Abstract
The capacity of stem and progenitor cells to stimulate cardiac regeneration has been studied for almost 20 years, with very promising preclinical data and mixed clinical results. Several cell types have been studied, identified by their cell surface markers, differentiation capacity and their secreted growth factors. Bone marrow derived mesenchymal stem cells (MSCs) have been found to have potent regenerative capacity, through multiple mechanisms, including mesoderm lineage differentiation, immunomodulation, and paracrine stimulation. MSCs also secrete exosomes and microvesicles, which themselves contain potent angiogenic cytokines or mRNA molecules with effects on their local milieu. This concise review summarizes the mechanisms of MSC‐based cardiac regeneration and highlighting results from molecular and preclinical studies. We also discuss clinical trial results to date, and ongoing studies. Furthermore, we discuss novel approaches for the enhancement of MSC based cardiac regeneration, such as genetic modification. stem cells translational medicine 2018;7:543–550
Keywords: Cardiac, Mesenchymal stem cells
Significance Statement.
This concise review summarizes results from experiments using a specific type of stem cells, called mesenchymal stem cells, which have shown a capacity to repair and regenerate the heart following injury. This article summarizes the mechanisms by which these cells act, and discusses ongoing research in how to improve their effect.
Introduction
Cardiovascular diseases remain one of the leading causes of death worldwide. Myocardial infarction (MI) from atherosclerotic plaque rupture remains the most common cause, frequently leading to the development of heart failure (HF) 1, 2. In industrialized countries, the prevalence of HF is high, affecting 1%–3% of total population, representing one of health care's most expensive diagnoses 3.
As a result of a pathological stimulus, the left ventricle undergoes a robust plasticity response known as pathological remodeling 4. This process refers to the change in cardiomyocyte biology and cardiac structure post insult, and is the culmination of a series of transcriptional, signaling, structural, electrophysiological, and functional events occurring within the cardiomyocyte, along with a range of events which occur in fibroblasts, vascular smooth muscle cells, endothelial cells, and leukocytes 5. While these changes are aimed at stabilizing the heart in the short term, the long‐term consequence is an inexorable progression to pump failure and death. Current therapy involves beta blockade, angiotensin converting enzyme inhibition, aldosterone blockade 6, and biventricular pacing strategies 7, 8. These strategies primarily work by reducing pathological left ventricle (LV) remodeling via inhibition of “neuro‐hormonal activity,” which include sympathetic and renin‐angiotensin‐aldosterone activation. Despite medical therapy, the mortality and morbidity from HF secondary to MI remains unacceptably high. For example, recent data in Ontario, Canada, demonstrates that the 1‐year mortality for a diagnosis of congestive heart failure (CHF), regardless of the etiology is approximately 25% 9.
Given the limited capacity for self‐renewal, the concept of cell‐based strategies to “regrow” lost cardiomyocytes or to promote endogenous repair became popular in the late 1990s. Since then, the field of regenerative medicine has dramatically expanded, with a growing body of the literature to support the safety and efficacy of this approach. However, there lacks definitive clinical data to move this field into mainstream medical practice. This review will focus upon a well‐studied and safe stem cell subpopulation known as mesenchymal stem cells (MSCs). We will further focus upon the use of MSCs as a therapeutic strategy to reverse deleterious LV remodeling, and outline current and future clinical trials using this regenerative approach.
MSC Differentiation
MSCs are a subset of bone marrow cells that can be isolated from other bone marrow derived mononuclear cells (BM‐MNCs) by their rapid adherence to plastic tissue culture dishes. Following culture, the remaining cells typically express markers CD29 (integrin ß‐1), CD44 (hCAM), CD90 (thy‐1), CD105, and CD117 (c‐kit) and are negative for the hematopoietic and vascular markers CD34, CD45, and CD11b 10, 11.
Using growth‐factor rich selective media, MSCs have been shown to be able to differentiate into multiple mesoderm lineages and differentiated cell types, including osteoblasts 12, adipocytes 13, skeletal muscle myocytes/myotubes 14, pancreatic islet cells 15, and cardiomyocytes 16, 17. If delivered in vivo, they have been shown to engraft and transdifferentiate into cardiomyocytes, repairing the infarcted myocardium 18, 19. Further studies challenged these findings, as very limited engraftment was found, although there was still benefit on overall myocardial function in small animal models 20, 21. In pigs, 2 weeks following coronary injection, only 2% of cells were found in the heart, and there was no evidence of cardiomyocyte differentiation 22. Overall, animal studies have shown that MSCs can improve cardiac function, but likely not exclusively through replacement of injured contractile cardiomyocytes. Figure 1 summarizes the mechanisms listed below.
Figure 1.
