cells 12 hours after coculture with ATRA. Rapamycin pretreatment attenuated the activation of p70S6k1 in MSCs cocultured with ATRA. Phosphorylated p70S6k1 was not detected at 3 or 6 hours of ATRA stimulation (data not shown). Thus, one consequence of ATRA treatment was activation of p70S6k1, which appeared essential to the reparative activity of the MSCs.
Over‐Expression of p70S6k1 in MSCs/ATRA Improved Lung Tissue Damage
To further establish the role of p70S6k1 in the MSC/ATRA effects on damaged lung tissue, p70S6k1 was over‐expressed in MSCs followed by adoptive transfer into mice 21 days after PPE. As shown in Figure 3A, p70S6k1 protein levels were upregulated following lentivirus transfection compared with empty vector‐treated cells. Morphometric and physiological analyses revealed significantly lower MLI and Cst values and significantly higher S values in lungs of mice, which received p70S6k1 over‐expressing MSCs compared with mice that received WT MSCs (Fig. 3B, 3C).
Figure 3.
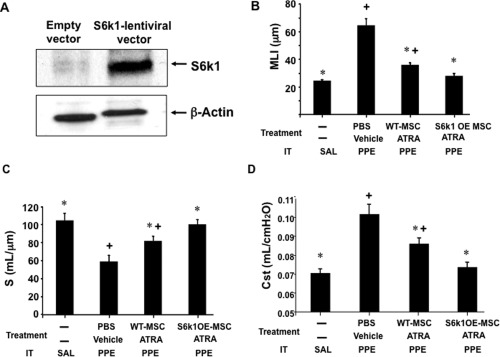
The effects of p70S6k1 over‐expressing MSCs on MSC/ATRA‐dependent lung tissue repair. (A): Immunoblot analysis of whole protein levels of p70S6k1 in MSCs following lentiviral transfection. Values of MLI (B), alveolar surface area (S) (C), and Cst (D) in mice that received transfer of WT‐MSCs or p70S6k1 over‐expressing (S6k1OE) MSCs with ATRA administration following elastase (PPE) or saline (SAL) instillation (IT). n = 8 in each group. *, p < .05 versus PBS/vehicle‐PPE, +, p < .05 versus saline. Abbreviations: ATRA, all‐trans retinoic acid; MLI, mean linear intercept; MSCs, mesenchymal stem cells; PBS, phosphate buffered saline; PPE, porcine pancreatic elastase; WT, wild‐type.
Effects of ATRA Treatment on the Number of Transferred MSCs in Lung Tissue
To determine the effects of ATRA treatment on the survival of MSCs in lung tissue, labeled MSCs were tracked and the number of cells in the lungs was monitored. tdTomato+ MSCs (1 × 106 cells) were transferred into mice following elastase instillation, and the numbers of positive cells among recovered lung cells were analyzed by flow cytometry. As shown in Figure 4A and 4B, the numbers of tdTomato+ MSCs were significantly increased in mice which received ATRA compared to recipients of vehicle at 48 hours, 72 hours, and 7 days after MSC transfer. However, when MSCs were transferred into mice following saline instillation, numbers of tdTomato+ MSCs were significantly decreased regardless of ATRA treatment.
Figure 4.
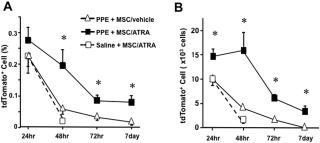
Increased numbers of labeled MSCs in lungs following transfer. Numbers of transferred tdTomato+ MSCs in lungs were analyzed by flow cytometry. Recipient mice received elastase instillation 21 days prior to MSC transfer, and ATRA (PPE + MSC/ATRA) or vehicle (PPE + MSC/vehicle) were administered following the transfer until the last experimental days. A group of mice received saline instillation 21 days prior to MSC transfer and ATRA treatment (saline + MSC/ATRA). (A): Ratio of tdTomato+ cells to total lung cells. (B): Estimated absolute numbers of tdTomato+ cells per lung. n = 6 in each group. *, p < .05 versus PPE + MSC/vehicle and saline + MSC/ATRA. Abbreviations: ATRA, all‐trans retinoic acid; MSCs, mesenchymal stem cells; PPE, porcine pancreatic elastase.
