Abstract
Previous studies have shown that lifestyle modification can successfully prevent or delay development of type 2 diabetes. This trial aimed to test if an underserved, low-income population would engage in a digital diabetes prevention program and successfully achieve lifestyle changes to reduce their risk of type 2 diabetes.
Participants were recruited from three health care facilities serving low-income populations. The inclusion criteria were: a recent blood test indicating prediabetes, body mass index (BMI) > 24 kg/m2, age 18–75 years, not pregnant, not insured, Medicaid insured or Medicaid-eligible, internet or smartphone access, and comfort reading and writing in English or Spanish. A total of 230 participants were enrolled and started the intervention. Participants' average age was 48 years, average BMI = 34.8, average initial HbA1c = 5.8, 81% were female, and 45% were Spanish speaking. Eighty percent had Medicaid insurance, 18% were uninsured, and 2% were insured by a medical safety net plan.
Participants completed a health assessment including measured anthropometrics, HbA1c test, and self-report questionnaires at baseline, 6 and 12 months. The 52-week digital diabetes prevention program included weekly educational curriculum, human health coaching, connected tracking tools, and peer support from a virtual group. Qualitative data on implementation was collected with semi-structured interviews with key informants to understand the barriers, keys to success, and best practices in the adoption of the program within the clinical setting.
This paper describes the study design and methodology of a digital diabetes prevention program and early lessons learned related to recruitment, enrollment, and data collection.
Keywords: Diabetes prevention, Minority health, Clinical trial
1. Introduction
The landmark Diabetes Prevention Program (DPP) trial demonstrated that lifestyle modification to promote healthful dietary intake, increased physical activity, and sustained weight loss is successful and more effective than prescription medication to prevent or delay the onset of Type 2 diabetes [1,2]. The success of the DPP lifestyle intervention in the original trial and the long-term salutary benefits found in the DPP Outcomes Study (DPP-OS) have firmly established the role of behavioral intervention as effective, safe, and sustainable for diabetes prevention [3]. With the preponderance of evidence supporting the DPP, policy efforts are successfully improving provider infrastructure and expanding health insurance coverage to make diabetes prevention more accessible to at-risk populations [4,5]. Translational efforts have disseminated the DPP through various modes of delivery, including in-person groups and online/digital formats. On average, these translational DPP efforts have achieved positive results in replicating the goals of the DPP, and have expanded the reach of lifestyle modification nationwide [6,7]. In particular, technology-enabled and digital versions of the DPP (utilizing remote coach access, internet platforms, telecommunications and smartphone apps) have achieved positive clinical efficacy, with several meta-analyses showing clinically meaningful weight-loss in the range of 3–4 kg [8], and digital programs performing comparable to or better than in-person programs in achieving meaningful weight loss and glycemic control [9,10].
While diabetes prevention efforts continue to proliferate, the incidence of Type 2 diabetes and obesity remain disproportionately higher among Americans from lower income brackets, particularly low-income Americans from underrepresented racial and ethnic groups [11,12]. As with all components of health care, concerted efforts are needed to ensure low-income individuals have equitable access to diabetes prevention programs. In fact, many DPP adaptations targeting low-income communities to better address socioeconomic challenges have been developed and tested, with modest but promising results [[13], [14], [15], [16], [17]]. Persistent limitations to many of the DPP adaptations is the dependency on face-to-face contact, location-based meetings, and time-restricted options for group sessions. Limited flexibility around work schedules, access to reliable transportation, or access to affordable childcare options are reported to pose challenges for potential participants to attend the required in-person DPP sessions [18,19]. People in rural areas face similar access obstacles when locations for DPP group sessions may be logistically prohibitive [20].
To address these logistical and scheduling barriers, remotely delivered DPP solutions using telecommunications and internet delivery present a viable option for hard-to-reach populations. While lower-income Americans continue to lag behind higher income groups in technology adoption and use of technology for health care [21], now over 60% of low-income Americans own a smartphone, and over half own a laptop and have broadband internet access at home [22]. Furthermore, a recent study found that 71% of surveyed low-income patients receiving care through medical safety net services (i.e., subsidized medical care for income limited, uninsured and underinsured individuals) were interested in using digital tools for health care communication [23]. Technology-enabled DPPs are now emerging to serve vulnerable populations with evidence of good engagement levels, improved knowledge, and increased behavioral intentions [24,25].
