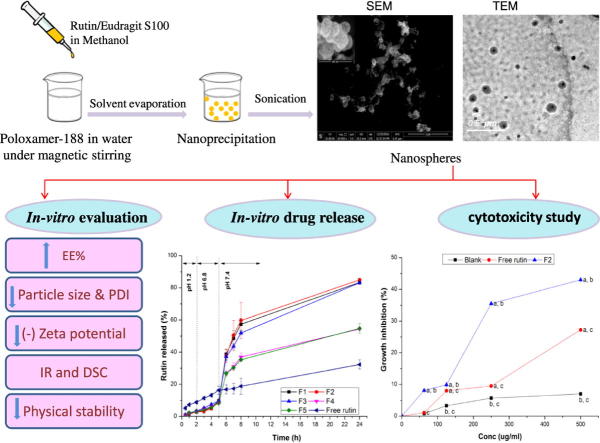
Graphical abstract
Keywords: Rutin, pH sensitive nanospheres, Colon targeting, Cytotoxicity, Anti-cancer, HCT-116 cell line
Abstract
The objective of this study was to target rutin, in a more solubilized form, to the colon aiming at treatment of colon carcinoma. pH sensitive nanospheres were prepared by the nanoprecipitation technique employing Eudragit S100. Different drug: polymer ratios as well as different concentrations of the stabilizer Poloxamer-188 were used. The developed rutin nanospheres exhibited entrapment efficiency ranging from 94.19% to 98.1%, with a zeta potential values <−20 mV. They were spherical in shape and their sizes were in the nanometric dimensions. The in vitro release study of nanospheres formulations revealed enhancement of aqueous solubility of rutin and indicated drug targeting to the colon. The selected formulations were stable after storage for 6 months at ambient room and refrigeration temperatures. In vitro cytotoxic study was conducted on human colon cancer (HCT-116) as well as normal human fibroblasts (BHK) cell lines, employing Sulphorhodamine-B assay. Rutin nanospheres showed significantly (P = .001) higher area under inhibition percentage curve, when compared to free drug, revealing more than 2-fold increase in rutin cytotoxic activity. These results reveal that Eudragit S100 nanospheres could be a potential drug delivery system to the colon with enhanced solubility and hence improved the cytotoxic activity of rutin.
Introduction
Nanoparticles term is generally used for solid colloidal particles having a size ranging from 1 to 1000 nm. The term polymeric nanoparticles is generally given for any type of polymer nanoparticle, especially for nanospheres and nanocapsules [1]. Nanocapsules are systems in which the drug is confined to a cavity consisting of a liquid core (oil or water) surrounded by a solid material shell, while nanospheres are matrix particles in which the entire mass of the particles is solid and the drug is physically and uniformly dispersed [1], [2]. The main goals of nanoparticles formulations as a delivery system are to control surface properties, particle size and release of the drug to achieve the site-specific action of the drug at the optimal rate and dose regimen [2].
Various polymers were employed for the formulation of drug loaded nanoparticles in order to increase its efficacy and minimize its side effects. The nature, surface charge, and properties of the polymers control the formulation parameters, such as drug release and stability [3]. The most frequently methods involved for preparation of nanoparticles fall into two major classes: polymerization of monomers and dispersion of polymers (salting out, emulsification-diffusion and nanoprecipitation) [4]. The nanoprecipitation method, developed by Fessi et al. [5], is the most famous technique that produce small and low polydisperse nanoparticle population. It is a simple and fast method used for the preparation of both nanospheres and nanocapsules. This method, also called solvent displacement method [1], requires two miscible solvents. Briefly, both the drug and polymer should be dissolved in the same solvent (named as the solvent) but not in the other solvent (named as the anti-solvent). Nanoprecipitation takes place by a rapid desolvation of the polymer when the polymer solution is added to the anti-solvent, resulting in precipitation of the polymer, with immediate entrapment of the drug [6]. This technique is basically suitable for hydrophobic drugs due to the miscibility of the solvent with the anti-solvent [7]. Eudragit polymers (polymethacrylate polymers) are widely used in the preparation of polymeric nanoparticles [8]. It is true that, pH-sensitive polymeric nanoparticles are promising for oral drug delivery, especially for peptide/protein drugs as well as poor water soluble drugs [9]. Among several types of Eudragit polymers, Eudragit S100 is pH sensitive anionic copolymers based on methacrylic acid and methyl methacrylate in the ratio 1:2. It does not degrade below pH 7 [10]. In other words, this polymer does not dissolve in stomach and intestinal pH, yet it dissolves in the pH of the colon (pH > 7) due to the ionization of its carboxyl functional groups and consequently the drug can be released in the colon [11]. Eudragit S100 is widely employed for drug targeting to the colon [10], [11], [12], [13], [14] to avoid the rapid dissolution of the drug during the initial passage of nanoparticles through the gastric cavity and upper small intestine. Several reports have provided some profound insights about the potential of pH sensitive delivery system for targeting of therapeutic agents [15], [16], [17].
Colon cancer is the third most common cancer around the world, causing 655,000 deaths globally every year and it is the second leading cause of deaths associated with cancer in the western world [18]. After conventional oral administration, drugs are either absorbed from GIT into systemic circulation, leading to undesired side effect, or degraded in GIT before even reaching colon. To overcome this problem, a colon specific drug delivery approach is required for effective targeting the drug to cancer cells with a lower dose and less systemic side effects. Flavonoids are polyphenolic compounds which belong to a class of phytochemicals characterized by the presence of phenolic ring in their structures. A previous large study demonstrated an inverse relationship between total intake of flavonoid and cancer incidence [19]. Among these compounds, rutin (3-rhamnosyl-glucosylquercetin) exerts in vitro toxic effects on cancer cell lines, including colon cancer cells of human [20], [21] as well as in vivo anti-tumor and anti-angiogenic activities [22]. Rutin exerts its chemo preventive effect on cancer cells by arresting cell cycle and/or apoptosis, as well as inhibition of proliferation, angiogenesis, and/or metastasis in addition to exhibiting anti-inflammatory and/or anti-oxidant effects [23].
It has been reported that rutin has the ability of binding to proteins in the small intestine resulting in its absorption and subsequently its up taking to the systemic circulation [24]. Colonic microbiota has an important role in the hydrolysis of rutin with the release of the aglycon part, namely quercetin which has a protective effect against cancer [25]. The internalization of rutin by human colon adenocarcinoma cell line was reported to take place through its absorption by the basolateral and apical membranes [26]. In the light of these reported findings, rutin has potential advantages to be targeted, by drug delivery systems, to the colon for treatment of colon cancer. Although rutin has anti-cancer activity, but it has not been clinically explored because of its poor solubility [27]. Thus for clinical application of rutin for treatment of colon cancer, it is necessary to deliver rutin intact to the colon by minimizing its absorption through stomach and intestine, in addition to enhancing its aqueous solubility. The main objective of the present work was to prepare and characterize rutin-loaded pH sensitive nanospheres, using Eudragit S100, for developing an oral formulation that can target rutin, in a more solubilized form, to the colon aiming at increasing the drug cytotoxic activity.
Material and methods
Material
Chemicals
Rutin was provided as a kind gift sample from Kahira Pharmaceuticals and Chemical industries Co. (Cairo, Egypt). Eudragit S100 was purchased from Evonik industries (Marl, Germany). Poloxamer-188 and methanol were procured from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemical reagents used in this study were of analytical grade.