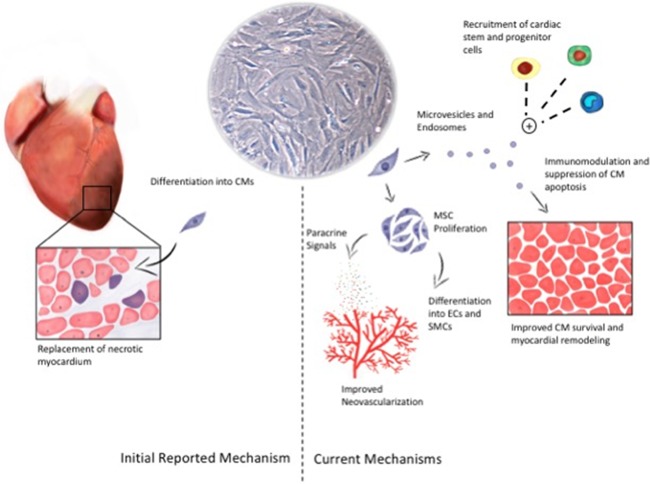
Mechanisms of MSC‐mediated cardiac regeneration. The initial reported mechanisms of MSCs' impact on cardiac regeneration were via replacement of necrotic contractile myocardium with differentiated cardiomyocytes (CMs; left side of figure). The relative contribution of this mechanism is likely quite small, with greater contribution from paracrine mechanisms, whether from secreted paracrine signals or encapsulated signals in microvesicles or endosomes (right side of figure). Together, these processes lead to improved cardiomyocyte survival, reduced inflammation, and preserved myocardial function. Abbreviations: CM, conditioned medium; MSC, mesenchymal stem cell; SMC, smooth muscle cell.
Paracrine Effect
MSCs also secrete multiple cytokines and growth factors, together termed their “secretome,” which contribute to their paracrine therapeutic effect. These factors are released in soluble form, or in exosomes and in extracellular vesicles (EVs), and can be sampled by collecting the medium in which the cells are cultured, so‐called “conditioned medium” (CM) 23. Over 30 systematic proteomic studies on MSC CM have been conducted, reporting a multitude of growth factors that could have potent paracrine effects. These include hepatocyte growth factor (HGF) 20, interleukin‐1 (IL1) and −6 (IL6) 24, stem‐cell derived factor‐1 (SDF‐1) 25, and several others 23. Within the EVs or exosomes, several mRNAs have been found, such as miR221 and miR‐19a, which are involved with suppressing apoptosis or stimulating Akt (a potent survival mediator) in various cell types 26, 27, including cardiomyocytes.
Several groups have shown benefit of MSC‐derived growth factors and CM for cardiac repair and regeneration. In a rat model of acute MI, CM from cultured MSCs were able to preserve myocardial contractile capacity, inhibit apoptosis of cardiomyocytes, and allow the formation of new vessels in damaged tissues 28. This study also showed upregulation of vascular endothelial growth factor (VEGF) and IL‐1ß in CM from MSCs cultured under hypoxic conditions, suggesting that hypoxia might stimulate production of these vasculoprotective and anti‐apoptotic cytokines. With MSCs engineered to overexpress Akt, CM from hypoxia‐treated cells was able to prevent in vitro apoptosis of rat cardiomyocytes, and in vivo lead to reduced infarct size and preserved LV contractility 29, 30.
The paracrine factors secreted by MSCs likely exert a pleiotropic effect on the myocardium, with improved local angiogenesis, cardiac stem‐cell stimulation, and reduced cardiomyocyte death. There is also evidence of reduced fibroblast activation and cell‐mediated immune response, with corresponding reduction in myocardial fibrosis.
Various preclinical studies have been shown to enhance the ability of MSCs to secrete soluble angiogenic markers, such as VEGF and Placental growth factor (PLGF) 20. MSCs transduced with GATA‐4, a GATA zinc finger transcription factor family member, showed increased production of insulin‐like growth factor‐1 (IGF‐1) and VEGF 26. Injection of the cells into a rat model of MI increased peri‐infarct neovessel formation and reduced overall infarct size 31. In a swine model of MI, human MSC CM was injected intravenously and lead to increased capillary density and preserved cardiac function 32. By echocardiography, animals who received CM had preserved wall thickness, fractional area shortening, ejection fraction, stroke volume, and stroke work compared to those who received a non‐CM product. Further evaluation of MSC CM showed an abundant production of Cysteine‐rich protein 61 (Cyr61), a secreted extracellular matrix related protein that can modulate cell adhesion, migration, proliferation, differentiation, apoptosis, and senescence through interaction with cell surface integrin receptors and heparan sulfate proteoglycans 33. When the production of this protein was inhibited in MSCs, the angiogenic benefit of CM was abrogated. It is unclear from these studies what the exact targets of Cyr61 are, and whether this is a separate mechanism from the VEGF and IGF‐mediated one discussed above.