The purpose of this study is to evaluate a digitally-delivered version of the DPP that was specially adapted for lower-income populations. This evaluation collects both quantitative and qualitative data on the experience of patients utilizing the digital DPP to evaluate clinical outcomes and to better understand the clinic implementation of the program in facilities serving low-income populations. This paper outlines the methods used in conducting the trial and shows the results of preliminary baseline data analysis.
2. Methods
2.1. Study design
The trial is a non-randomized, controlled trial with historical, matched controls serving as the comparison group. A non-randomized design was chosen because sufficient resources were not available for a randomized clinical trial, and because there were no alternative programs available for the targeted population with prediabetes. Investigators were concerned that sites and participating clinicians would be less receptive to refer patients to the study if there was a control arm since some of their patients might not receive any services. An ethical and practical alternative with the resources available was to offer the program to all eligible patients that were identified, and utilize a historical comparison group.
A total of 230 participants were recruited over a 14-month period from three health care facilities serving Medicaid-insured, safety net insured, or uninsured individuals. The three facilities include a federally qualified health center located in Southern California, an outpatient clinic located within a large public teaching hospital in Southern California, and a clinic serving large numbers of Medicaid patients operating within a large, not-for-profit, integrated healthcare network in the state of Washington. All qualified participants enrolled in the trial were offered the digital DPP program. A comparison group was created using the same criteria used to determine study eligibility and was abstracted from de-identified records of patients who did not enroll in the trial. The study protocol was reviewed and approved by Western Institutional Review Board and the Health Sciences Institutional Review Board at University of Southern California.
2.2. Recruitment and eligibility criteria
Participants were initially identified through their electronic health record (EHR) or through referral from their primary care physician. Study coordinators made contact on behalf of the study through secure email, secure text messaging, or in-person meetings in the clinics. Coordinators had private conversations with all identified/referred participants to elicit their interest in participation and ensure that the following eligibility criteria were met: evidence of prediabetes defined by either a fasting blood glucose test of 100–125 mg/dL, glycosylated hemoglobin (HbA1c) test of 5.7–6.4%, or an oral glucose tolerance test result of 140–199 mg/dL performed within 6 months of the EHR review date; age 18–75 years at time of screening; insured through Medicaid, a federally subsidized Affordable Care Act marketplace plan, or uninsured; able to speak and read English or Spanish at a 5th grade level or higher; body mass index (BMI) greater than or equal to 24 kg/m2; able to access and use the internet at least once a week; and able and willing to give informed consent to participate. Potential participants were excluded if they had any of the following: diagnosis of Type 1 or 2 Diabetes; taking insulin, metformin, or other hypoglycemic agent; pregnant or planning to become pregnant during the trial period; currently active/acute medical or psychiatric condition that would preclude program participation (i.e., under treatment for acute myocardial infarction, unstable hypertension); any physical limitation that would preclude unsupervised exercise (e.g., severe bone or joint pain); current or suspected drug or alcohol misuse; or instability in living situation that would preclude full participation.