Cell culture
Human colon cancer (HCT-116) cell lines as well as normal human fibroblasts (BHK) cell line were supplied by the Cancer cell line special unit, National Cancer Institute (Cairo, Egypt). Roswell Park Memorial Institute (RPMI) 1640 medium, heat-inactivated fetal bovine serum, glutamine, and gentamycin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Methods
Preparation of rutin- loaded Eudragit S100 nanospheres
Eudragit S100 nanospheres were prepared by the nanoprecipitation method adopted by Fessi et al. [5] with slight modification. Briefly, different weight ratios of Eudragit S100 and rutin were weighed accurately, where the net weight was 100 mg. They were then dissolved in a sealed vial containing 2 mL methanol, as a water miscible organic solvent, in an ultrasonic bath (BRANSONIC®, 2510E-DTH, Danbury, USA) for 10 min. This organic phase was added drop wise (0.5 mL/min) into 8 mL distilled water containing different concentrations of Poloxamer-188, used as a stabilizer, under magnetic stirring at 500 rpm. Nanospheres were formed spontaneously, and turned into a milky colloidal dispersion. Stirring process was continued for further 1 h to evaporate the residual organic solvent. Finally, the nanospheres suspension was sonicated in an ultrasonic bath for 30 min to aid size reduction. Composition of different rutin-loaded nanospheres formulations is listed in Table 1.
Table 1.
Composition, EE%, DL% and physico-chemical properties of rutin-loaded Eudragit S100 nanospheres (n = 3; data are expressed as the mean ± SD).
| Formulae code | Drug: polymer (weight ratio)* | Poloxamer-188 (w/v%) | EE% | DL% | Particle size (nm)** | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|---|---|
| F1 | 1:1 | 0.25% | 97.99 ± 0.05 | 49.49 ± 0.01 | 154.90 ± 44.40a,b | 0.31 ± 0.09 | −21.80 ± 4.06 |
| F2 | 1:1 | 0.50% | 98.10 ± 0.50 | 49.52 ± 0.13 | 130.30 ± 35.29a | 0.29 ± 0.10 | −22.90 ± 5.18 |
| F3 | 1:1 | 0.75% | 97.40 ± 0.10 | 49.34 ± 0.02 | 141.30 ± 48.81a | 0.31 ± 0.15 | −25.20 ± 4.29 |
| F4 | 1:2 | 0.25% | 96.23 ± 0.84 | 32.46 ± 0.19 | 207.60 ± 56.60a,b | 0.48 ± 0.01 | −21.70 ± 4.78 |
| F5 | 1:2 | 0.50% | 96.33 ± 0.66 | 32.49 ± 0.15 | 190 ± 46.60a,b | 0.47 ± 0.15 | −22.10 ± 4.38 |
| F6 | 1:2 | 0.75% | 95.85 ± 0.45 | 32.38 ± 0.10 | 350.80 ± 73.17b | 0.46 ± 0.01 | −20.50 ± 4.78 |
| F7 | 1:4 | 0.25% | 94.19 ± 0.33 | 19.06 ± 0.05 | 850 ± 205.70c,d | 0.42 ± 0.11 | −26.70 ± 5.59 |
| F8 | 1:4 | 0.50% | 94.32 ± 0.14 | 19.08 ± 0.02 | 716.20 ± 74c | 0.44 ± 0.08 | −27.30 ± 5.81 |
| F9 | 1:4 | 0.75% | 94.80 ± 0.71 | 19.16 ± 0.11 | 968.6 ± 261.30d | 0.48 ± 0.03 | −26.90 ± 6.34 |
The net weight of the drug and polymer is 100 mg in all the developed formulations.
Means assigned with the same letter are statistically non-significant different, while different letters denote a statistically significant difference between means at P < 0.05.
Characterization of rutin-loaded nanospheres
Estimation of rutin entrapment efficiency (EE%) and drug loading (DL%) percentages
Nanospheres suspension was centrifuged at 10,000 rpm, 4 °C for 40 min [14] using cooling centrifuge (Union 32R, Hanil Co., Gyeonggi-do, Republic of Korea). Nanospheres pellets were then washed three times with distilled water and re-centrifuged. An aliquot from the collected supernatant was filtered through Millipore filter (0.45 um) and was further diluted with methanol. The free drug content was estimated in the filtrate using a UV–vis spectrophotometer (Shimadzu UV–Visible recording spectrophotometer, 2401/PC, Tokyo, Japan) at 257 nm [28]. Amount of entrapped drug was calculated by subtracting the amount of free drug from the total amount of drug added in the formulation. The percentage of drug entrapment, expressed as entrapment efficiency (E.E.%) and drug loading (D.L.%) percentages were calculated according to the following equations:
| (1) |
| (2) |
Particle size, polydispersity index (PDI), and zeta potential determination
The separated and washed nanospheres pellets were re-suspended in 10 mL distilled water and were then appropriately diluted with double distilled water (1:40, v/v). The obtained diluted suspensions were analyzed for particle size and PDI by dynamic light scattering (DLS) using Zeta-Sizer (Malvern, Nano Series ZS90, Malvern Instruments, Ltd., Worcestershire, UK). Zeta potential was estimated using the same instrument. All studies were repeated in triplicate, from three independent samples, at 25 °C.
Transmission electron microscopy (TEM)
A drop of the diluted nanospheres suspension was placed on a carbon-coated copper grid and air-dried at room temperature for 10 min. The sample was subsequently negatively stained with one drop of 1% (w/v) phosphotungstic acid solution applied on the same carbon grid and left to stand for 2 min. The excess of solution was removed with filter paper, before being loaded to TEM (JEOL Co., JEM-2100, Tokyo, Japan).
Scanning electron microscopy (SEM)
Few drops of the diluted nanospheres suspension were placed on a clean glass surface and allowed to be dried overnight in air. The shape and surface morphology of the dried nanoparticles were examined by scanning electron microscopy (QUANTA FEG 250, Oregon, USA).
Fourier transforms infrared (FT-IR) spectroscopy analysis
The chemical integrity and possible chemical interaction between rutin and Eudragit S100 can be estimated by FT-IR analysis using FT-IR spectrophotometer (JASCO 6100, Tokyo, Japan). Rutin, Eudragit S100 as well as the freeze dried nanospheres were mixed separately with KBr and compressed by applying pressure of 200 kg/cm2 for 2 min in hydraulic press to prepare the pellets. Each KBr pellet of the sample was scanned against a blank KBr pellet background at wave number range 4000–400 cm−1.
Differential scanning calorimetry (DSC) analysis
The physical state of the drug inside the nanospheres was assessed by the DSC analysis (Shimadzu DSC-50, Tokyo, Japan) after lyophilization of the investigated nanospheres. The main components of the nanospheres; rutin and the physical mixture (drug: Eudragit S100 1:1, w/w) were also investigated. About 5 mg of each sample was placed separately into a sealed aluminium pan and heated under nitrogen atmosphere from 25 °C to 300 °C with a heating rate of 10 °C/min. An empty aluminium pan was used as the reference pan.
In vitro drug release study
In vitro drug release experiment
In vitro release of rutin, from the selected nanospheres formulations as well as the free drug suspension, was evaluated by dialysis bag diffusion technique using a thermo-stated shaking water bath (Memmert, SV 1422, Schwabach, Germany). The pre-separated and washed rutin-loaded nanospheres pellets of the selected formulations, as well as the free rutin, were re-suspended in distilled water and placed in cellulose dialysis bag (Dialysis tubing cellulose membrane, Sigma Co., USA; Molecular weight cutoff 12,000–14,000) and sealed at both ends. The dialysis bag was immersed in a well closed glass bottle, filled with 100 mL release medium, and maintained at 37 °C ± 0.5 °C with a rotating speed of 100 rpm.
To attain gastrointestinal transit condition, pH of the dissolution medium was changed at various time intervals. Initially, in vitro release was performed in a release media of 0.1 N HCl solution (pH adjusted to 1.2), mimicking the stomach condition for 2 h. The dialysis bag was then transferred to a release media of phosphate buffer solution (pH 6.8), mimicking the intestine condition, for 3 h. Finally, the dialysis bag was immersed in a release media of phosphate buffer solution (pH 7.4), mimicking the colon condition till 24 h [17]. All the release media contain 0.5% (w/v) of Tween 80 to maintain sink condition for rutin [27]. At predetermined time intervals, 2 mL sample was withdrawn and replaced with fresh release medium to assure the sink condition during the experiment. The collected samples were filtered through 0.22 um membrane filter (Millipore), and analyzed spectrophotometrically at λmax 255, 266, and 270 for the release media pH 1.2, 6.8, and 7.4, respectively, using the regression equation of a standard curve developed in the same medium. The cumulative release percentages were calculated as the ratio of the amount of drug released to the initial amount of drug in the dialysis bag, at each time interval, using Microsoft Excel Program (Microsoft Excel 2007). The experiments were repeated in triplicate and the results were represented as mean value ± S.D. The cumulative percentage drug release versus time curves were plotted and the release efficiencies were calculated [29].
