Abstract
Stroke is a major cause of death and long‐term disability, affecting one in six people worldwide. The only currently available approved pharmacological treatment for ischemic stroke is tissue plasminogen activator; however, relatively few patients are eligible for this therapy. We hypothesized that intravenous (IV) infusion of banked unrelated allogeneic umbilical cord blood (UCB) would improve functional outcomes in patients with ischemic stroke. To investigate this, we conducted a phase I open‐label trial to assess the safety and feasibility of a single IV infusion of non‐human leukocyte antigen (HLA) matched, ABO matched, unrelated allogeneic UCB into adult stroke patients. Ten participants with acute middle cerebral artery ischemic stroke were enrolled. UCB units were matched for blood group antigens and race but not HLA, and infused 3–9 days post‐stroke. The adverse event (AE) profile over a 12 month postinfusion period indicated that the treatment was well‐tolerated in these stroke patients, with no serious AEs directly related to the study product. Study participants were also assessed using neurological and functional evaluations, including the modified Rankin Score (mRS) and National Institute of Health Stroke Scale (NIHSS). At 3 months post‐treatment, all participants had improved by at least one grade in mRS (mean 2.8 ± 0.9) and by at least 4 points in NIHSS (mean 5.9 ± 1.4), relative to baseline. Together, these data suggest that a single i.v. dose of allogeneic non‐HLA matched human UCB cells is safe in adults with ischemic stroke, and support the conduct of a randomized, placebo‐controlled phase 2 study. stem cells translational medicine 2018;7:521–529
Keywords: Cellular therapy, Umbilical cord blood, Stem cells, Clinical trials, Cord blood, Human cord blood, Umbilical cord
Significance Statement.
Data from this phase I study suggest that it is safe and feasible to infuse banked, nonhuman leukocyte antigen matched, unrelated allogeneic, umbilical cord blood into adults during the 3–10 day window following an acute ischemic stroke in the middle cerebral artery distribution.
Introduction
Stroke remains a major cause of death and long‐term disability, and is associated with a one in six lifetime risk worldwide. Approximately 795,000 Americans suffer a stroke each year, 140,000 of which are fatal, making stroke a leading cause of death in the United States 1, 2, 3. Although stroke can occur at any age, most (∼75%) occur among individuals over the age of 65, and risk of stroke more than doubles for each decade after the age of 55 1.
The majority (85%) of strokes are ischemic and occur when blood flow to a region of the brain is reduced beyond a critical threshold. Rapid restoration of blood flow to the ischemic penumbra is the most robust predictor of good clinical prognosis after ischemic stroke 2. Following vascular occlusion, a complex chain of events occurs at the molecular level including energy depletion, glutamate‐induced excitotoxicity and calcium overload, loss of transmembrane ionic gradients, and free radical production. Together, these result in neuronal dysfunction and ultimately lead to immediate and irreversible necrotic cell death within the ischemic core 4, 5, 6. In contrast, the tissue surrounding the core, the ischemic penumbra, undergoes delayed programed death 3, suggesting that early intervention may facilitate recovery to this region.
Neuroinflammation plays a significant role in the pathophysiology of stroke. In the healthy brain, microglia, the resident immune cells of the brain, help to maintain tissue integrity and neuronal function by continually surveying the brain for changes in the microenvironment that could upset homeostasis 7. Within minutes following ischemic stroke, microglia become activated, and this activation peaks several days later, and persists for weeks 8, 9. In the setting of acute brain injury, activated microglia may play a paradoxical role, releasing factors which both exacerbate the inflammatory response and secondary neuronal injury, as well as trophic factors that mediate tissue repair and regeneration 10. To this end, Hu et al. showed that reparative microglia populations are more active during the acute phase of stroke and exhibit enhanced phagocytic activity, secrete fewer inflammatory mediators, and promote the survival of cortical neurons. In contrast, a more detrimental microglia phenotype, characterized by reduced phagocytosis and increased secretion of proinflammatory mediators, is more prominent during the subacute and chronic phases of stroke 11. This suggests that maintaining the nuanced balance between protective and toxic microglia phenotypes could benefit recovery following ischemic brain injury.
To date, there is no Food and Drug Administration (FDA)‐approved pharmacological treatment targeting neuroprotection in acute ischemic stroke, and the only approved therapy to promote early reperfusion is i.v. administration of tissue plasminogen activator (tPA) 12. Given the short window for administration post‐stroke and increased risk of bleeding, tPA is used in only about 20% of patients with ischemic stroke, and cannot be used to treat hemorrhagic stroke 13, 14. Recent data also suggest the utility of mechanical reperfusion via endovascular intervention; however, therapy is limited to patients with proximal occlusion and specialized treatment facilities, and has greatest benefit when performed within 6 hours following stroke onset 15, 16, 17, 18, 19. In addition to mechanical thrombectony and tPA 20, 21, decompressive hemicraniectomy in selected patients with malignant cerebral edema within 48 hours after stroke onset has been demonstrated to reduce mortality following ischemic stroke, as has the establishment of specialized stroke care units 22. Numerous clinical trials conducted during the past 2 decades have tested a variety of pharmacological interventions to reduce tissue injury and improve functional outcomes following acute stroke, but their outcomes have not been as promising as desired 23, 24, 25.