2.3. Intervention
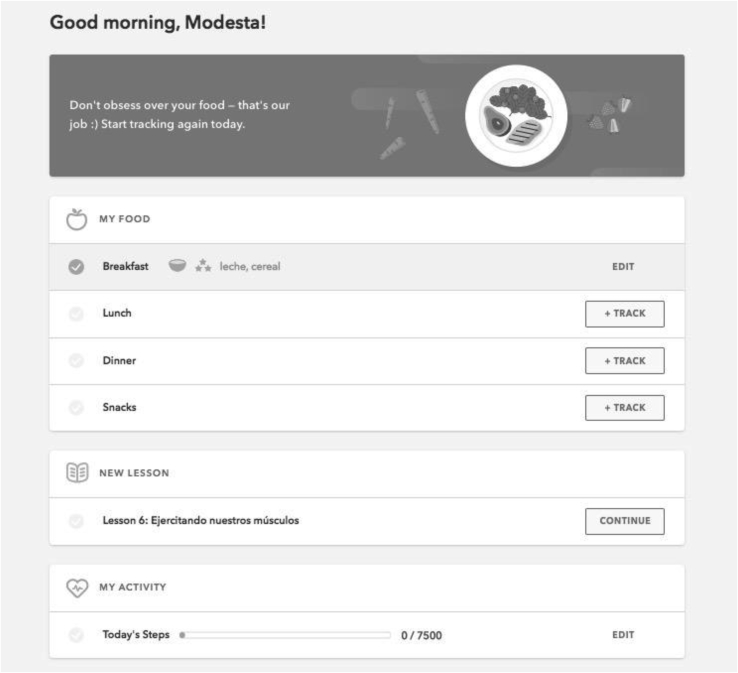
All enrolled participants received a language-and-literacy adapted version of the Omada Health Program. The Omada Health Program is a digital intensive lifestyle intervention that includes virtual group support, personalized health coaching, weekly lessons, and digital tracking tools. The program is a Diabetes Prevention Program (DPP) and is recognized by the Diabetes Prevention Recognition Program (DPRP) [4]. The program is 12 months long, with an initial intensive 16-week phase (considered the “Core” program by DPRP standards) followed by a 36-week maintenance phase. Participants are placed into small virtual groups with peers. They start the program at the same time, and are assigned to the same, live health coach. Each group has a private online social network where they can discuss goals, challenges, progress, and provide social support to one another at any time, similar to a private chat board or discussion board. Users asynchronously complete weekly health education lessons each week. The lessons are available on the digital platform and can be accessed through internet or smartphone. User communicate with their health coach and receive individualized counseling through private messaging on the platform; coaches also facilitate discussions on the group chat board. Users track meals using digital online tracking tools, track weight loss and physical activity using a wireless weight scale and pedometer, and monitor their engagement and weight loss progress. See Fig. 1, Fig. 2 for visual examples of the program.
Fig. 1.
Sample home page of the Omada Health Program.
Fig. 2.
Sample lesson page.
The linguistically adapted version includes all components of the standard digital DPP but has enhanced features for lower-literacy accessibility. In previous iterative development work, the following adaptations were made: 1) revision of the curriculum text and reading content to a 4th-5th grade reading level; 2) cultural adaptions of the curriculum, health coach messaging, recipes and meal plans to be more economically, culturally, and environmentally sensitive, based on feedback from a series of focus groups; and 3) Spanish translation of the curriculum. These adaptations were pilot tested in a sample of 40 participants, and the program was found to be feasible and acceptable to the target audience [26]. Two of the three clinical sites offered the Spanish and English versions of the program, and one site offered only English.
After study eligibility and study enrollment activities were completed, site study coordinators assisted participants in finding the Program enrollment homepage and setting up a user account. Once that step was completed, the program conducted all outreach by email to participants to notify them of their program start date, introduced them to their coach with a link to an online video welcome, and sent emails or app push notifications (when enabled) to alert them when lessons were open for access, or when group/coach communications were posted. Participants could access the program at any time on any internet-enabled device.
2.4. Matched comparison group
A comparison group of patients with similar demographics were selected from each site to be used as historical controls in a matched control design [27]. EHR records were abstracted for patients meeting the following inclusion criteria: (1) prediabetes as defined by fasting blood glucose of 100–125 mg/dL or HbA1c 5.7–6.4% within the past 12 months, (2) age 18 and older, (3) insured by Medicaid, safety net insurance, or uninsured, (4) speaks/reads English or Spanish, (5) BMI of 24 or greater; and the following exclusion criteria (1) evidence of a diagnosis of Type 1 or Type 2 Diabetes, (2) evidence of prescription for insulin, metformin or any other hypoglycemic agent, and (3) evidence of current pregnancy. The comparison group was comprised of patients who were matched to age, gender race/ethnicity, and baseline BMI as closely as possible to the participants receiving the intervention. All records of BMI and blood glucose test results were abstracted for a 12 month period, where available. Sites were allowed to search for the matched control cases within 12 months prior to the start of enrollment or concurrent with enrollment period of the trial. This approach allowed a maximum 24-month window to find a matched comparison group.