Drug release kinetics
In vitro release data were analyzed kinetically to find out the mechanism of drug release from nanospheres. The obtained data was fitted with zero-order, first-order, Higuchi, Hixson-crowell erosion equation, and Korsmeyer-Peppas equation. Linear regression analysis for the release data was done, using Microsoft Excel Program, to determine the proper release model which was assessed on the basis of the regression coefficient (R2). Release model having R2 value close to one was considered as best fit model.
Stability study
Stability study was performed to evaluate the effect of storage conditions on the physicochemical parameters of the selected nanospheres formulations, in order to assess most suitable storage conditions. The selected rutin-loaded nanospheres formulations were stored in a sealed glass vials at ambient room temperature (20–25 °C) and refrigeration temperature (4–8 °C), protected from light, for 6 months. The stored formulations were evaluated for their physical appearance, EE%, particle size, PDI as well as zeta potential and compared to those of the freshly prepared formulations. The percentage of rutin retained was calculated using the following equation:
| (3) |
Formulations showing a high drug retention% (>90%) were considered to be stable [30]. The experiments were performed in triplicate.
In vitro cytotoxicity of rutin-loaded nanospheres
This evaluation took place by comparing the cytotoxic activity of rutin, rutin-loaded nanospheres, and blank nanospheres on human colon cancer (HCT-116) cell lines and on normal human fibroblasts (BHK) cell line as well.
Cell culture
Human colon cancer (HCT-116) cell lines as well as normal human fibroblasts BHK cell line, were maintained at the cancer cell line special unit, National Cancer Institute, and were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine and 50 µg/mL gentamicin in a 37 °C humidified incubator and 5% CO2 atmosphere. Cell viability was assessed by the trypan blue dye exclusion method [31] at the beginning of the experiment and was always greater than 98%.
Sulphorhodamine-B (SRB) assay of cytotoxic activity
Potential cytotoxicity of different samples was tested employing sulphorhodamine-B (SRB) assay [32]. SRB is a bright pink aminoxanthrene dye with two sulphonic groups. It is a protein stain that binds to the amino groups of intracellular proteins under mildly acidic conditions to provide a sensitive index of cellular protein content. Cells were seeded in 96-well microtiter plates at a concentration of (104 cells/well) in a fresh medium and left to attach to the plates for 24 h in 5% CO2 atmosphere at 37 °C. After 24 h, cells were incubated with the appropriate concentration ranges (0, 62.5, 125, 250, 500 µg/mL) of either rutin or rutin-loaded nanospheres suspension, completed to total of 200 µL volume/well using fresh medium and incubation was continued for 48 h at 37 °C and in atmosphere of 5% CO2.The investigated concentration range was selected depending on a previous report on the same assay conducted with rutin and rutin formulations on human colon adenocarcinoma [20]. The same was performed for the blank nanospheres for comparative evaluation. Triplicate wells were prepared for each individual dose.
Following 48 h treatment, the cells were fixed with 50 µL cold 50% trichloroacetic acid for 1 h at 4 °C. Wells were washed 5 times with distilled water; air dried, and then stained for 30 min at room temperature with 50 µL 0.4% SRB stain dissolved in 1% acetic acid. The wells were then washed 4 times with 1% acetic acid. The plates were air-dried and the dye was solubilized with 10 mM tris EDTA (pH 10.5) for 5 min on a shaker (Orbital shaker OS 20, Boeco, Germany) at 1600 rpm. The color intensity was measured spectrophotometrically at 540 nm with an ELISA microplate reader (Meter tech., 960, USA). For each concentration, triplicate wells were prepared. The cytotoxicity was determined as a percentage of the viable treated cells in comparison with the number of viable untreated control cells. The cell viability (survival fraction%) was calculated according to the formula [33]:
| (4) |
Inhibition percentage [1 − (surviving fraction) × 100] was calculated and plotted against drug concentration. Area under growth inhibition percentage versus drug concentration curve was determined employing the trapezoidal rule.
Statistical analysis
Data were represented as mean values ± SD (standard deviation). Statistical analysis was assessed by SPSS software (version 22; IBM Corporation, Armonk, NY, USA). The significance of differences between the mean values was performed by one-way analysis of variance (ANOVA), followed by Fisher’s LSD post-hoc test. Difference at P < 0.05 was considered to be significant.
Results and discussion
Rutin-loaded nanospheres were successfully prepared using nanoprecipitation method. Milky colloidal dispersions were obtained and then characterized by several means.
Characterization of rutin-loaded nanospheres
Entrapment efficiency (EE%) and drug loading (DL%) percentages
Results tabulated in Table 1, revealed that the EE% of rutin was sufficiently high, ranging between 94.19% ± 0.33 and 98.1 ± 0.5. It could be concluded that EE% was high as both polymer and drug have a high affinity to the same solvent. On the other hand, it has been reported that low EE% was revealed when there was high affinity of polymer and drug to the different solvents [8]. The high EE% can be attributed to two factors. First, rutin is poorly water soluble drug and it has high affinity to the same organic solvent in which the polymer is dissolved, thus there is no leakage of the hydrophobic drug to the aqueous phase during preparation. This results in improved entrapment into the polymer matrix, as previously explained for the hydrophobic drugs [34]. The second factor is that there is a possible interaction between the rutin and Eudragit S100, indicating intermolecular hydrogen bond formation, as it will be discussed later. The drug/polymer ratio has no noticeable effect on the entrapment efficiency. Concerning DL%, Table 1 revealed that DL% ranged between 19.06 ± 0.05 and 49.52 ± 0.13.
Particle size, PDI and zeta potential
The particle size is an important parameter where it affects drug release, biodistribution, cellular uptake as well as the stability of the formulations. Larger particles have a high tendency to aggregate compared to smaller ones resulting in sedimentation. From Table 1, it is obvious that, the particle size is significantly increased as drug: polymer ratio increased from 1:1 to 1:4, at the same stabilizer concentration (P = 0.001). The particle size of rutin-loaded Eudragit S100 nanospheres is in nanometric size range (130.3 ± 35.29–350.80 ± 73.17) for the drug/polymer ratio 1:1 and 1:2 (F1–F6). Upon increasing the drug/polymer ratio to 1:4 (F7–F9), the particle size approaches to one micron (716.20 ± 74.29–968.60 ± 261.30 nm). This increase in particle size of nanospheres may be due to increasing viscosity of the polymer organic phase solution which hinders its dispersability into the aqueous phase, resulting in the formation of larger nanodroplets. Similar results have been reported previously [35]. Table 1 also depicted that, increasing concentration of stabilizer (poloxamer-188) from 0.25% to 0.5% led to a relative decrease in particle size, however this decrease is insignificant (P = 0.192–.861) but further increase in poloxamer-188 concentration to 0.75% resulted in a significant increase in particle size (P = 0.02), at the drug: polymer ratio 1:4, however this increase is insignificant (P = 0.121–0.917) for the polymer ratio 1:1 and 1:2. Block copolymer like poloxamer-188 consists of one hydrophobic poly propylene oxide (PPO) block, serving to anchor this macromolecule on the colloid surface, and two hydrophilic poly ethylene oxide (PEO) blocks, which extend into the surrounding liquid, providing a steric repulsion between particles, thus prevents particle aggregation [36]. On the other hand, excess of stabilizer concentration results in an increased interaction between stabilizer molecules, resulted in further adsorption on nanoparticles surfaces and thus formation of multiple layer with increasing in particle size [36]. The same findings, concerning the effect of stabilizer concentration on the particle size of nanoparticles, have been previously reported [13].