The immunomodulatory effects of MSCs are the result of cell‐to‐cell contact, production of inhibitory molecules, and induction of regulatory T‐cells 34. MSCs have been shown to suppress inflammatory reactions in various tissues via interference with multiple types of signals. In a mouse model of asthma, MSCs suppressed Th2‐mediated inflammation via transforming growth factor‐β (TGF‐β) secretion as well as activation of the STAT6 pathway via IL‐4 and IL‐13 35. In a model of interstitial lung disease, however, inflammation was suppressed by MSCs via tumor necrosis factor 1 (TNF‐α) and IL1R 36. In the mouse heart, following creation of acute MI, MSCs were shown to inhibit inflammation via production of TNF‐α‐induced protein 6 (TNAIP6). This study showed that induction of this molecule was associated with decreased proteolytic injury to the heart, reduced fibrosis and overall preserved cardiac function 37.
The paracrine factors secreted by MSCs also exert their effect via the stimulation of local cardiac stem cells or cardiac progenitor cells (CPCs). Nakanishi et al. showed that MSC CM promoted proliferation and migration of isolated CPCs and prevented hypoxia‐induced apoptosis 38. Interestingly, isolated CPCs grown in MSC conditioned medium also showed upregulation of cardiomyocyte‐related genes such as beta‐myosin heavy chain (ß‐MHC) and atrial natriuretic peptide 38. A subsequent study showed that the paracrine effects of the MSCs are mediated even distantly, as skeletal muscle injection showed similar results in a rat model of acute MI. MSCs or CM implanted into skeletal muscle lead to improved left ventricular function and cardiomyocyte regeneration, supported by a doubling of the expression of cell cycle markers Ki67 and phosphohistone H3 39. Consequently, there was a 13% reduction in mean myocyte diameter. In recipient animals, there were significantly increased serum levels of HGF, leukemia inhibitory factor, and macrophage colony‐stimulating factor. Examination of the myocardium also confirmed increased presence of c‐Kit+, CD31+, and CD133+ progenitor cells. The authors suggest that in addition to recruiting local CPCs, the paracrine factors may also be recruiting more bone marrow derived progenitors, which engraft and also exert their regenerative effect on the ischemic myocardium 39.
EVs and Exosomes
The use of EVs and exosomes, without the cells themselves, is a growing practice for regenerative therapy. EVs have a size between 100 nm and 1 µm and derive from the detachment of cytoplasmic protrusions. EVs from MSCs express CD13, CD29, CD44, CD73, and CD105, similar to MSCs themselves 40, 41, 42. Exosomes have a size ranging between 30 and 100 nm and originate from fusion of endosomes with the plasma membrane, which are released by exocytosis. Both contain nucleic acids, coding mRNA and noncoding RNA. Coding mRNAs present in EVs include transcripts related to control of transcription, cell proliferation, and immune regulation 42, 43. Among the noncoding RNAs contained in released MSC‐EVs, there are selected patterns of miRNAs 44, 45, which can be transferred to target cells and downregulate mRNA translation and protein expression 46, 47.
Recent studies suggest that the therapeutic effect of MSCs is in large part due to secreted EVs and exosomes 48. In particular, the CM of human embryonic stem cell‐derived MSCs injected in a porcine model of myocardial I/R was able to limit infarct size and improve systolic function via reduction of TGF‐β signaling and apoptosis 49. Fractionation analyses then revealed that cardioprotection was mediated by components with a size between 100 and 220 nm, suggesting the presence of large particles rather than secreted cytokines. The same group then showed that highly purified exosomes isolated from CM of the same MSCs had a radius of 55–65 nm and induced significant cardioprotection when injected in a murine MI model 50. Interestingly, this effect was only produced by intact, not lysed, exosomes 51.
A recent study showed that murine MSCs released exosomes enriched with miR‐22, which were internalized by cocultured cardiomyocytes. MiR‐22 prevented CM apoptosis via interaction with methyl CpG binding protein 2 (Mecp2) 52. Another group showed that MSCs transduced with GATA‐4 produced exosomes with high levels of several miRNAs, among them miR‐221 and miR‐19a 27. These mRNAs reduced apoptosis of ischemic cardiomyocytes via inhibition of p53‐upregulated modulator of apoptosis, a subclass of the Bcl‐2 protein family 53, and inhibition of Phosphatase and tensin homolog (PTEN) with resultant activation of Akt and extracellular signal‐regulated kinase (ERK) pathways 27.
Clinical Trials of MSCs for Ischemic Heart Disease
While most cell therapy trials for ischemic heart disease (IHD) have concentrated on BM‐MNCs, isolated MSCs have also been used in trials of acute and chronic IHD. Table 1 summarizes these studies.
Table 1.