2.5. Measurements
After eligibility was confirmed and participants provided informed consent in their preferred language, participants completed a set of clinical and psychosocial measurements with the study coordinator. These measures were only collected on participants receiving the intervention; matched comparator subjects did not complete these measurements.
Body Composition. Height and weight were collected by the study coordinators using calibrated stadiometers and weight scales in the health care clinics; from these data, body mass index is calculated from weight in kilograms divided by height in meters squared (kg/m2). Additionally, once enrolled in the DPP, participants were mailed a wireless weight scale (BodyTrace, Inc., New York, NY) that was linked to their online program account. Participants were encouraged to weigh themselves every day at the same time of day, preferably in the morning before eating or dressing. The scale automatically and securely transmitted weights to the program database using the cellular GSM network. The participant's program starting weight is captured before they began receiving any educational or support content from the program.
Blood Glucose. Glycosylated hemoglobin (HbA1c) was measured in percentage (NGSP/DCCT) units using self-administered AccuBase A1c test kits (DTI laboratories, Thomasville, GA). The kit includes an FDA-cleared, whole blood fingerstick test that uses a capillary tube blood collection method. Study site coordinators dispensed an A1c kit to participants at the baseline, 6 month, and 12 month visits. Participants are not required to fast prior to taking the blood sample for an A1c test, and can give the sample at any time of day. Participants completed the fingerstick blood sample collection independently and mailed the preserved blood samples to a central processing lab for analysis. A central coordinator was notified of the results, who relayed the information to the specific site coordinators.
Blood Pressure and Lipids. One clinical site also collected resting blood pressure and blood lipids measurements on a subsample of participants at all time points. Blood pressure was measured after a brief resting period, in a seated position with both feet on the floor, using an automated blood pressure monitor with arm cuff, with the arm elevated to heart level (Omron, model HEM-712C, Kyoto, Tokyo). Blood lipids were measured using a non-fasting, whole blood fingerstick with a capillary tube, processed with the Cholestech LDX Analyzer (Alere Inc., Waltham, MA). The analyzer meets National Cholesterol Education Program (NCEP) performance goals and is certified by the Centers for Disease Control and Prevention Lipid Standardization Program [28].
Patient-Reported Outcomes. Site study coordinators conducted interviews to collect demographic information at baseline including marital status, household size, gender identity, race/ethnic identity, preferred language, educational status, and current employment status. Measures of psychosocial and environmental factors that may contribute to program efficacy were collected through an interviewer-administered survey (conducted by the site coordinator) prior to the start of the program, and will be re-administered at 6 months and 12 months.
Self-rated health was assessed at baseline and re-assessed at both 6 months and 12 months. The single-item measure prompts readers to rate their health as excellent, very good, good, fair, or poor, and has been shown to generate an accurate reflection of actual health status [29].
Language proficiency in English and Spanish was ascertained with 8 items (4 for English language proficiency, 4 for Spanish) that were taken from U.S. Census and adapted in previous culturally-sensitivity measurement research [30,31]. The items ask the respondent to rate their proficiency in speaking, understanding, reading, and writing in the language of interest. A cutpoint of 3 is recommended to identify people with limited English proficiency [31].
Health literacy was measured with the single item health literacy question [32]. This single-item asks “How confident are you filling out medical forms by yourself?” and users respond on a numeric scale of 1 (extremely) to 5 (not at all), with scores of 3 or higher identifying individuals with inadequate health literacy. This single item has been validated against established health literacy assessment measures [32].
Health care utilization was measured with a scale from the Hispanic Community Health Study/Study of Latinos [33]. Respondents were asked to identify where they received most of their care, whether they encountered a time when they needed care but could not get it, reasons why, and if they could not obtain specific elements of care due to financial constraints.