The homogeneity of particle size distribution is assessed by polydispersity index (PDI) value. PDI of rutin-loaded Eudragit S100 nanospheres ranged between 0.29 and 0.48, i.e. < 0.5, indicating a narrow size distribution [37]. Formulations (F1-F5) were selected for further studies as they possessed smaller particle sizes ≤ 207.6 nm as well as relatively higher EE%.
All rutin-loaded Eudragit S100 nanospheres showed a negative zeta potential value that ranged between −20.1 ± 4.78 and -27.3 ± 5.81 mV. These results are attributed to the free acrylic acid groups of Eudragit S100, as an anionic polymer [12]. The magnitude of zeta potential indicates the potential stability of colloidal system [38]. Usually, the possibility of particle aggregation is much lower for charged particles with zeta potential >|20| [39], thus all the investigated nanospheres formulations showed a good physical stability.
TEM and SEM
The nanosphere particles were spherical in shape with smooth surfaces (Fig.1). The micrographs also revealed no aggregation of the particles, with particle size in the nano scale, confirming the results obtained from particle size determination. Furthermore, at higher magnification of SEM micrograph, it was depicted that the surface of nanospheres has non homogenous texture confirming that rutin is dispersed in the entire mass of the solid particles.
Fig. 1.
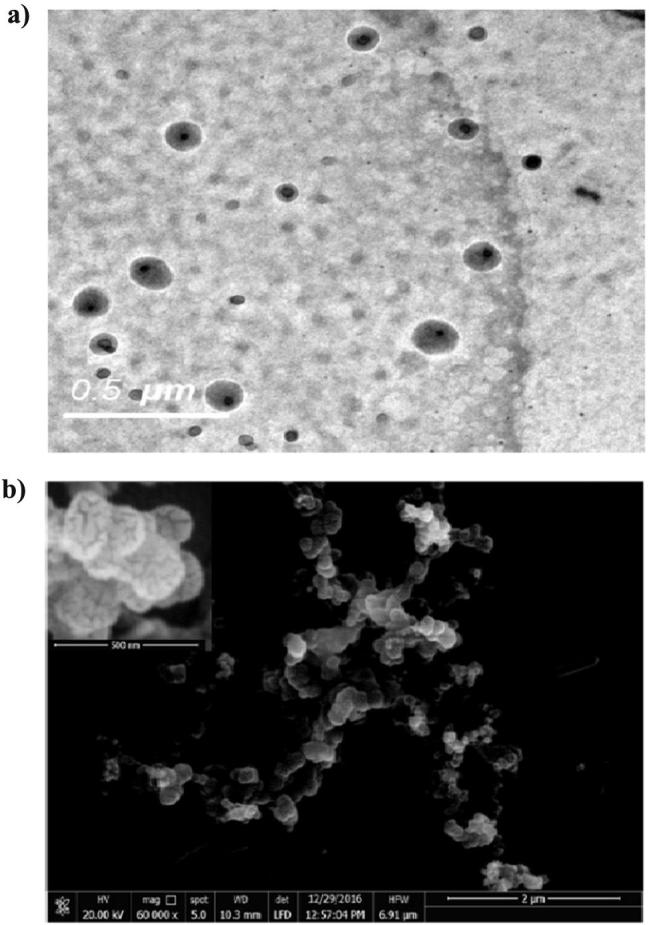
Micrographs of rutin-loaded nanospheres (F2) revealed by TEM (a) and SEM (b). Inset is SEM micrograph with a high magnification power (240,000×).
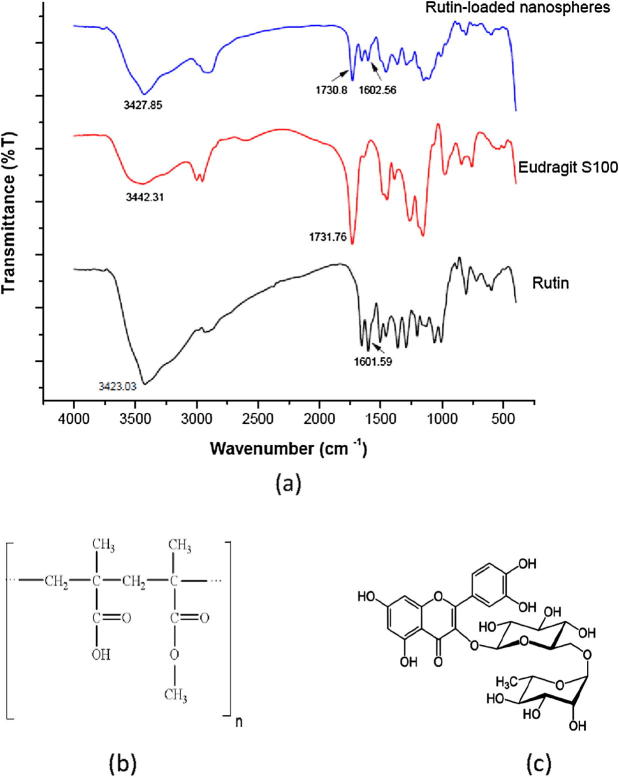
Fourier transforms infrared (FT-IR) spectroscopy
As illustrated in Fig. 2, rutin had characteristic bands observed at 3423.03 cm−1 (OH bonded), 2989.12 cm−1 (C—H stretch), 1655.59 cm−1 (C O stretch) and 1601.59 cm−1 (aromatic structure). These peaks were shifted, in the rutin-loaded nanospheres spectrum, to 3427.85 cm−1 (OH bonded), 2909.09 cm−1 (C—H stretch), 1654.62 cm−1 (C O stretch) and 1602.56 cm−1 (aromatic structure). For Eudragit S100, the peak at 3442.31 cm−1 (OH bonded) was shifted to a lower frequency at 3427.85 cm−1 in the spectrum of rutin-loaded nanospheres. This is one of the basic IR characteristics of hydrogen bonds formation [40]. Moreover, the intensity of the peak at 1731.76 cm−1 (C O stretch) of Eudragit S100 was decreased after being incorporated in nanospheres formulation, as indicated in IR spectrum of rutin-loaded nanospheres (1730.8 cm−1). This indicates intermolecular hydrogen bond formation between the drug and Eudragit S100. Concerning rutin, the intensity of the peak at 3423.03 cm−1 (OH bonded), was decreased after being loaded into nanospheres, as depicted in IR spectrum of rutin-loaded nanospheres (3427.85 cm−1). This decrease in peak intensity is due to the intermolecular hydrogen bonding between the drug and Eudragit S100, indicating the chemical stability of the drug inside the nanospheres [8]. These positional as well as morphological changes in the peaks confirms the presence of interaction between the drug and polymer [41]. This can account for the high EE% of rutin into nanospheres. It is worthy to note that, the presence of rutin aromatic structure peak in IR spectra of nanospheres provides additional confirmation for the incorporation of drug into nanospheres since this peak is absent in IR spectrum of Eudragit S100 which lacks the aromatic ring in its structure .
Fig. 2.
FT-IR spectra of rutin, Eudragit S100 and rutin-loaded nanospheres, F2 (a). Chemical structure of Eudragit S100 (b) and rutin (c).
Differential scanning calorimetry (DSC) analysis
DSC is one of the most general methods to assess the drug physical state in the final formulation which can govern the release characteristics of the drug [13]. In addition, DSC is one of the most important methods to assess the physico-chemical interaction between drug and polymer in a formulation [42]. The thermogram of rutin (Fig. 3.) revealed a sharp endothermic peak at 179.64 °C, corresponding to the melting point of rutin. Eudragit S100 thermogram revealed two broad endothermic peaks at 87.13 °C and 226.90 °C. DSC thermogram of rutin-Eudragit S100 physical mixture revealed no shifting in the endothermic melting peak of rutin, where it is appeared at 179.46 °C; this indicates the absence of solid-state interaction between the drug and polymer, this implies the compatibility of Eudragit S100 with rutin.