More recently, significant efforts have been made to develop cell‐based therapies that improve recovery following ischemic stroke 2, 26, 27, 28. Delivery of exogenous human stem cells into animal models for stroke have shown that stem cells survive, are capable of migration and immunomodulation, secrete trophic factors thought to enhance neurogenesis and angiogenesis, reduce infarct volume, and improve outcomes 29, 30, 31, 32, 33. In particular, cells derived from umbilical cord blood (UCB) confer several advantages and have been widely used for over 2 decades as a blood stem cell donor graft for allogeneic, unrelated donor, hematopoietic stem cell transplantation 28, 34, 35. One major advantage of UCB stem cells is that they are immunologically tolerant, rendering them less reactive to human leukocyte antigen (HLA)‐mismatch than bone marrow or mobilized peripheral blood grafts 36. Furthermore, human UCB is a readily available, cryopreserved, banked product that is a preferable option for medically fragile stroke patients because it does not require collection of autologous cells via bone marrow harvest or peripheral stem cell collection.
To date, numerous phase I/II trials conducted in ischemic stroke patients have reported favorable safety profiles for both autologous stem cells from bone marrow‐derived mononuclear cells and mesenchymal stem cells, as well as with several modified cell lines 37, 38, 39. In the majority of these studies, cells were delivered intravenously, although intra‐arterial (IA), intrathecal, and intracranial routes have also been used 27. Additionally, limited pilot clinical studies support the safety of infusion of both expanded and nonexpanded allogeneic cells derived from bone marrow or umbilical cord in stroke patients who were not pretreated with immunosuppression or myeloablation 27.
UCB cells were used in this study because of their superior immunotolerance and availability for infusion compared with bone marrow cells. We hypothesized that infusion of unrelated donor allogeneic UCB in ischemic stroke patients would improve recovery by downregulating inflammation and promoting neuroprotection and plasticity, as demonstrated by reduced neurological, physical, and functional deficits. The purpose of this phase I study was to investigate if a single IV infusion of non‐HLA matched human unrelated donor UCB cells was safe and feasible in adult participants with normal immune function who had experienced an acute ischemic stroke.
Materials and Methods
Study Design and Overview
Cord blood infusion for adults with ischemic stroke (CoBIS) 1 was a phase I, multisite, open‐label, prospective clinical trial studying the safety and feasibility of a single allogeneic UCB i.v. infusion into 10 adults who had each experienced an ischemic stroke. This study was conducted at Duke University and Houston Methodist Neurological Institute, and UCB was obtained from the Carolinas Cord Blood Bank, Durham, NC or the MD Anderson Cord Blood Bank, Houston, Texas, respectively. The study was approved by each clinical site and cord blood bank's local institutional review board, and registered under investigational new drug (IND) #16274 and http://www.ClinicalTrials.gov identifier NCT03004976.
Participants were treated with a single i.v. infusion of allogeneic human unrelated donor banked UCB cells within a window of 3–10 days following an acute ischemic stroke event. The study was designed such that subjects 1 and 2 were infused days 7–10 post‐stroke; subjects 3–5 were infused on days 5–10 post‐stroke; and subjects 6–10 were infused 3–10 days post‐stroke. There was a 1 month interval between enrollment of subjects 1, 2, and 3 to allow for Data Safety Monitoring Board (DSMB) review. Subsequent subjects (4–10) were enrolled and treated without prescribed hold intervals between subjects, though enrollment was temporarily suspended for DSMB review prior to the enrollment of subject 7 per study protocol. Subjects were assessed clinically by a trained neurologist and were strongly encouraged to participate in rehabilitative therapy. Adverse events (AEs) were documented and reviewed by the study team at scheduled visits and via telephone.
Participants
Eligible patients were male and female adults 18–90 years of age who experienced an acute, cortical ischemic stroke in the middle cerebral artery (MCA) distribution that was verified by a diffusion‐weighted imaging (DWI) abnormality on magnetic resonance imaging (MRI). Patients who received tPA or underwent mechanical reperfusion were eligible to participate, but immunocompromised patients and patients being treated with immunosuppressive drugs were excluded. Patients with a medical history of neurological or orthopedic pathology resulting in a modified Rankin Score (mRS) 40 score >1 prior to stroke, or with a pre‐existing cognitive deficit were ineligible for the study. Additional exclusion criteria included patients with clinically significant hemorrhage associated with the stroke; evidence of midline shift, edema, or mass effect placing patient at increased risk for secondary deterioration; required craniotomy or mechanical ventilation; and serious psychiatric or neurological disease which could alter evaluation on functional or cognitive scales. Subjects were also excluded from the study if they had an active malignancy or autoimmune disease requiring immunosuppressive therapy within 3 years prior to the start of screening (excluding skin cancers other than melanoma), known coagulopathy, or concurrent illness or condition that might interfere with treatment or safety evaluations. A detailed list of inclusion/exclusion criteria is provided in Supporting Information Table S1. In addition, subjects must have had neurological deficit, defined as National Institutes of Health Stroke Scale (NIHSS) 41 scores of 8–15 (right hemisphere) and 8–18 (left hemisphere) at the time of enrollment, with no more than a 4 point increase (worsening of score) over baseline in the 24 hours prior to infusion to receive treatment.
DSMB Review
A DSMB was formed and charter established. Members of the study's DSMB were neurologists and a physician with experience in cell therapy; faculties of participating clinical sites were recused. Reviews were conducted after each of the first three subjects, and after the cohort of subjects 4–6 was treated. The DSMB was notified immediately for all serious AEs directly related to the study product throughout the study. Additionally, a total safety report was prepared and assessed by the DSMB annually.