Program Engagement. It was unclear if lower-income people would be able to easily access a technology-enabled program. Therefore, we captured program usage features to determine if participants were able to utilize all components. The web-supported platform was capable of capturing frequency of user log-ins, number of times they measure weight on a connected scale, number of curriculum lessons completed, use of the food and activity tracking logs, and frequency of communication on the group messaging board and with the health coach. The National DPP has quality metrics anchored on lesson completion [6], and thus, we will be able to benchmark this sample to national norms.
Qualitative Interviews. Qualitative data on implementation were collected with semi-structured interviews with key informants at the clinics to understand the barriers, keys to success, and best practices in the adoption of the program within the clinical setting. Study investigators visited each of the three sites and interviewed key staff including the site's program manager, the enrollment coordinator, and individual providers who referred their patients to the trial. The aim of the semi-structured interviews was to understand the implementation process from the staff's perspectives. The interview questions asked about each site's motivation for participating in the trial, their understanding of the trial, expectations, recruitment strategies, and challenges or obstacles. Some examples of questions asked to probe the staff were: “How well did you understand the study project during its planning phase, that is, leading up to recruitment?”, “How did you try to recruit patients into the program?”, “In your opinion, what was the most successful strategy for recruiting patients?” and “What were the obstacles?” The referring clinicians were also asked about their communication with the study staff, any follow-up with their patients regarding the trial, and their thoughts on whether the program was beneficial for their patients.
Finally, five focus groups of enrollees were conducted, one each at each of the study locations conducted in English and two focus groups in Spanish. To get a representation of participants across the spectrum of program engagement, we recruited participants with 5 or fewer DPP lessons completed, and those with 9 or more lessons completed. The focus groups queried enrollees about the challenges in participating in the program and contributors to successful participation. Qualitative data from focus groups were collected from semi-structured discussion guides by one facilitator and one note taker. The focus groups were audio-recorded and translated to English or translated verbatim. Two reviewers analyzed and coded the transcripts for common and unique themes. The research team reviewed the themes and identified common themes if they surfaced in two or more focus groups. When a theme emerged in only one group, it was identified as a unique theme.
2.6. Sample size calculation
The study was powered to detect a statistically significant, pre-post difference of 3% weight loss in the treated group relative to a 0% weight reduction expected in the comparison group. Based on similar studies with comparable populations [34], we estimated a 4% standard deviation across groups. With alpha = 0.05 and power = 0.8, we estimate a minimal sample size of 40 per group was necessary to detect a 3% difference in weight. At the time the study was conceived (2015), estimated churn rates for Medicaid varied between 20 and 50% [35,36], from which we expected a 30–40% loss-to-follow up rate. Factoring in the potential to analyze subgroups of the sample based on language and sites, we planned to recruit up to 100 participants at the two sites with a bilingual population and 40 at the English-only site, and after adjusting for loss-to-follow-up rate, expect to recruit and retain an analyzable sample size of 200.
3. Results
Study recruitment, screening, and enrollment took place at the three sites between February 2016 and March 2017. Across all sites, and through referrals from clinicians and regular reviews of available information in the EHR (primarily BMI, any lab test results for blood glucose, and age), a total of 273 participants were identified as potentially eligible from the EHR and were approached and screened; of these, 14 were determined to be ineligible for the following reasons: five did not meet the BMI criteria; three were pregnant; two were prescribed metformin for prediabetes, two expressed discomfort reading and writing; one person lacked reliable internet access; and one was already in a weight management program. An additional seven participants declined to continue after the screening interview, stating they were either too busy to participate or no longer interested. Of the remaining 253 that were interested and eligible, 23 did not complete the online registration process for the digital diabetes prevention program. Thus, out of the 273 approached, 230 individuals (84%) were placed in virtual groups assigned a health coach and started the intervention.
Table 1 shows the baseline demographic characteristics of program participants. Overall, the participants were predominantly women in their late-forties. Two-thirds of all participants identified with Hispanic/Latino ethnicity and 61% of all participants were born outside the U.S. Among them, the average number of years living in the U.S was 25.0 (SD = 10.2). Forty six percent of participants endorsed Spanish as their preferred language (71% of Hispanic/Latino participants), and 42% of the sample were identified as having limited English proficiency.