Discussion
In the present study, the significant benefits of combining MSCs and ATRA in an experimental model of emphysema were demonstrated, as well as a critical role of the p70S6k1‐signaling pathway in this process. First, we demonstrated that treatment with either MSCs or ATRA alone showed modest effects on physiological and histological outcomes expressed as Cst, MLI, and S values. However, when MSCs were adoptively transferred, followed by ATRA administration, the improvements were greater. To address the role of p70S6k1 in this reparative process, S6k1−/− MSCs were transferred followed by ATRA treatment and compared to the benefits of transfer of WT MSCs on elastase‐induced emphysema. In contrast to the benefits of WT MSCs, S6k1−/− MSCs had limited effects on damaged lungs. Addition of ATRA to MSCs in vitro resulted in increased p70S6k1 phosphorylation in WT MSCs. Rapamycin inhibits activation of the mTOR‐p70S6k1 pathway 21, 25 and when added to ATRA‐treated MSCs, phosphorylation of p70S6k1 was attenuated. To confirm the role of p70S6k1 activation, mice were treated with rapamycin. Similar to the results of transfer of S6k1−/− MSCs, rapamycin administration prevented the reversal of lung tissue damage seen with WT MSCs/ATRA. As a corollary to these findings, transfer of p70S6k1‐overexpressing MSCs into PPE‐treated mice, followed by ATRA administration, normalized lung function, and histological findings. An additional feature of ATRA treatment of MSCs was the enhanced accumulation and prolonged residence time of labeled and transferred cells in the lung following elastase but not vehicle instillation.
Massaro et al. initially reported that ATRA administration repaired damaged lung tissue in a rat model of emphysema 14. This study fostered clinical trials with ATRA or pharmacologically modified retinoid receptor antagonists, and beneficial clinical effects were not observed 18. In animal models of emphysema, findings following treatment with ATRA alone have been inconsistent. Unlike in rats 14, positive results with ATRA were not seen in elastase or cigarette smoking‐induced mouse models of emphysema 16, 17. In the current study, only modest levels of tissue repair were seen with ATRA treatment alone.
Stem cell therapy is now a focus of attention in a variety of difficult to treat diseases. MSCs are stem cells, which can be differentiated into different tissues such as bone, cartilage, or adipose tissue 37. As MSCs express low levels of HLA class II molecules, exogenous MSCs are able to escape allorecognition; thus, MSCs can be transferred between different species 37, 38. Based on these properties, exogenous MSCs have been used to engraft and repair damaged tissue. However, recent studies revealed that the primary function of the transferred MSCs were immunomodulatory through the release of numerous factors 12, 39. MSCs have been studied in many models of lung disease 40, including the ability to reverse the consequences of induced lung damage and emphysema. Katsha et al. showed that intratracheal instillation of bone marrow‐derived MSCs reduced MLI values in elastase‐induced emphysema in mice 9. Their study also suggested that factors secreted from MSCs such as hepatocyte growth factor, epidermal growth factor, or secretory leukocyte protease inhibitor may play roles in the repair process. MSCs derived from a different tissue, such as adipose tissue, showed benefits when transferred into mice, which developed emphysema as a consequence of cigarette smoke exposure, and also in elastase‐induced emphysema in rats 8, 41. It is likely that treatment with MSCs from different sources, generations, or culture conditions can result in variable outcomes 10. To counter this, we used a standardized and established MSC protocol and showed that transfer of MSCs alone had limited efficacy. Administration of ATRA to the MSC recipients enhanced the beneficial effects. MSCs were shown to be short‐lived in the recipients after administration 8. As p70S6k1 has been associated with cell survival and migration of mesenchymal cells 42, 43, we hypothesized that ATRA treatment may extend the survival and/or enhance the accumulation of MSCs to further augment improvements to damaged lung tissue. Based on the cell tracking studies of labeled MSCs, the number of cells and lung residence time were increased in the recipients that received ATRA treatment (in mice that received PPE but not vehicle alone). As the beneficial effects of MSCs have been associated with their secretion of numerous factors in a paracrine manner 12, a longer presence in the lung may be a major contributor to the repair of damaged lung tissue.
S6k is a major downstream target of mTOR, and this pathway plays an important role in protein synthesis, cell proliferation, and cell cycle progression 44. mTOR plays a critical role in lung development 25, and upregulation of mTOR was shown to protect against development of cigarette smoke‐induced emphysema 21. In the current study, failure to show lung tissue improvement by S6k1−/− MSCs, as well as the inhibition of the effects of WT MSCs by rapamycin supported the critical role of the mTOR/p70S6k1 pathway in the MSC/ATRA‐induced improvement of damaged lung tissue. p70S6k1 was phosphorylated in MSCs following ATRA stimulation, which was blocked by rapamycin pretreatment, demonstrating that activation of mTOR/p70S6k1 is a downstream event of ATRA as observed in a study of neurons 45. The importance of the role of p70S6k1 in MSCs on lung tissue repair was further demonstrated with greater improvements shown following the transfer of p70S6k1‐overexpressing MSCs.