Selection of UCB Units
UCB units were selected from an accredited United States public cord bank (Carolinas Cord Blood Bank or MD Anderson Cord Blood Bank) based on blood type and race, provided they satisfied the following criteria: (a) a targeted cell dose ranging between 5 × 106 and 5.0 × 107 total nucleated cell count (TNCC) per kg, based on the precryopreservation TNCC, (b) viability ≥85%, (c) sterility cultures that were negative for growth, and (d) maternal infectious disease markers tested on the maternal donor or UCB product that were negative. Screening of maternal blood collected within the 7 days before or after delivery was used for UCB donor infectious disease screening. Maternal testing was performed in a Clinical Laboratory Improvement Amendments certified donor‐testing laboratory and included hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti‐HBc), hepatitis C antibody, human immunodeficiency virus (HIV)‐I and HIV‐2 antibodies, human T cell lymphotropic virus (HTLV)‐I and HTLV‐II antibodies, cytomegalovirus (CMV), and syphilis. Additional screening dependent on the timing of the UCB collection included nucleic acid testing (NAT) for HIV, hepatitis C virus, hepatitis B virus (HBV), and West Nile virus, as well as serological testing for Chagas disease. The donor mother must have screened negative for all tests, except for CMV or anti‐HBc. Units from mothers that tested positive for anti‐HBc but negative for HBsAg and HBV NAT were considered acceptable. Following UCB selection, units were thawed into Dextran 40 (Hospira, Inc., Lake Forest, IL) + 5% human serum albumin (Grifols Biologicals, Inc., Los Angeles, CA), washed on the Sepax 2 RM automated cell processor (Biosafe, Geneva, Switzerland), tested, released, and infused intravenously using common standard operating procedures common to all sites.
Procedures
Allogeneic UCB Infusion
Subjects were not HLA‐matched and did not receive immunosuppressive or myeloablative medications between the time of consent and the infusion. On the day of treatment, UCB was thawed and washed in an automated fashion using a Sepax 2 RM device. The washed product was deposited into a transfer bag on the washing kit (Biosafe, Geneva, Switzerland) and was in 50 ml Dextran 40 and 5% human serum albumin. Thawed UCB units were tested for enumeration of TNCC, viable CD34+ cells, colony forming units, cell viability via trypan blue, confirmatory HLA type, and sterility cultures. A final 50 ml volume of the cellular product was delivered to the participant in the transfer bag using a container validated to maintain 20°C–24°C. Once thawed, UCB units have an expiry of 4 hours at ambient temperature.
Stroke participants were premedicated with diphenhydramine 0.5 mg/kg/dose IV (maximum 50 mg), hydrocortisone 1 mg/kg/dose i.v. (maximum 100 mg), and acetaminophen 10–15 mg/kg (maximum 650 mg) by mouth per os (PO) or per rectum (PR) 30–60 minutes prior to the UCB infusion. Antihypertensive medication was available at the patient's bedside because of the potential risk that hydrocortisone and residual dimethyl sulfoxide in the cell product would elevate blood pressure. A peripheral IV was used to administer allogeneic UCB over a period of 5–30 minutes, at a maximum rate of 5 ml/kg per hour; this was performed under direct physician supervision. Participants received IV hydration of normal saline infused at a minimum of 75 ml/hour for 2–4 hours postinfusion.
Safety Evaluation
Safety was evaluated during the infusion, the first 24 hours postinfusion, and at scheduled visits or telephone calls 30 days (±7 days), 3 months (90 ± 14 days), 6 months (±14 days), and 12 months (±14 days) after treatment.
The day of treatment, urine output was monitored, and vital signs (heart rate, blood pressure, temperature, respiratory rate, oxygen saturation) were reviewed preinfusion, every 5 minutes during the infusion, every 15 minutes for 1 hour postinfusion, every 30 minutes for 2 hours postinfusion, and then hourly until 6 hours postinfusion. Subjects were observed during the infusion, and 6 and 24 hours postinfusion to document AEs and any changes to their functional statuses.
Additional safety monitoring included assessing alloimmunization by direct and indirect Coombs and HLA reactive antibody testing, prior to and 3 months postinfusion. Clinical symptoms for graft‐versus‐host disease (GVHD), infection, and hypersensitivity were monitored at the 3 month visit, and at 6 and 12 months by remote follow‐up. Additional AEs were identified in person at the 3‐month clinic visit, and through phone interviews with participants at 1, 6, and 12 months post‐treatment.
For analysis, verbatim AE terms were mapped onto standard terminology defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and summarized according to severity and relationship to the intervention, as judged by the investigator. For the purposes of this study, grade 1 and 2 AEs were defined as mild, grade 3 AEs were moderate, and grade 4 AEs were severe. No grade 5 AEs (death) occurred. AEs were considered serious if they resulted in any of the following: death, a life‐threatening AE, in‐patient hospitalization or prolongation of existing hospitalization ≥24 hours, a persistent or significant incapacity, substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect.
Clinical Assessments
General physical and neurological examinations were conducted by study team neurologists during screening, the day of treatment (before and after the infusion), and at the 3 month follow‐up visit. Neurological evaluations were also performed so as to assess the impact of treatment: mRS for disability 42, 43 NIHSS for determining neurological impairment 41, and Barthel Index (BI) for activities of daily living 44.
The mRS is a commonly used ordinal scale for measuring the degree of disability or dependence in the daily activities following stroke, and has become the most widely used clinical outcome measure for stroke clinical trials. The mRS is scored from 0 (no symptoms) to 6 (death).
The NIHSS is a validated scoring system to assess the level of neurological impairment following a stroke. It consists of a 15‐item neurologic examination that evaluates the impact of acute cerebral infarction on the levels of consciousness, language, neglect, visual‐field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss. A trained observer rates the subject's ability to answer questions and perform activities. Ratings for each item are scored on 3‐ or 5‐point scales (0 is normal), with an allowance for untestable items. A subject with a completely normal neurological exam will have an NIHSS score of 0. The maximum NIHSS score is 42.
The BI is a standard measure used to assess the disability of stroke patients. The scale consists of 10 items addressing self‐care (feeding, grooming, dressing), ability to use the bathroom, and acts of mobility. A higher score reflects greater independence, with a maximum score of 100.