Fig. 3.
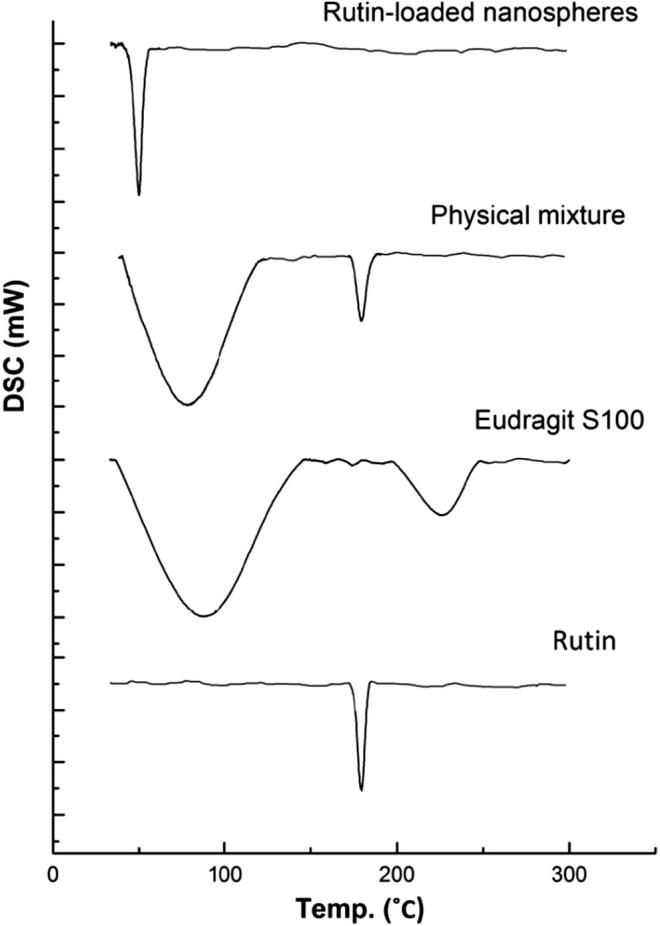
DSC thermograms of rutin, Eudragit S100, physical mixture of rutin with Eudragit S100 (1:1 w/w) and rutin-loaded nanospheres (F2).
The melting endothermic peak of rutin was not detected in the thermogram of rutin-loaded Eudragit S100 nanospheres, indicating the absence of drug in a crystalline state. Thus, it can be concluded that, rutin was present in an amorphous state, after being loaded in Eudragit S100 nanospheres, and could have been dispersed homogenously in the polymer matrix [35]. The sharp endothermic peak that appeared at 49.9 °C, might be the melting peak of poloxamer-188 where it was reported that poloxamer-188 exhibits a melting peak at 55 °C [43]. This can account for the presence of poloxamer-188 at the surface of nanospheres [7].
In vitro drug release study
In vitro drug release experiment
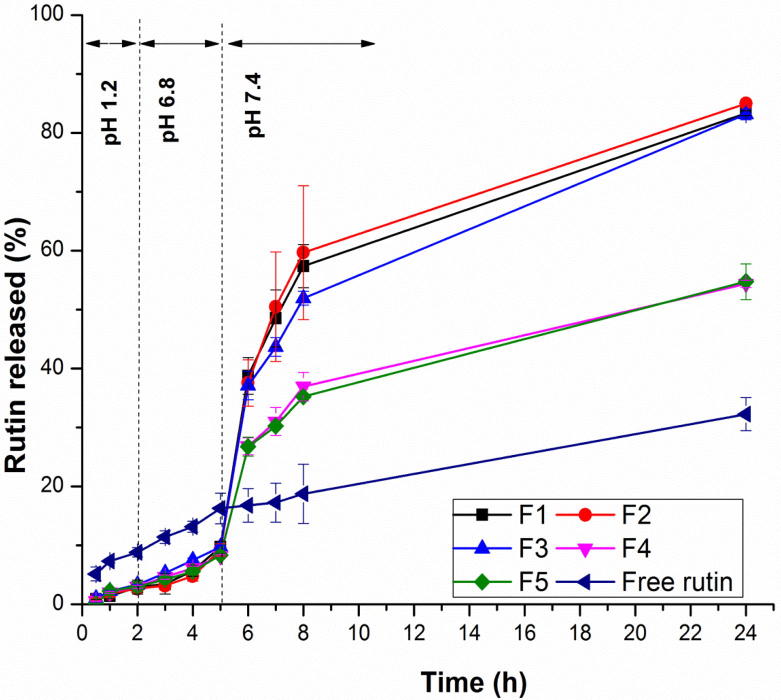
The in vitro drug release was performed to evaluate the potential of the pH- sensitive nanospheres to target rutin to the colon. Rutin-loaded Eudragit S100 nanospheres formulations, namely F1-F5 were selected for in vitro drug release study as they possessed the highest EE% as well as smallest particle size diameter. Fig. 4 and Table 2 revealed that the initial drug release was negligible (less than 3.5%) up to 2 h at pH 1.2, for the nanospheres formulations, indicating that rutin is not released at gastric pH from pH-sensitive nanospheres, compared to the free drug which showed a release of 8.822% at the end of 2 h. The minute amount of the drug released from nanospheres formulations at the end of 2 h may be due to the adsorbed drug on the surface of nanospheres. Only a slight amount of rutin was released, from nanospheres formulations, at pH 6.8 (less than 10%) up to 5 h, compared to that released from the free drug (16.285%). The drug release from nanospheres at pH 6.8 may be due to the pore formation after the polymer swelling [44]. Statistical analysis, by ANOVA, revealed a significant difference (P = 0.001) in the cumulative drug released percentage between the free drug and all of the nanospheres formulations, at both gastric and intestinal pH, while there was insignificant difference among the five formulations themselves (P = 0.056–0.993). On the other hand, a substantial amount of rutin was released, from nanospheres formulations, at the higher colonic pH value of 7.4 because Eudragit S100 is an acrylic polymer i.e. can dissolve rapidly upon de-protonation of carboxylic acid groups at pH > 7. Hence, the drug release profiles of Eudragit S100 nanospheres revealed a significant pH sensitivity [45]. It has been also reported that swelling as well as erosion occurred simultaneously from acrylic Eudragit polymer matrices, upon increasing pH, due to increasing the ionization of methacrylic acid moiety present in Eudragit. This induces electrostatic repulsion forces between Eudragit polymer chains, thus disrupt the matrix and increase both swelling and erosion at higher pH [46]. The drug release from these matrices was related directly to swelling and erosion [46]. Thus, Eudragit S100 has an important role to avoid rutin dissolution during the initial transit of the nanospheres through gastric cavity and the upper small intestine. All nanospheres formulations also revealed a sustained release of rutin up to 24 h at colonic pH.
Fig. 4.
In vitro drug release profile of rutin and various formulations of rutin-loaded nanospheres in gradually pH-changing buffer at 37 °C up to 24 h. Each data point represents mean ± SD (n = 3).
Table 2.
Cumulative amount released (%) of rutin from either free form or various formulations of rutin-loaded nanospheres in gastric, intestinal and colonic pH, at 37 °C (n = 3; data are expressed as the mean ± SD).
| Formula code | Cumulative amount of rutin released (%) |
||
|---|---|---|---|
| Gastric pH (1.2) | Intestinal pH (6.8) | Colonic pH (7.4) | |
| F1 | 2.93 ± 1.17a | 9.72 ± 0.97a | 83.34 ± 0.42a |
| F2 | 2.68 ± 0.61a | 9.26 ± 1.03a | 84.99 ± 0.49a |
| F3 | 3.35 ± 0.03a | 9.75 ± 0.28a | 83.14 ± 0.90a |
| F4 | 3.00 ± 0.24a | 8.22 ± 0.22a | 54.38 ± 0.60b |
| F5 | 3.01 ± 0.88a | 8.36 ± 0.45a | 54.73 ± 3.02b |
| Free rutin | 8.82 ± 0.80b | 16.28 ± 2.59b | 32.27 ± 2.79c |
*Means assigned with the same letter, in the same column are statistically non-significant different, while different letters, in the same column; denote a statistically significant difference between means at P < 0.05.