Summary of key clinical trials of MSC therapy for ischemic heart disease
| Study | n | Cell source | Cell dose (× 106) | Design | Delivery | Key findings |
|---|---|---|---|---|---|---|
| Acute myocardial infarction | ||||||
| Chen et al. 53 | 69 | Autologous BM | 4800–6000 | RPCT | IC | Improved LVEF, perfusion and wall motion |
| Katritsis et al. 54 | 22 | Autologous BM | 1–2 | Open | IC | Improved wall motion and perfusion |
| Hare et al. 55 | 53 | Allogeneic BM (Provacel) | 0.5/1.6/5 per kg | RPCT | IV | Safety; improved LVEF and remodeling |
| Houtgraaf et al. 56 | 14 | Autologous BM | 20 | RPCT | IC | Improvement in perfusion and myocardial scar |
| Gao et al. 57 | 41 | Autologous BM | 3.1 | RPCT | IC | No difference in viability, perfusion or LVEF |
| SEED‐MSC 58 | 80 | Autologous BM | 72 ± 9 | Open | IC | Improved LVEF |
| Chronic ischemic heart disease | ||||||
| Chen et al. 53 | 22 | Autologous BM | 5 | Open | IC | Increased LVEF and improved symptoms |
| Mohyeddin‐Bonab et al. 59 | 8 | Autologous BM | 5.6 | Open | IC/IM | Improved LVEF, reduced infarct size |
| Friis et al. 60 | 31 | Autologous BM | 22 | Open | IM | Improved LVEF and exercise capacity |
| POSEIDON 61 | 30 | Allogeneic/autologous BM | 20/100/200 | Randomized open | IM | Safe |
| Mathiasen et al. 62 | 60 | Autologous BM | 83 | RPCT | IM | Improved LVEF and muscle mass |
| Perin et al. 63 | 60 | Allogeneic BM | 25/75/150 | RPCT | IM | Safety, feasible |
| Qayyum et al. (2017) | 60 | Autologous adipose tissue | 72 ± 45 | RPCT | IM | No difference in exercise capacity |
| TAC‐HFT 64 | 65 | Autologous BM | 40 | RPCT | IM | Improved exercise tolerance and reduced infarct size. |
| PROMETHEUS 65 | 9 | Autologous BM | 20–40 | RPCT | IM | Increased LVEF and decreased scar. |
Abbreviations: BM, bone marrow; IC, intracoronary; IM, intramyocardial; IV, intravenous; LVEF, left ventricular ejection fraction; MSC, mesenchymal stem cell; RPCT, randomized placebo‐controlled trial.
Chen et al. 53 randomized 69 patients after acute MI and injected 48–60 × 109 MSCs into the infarct related coronary artery 10 days following reperfusion and stenting. At 3 and 6 months follow‐up, they found a significant difference in the improvement of left ventricular ejection fraction (LVEF) in the MSC group compared to placebo (17% vs. 5%), in addition to reduced infarct size 66. In 2009, Hare et al. 55 published a dose escalation study of allogenic MSCs (0.5 × 106/kg, 1.6 × 106/kg, and 5 × 106/kg) in patients post‐percutaneous coronary intervention (PCI) for acute MI. Although safety was the primary endpoint, they also performed efficacy measurements. Notably, they found an increase in LVEF at 3, 6, and 12 months in the MSC group compared to the placebo group. At 12 months post injection, the improvement was of 5.2% versus 1.8% by cardiac magnetic resonance imaging (MRI), with the greatest benefit being in patients with and anterior MI. Lee et al. 58 randomized 69 patients post MI to receive 7.2 ± 0.9 × 106 autologous MSCs or placebo and found a similar result at 6 months, with an improvement in LVEF of 5.2% versus 1.6% at 6 months (using SPECT). The APOLLO trial was a double blond placebo‐controlled trial comparing adipose‐derived MSCs (also known as adipose‐derived regenerative cells) compared to placebo post ST‐elevation MI. Fourteen patients were enrolled in a 3:1 randomization to receive intracoronary injection of either 20 × 106 cells or placebo. After 6 months, they found a reduction in LV infarction percentage (15.3% ± 2.6% vs. 31.6% ± 5.3%) by cardiac MRI in the cell‐treated group, in addition to an improvement in the MIBI‐SPECT perfusion defect. In contrast, Gao et al. injected 3.0 × 106 MSCs 14 days postacute MI, and found no significant difference in LVEF between MSC‐treated and placebo‐treated groups up to 24 months post injection 57.