Neuroimaging
To be eligible, participants were required to have evidence of an MCA ischemia with cortical involvement confirmed by MRI as a DWI abnormality. When the UCB infusion was initiated >24 hours after the baseline brain MRI, a noncontrast head computerized tomography (CT) was obtained within 24 hours prior to infusion, to evaluate for pretreatment hemorrhage, increasing edema, or midline shift. Each institution performed initial (baseline) and 3 month postinfusion brain MRI imaging, using standard stroke imaging protocols and imaging equipment.
Endpoints
The primary endpoint was safety, as determined by the incidence of study‐related AEs and proportion of participants experiencing GVHD during the 12‐month postinfusion period, and the frequency of unexpected complications detected by brain MRIs at 3 months postinfusion. The secondary endpoint was to assess change in neurological function from baseline to 3 months postinfusion.
Results
Participant Characteristics
Ten adult patients (6 Caucasians, 3 African Americans, and 1 American Indian/Native), with a median age of 65.5 years (range 45–79), were enrolled in the CoBIS phase I study between July, 2015 and February, 2016 (Table 1). Both sexes were eligible to participate in this study, but only males elected to enroll. All participants were independent prior to the stroke; 9 had an historic mRS of 0 (no symptoms at all) and 1 participant had a mRS of 1 (no significant disability despite symptoms; able to carry out all usual activities), due to a bilateral below‐the‐knee amputation. At infusion (baseline), six participants had a mRS of 4 (moderate severe disability; unable to walk without assistance and attend to own bodily needs without assistance) and four participants had a mRS of 5 (severe disability; bedridden, incontinent and requiring constant nursing care and attention) (Table 2). The mean NIHSS score at baseline was 11.2 ± 1.6 (range 9–14). The majority of participants had risk factors for stroke including hypertension, hyperlipidemia, diabetes mellitus, and smoking history.
Table 1.
Baseline characteristics of participants and infused autologous cord blood units (n = 10)
| Participant characteristics | |
|---|---|
| Sex, no. (%) | |
| Male | 10 (100.0%) |
| Female | 0 (0.0%) |
| Age, years, median (range) | 65.5 (45–79) |
| Race, no. (%) | |
| White | 6 (60.0%) |
| Black/African American | 3 (30.0%) |
| American Indian/Native American | 1 (10.0%) |
| Ethnicity, no. (%) | |
| Hispanic | 0 (0.0%) |
| Not hispanic | 10 (100.0%) |
| Umbilical cord blood characteristics, median (range) | |
| TNCC infused, × 109 | 1.68 (0.84–2.92) |
| Cell dose infused, × 107/kg | 1.54 (0.83–3.34) |
| Viable CD34+ dose infused, × 105/kg | 2.03 (0.10–6.80) |
Abbreviation: TNCC, total nucleated cell count.
Table 2.
Neurological evaluation results
| Participant | Time of infusion (days post‐stroke) | mRS | NIHSS | BI | |||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | ||
| 1 | 8 | 5 | 4 | 11 | 7 | 5 | 15 |
| 2 | 9 | 4 | 2 | 11 | 6 | 10 | 90 |
| 3 | 6 | 4 | 2 | 11 | 5 | 30 | 85 |
| 4 | 7 | 4 | 2 | 10 | 3 | 20 | 95 |
| 5 | 3 | 4 | 2 | 11 | 3 | 30 | 95 |
| 6 | 9 | 4 | 3 | 10 | 5 | 15 | 90 |
| 7 | 7 | 5 | 4 | 14 | 9 | 10 | 25 |
| 8 | 8 | 5 | 4 | 14 | 8 | 0 | 35 |
| 9 | 7 | 5 | 2 | 11 | 3 | 30 | 80 |
| 10 | 4 | 4 | 3 | 9 | 4 | 35 | 95 |
| Median | 7 | 4 | 2.5 | 11 | 5 | 17.5 | 87.5 |
| Range | 3–9 | 4–5 | 2–4 | 9–14 | 3–9 | 0–35 | 15–95 |
Baseline scores are recorded on the day of infusion before administration of premedications and study product. mRS, modified Rankin Scale; scored 0 (asymptomatic) to 6 (death). NIHSS, National Institutes of Health Stroke Scale; scored 0 (normal) to 42 (highly symptomatic). BI, Barthel Index; scored 0 (highly dependent) to 100 (independent).
Allogeneic UCB Infusion
Allogeneic UCB units were retrieved from two U.S. public banks and screened for sterility and risk of infection or genetic disease transmission as described in the “Materials and Methods” section. All subjects were infused 3–9 days post‐stroke (Table 2). On the day of infusion, clinical laboratory tests and neuroimaging were reviewed prior to infusion. In the event that the UCB infusion was scheduled to be initiated >24 hours after the baseline MRI, a noncontrast CT was obtained prior to the infusion. In addition, a general physical and neurological exam was performed by a study team neurologist before and following infusion. Per protocol, participants with a greater than 4 point increase (worsening) in their NIHSS scores from baseline to 24 hours prior to infusion were ineligible for infusion. All remaining participants eligible for treatment received a single i.v. infusion of allogeneic UCB cells selected to match for ABO/Rh and race but not for HLA, administered 3–10 days post‐stroke. Characteristics of the thawed UCB administered to participants are shown in Table 1. The median TNCC and viable CD34+ cell doses infused were 1.54 × 107 TNCC (range: 0.83–3.34 × 107 TNCC/kg) and 2.03 × 105 CD34+ cells/kg (range: 0.1–6.8 × 105 CD34+ cells/kg), respectively.