It is obvious that the release rate of rutin from nanospheres decreased as the polymer concentration increased, where F1- F3 depicted a statistically significant higher cumulative percentage drug released, at colonic pH, compared to both F4 and F5 of higher polymer content (P = 0.001). This may be due to the larger particle size of F4 and F5, resulted from the higher polymer content, this in turn results in reduced surface area available for the drug release [11]. Furthermore, higher content of Eudragit S100 may results in formation of a stringent barrier due to the development of a higher viscous polymeric solution, so it is difficult to the drug to comes out from the formulation [14].
It was observed that the cumulative percentage release of free rutin (32.272%), after 24 h, is significantly lower than that of all nanospheres formulations (P = 0.001), where 83.136–84.986% of rutin released from F1–F3, and 54.382%, 54.735% released from F4 and F5, respectively. These findings can be additionally clarified by comparing the release efficiency of rutin, in the colon, from all the investigated formulations to that released as a free drug; where the release efficiency of rutin released as a free drug (24.20% ± 2.69) was statistically significant lower than that of rutin released from F4 and F5 (42.68% ± 0.73 and 42.03% ± 0.80, respectively) (P = 0.001) which in turn were statistically significant (P = .001) lower than that of rutin released from F1, F2 and F3 (65.61% ± 1.34, 67.36% ± 3.92 and 62.75% ± 0.26, respectively). In the other words the solubility of rutin, released in the colonic pH, was enhanced after entrapping into nanospheres, especially F1-F3, by about 2.5 times. Thus, it can be concluded that pH sensitive nanospheres formulations not only target the entrapped rutin into colon, but also enhance its solubility due to the nanosized drug particles in an amorphous state as indicated by DSC analysis.
Drug release kinetics
Mathematical modeling of the drug release profiles to different kinetic equations indicated that the regression coefficient (R2) for all the investigated formulations was not ideal (0.67–0.94). Thus, it is speculated that there may be more than one mechanism involved in the drug release. Consequently, the obtained data of the drug release profile was fitted into Korsmeyer-Peppas equation, where 60% of release data was incorporated, to find out the (n) value in order to assess the mechanism of drug release. The (n) value was >0.85, for all the investigated formulations, indicating that the release mechanism is super case II release. The same finding was previously reported [11]. Super case II release takes place by simultaneous mechanisms involving diffusion, polymer relaxation (due to swelling) and erosion (due to dissolution), but polymer erosion is the main mechanism involved in the release of the drug [47]. This confirms the fact that the drug release from acrylic polymers is controlled by swelling (polymer relaxation) and erosion of matrix (due to dissolution of polymer) [46], as previously discussed. Therefore, it was concluded that pH sensitive nanospheres were able to protect the drug from being released before reaching the colon, indicating good potential for site specific controlled drug delivery to the colon.
Stability study
F1, F2 and F3 were selected for stability study as they revealed higher release efficiencies compared to those of F4 and F5. After 6 months storage, deposits formed on the base of container were easily re-dispersed by manual shaking. Neither aggregation nor irregularity was observed during the storage period, this may be due to the presence of surfactant that prevents the agglomeration of the nanoparticle suspension over long storage period [1]. Table 3 depicted a high rutin retained% at both storage conditions, where its value ranged between 94.63% ± 7.09 and 98.49% ± 0.59, this may be due to the high affinity of rutin to Eudragit S100. Hence, the investigated formulations were stable at both storage conditions as the drug retention% value >90% [30]. Statistical analysis revealed insignificant reduction (P = 0.161–0.357) in rutin retained% value at ambient room temperature, compared to that at refrigeration temperature. Table 3 also depicted that larger particles, for F1 and F3, were significantly (P = 0.008–0.045) observed in case of storage at ambient room temperature, compared to those freshly prepared. However, the particle size was still in nanoscale. On the other hand, F2 revealed insignificant (P = 0.276–0.580) increase in particle size at both storage conditions, compared to that freshly prepared. This may be due to the optimum concentration of stabilizer attained in F2 as discussed before. Therefore, F2 was selected to be evaluated for cytotoxic activity.
Table 3.
Stability testing parameters of the optimized nanospheres formulations, stored at different temperatures for 6 months (n = 3; data are expressed as the mean ± SD).
| Formulae code | Temperature | Rutin retained% | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|
| F1 | A | 100 ± 0a | 154.90 ± 44.40a | 0.31 ± 0.09 | −21.80 ± 4.06 |
| B | 95.40 ± 1.37b | 307.80 ± 42.30b | 0.42 ± 0.10 | −22.50 ± 5.90 | |
| C | 96.29 ± 0.6b | 159.20 ± 59.67a | 0.44 ± 0.08 | −20.50 ± 5.65 | |
| F2 | A | 100 ± 0a | 130.30 ± 35.29a | 0.29 ± 0.10 | −22.90 ± 5.18 |
| B | 96.63 ± 2.56b | 176.70 ± 54.35a | 0.33 ± 0.11 | −24.4 ± 4.66 | |
| C | 98.49 ± 0.59a,b | 154.20 ± 48.95a | 0.46 ± 0.07 | −21.1 ± 5.96 | |
| F3 | A | 100 ± 0 a | 141.30 ± 48.81a | 0.31 ± 0.15 | −25.20 ± 4.29 |
| B | 94.63 ± 7.09a | 340.60 ± 128.30b | 0.31 ± 0.13 | −23.9 ± 4.58 | |
| C | 97.82 ± 0.67a | 290.30 ± 113.2b | 0.41 ± 0.12 | −20.00 ± 5.61 | |
A = Freshly prepared formulae.
B = Formulae after storage at ambient room temperature (20–25 °C) for six months.
C = Formulae after storage at refrigeration temperature (4–8 °C) for six month.
*Means assigned with the same letter, in the same column for each formula, are statistically non-significant different while different letters, in the same column for each formula; denote a statistically significant difference between means at P < 0.05.
Considering PDI, the particle size distributions were homogenous after 6 months storage at both storage conditions, as PDI values were less than 0.5 [37], for the three investigated formulations. No significant changes in zeta potential values were observed after 6 months storage at both storage conditions, for all investigated formulations, compared to the freshly prepared ones, proving good stability of the nanospheres formulations.
In vitro cytotoxicity of rutin-loaded nanospheres
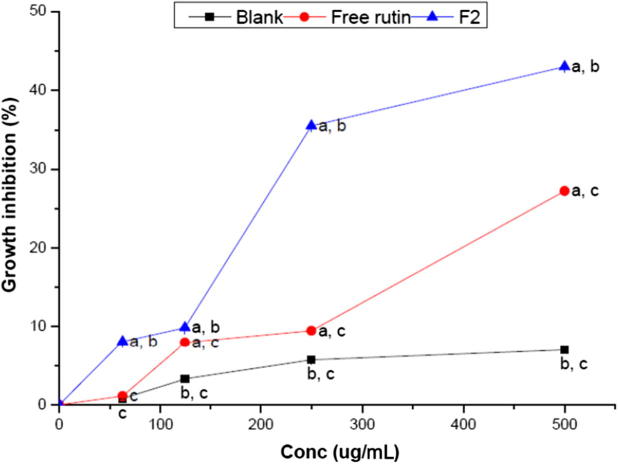
The cytotoxic activity of rutin, rutin-loaded nanospheres (F2) and blank nanospheres on the human colon cancer HCT-116 cell line, as well as on normal human fibroblasts BHK cell line, was assessed with SRB assay, after 48 h of incubation. The results revealed that there was no cytotoxic effect of any of the investigated groups against normal human cell lines. Considering rutin-loaded nanospheres, they showed insignificant effect (P = 0.178–0.980) on the proliferation of normal cells in a dose–dependent manner (Fig. 5 and Table 4), indicating the safety of the components of developed rutin-loaded nanospheres on normal cells. This is in a good agreement with that reported by Yoo et al. [48], where Eudragit S100 had no self cytotoxic effect on normal cell line. On the other side, rutin nanosphere exhibited cytotoxic activity on human colon cancer cells, revealed in the same fig. and table, where increasing its concentration led to a significant (P = 0.001) decrease in the cell viability%. Fig. 6 and Table 5 revealed that the free rutin, exhibited a low anti-cancer activity (growth inhibition percentage was less than 30% at highest concentration investigated). This could be attributed to the poor water solublity of rutin (12.5 mg/100 mL of water) compound, thus the non-encapsulated rutin was not completely dissolved in the culture medium that is composed of water as the main compartment. Hence, rutin revealed very low cytotoxic activity. These results comes in accordance with previously reported study [20]. Considering blank nanospheres, the results revealed that it had a negligible effect.