In the treatment of chronic ischemia and ischemic cardiomyopathy, early studies were quite small, but since 2011 some larger randomized studies have emerged with interesting results. The route of delivery, rather than intracoronary or intravenous, is predominantly intramyocardial. Friis et al. 60 conducted a safety study and enrolled 31 patients with stable, moderate‐severe angina with no further revascularization options. MSCs (mean: 21.5 × 106, range 3–62 × 106) were delivered by intramyocardial injection, and all recipients were followed for 6 months. There were no ventricular arrhythmias or other major adverse cardiac events (MACE) associated with the cells. SPECT analysis showed no difference in the perfusion score, and cardiac MRI showed improvement in LVEF from 55.9% to 57.9% (p < .001). Clinically there was an improvement in exercise capacity and Canadian Cardiovascular Society (CCS) class of angina, although these results were not placebo‐controlled. In 2015, Mathiasen et al. published results of the MSC‐HF trial, a randomized controlled trial for patients with symptomatic ischemic cardiomyopathy (LVEF < 45%) 62. Sixty patients were enrolled and randomized in a 2:1 fashion, and MSC recipients received a mean of 8.3 × 107 autologous cells via intramyocardial injection. At 6 months of follow‐up, LV end‐systolic volume (LVESV) was significantly reduced in the MSC group compared to placebo (−13.0 ml; p = .001). Compared with placebo, there were also significant improvements in LVEF of 6.2% (p < .0001), stroke volume of 18.4 ml (p < .0001), and myocardial mass of 5.7 g (p = .001).
Meta‐analyses of trials using bone marrow derived progenitor and stem cells, with a total sample size of 2,602, albeit not focused exclusively on MSCs, have shown the limitation of trials to date 67, 68. All have been relatively under powered studies and have used diverse protocols. The exact cell type and number of cells have been quite variable, as exemplified in the MSC trials using a 10,000‐fold difference in the amount of delivered cells. Not unexpectedly, one meta‐analysis showed that cell number was an independent predictor of outcome on LV function, with trials using greater than 50 × 106 cells having more efficacy than those using less 68. Regardless, the data for BM‐derived cells overall show a small but significant benefit in LVEF (+2.92%), reduction in infarct size (−2.25%), and LVESV (−6.37 ml) compared with standard therapy 68. Furthermore, a 2014 meta‐analysis comparing various selected stem cell populations performed an analysis of MSC efficacy for acute MI specifically, and found that MSCs lead to an overall benefit in LVEF of 4.41% compared to placebo control, an effect that was statistically significant (p = .01) 67.
There are many other limitations to the clinical trials conducted to date, including differences in the timing of cell delivery, delivery method (intramyocardial, intracoronary, or intravenous delivery), follow up, and cell processing. The trials were mostly locally driven translational studies in the absence of standard procedures across the trials. Unfortunately, the heterogeneity of the trials has reduced the impact of their data, especially in the meta‐analyses, ultimately creating more confusion than clarity.
Currently, there are over 25 trials of MSC delivery for cardiac regeneration registered with http://www.clinicaltrials.gov, including for acute MI and ischemic cardiomyopathy. There are also trials using MSCs for nonischemic conditions, such as anthracycline‐medicated cardiomyopathy. Many are phase IIa/b, with LV function or MACE as primary outcomes. There are still no larger scale efficacy trials, likely due to regulatory, fiscal, and institutional limitations. Table 2 summarizes the ongoing trials.
Table 2.
Summary of ongoing clinical trials using MSCs for ischemic heart disease
| Study | n | Cell source | Condition | Design | Delivery | ClinicalTrials ID |
|---|---|---|---|---|---|---|
| Acute myocardial infarction | ||||||
| RELIEF | 135 | Autologous BM | Acute MI | Phase III | IC | NCT01652209 |
| CIRCULATE | 105 | Allogeneic BM | Acute MI | Phase II/III | IC | NCT03404063 |
| HUC‐HEART | 79 | Autologous/allogeneic BM | Pre‐CABG | Phase I/II | IM | NCT02323477 |
| Kumar et al. | 20 | Allogeneic BM | Acute MI | Phase I/II | IV | NCT00883727 |
| Perin et al. | 25 | Allogeneic BM | Acute MI | Phase I/II | IM | NCT00555828 |
| Skerrett et al. | 220 | Allogeneic BM (PROCHYMAL) | Acute MI | Phase II | IV | NCT00877903 |
| Musialek et al. | 115 | Allogeneic BM (Cardiocell) | Acute MI | Phase II/III | IC | NCT03404063 |