Safety
The primary endpoint of this open label phase I trial was safety based on the frequency of study‐related AEs and the proportion of subjects experiencing GVHD during the 12‐month follow‐up period. A total of 113 AEs were reported in 10 participants, with a median of 10.5 events per participant (range: 3–32) (Fig. 1). Sixty‐two events were graded as mild, 42 as moderate, and 9 as severe (Fig. 1). There were no AEs that were determined to be definitely or probably related to investigational treatment, and only one AE that was determined to be possibly related to the investigational treatment: pruritis of moderate severity. This AE was pruritis of moderate severity, was expected, and resolved the same day. Eight serious AEs were reported in two subjects and were determined to be unrelated to study therapy. Six of these occurred in one patient who required hospitalization on five occasions for the treatment of sepsis secondary to a urinary tract infection associated with a chronic indwelling suprapubic urinary catheter, and once for chronic paralytic ileus. The serious adverse event (SAE) incidence rate, defined as the number of participants experiencing an event divided by the number receiving treatment, was 20% (2/10 participants experienced an SAE). There were no reports of subjects experiencing GVHD in the 12 months following treatment. The frequency of AEs according to the CTCAE classification is shown in Figure 2. A summary of AEs per participant is provided as Supporting Information Table S2.
Figure 1.
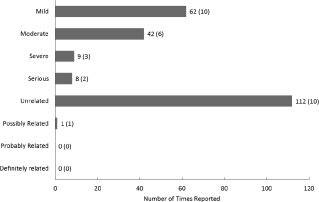
Frequency of adverse events (AEs). AEs classified in terms of severity, seriousness, and relationship to the investigational treatment. A total of 113 AEs were reported; eight of these were also classified as serious adverse events. The only related AE was expected. Bars denote total reported AEs. The number of participants reporting an event is in parentheses. A summary of per participant AEs is included as Supporting Information.
Figure 2.
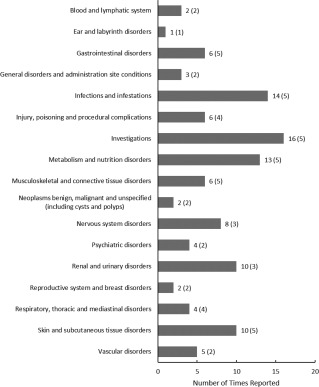
Frequency of adverse events (AEs) according to CTCAE classification. Bars denote total reported AEs. The number of participants reporting an event is in parentheses.
Neurological Outcomes
The mRS is the most widely used clinical outcome measure used by stroke clinical trials to measure the degree of disability or dependence in the daily activities of individuals following a stroke 40. We used the mean change in mRS score from baseline (infusion) to 3 months postinfusion as a secondary endpoint for the study. The median mRS among study participants was 4.4 ± 0.5 at baseline (Table 2). Three months later, all participants had improved at least one grade in mRS relative to baseline (time of infusion) with a mean mRS of 2.8 ± 0.9 (range 1–3 points), indicating improved outcome. Fifty percent of subjects exhibited a 1 grade improvement in mRS, 40% improved by 2 grades, and 10% (1 patient) by 3 grades. The NIHSS was used to measure impairments due to stroke at baseline and 3 months after stroke and similarly demonstrated favorable outcomes from time of infusion (mean 11.2 ± 1.6; range 9–14) to 3 month assessment (mean 5.3 ± 2.2; range 3–9), with improvement by at least 4 points (mean 5.9 ± 1.4; range 4–9) (Table 2). In addition, all participants showed improvement in activities of daily living as indicated by an increase in points (mean 52.0 ± 24.7; range 10–80) from baseline to 3 months using the BI scale. MRIs performed at 3 months postinfusion revealed normal evolution of the stroke with no significant increase in infarct volume, unexpected bleeding, or other safety concerns, when qualitatively compared to baseline scans.
Discussion
This phase I open‐label study investigated the safety of a single IV infusion of banked, non‐HLA matched, unrelated, human, allogeneic UCB in adults following ischemic stroke. A dose of 0.83 × 107 to 3.34 × 107 TNCC/kg UCB was delivered 3–9 days following an ischemic stroke to 10 male participants (median age: 65.5 years, age range: 45–79 years) who were not treated with immunosuppressive or myeloablative medications prior to infusion. The dose needed for a therapeutic effect in stroke is not known and doses were selected that were known to be safe and feasible with one cord blood donor. Participants treated with IV or intra‐arterial (IA) tPA, or those who underwent endovascular reperfusion following stroke were eligible for the study. Safety was assessed at 24 hours and at 3, 6, and 12 months postinfusion. Assessment of AEs over the 12 month period following infusion indicated that UCB was safe and well tolerated by these ischemic stroke patients. The majority (54.8%) of AEs were of mild intensity. One AE, which resolved the same day was possibly related to the cord blood infusion, but was expected. None of the eight SAEs reported, which occurred in only two participants, were related to the infusion. In addition, no incidents of GVHD were observed in any of the participants during the 12 month follow‐up period. Together, these data suggest that UCB is safe and well‐tolerated in ischemic stroke patients.
There is not a consensus in the literature as to the time course for recovery following stroke or the trajectory of recovery for ischemic versus hemorrhagic stroke 45. Most ischemic stroke patients will show the greatest degree of improvement within the first 3 months following stroke, with the greatest rate of recovery occurring during the first month 46, 47, 48. Recovery generally plateaus at 6 months post‐stroke, although functional improvement may continue for over a year. In our phase I CoBIS study, neurological, physical, and functional deficits in participants were evaluated using standard impairment scales.