Fig. 5.
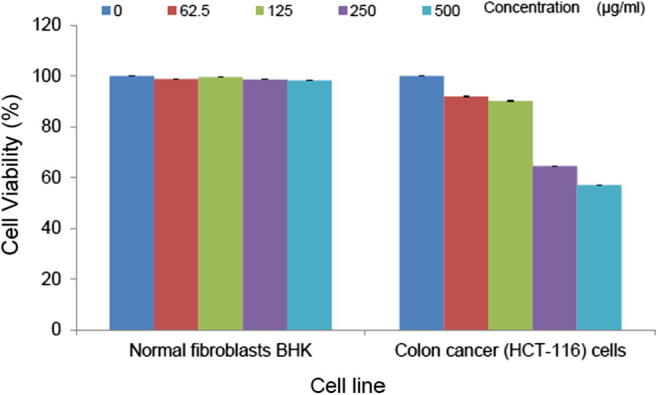
Cytotoxic effect of rutin-loaded nanospheres on normal human fibroblasts (BHK) and on human colon cancer (HCT-116) cell lines. Cell viability at the indicated concentrations of rutin nanospheres was performed employing sulphorhodamine-B assay. Each data point represents mean ± SD (n = 3).
Table 4.
Cytotoxic effect of rutin-loaded nanospheres (F2) on normal human fibroblasts (BHK) and on human colon cancer (HCT-116) cell lines. Cell viability at the indicated concentrations of rutin nanospheres was performed employing sulphorhodamine-B assay. Each data point represents mean ± SD (n = 3).
| Conc (ug/ml) | Normal fibroblasts BHK viability (% ± SD) | Colon cancer (HCT-116) cells viability (% ± SD) |
|---|---|---|
| 0 | 100 ± 0.03a | 100 ± 0a |
| 62.5 | 99.81 ± 0.05a | 91.9 ± 0.11b |
| 125 | 99.9 ± 0.02a | 90.1 ± 0.10c |
| 250 | 99.83 ± 0.14a | 64.5 ± 0.12d |
| 500 | 99.82 ± 0.15a | 57.03 ± 0.15e |
*Means assigned with the same letter, in the same column are statistically non-significant different, while different letters, in the same column; denote a statistically significant difference between means at P < 0.05.
Fig. 6.
Growth inhibition percentage of rutin, selected rutin-loaded nanospheres (F2) and blank nanospheres suspensions against colon cancer (HCT-116) cell line, as indicated by sulphorhodamine-B assay. Each data point represents mean ± SD (n = 3). a: significantly different (P < 0.05) from blank nanospheres. b: significantly different (P < 0.05) from rutin suspension. c: significantly different (P < 0.05) from rutin-loaded nanosphere (F2).
Table 5.
Growth inhibition percentage of rutin, selected rutin-loaded nanospheres (F2) and blank nanospheres suspensions against colon cancer (HCT-116) cell line, as indicated by sulphorhodamine-B assay. Each data point represents mean ± SD (n = 3).
| Treatment | Growth inhibition percentage at different rutin concentrations (ug/mL)* |
Area under inhibition percentage versus concentration curve | |||
|---|---|---|---|---|---|
| 62.5 | 125 | 250 | 500 | ||
| Blank** | 0.8 ± 0.20a | 3.3 ± 0.01a | 5.7 ± 0.07a | 6.79 ± 0.14a | 4009 ± 50a |
| Free rutin | 1.1 ± 0.21a | 8.00 ± 0.10b | 9.5 ± 0.13b | 27.23 ± 0.37b | 6004.16 ± 48b |
| F2 | 8.1 ± 0.02b | 9.9 ± 0.10c | 35.5 ± 0.23c | 43 ± 0.44c | 13461.46 ± 33.4c |
Means assigned with the same letter, in the same column are statistically non-significant different, while different letters, in the same column; denote a statistically significant difference between means at P < 0.05.
The volume taken from the blank nanospheres suspension is equal to that taken from F2 suspension.
Rutin-loaded nanospheres showed a significant (P = 0.001) higher growth inhibiting activity against human adenocarcinoma HCT-116 cell line, compared to free rutin and blank nanospheres, at all concentrations investigated. Furthermore, upon comparing area under inhibition percentage versus concentration curves (AUC), we can deduce that rutin loaded nanospheres exhibited statistically higher (P = 0.001) AUC (13461.46 ± 33.4) compared to that of free rutin and blank nanospheres (6004.16 ± 48 and 4009 ± 50, respectively). Thus, loading of rutin into nanospheres led to increasing its cytotoxic activity by more than two folds. The enhancement of rutin-loaded nanospheres growth inhibiting activity could be justified by its ability to reach the cancer cells in an effective concentration when loaded into nanospheres. This can be attributed to the presence of rutin in a more solubilized form. Moreover, poloxamers could result in severe sensitization of multi-drug resistant tumors to different anti-cancer agents by affecting their cellular functions, such as ATP synthesis, mitochondrial respiration, drug efflux transporters, and gene expression [49] .