| AMICI | 105 | Allogeneic BM | Acute MI | Phase II | IC | NCT01781390 |
| ESTIMATION | 50 | Autologous BM | Postacute MI | Phase III | IM | NCT01394432 |
| Chronic ischemic heart disease | ||||||
| Jerome et al. | NYD | Autologous BM | Ischemic CM (LVAD) | Phase I | IM | NCT02460770 |
| MESAMI2 | 90 | Autologous BM | Chronic ischemic CM | Phase II | IM | NCT02462330 |
| Dai et al. | 45 | Autologous BM | Chronic ischemic CM | Phase I/II | Collagen scaffold | NCT02635464 |
| CONCERT‐HF | 144 | Autologous BM | Ischemic CM | Phase II | IM | NCT02501811 |
| Antonitsis et al. | 30 | Allogeneic BM | Ischemic CM needing CABG | Phase I | IM | NCT01753440 |
| Antonitisis et al. | 5 | Allogeneic BM | Ischemic CM with LVAD | Phase I | IM | NCT01759212 |
| Kastrup et al. | 10 | Allogeneic adipose tissue | Ischemic CM | Phase I | IM | NCT02387723 |
| Kastrup et al. | 81 | Allogeneic adipose tissue | Ischemic CM | Phase II | IM | NCT03092284 |
| SCIENCE | 138 | Allogeneic adipose tissue | Ischemic CM | Phase II | IM | NCT02673164 |
| UCMSC‐Heart | 40 | Allogeneic UC | Ischemic CM | Phase I/II | IC | NCT02439541 |
| TRIDENT | 40 | Allogeneic BM | Ischemic CM | Phase II | IM | NCT02013674 |
| DREAM HF‐1 | 600 | Allogeneic BM (rexlemestrocel‐L) | Ischemic CM | Phase III | IM | NCT02032004 |
| SEESUPIHD | 64 | Allogeneic UC | Ischemic CM | Phase I/II | IC | NCT02666391 |
| TPAABPIHD | 200 | Autologous BM | Ischemic CM | Phase I/II | NYD | NCT02504437 |
| Maskon et al. | 80 | Autologous BM | Ischemic dilated CM | Phase II | IC | NCT01720888 |
| Harjula et al. | 60 | Autologous BM | Ischemic CM needing CABG | Phase II | IM | NCT00418418 |
| TAC‐HFT‐II | 55 | Autologous BM ± CSC | Ischemic CM | Phase I/II | IM | NCT02503280 |
| TEAM‐AMI | 124 | Autologous BM | Ischemic CM | Phase II | IC | NCT03047772 |
| Nonischemic cardiomyopathy | ||||||
| Hu et al. | 30 | Umbilical cord | Idiopathic dilated CM | Phase I | IM | NCT01219452 |
| Olson et al. | 45 | Allogeneic BM | Anthracycline‐mediated CM | Phase I | IV | NCT02408432 |
| Fernandez‐Avilez et al. | 70 | Autologous BM | Idiopathic dilated CM | Phase I/II | IM | NCT01957826 |
| Bartolucci et al. | 30 | Allogeneic UC | Dilated CM | Phase I/II | IV | NCT01739777 |
Abbreviations: BM, bone marrow; CABG, coronary artery bypass grafting; CM, cardiomyopathy; CSC, cardiac stem cells; IC, intracoronary; IM, intramyocardial; IV, intravenous; LVAD, left ventricular assist device; MI, myocardial infarction; MSC, mesenchymal stem cell; NYD, not yet determined; UC, umbilical cord.
Future Directions
Generating reliable and effective cell‐based therapy for IHD requires optimization of the product, delivery method, and recipient selection. Many preclinical studies have shown benefit of cell modification to enhance their survival, proliferative capacity, and secretion of paracrine factors. These include genetic manipulation, in vitro preconditioning (with hypoxia or with pharmaceutical agents, for example), or pretreatment with growth factors or other cytokines 69. Gene delivery of Akt 29 or haem‐oxygenase 1 (HO‐1) 70 in MSCs prior to transplantation have shown benefit in cell survival, with resulting improvement in rat myocardial function postdelivery. Similarly, transfection of MSCs with anti‐apoptotic genes such as bcl‐2 71, bcl‐xL 72, connexin43 73, and survivin 74 have been found to improved MSC survival in vivo, and result in moderate improvement of LVEF in rats.
Conclusion
While there are many preclinical approaches that have shown promise, there is a great need for larger scale clinical trials showing efficacy. MSC‐based cell therapy, either using the cells themselves or their derived products, offers promise, and may provide more convincing data compared to a more heterogeneous cell population such as BM‐MNCs. Investment into this field is imperative to the development of feasible treatments, and requires engagements from both the public and private sector. Without a manufactured product per se, there are limitations to the generation of a marketable product, but regardless, from a therapeutic point of view, harnessing stem cell biology may hold great promise.
Author Contributions
M.R.W. contributed as the lead author on this review. A.A. was a contributing author. K.A.C. was the senior author. No other authors or writers were involved with this manuscript.
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest for any of the authors.
Acknowledgments
We acknowledge the help of Jean‐Francois Desjardins in the preparation of this manuscript.