Conclusion
The findings demonstrated that a combination of MSCs and ATRA was greater than either alone in inducing the improvement of elastase‐induced emphysema in mice (Fig. 5). This ATRA‐dependent enhancement of MSCs function appeared linked to p70S6k1 phosphorylation, as confirmed by the failure of p70S6k1‐deficient MSCs and the benefits of p70S6k1 over‐expressing MSCs. The specific roles of p70S6k1 activation on MSCs function now need further elucidation as to which reparative factors are released, which cell types initiate lung tissue repair, and what role prolongation of lung MSCs residence time combine to reverse established lung disease.
Figure 5.
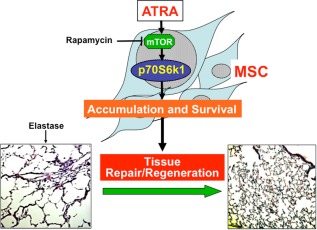
Proposed mechanisms of MSC/ATRA‐dependent repair effects on damaged lung tissue in emphysema. ATRA resulted in the activation of p70S6k1 in exogenous MSCs and increased accumulation and survival time in the lung, promoting lung tissue repair/regeneration. Abbreviations: ATRA, all‐trans retinoic acid; MSC, mesenchymal stem cell; mTOR, mammalian target of rapamycin.
Author Contributions
K.T.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing and financial support. F.N., J.D., S.A., S.H.‐Kim, Y.Y. J. and Y.S.: Collection and/or assembly of data. M.O.: Conception and design. N.T. and E.R.S.: Conception and design, and manuscript writing. E.W.G.: Aided in design, data analysis and interpretation and manuscript writing.
Disclosure of Potential Conflicts of Interest
E.R.S. disclosed employment with Sanofi. The other authors indicated no potential conflicts of interest.
Acknowledgments
The authors thank Cindy Chavez for assistance in preparing the manuscript. This research was supported by the ATS/Alpha‐1 Foundation Research Grant AAT‐10‐001 in Alpha‐1. MSCs provided by the TAMHSC, Institute for Regenerative Medicine at Scott & White Hospital were supported by a grant from the National Center for Research Resources of the National Institute of Health, Grant P40RR017447.
References
- 1. World Health Organization . Chronic respiratory diseases: Chronic obstructive pulmonary disease (COPD). Available at http://www.who.int/respiratory/copd/en/. Accessed August 2, 2017.
- 2. Forey BA, Thornton AJ, Lee PN. Systematic review with meta‐analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 2011;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gooptu B, Ekeowa UI, Lomas DA. Mechanisms of emphysema in alpha1‐antitrypsin deficiency: Molecular and cellular insights. Eur Respir J 2009;34:475–488. [DOI] [PubMed] [Google Scholar]
- 4. Nathan SD, King CS. Organ donors: Making the most of what is offered. Chest 2015;148:303–305. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Chen X, Cao W et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 2014;15:1009–1016. [DOI] [PubMed] [Google Scholar]
- 6. Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014;6:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trounson A. New perspectives in human stem cell therapeutic research. BMC Med 2009;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schweitzer KS, Johnstone BH, Garrison J et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 2011;183:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsha AM, Ohkouchi S, Xin H et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase‐induced emphysema model. Mol Ther 2011;19:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antunes MA, Abreu SC, Cruz FF et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res 2014;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz LA, Dutreil M, Fattman C et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiss DJ. Concise review: Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 2014;32:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das BC, Thapa P, Karki R et al. Retinoic acid signaling pathways in development and diseases. Bioorg Med Chem 2014;22:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase‐induced pulmonary emphysema in rats. Nat Med 1997;3:675–677. [DOI] [PubMed] [Google Scholar]
- 15. Dollé P. Developmental expression of retinoic acid receptors (RARs). Nucl Recept Signal 2009;7:e006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujita M, Ye Q, Ouchi H et al. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax 2004;59:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. March TH, Bowen LE, Finch GL et al. Effects of strain and treatment with inhaled all‐trans‐retinoic acid on cigarette smoke‐induced pulmonary emphysema in mice. COPD 2005;2:289–302. [PubMed] [Google Scholar]
- 18. Roth MD, Connett JE, D'Armiento JM et al. Feasibility of retinoids for the treatment of emphysema study. Chest 2006;130:1334–1345. [DOI] [PubMed] [Google Scholar]