Conclusions
In the present investigation, rutin-loaded pH sensitive Eudragit S100 nanospheres, were successfully developed using the nanoprecipitation technique. The developed nanospheres possessed suitable physicochemical parameters. The release profile of rutin-loaded nanospheres depicted significant pH sensitivity that can target rutin into the colon, as well as a significant enhanced solubility of the hydrophobic drug rutin. The optimum formula exhibited more than 2-fold increase in cytotoxic activity compared to free drug suspension, employing human colon cancer HCT 116 cell line. Thus, the developed pH sensitive nanospheres could be a potential carrier for colon targeting of rutin, with enhancement of its cytotoxic activity against colon carcinoma. These promising in vitro study results encourage us to perform the biological evaluation of the developed nanospheres. It would be interesting to consider the in vivo study through collaboration with the pharmacological department in a future work.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The authors would deeply thank the Project's Sector at the National Research Centre, Cairo, Egypt for funding this work through the research group project fund number: 11010303.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Rao J.P., Geckeler K.E. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36(7):887–913. [Google Scholar]
- 2.Mohanraj V., Chen Y. Nanoparticles–a review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- 3.Heller J. Biodegradable polymers in controlled drug delivery. Crit Rev Ther Drug Carrier Syst. 1984;1(1):39–90. [PubMed] [Google Scholar]
- 4.Galindo-Rodriguez S., Allemann E., Fessi H., Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21(8):1428–1439. doi: 10.1023/b:pham.0000036917.75634.be. [DOI] [PubMed] [Google Scholar]
- 5.Fessi H., Puisieux F., Devissaguet J.P., Ammoury N., Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–R4. [Google Scholar]
- 6.Bilati U., Allemann E., Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24(1):67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Yadav S.K., Mishra S., Mishra B. Eudragit-based nanosuspension of poorly water-soluble drug: formulation and in vitro-in vivo evaluation. AAPS Pharm Sci Tech. 2012;13(4):1031–1044. doi: 10.1208/s12249-012-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jana U., Mohanty A.K., Manna P.K., Mohanta G.P. Preparation and characterization of nebivolol nanoparticles using Eudragit® RS 100. Colloids Surf B Biointerf. 2014;113:269–275. doi: 10.1016/j.colsurfb.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang X.Q., Zhang Q. PH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 2012;82(2):219–229. doi: 10.1016/j.ejpb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Chawla A., Sharma P., Pawar P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: formulation, optimization and in vitro evaluation. Acta Pharm. 2012;62(4):529–545. doi: 10.2478/v10007-012-0034-x. [DOI] [PubMed] [Google Scholar]
- 11.Madhavi M., Madhavi K., Jithan A.V. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J Pharm Bioallied Sci. 2012;4(2):164–171. doi: 10.4103/0975-7406.94825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subudhi M.B., Jain A., Jain A., Hurkat P., Shilpi S., Gulbake A., et al. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-Fluorouracil. Materials. 2015;8(3):832–849. doi: 10.3390/ma8030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prajakta D., Ratnesh J., Chandan K., Suresh S., Grace S., Meera V., et al. Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol. 2009;5(5):445–455. doi: 10.1166/jbn.2009.1038. [DOI] [PubMed] [Google Scholar]
- 14.Pandey S., Swamy S.M.V.I., Bhandari A., Koli A., Gupta A., Yadav J.S. Design development and statistical optimization of capecitabine loaded pH sensitive nanoparticle for colon targeted delivery: cell line study. Int J Pharm Res. 2015;7(4):71–79. [Google Scholar]
- 15.Karimi M., Eslami M., Sahandi-Zangabad P., Mirab F., Farajisafiloo N., Shafaei Z., et al. PH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(5):696–716. doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv Y., Hao L., Hu W., Ran Y., Bai Y., Zhang L. Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting. Sci Rep. 2016;6:29321. doi: 10.1038/srep29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makhlof A., Tozuka Y., Takeuchi H. PH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm. 2009;72(1):1–8. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Dev R.K., Bali V., Pathak K. Novel microbially triggered colon specific delivery system of 5-Fluorouracil: statistical optimization, in vitro, in vivo, cytotoxic and stability assessment. Int J Pharm. 2011;411(1–2):142–151. doi: 10.1016/j.ijpharm.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 19.Knekt P., Jarvinen R., Seppanen R., Hellovaara M., Teppo L., Pukkala E., et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol. 1997;146(3):223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 20.Jantrawut P., Akazawa H., Ruksiriwanich W. Anti-cancer activity of rutin encapsulated in low methoxyl pectin beads. Int J Pharm Pharm Sci. 2014;6(3):199–202. [Google Scholar]
- 21.Kuntz S., Wenzel U., Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38(3):133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Castro A.J., Domínguez F., García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 2013;44(5):346–351. doi: 10.1016/j.arcmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Araujo J.R., Goncalves P., Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011;31(2):77–87. doi: 10.1016/j.nutres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Carbonaro M., Grant G. Absorption of quercetin and rutin in rat small intestine. Ann Nutr Metab. 2005;49(3):178–182. doi: 10.1159/000086882. [DOI] [PubMed] [Google Scholar]
- 25.Amaretti A., Raimondi S., Leonardi A., Quartieri A., Rossi M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients. 2015;7(4):2788–2800. doi: 10.3390/nu7042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X., Song J., Shi X., Miao S., Li Y., Wen A. Absorption and metabolism characteristics of rutin in Caco-2 cells. Sci World J. 2013;2013:1–8. doi: 10.1155/2013/382350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banjare L., Ghillare N. Development of biocompatible nanoparticles for sustained topical delivery of Rutin. Int J Pharm Biol Arch. 2012;3(2):326–332. [Google Scholar]
- 28.Soni H., Singhai A. Formulation and development of hydrogel based system for effective delivery of rutin. Int J Appl Pharm. 2013;5(1):5–13. [Google Scholar]
- 29.Khan K.A. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 30.Abd El-Alim S.H., Kassem A.A., Basha M. Proniosomes as a novel drug carrier system for buccal delivery of benzocaine. J Drug Del Sci Tech. 2014;24(5):452–458. [Google Scholar]
- 31.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. Appendix 3: Appendix 3B; 2001. http://www.ncbi.nlm.nih.gov/pubmed/18432654. [DOI] [PubMed]
- 32.Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 33.Mineura K., Izumi I., Watanabe K., Kowada M. Enhancement of ACNU cytotoxicity by pretreatment withO 6-methylguanine in ACNU-resistant brain tumors. J Neurooncol. 1994;19(1):51–59. doi: 10.1007/BF01051048. [DOI] [PubMed] [Google Scholar]
- 34.Barichello J.M., Morishita M., Takayama K., Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25(4):471–476. doi: 10.1081/ddc-100102197. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi A., Jana S., Sen K.K. In-vitro release of acyclovir loaded Eudragit RLPO® nanoparticles for sustained drug delivery. Int J Biol Macromol. 2014;67:478–482. doi: 10.1016/j.ijbiomac.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Jain D., Athawale R., Bajaj A., Shrikhande S., Goel P.N., Gude R.P. Studies on stabilization mechanism and stealth effect of poloxamer 188 onto PLGA nanoparticles. Colloids Surf B: Biointerf. 2013;109:59–67. doi: 10.1016/j.colsurfb.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Kassem A.A., Mohsen A.M., Ahmed R.S., Essam T.M. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J Mol Liq. 2016;218:219–232. [Google Scholar]
- 38.Mohsen A.M., Asfour M.H., Salama A.A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug Dev Ind Pharm. 2017;43:2043–2054. doi: 10.1080/03639045.2017.1361968. [DOI] [PubMed] [Google Scholar]
- 39.Aditya N., Shim M., Lee I., Lee Y., Im M.-H., Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem. 2013;61(8):1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W., Dehghani-Sanij A.A., Blackburn R.S. IR study on hydrogen bonding in epoxy resin–silica nanocomposites. Prog Nat Sci. 2008;18(7):801–805. [Google Scholar]
- 41.Kamel R., Basha M. Preparation and in vitro evaluation of rutin nanostructured liquisolid delivery system. Bull Fac Pharm Cairo Univ. 2013;51(2):261–272. [Google Scholar]
- 42.Barzegar-Jalali M., Alaei-Beirami M., Javadzadeh Y., Mohammadi G., Hamidi A., Andalib S., et al. Comparison of physicochemical characteristics and drug release of diclofenac sodium–eudragit® RS100 nanoparticles and solid dispersions. Powder Technol. 2012;219:211–216. [Google Scholar]
- 43.Patel H.R., Patel R.P., Patel M. Poloxamers: a pharmaceutical excipients with therapeutic behaviors. Int J PharmTech Res. 2009;1(2):299–303. [Google Scholar]
- 44.Gaur P.K., Mishra S., Bajpai M. Formulation and evaluation of controlled-release of telmisartan microspheres: in vitro/in vivo study. J Food Drug Anal. 2014;22(4):542–548. doi: 10.1016/j.jfda.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai J., Nagai T., Wang X., Zhang T., Meng M., Zhang Q. PH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int J Pharm. 2004;280(1):229–240. doi: 10.1016/j.ijpharm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Al-Taani B.M., Tashtoush B.M. Effect of microenvironment pH of swellable and erodable buffered matrices on the release characteristics of diclofenac sodium. AAPS Pharm Sci Tech. 2003;4(3):110–115. doi: 10.1208/pt040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asghar L.F.A., Chandran S. Design and evaluation of matrix base with sigmoidal release profile for colon-specific delivery using a combination of Eudragit and non-ionic cellulose ether polymers. Drug Deliv Transl Res. 2011;1(2):132–146. doi: 10.1007/s13346-011-0016-4. [DOI] [PubMed] [Google Scholar]
- 48.Yoo J.W., Giri N., Lee C.H. PH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403(1–2):262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54(5):759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]