Although this trial was designed to demonstrate safety and provide preliminary data on change in neurological function after administration of unrelated donor UCB, the open label study design and small patient population precludes making definitive statements regarding benefit. However, of note, the trend toward recovery was greater than described in previously published historical cohorts. For example, Lai and Duncan 48 found that 62% of participants improved at least 1 grade in the mRS at 3 months compared with baseline, whereas 100% of CoBIS 1 participants (n = 10) improved at least one grade, 40% by 2 grades, and 10% (1 patient) by 3 grades (Table 2). This trial's 3 month postinfusion mRS results were better than what would be expected in patients with comparable injury 47. Similarly, all CoBIS 1 participants demonstrated improved NIHSS scores from time of infusion to 3 month assessment, with an improvement of at least 4 points (Table 3). Improvement in activities of daily life as measured by an increase in points on the BI was similarly observed (Table 2). Together, these observations suggest that allogeneic UCB may benefit patients with acute ischemic stroke, although additional studies with concurrent controls are required to test this hypothesis. We observed no interaction between the effects of the cell based intervention and reperfusion therapy (tPA and/or mechanical thrombectomy), although the small sample size of this phase I trial makes this difficult to evaluate. We plan to specifically address this in our phase 2 study.
While the exact pathways by which UCB cells lead to recovery following brain injury have yet to be elucidated, animal models suggest several potential mechanisms. Transplanted cells may migrate to the ischemic area and deliver trophic factors that provide anti‐inflammatory and neuroprotective effects, and improve the potential for host brain cell survival. For example, human UCB cells release brain‐derived neurotrophic factor and vascular endothelial growth factor, which have been shown to play a role in neurogenesis and angiogenesis in rodent models of brain injury 29, 30, 31. These factors may facilitate plasticity of the injured brain by enhancing synaptogenesis, neovascularization, and endogenous repair mechanisms, and by inducing migration and proliferation of endogenous neural stem cells 30.
The optimal delivery route for stem cells in the setting of stroke remains a significant translational issue and evidence is inconclusive as to whether the route of cell transplantation correlates with the extent of recovery 28, 49, 50, 51, 52, 53, 54. In this study, we used an IV route of administration, as this is the least invasive and safest, and preclinical studies have demonstrated functional improvements 29. However, various more invasive routes of delivery have also been reported, including IA, intrathecal, and direct stereotactic parenchymal implantation 29. Several groups comparing IV and IA delivery in preclinical models of stroke observed no differences in functional or structural outcomes between the two 29. Given that the IV infusion of stem cells is safe, easily delivered, and inexpensive compared to other routes of administration, and is advantageous should repeat treatments prove beneficial, we felt it to be the best delivery route for this study.
At the time of this manuscript's preparation, there were a small number of clinical trials being conducted to investigate the use of allogeneic stem cells as a treatment for ischemic stroke 27. However, more clinical studies have been conducted using autologous stem cells in the setting of other brain injuries. In 2010, Yang et al. reported the safety of allogeneic human UCB infusion in patients without prior immunosuppression in a variety of degenerative conditions including paraplegia, multiple sclerosis, amyotrophic lateral sclerosis, and traumatic brain injury 55. Others have demonstrated that infusion of autologous stem cells derived from bone marrow and peripheral blood are safe in participants with acute (1–9 days), subacute (∼10–30 days), and chronic (6–60 months) stroke 56, 57, 58, 59, 60, 61, 62, 63.
In addition to stem cells, modified cell lines and cell products have been investigated as stroke therapies. The first of these studies was conducted in 2000, when human neuronal cells derived from a teratocarcinoma cell line implanted into the infarcts of participants with basal ganglia stroke were shown to be safe, but did not improve outcome 64, 65. Later, it was demonstrated that modified SB623 stromal cells transplanted into the brains of chronic stroke patients were safe and associated with improvement in clinical outcomes at 12 months 38. More recently, the PISCES phase I trial demonstrated that intracerebral implantation of CTX0E03 cells, a genetically modified, immortalized, human neuronal stem cell line, was safe and associated with improved neurological function in participants with chronic stroke 39. In contrast, MultiStem, a proprietary stem cell product derived from mononuclear bone marrow cells, did not improve 90‐day outcomes in the setting of acute stroke, although treatment was associated with lower rates of mortality and life‐threatening SAEs 66, 67.
UCB stem cells confer a number of advantages compared to bone marrow stem cells and cell lines 28. UCB stem cells are biologically closer to embryonic stem cells, offer improved plasticity and faster growth rates, are more immunologically tolerant, and are readily available without the need for invasive procedures in compromised patients. Furthermore, we based this study on the hypothesis that i.v. infusion of allogeneic UCB cells in patients with stroke do not need to durably engraft but rather, to circulate and temporarily survive for sufficient time to alter the paracrine signaling of endogenous cells. Permanent engraftment is not a goal, and as such, myelo‐ and/or immunoablation is not a prerequisite for treatment. Although other groups have reported favorable results following surgical implantation of cells directly into the brain parenchyma, IV infusion of umbilical stem cells in stroke patients offers a less invasive, simpler, and more cost‐effective delivery method. The results from our phase I study suggest that IV infusion of allogeneic UCB is both safe and feasible in patients following acute ischemic stroke, and may offer an alternative therapy outside the narrow window for available reperfusion strategies. Several notable limitations of this study include its small sample size and open‐label, nonrandomized design. Accordingly, we have initiated a larger phase 2 randomized, placebo‐controlled, double‐blind trial to evaluate the ability of allogeneic human UCB to improve functional outcomes in acute ischemic stroke patients.
Conclusion
This phase I trial suggests that IV infusion of non‐HLA matched allogeneic, unrelated donor UCB in adults after acute ischemic stroke is safe, well‐tolerated, and feasible. In addition, improvements in functional outcome were observed in all participants by 3 months postinfusion.