- 19. Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol 2011;226:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tavares MR, Pavan IC, Amaral CL et al. The S6K protein family in health and disease. Life Sci 2015;131:1–10. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida T, Mett I, Bhunia AK et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke‐induced pulmonary injury and emphysema. Nat Med 2010;16:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang X, Li K, Tao X et al. Effects of phosphoinositide 3‐kinase on protease‐induced acute and chronic lung inflammation, remodeling, and emphysema in rats. Chest 2013;143:1025–1035. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Huang S, Zhang J et al. Mesenchymal stem cells ameliorate inflammatory cytokine‐induced impairment of AT‐II cells through a keratinocyte growth factor‐dependent PI3K/Akt/mTOR signaling pathway. Mol Med Rep 2016;13:3755–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie J, Liu B, Chen J et al. Umbilical cord‐derived mesenchymal stem cells alleviated inflammation and inhibited apoptosis in interstitial cystitis via AKT/mTOR signaling pathway. Biochem Biophys Res Commun 2018;495:546–552. [DOI] [PubMed] [Google Scholar]
- 25. Land SC, Scott CL, Walker D. mTOR signalling, embryogenesis and the control of lung development. Semin Cell Dev Biol 2014;36:68–78. [DOI] [PubMed] [Google Scholar]
- 26. Kim YW, Lee WH, Choi SM et al. DA6034 promotes gastric epithelial cell migration and wound healing through the mTOR pathway. J Gastroenterol Hepatol 2012;27:397–405. [DOI] [PubMed] [Google Scholar]
- 27. Yang L, Miao L, Liang F et al. The mTORC1 effectors S6K1 and 4E‐BP play different roles in CNS axon regeneration. Nat Commun 2014;5:5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosner M, Hengstschläger M. Nucleocytoplasmic localization of p70 S6K1, but not of its isoforms p85 and p31, is regulated by TSC2/mTOR. Oncogene 2011;30:4509–4522. [DOI] [PubMed] [Google Scholar]
- 29. Kawasome H, Papst P, Webb S et al. Targeted disruption of p70 (s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc Natl Acad Sci USA 1998;95:5033–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ge Y, Wu AL, Warnes C et al. mTOR regulates skeletal muscle regeneration in vivo through kinase‐dependent and kinase‐independent mechanisms. Am J Physiol Cell Physiol 2009;297:C1434–C1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tibboel J, Keijzer R, Reiss I et al. Intravenous and intratracheal mesenchymal stromal cell injection in a mouse model of pulmonary emphysema. COPD 2014;11:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 2012;122:2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Ghazanfari R, Zacharaki D et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann NY Acad Sci 2016;1370:109–118. [DOI] [PubMed] [Google Scholar]
- 34. Wu R, Hu TC, Rehemtulla A et al. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clin Cancer Res 2011;17:7359–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsia CC, Hyde DM, Ochs M et al. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010;181:394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitzner W. Use of mean airspace chord length to assess emphysema. J Appl Physiol 2008;105:1980–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frenette PS, Pinho S, Lucas D et al. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping‐stone for regenerative medicine. Annu Rev Immunol 2013;31:285–316. [DOI] [PubMed] [Google Scholar]
- 38. Le Blanc K, Tammik C, Rosendahl K et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890–896. [DOI] [PubMed] [Google Scholar]
- 39. Eggenhofer E, Luk F, Dahlke MH et al. The life and fate of mesenchymal stem cells. Front Immunol 2014;5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tzouvelekis A, Ntolios P, Bouros D. Stem cell treatment for chronic lung diseases. Respiration 2013;85:179–192. [DOI] [PubMed] [Google Scholar]
- 41. Furuya N, Takenaga M, Ohta Y et al. Cell therapy with adipose tissue‐derived stem/stromal cells for elastase‐induced pulmonary emphysema in rats. Regen Med 2012;7:503–512. [DOI] [PubMed] [Google Scholar]
- 42. Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J 2012;441:1–21. [DOI] [PubMed] [Google Scholar]
- 43. Madala SK, Sontake V, Edukulla R et al. Unique and redundant functions of p70 ribosomal S6 kinase isoforms regulate mesenchymal cell proliferation and migration in pulmonary fibrosis. Am J Respir Cell Mol Biol 2016;55:792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asnaghi L, Bruno P, Priulla M et al. mTOR: A protein kinase switching between life and death. Pharmacol Res 2004;50:545–549. [DOI] [PubMed] [Google Scholar]
- 45. Chen N, Napoli JL. All‐trans‐retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane‐associated RARalpha. FASEB J 2008;22:236–245. [DOI] [PubMed] [Google Scholar]