References
- 1. Levy D, Kenchaiah S, Larson MG et al. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 2. Dahlöf B, Devereux RB, Kjeldsen SE et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 3. Chow CM, Donovan L, Manuel D et al. Regional variation in self‐reported heart disease prevalence in Canada. Can J Cardiol 2005;21:1265–1271. [PubMed] [Google Scholar]
- 4. Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 2008;358:1370–1380. [DOI] [PubMed] [Google Scholar]
- 5. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013;128:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKelvie RS, Moe GW, Ezekowitz JA et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: Focus on acute and chronic heart failure. Can J Cardiol 2013;29:168–181. [DOI] [PubMed] [Google Scholar]
- 7. Tang ASL, Wells GA, Talajic M et al. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 8. Wells G, Parkash R, Healey JS et al. Cardiac resynchronization therapy: A meta‐analysis of randomized controlled trials. Can Med Assoc J 2011;183:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeung DF, Boom NK, Guo H et al. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. Can Med Assoc J 2012;184:E765–E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 11. Khan I, Ali A, Akhter MA et al. Epac‐Rap1‐activated mesenchymal stem cells improve cardiac function in rat model of myocardial infarction. Cardiovasc Ther 2017;35:e12248. [DOI] [PubMed] [Google Scholar]
- 12. Caplan AI, Correa D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop Res 2011;29:1795–1803. [DOI] [PubMed] [Google Scholar]
- 13. Casado‐Díaz A, Anter J, Dorado G et al. Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J Nutr Biochem 2016;32:151–162. [DOI] [PubMed] [Google Scholar]
- 14. Park S, Choi Y, Jung N et al. Myogenic differentiation potential of human tonsil‐derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med 2016;37:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monfrini M, Donzelli E, Rodriguez‐Menendez V et al. Therapeutic potential of mesenchymal stem cells for the treatment of diabetic peripheral neuropathy. Exp Neurol 2017;288:75–84. [DOI] [PubMed] [Google Scholar]
- 16. Makino S, Fukuda K, Miyoshi S et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999;103:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hafez P, Jose S, Chowdhury SR et al. Cardiomyogenic differentiation of human sternal bone marrow mesenchymal stem cells using a combination of basic fibroblast growth factor and hydrocortisone. Cell Biol Int 2016;40:55–64. [DOI] [PubMed] [Google Scholar]
- 18. Kawada H. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004;104:3581–3587. [DOI] [PubMed] [Google Scholar]
- 19. Hatzistergos KE, Quevedo H, Oskouei BN et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 2010;107:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iso Y, Spees JL, Serrano C et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long‐term engraftment. Biochem Biophys Res Commun 2007;354:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toma C, Pittenger MF, Cahill KS et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93–98. [DOI] [PubMed] [Google Scholar]
- 22. Leiker M, Suzuki G, Iyer VS et al. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 2008;17:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallina C, Turinetto V, Giachino C. A new paradigm in cardiac regeneration: The mesenchymal stem cell secretome. Stem Cells Int 2015;2015:765846–765810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinnaird T. Bone marrow‐derived cells for enhancing collateral development: Mechanisms, animal data, and initial clinical experiences. Circ Res 2004;95:354–363. [DOI] [PubMed] [Google Scholar]
- 25. Kanki S, Segers VFM, Wu W et al. Stromal cell‐derived factor‐1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail 2011;4:509–518. [DOI] [PubMed] [Google Scholar]
- 26. Yu B, Gong M, Wang Y et al. Cardiomyocyte protection by GATA‐4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR‐221 in microvesicles. PLoS One 2013;8:e73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu B, Kim HW, Gong M et al. Exosomes secreted from GATA‐4 overexpressing mesenchymal stem cells serve as a reservoir of anti‐apoptotic microRNAs for cardioprotection. Int J Cardiol 2015;182:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi M, Li TS, Suzuki R et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 2006;291:H886–H893. [DOI] [PubMed] [Google Scholar]
- 29. Noiseux N, Gnecchi M, Lopez‐Ilasaca M et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 2006;14:840–850. [DOI] [PubMed] [Google Scholar]
- 30. Gnecchi M, He H, Liang OD et al. Paracrine action accounts for marked protection of ischemic heart by Akt‐modified mesenchymal stem cells. Nat Med 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Zuo S, He Z et al. Paracrine factors released by GATA‐4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol 2010;299:H1772–H1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Timmers L, Lim SK, Hoefer IE et al. Human mesenchymal stem cell‐conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011;6:206–214. [DOI] [PubMed] [Google Scholar]
- 33. Estrada R, Li N, Sarojini H et al. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol 2009;219:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavoie JR, Rosu‐Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie 2013;95:2212–2221. [DOI] [PubMed] [Google Scholar]
- 35. Nemeth K, Keane‐Myers A, Brown JM et al. Bone marrow stromal cells use TGF‐beta to suppress allergic responses in a mouse model of ragweed‐induced asthma. Proc Natl Acad Sci USA 2010;107:5652–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ortiz LA, DuTreil M, Fattman C et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee RH, Pulin AA, Seo MJ et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell 2009;5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakanishi C, Yamagishi M, Yamahara K et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun 2008;374:11–16. [DOI] [PubMed] [Google Scholar]