Author Contributions
D.T.L.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; E.R.B.: manuscript writing, collection and/or assembly of data, data analysis and interpretation; D.T.L. and E.R.B.: shared equally in manuscript writing; R.J.D. and M.F.: conception and design; J.J.V., J.R.W., E.S., and J.M.W.: collection and/or assembly of data, data analysis and interpretation; J.T.: data analysis and interpretation, manuscript writing; J.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript' first, followed by D.T.L etc.
Disclosure of Potential Conflicts of Interest
J.K. is Director of the Carolinas Cord Blood Bank and Medical Director of Cord: Use Cord Blood Bank. D.T.L. declared intellectual property rights with Aegis‐CN, LLC and Consultant/Advisory role with Zenith Insurance. M.F. declared research funding support for clinical trial in patients with intracerebral hemorrhage by Nico Corporation, Inc. The other authors indicated no potential conflicts of interest. J.M.W. is Assistant Director—MD Anderson Cord Blood Bank. J.T. is the President of the Board of Directors for the Community Data Roundtable, Consultant/Advisory role The EMMES Corporation, The Community Data Roundtable, AegisCN, received honoraria from GamidaCell and received research funding from Seattle Genetics.
Supporting information
Supporting Information Table 1
Supporting Information Table 2
Supporting Information
Acknowledgments
This work was funded by the Marcus Foundation. We thank the staffs of the Duke Stem Cell Transplant Laboratory, the Carolinas Cord Blood Bank and MD Anderson Cord Blood Bank for the preparation and testing of the cord blood units infused and Jessica Pritchard, Ph.D. for her contributions to the writing of this manuscript. This work would not have been possible without the participation of the participants and their families.
This article was published online on 12 May 2018. This article was authored by a member of the Cord Blood Association and is now indicated as such in the above logo. This notice is included in the online and print versions to indicate that both have been corrected on 27 May 18.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Kalladka D, Muir KW. Brain repair: Cell therapy in stroke. Stem Cells Cloning 2014;7:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feigin VL, Forouzanfar MH, Krishnamurthi R et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White BC, Sullivan JM, DeGracia DJ et al. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J Neurol Sci 2000;179:1–33. [DOI] [PubMed] [Google Scholar]
- 5. Hazell AS. Excitotoxic mechanisms in stroke: An update of concepts and treatment strategies. Neurochem Int 2007;50:941–953. [DOI] [PubMed] [Google Scholar]
- 6. Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med 2008;14:497–500. [DOI] [PubMed] [Google Scholar]
- 7. Patel AR, Ritzel R, McCullough LD et al. Microglia and ischemic stroke: A double‐edged sword. Int J Physiol Pathophysiol Pharmacol 2013;5:73–90. [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol 2013;2013:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denes A, Vidyasagar R, Feng J et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab 2007;27:1941–1953. [DOI] [PubMed] [Google Scholar]
- 10. Prinz M, Priller J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat Rev Neurosci 2014;15:300–312. [DOI] [PubMed] [Google Scholar]
- 11. Hu X, Li P, Guo Y et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012;43:3063–3070. [DOI] [PubMed] [Google Scholar]
- 12. Hacke W, Kaste M, Bluhmki E et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 13.Genentech USA. Full Prescribing Information: Activase (Alteplase) for Injection, for Intravenous Use. South San Francisco, CA: Genentech USA. Available at https://www.activase.com/. Accessed April 17, 2018.
- 14. Demaerschalk BM. Alteplase treatment in acute stroke: Incorporating food and drug administration prescribing information into existing acute stroke management guide. Curr Atheroscler Rep 2016;18:53. [DOI] [PubMed] [Google Scholar]
- 15. Berkhemer OA, Fransen PSS, Beumer D et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 16. Campbell BCV, Mitchell PJ, Kleinig TJ et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 17. Goyal M, Demchuk AM, Menon BK et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 18. Jovin TG, Chamorro A, Cobo E et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 19. Saver JL, Goyal M, Bonafe A et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 20. Brown DA, Wijdicks EFM. Chapter 16 ‐ decompressive craniectomy in acute brain injury In: Wijdicks EFM, Kramer AH, eds. Handbook of Clinical Neurology. Vol 140 Amsterdam, The Netherlands: Elsevier, 2017:299–318. [DOI] [PubMed] [Google Scholar]
- 21. Taylor B, Lopresti M, Appelboom G et al. Hemicraniectomy for malignant middle cerebral artery territory infarction: An updated review. J Neurosurg Sci 2015;59:73–78. [PubMed] [Google Scholar]
- 22. Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013. doi: 10.1002/14651858.CD000197.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veltkamp R, Gill D. Clinical trials of immunomodulation in ischemic stroke. Neurotherapeutics 2016;13:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kidwell CS, Liebeskind DS, Starkman S et al. Trends in acute ischemic stroke trials through the 20th century. Stroke 2001;32:1349–1359. [DOI] [PubMed] [Google Scholar]
- 25. Chollet F, Cramer SC, Stinear C et al. Pharmacological therapies in post stroke recovery: Recommendations for future clinical trials. J Neurol 2014;261:1461–1468. [DOI] [PubMed] [Google Scholar]
- 26. Gervois P, Wolfs E, Ratajczak J et al. Stem cell‐based therapies for ischemic stroke: Preclinical results and the potential of imaging‐assisted evaluation of donor cell fate and mechanisms of brain regeneration. Med Res Rev 2016;36:1080–1126. [DOI] [PubMed] [Google Scholar]
- 27. Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurg Focus 2016;40:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun JM, Kurtzberg J. Cord blood for brain injury. Cytotherapy 2015;17:775–785. [DOI] [PubMed] [Google Scholar]
- 29. Dulamea AO. The potential use of mesenchymal stem cells in stroke therapy–from bench to bedside. J Neurol Sci 2015;352:1–11. [DOI] [PubMed] [Google Scholar]
- 30. Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: A critical review. J Comp Neurol 2009;515:125–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vendrame M, Cassady J, Newcomb J et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose‐dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004;35:2390–2395. [DOI] [PubMed] [Google Scholar]
- 32. Willing AE, Lixian J, Milliken M et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res 2003;73:296–307. [DOI] [PubMed] [Google Scholar]
- 33. Nan Z, Grande A, Sanberg CD et al. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann NY Acad Sci 2005;1049:84–96. [DOI] [PubMed] [Google Scholar]
- 34. Zhou H, Chang S, Rao M. Human cord blood applications in cell therapy: Looking back and look ahead. Expert Opin Biol Ther 2012;12:1059–1066. [DOI] [PubMed] [Google Scholar]
- 35. Chen N, Newcomb J, Garbuzova‐Davis S et al. Human umbilical cord blood cells have trophic effects on young and aging hippocampal neurons in vitro. Aging Dis 2010;1:173–190. [PMC free article] [PubMed] [Google Scholar]
- 36. Kim YJ, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol 2011;79:112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeong H, Yim HW, Cho YS et al. Efficacy and safety of stem cell therapies for patients with stroke: A systematic review and single arm meta‐analysis. Int J Stem Cells 2014;7:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinberg GK, Kondziolka D, Wechsler LR et al. Clinical outcomes of transplanted modified bone marrow‐derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalladka D, Sinden J, Pollock K et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase I, first‐in‐man study. Lancet 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 40. Quinn TJ, Lees KR, Hardemark HG et al. Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials. Stroke 2007;38:2257–2261. [DOI] [PubMed] [Google Scholar]
- 41. Brott T, Adams HP Jr., Olinger CP et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 42. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 2007;38:1091–1096. [DOI] [PubMed] [Google Scholar]
- 43. Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19:1497–1500. [DOI] [PubMed] [Google Scholar]
- 44. Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 45. Kelly PJ, Furie KL, Shafqat S et al. Functional recovery following rehabilitation after hemorrhagic and ischemic stroke. Arch Phys Med Rehabil 2003;84:968–972. [DOI] [PubMed] [Google Scholar]
- 46. Kelly‐Hayes M, Wolf PA, Kase CS et al. Time course of functional recovery after stroke: The Framingham Study. J Neurol Rehabil 1989;3:65–70. [Google Scholar]
- 47. Hankey GJ, Spiesser J, Hakimi Z et al. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007;68:1583–1587. [DOI] [PubMed] [Google Scholar]
- 48. Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin assessment. Neuroepidemiology 2001;20:26–30. [DOI] [PubMed] [Google Scholar]
- 49. Janowski M, Walczak P, Date I. Intravenous route of cell delivery for treatment of neurological disorders: A meta‐analysis of preclinical results. Stem Cells Dev 2010;19:5–16. [DOI] [PubMed] [Google Scholar]
- 50. George PM, Steinberg GK. Novel stroke therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 2015;87:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei N, Yu SP, Gu X et al. Delayed intranasal delivery of hypoxic‐preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant 2013;22:977–991. [DOI] [PubMed] [Google Scholar]
- 52. Reitz M, Demestre M, Sedlacik J et al. Intranasal delivery of neural stem/progenitor cells: A noninvasive passage to target intracerebral glioma. Stem Cells Translational Medicine 2012;1:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iihoshi S, Honmou O, Houkin K et al. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res 2004;1007:1–9. [DOI] [PubMed] [Google Scholar]
- 54. Acosta SA, Tajiri N, Hoover J et al. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 2015;46:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang WZ, Zhang Y, Wu F et al. Safety evaluation of allogeneic umbilical cord blood mononuclear cell therapy for degenerative conditions. J Transl Med 2010;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Banerjee S, Bentley P, Hamady M et al. Intra‐arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Translational Medicine 2014;3:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moniche F, Gonzalez A, Gonzalez‐Marcos JR et al. Intra‐arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012;43:2242–2244. [DOI] [PubMed] [Google Scholar]
- 58. Savitz SI, Mattle HP. Advances in stroke: Emerging therapies. Stroke 2013;44:314–315. [DOI] [PubMed] [Google Scholar]
- 59. Vahidy FS, Alderman S, Savitz SI. Challenges enrolling patients with acute ischemic stroke into cell therapy trials. Stem Cells Dev 2013;22:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taguchi A, Sakai C, Soma T et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev 2015;24:2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borlongan CV. Bone marrow stem cell mobilization in stroke: A 'bonehead' may be good after all! Leukemia 2011;25:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Prasad K, Sharma A, Garg A et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014;45:3618–3624. [DOI] [PubMed] [Google Scholar]
- 63. Prasad K, Mohanty S, Bhatia R et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Ind J Med Res 2012;136:221–228. [PMC free article] [PubMed] [Google Scholar]
- 64. Kondziolka D, Wechsler L, Goldstein S et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000;55:565–569. [DOI] [PubMed] [Google Scholar]
- 65. Kondziolka D, Steinberg GK, Wechsler L et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J Neurosurg 2005;103:38–45. [DOI] [PubMed] [Google Scholar]
- 66. Mays R, Deans R. Adult adherent cell therapy for ischemic stroke: Clinical results and development experience using MultiStem. Transfusion 2016;56:6S–8S. [DOI] [PubMed] [Google Scholar]
- 67. Hess DC, Sila CA, Furlan AJ et al. A double‐blind placebo‐controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke 2014;9:381–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1
Supporting Information Table 2
Supporting Information