- 39. Shabbir A, Zisa D, Suzuki G et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 2009;296:H1888–H1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camussi G, Deregibus MC, Bruno S et al. Exosomes/microvesicles as a mechanism of cell‐to‐cell communication. Kidney Int 2010;78:838–848. [DOI] [PubMed] [Google Scholar]
- 41. Li T, Yan Y, Wang B et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013;22:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bruno S, Grange C, Collino F et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012;7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomasoni S, Longaretti L, Rota C et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev 2013;22:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collino F, Deregibus MC, Bruno S et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010;5:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valadi H, Ekström K, Bossios A et al. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 46. Kosaka N, Iguchi H, Yoshioka Y et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010;285:17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Liu D, Chen X et al. Secreted monocytic miR‐150 enhances targeted endothelial cell migration. Mol Cell 2010;39:133–144. [DOI] [PubMed] [Google Scholar]
- 48. Ranganath SH, Levy O, Inamdar MS et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012;10:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Timmers L, Lim SK, Arslan F et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2007;1:129–137. [DOI] [PubMed] [Google Scholar]
- 50. Lai RC, Arslan F, Lee MM et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 51. Arslan F, Lai RC, Smeets MB et al. Mesenchymal stem cell‐derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- 52. Feng Y, Huang W, Wani M et al. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR‐22. PLoS One 2014;9:e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen S, Liu Z, Tian N et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol 2006;18:552–556. [PubMed] [Google Scholar]
- 54. Katritsis DG, Sotiropoulou P, Giazitzoglou E et al. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace 2007;9:167–171. [DOI] [PubMed] [Google Scholar]
- 55. Hare JM, Traverse JH, Henry TD et al. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Houtgraaf JH, den Dekker WK, van Dalen BM et al. First experience in humans using adipose tissue‐derived regenerative cells in the treatment of patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol 2012;59:539–540. [DOI] [PubMed] [Google Scholar]
- 57. Gao LR, Pei XT, Ding QA et al. A critical challenge: Dosage‐related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol 2013;168:3191–3199. [DOI] [PubMed] [Google Scholar]
- 58. Lee JW, Lee SH, Youn YJ et al. A randomized, open‐label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci 2014;29:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mohyeddin‐Bonab M, Mohamad‐Hassani MR, Alimoghaddam K et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med 2007;10:467–473. [PubMed] [Google Scholar]
- 60. Friis T, Haack‐Sørensen M, Mathiasen AB et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J 2011;45:161–168. [DOI] [PubMed] [Google Scholar]
- 61. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mathiasen AB, Qayyum AA, Jørgensen E et al. Bone marrow‐derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo‐controlled trial (MSC‐HF trial). Eur Heart J 2015;36:1744–1753. [DOI] [PubMed] [Google Scholar]
- 63. Perin EC, Borow KM, Silva GV et al. A phase II dose‐escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res 2015;117:576–584. [DOI] [PubMed] [Google Scholar]
- 64. Heldman AW, DiFede DL, Fishman JE et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC‐HFT randomized trial. JAMA 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karantalis V, DiFede DL, Gerstenblith G et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 2014;114:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen SL, Fang WW, Ye F et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004;94:92–95. [DOI] [PubMed] [Google Scholar]
- 67. Liu B, Duan CY, Luo CF et al. Effectiveness and safety of selected bone marrow stem cells on left ventricular function in patients with acute myocardial infarction: A meta‐analysis of randomized controlled trials. Int J Cardiol 2014;177:764–770. [DOI] [PubMed] [Google Scholar]
- 68. Afzal MR, Samanta A, Shah ZI et al. Adult bone marrow cell therapy for ischemic heart disease: Evidence and insights from randomized controlled trials. Circ Res 2015;117:558–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karpov AA, Udalova DV, Pliss MG et al. Can the outcomes of mesenchymal stem cell‐based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif 2017;50:e12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tsubokawa T, Yagi K, Nakanishi C et al. Impact of anti‐apoptotic and anti‐oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase‐1 on myocardial ischemia. Am J Physiol Heart Circ Physiol 2010;298:H1320–H1329. [DOI] [PubMed] [Google Scholar]
- 71. Li W, Ma N, Ong LL et al. Bcl‐2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 2007;25:2118–2127. [DOI] [PubMed] [Google Scholar]
- 72. Xue X, Liu Y, Zhang J et al. Bcl‐xL genetic modification enhanced the therapeutic efficacy of mesenchymal stem cell transplantation in the treatment of heart infarction. Stem Cells Int 2015;2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang D, Shen W, Zhang F et al. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int 2010;34:415–423. [DOI] [PubMed] [Google Scholar]
- 74. Fan L, Lin C, Zhuo S et al. Transplantation with survivin‐engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail 2009;11:1023–1030. [DOI] [PubMed] [Google Scholar]