Table 1.
Baseline characteristics.
| Demographic | Participants (n = 230) |
|---|---|
| Female, n (%) | 186 (81%) |
| Average age in years, (SD) | 48 years (12) |
| Racial Identity, n (%) | |
| White (Hispanic or non-Hispanic) | 156 (68%) |
| Black or African-American | 16 (7%) |
| Asian | 11 (5%) |
| More than one race | 11 (5%) |
| Prefer not to answer | 36 (16%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 151 (66%) |
| Not Hispanic or Latino | 69 (30%) |
| Prefer not to answer | 10 (4%) |
| Preferred Language, n (%) | |
| English | 122 (53%) |
| Spanish | 106 (46%) |
| Health Insurance, n (%) | |
| Medicaid or MediCal | 181 (79%) |
| Uninsured | 44 (19%) |
| Safety net program | 5 (2%) |
| Education, n (%) | |
| Less than 8th grade | 46 (20%) |
| Some high school | 41 (18%) |
| High school diploma or GED | 46 (20%) |
| Some college | 60 (26%) |
| 2-year college degree | 11 (5%) |
| 4-year college degree | 17 (7%) |
| Some graduate level education | 6 (3%) |
| Prefer not to answer | 3 (1%) |
| Employment status | |
| Homemaker/Not employed for wages | 75 (33%) |
| Work part-time (<40 h) | 52 (23%) |
| Work full-time (≥40 h) | 66 (29%) |
| Retired | 10 (4%) |
| Unable to work/Disabled | 21 (9%) |
| Prefer not to answer | 6 (3%) |
At baseline, the average finger-stick A1c test result was 5.8% (SD = 0.39). Baseline BMI was 34.53 kg/m2 (SD = 7.86), with an average weight of 199.3 pounds (SD = 55.4) and median weight of 190 pounds. In the subset of 75 participants who completed blood pressure and lipids data collection, 38% had elevated blood pressure (systolic > 129 mmHg, diastolic > 85 mmHg), 36% had elevated total cholesterol (>200 mg/dL), 49% had high density lipoproteins (HDL) in the at-risk range (HDL<50 mg/dL), and 55% had triglycerides in the at-risk range (>150 mg/dL). Only 10% of participants reported self-rated health of Very Good or Excellent, 34% reported it as Good, 45% as Fair, and 11% as Poor.
On the one-item health literacy screener, 27% of the sampled rated themselves a three or higher, indicating inadequate health literacy. However, 23% rated themselves a two or “quite a bit” comfortable, and 49% rated themselves as “extremely” comfortable filling out medical forms on their own. In terms of health care utilization, 99% reporting receiving their health care in the US in the past year, and only 8.5% reported a time when they needed care but could not get it. The top reasons were (1) they couldn't get an appointment for care soon enough, and (2) they could not afford it. When asked about other elements of health care, 28% reported not getting dental care in the past year because they could not afford it, 27% reported not getting prescription glasses due to the cost, and 13% reported not getting prescription medications due to cost.
Table 2 shows participants' use of different technology devices and comfort with computers. The majority of the sample (60%) used a computer at least once a week. Similarly, overall comfort with using computers was high (61% reported feeling very comfortable).
Table 2.
Baseline technology use among program participants.
| Variable | Participants (n = 230) |
|---|---|
| Own mobile phone, n (%) | |
| Yes | 180 (78%) |
| No | 4 (2%) |
| Refused | 46 (20%) |
| Use wearable device, n (%) | |
| Yes | 16 (7%) |
| No | 162 (70%) |
| Refused | 52 (23%) |
| Frequency of computer use, n (%) | |
| Every day | 70 (33%) |
| Almost every day | 37 (17%) |
| About once a week | 22 (10%) |
| Once every few weeks | 19 (9%) |
| Rarely or never | 63 (30%) |
| Comfort with computers, n (%) | |
| Very uncomfortable | 23 (11%) |
| Somewhat uncomfortable | 30 (14%) |
| Very comfortable | 130 (61%) |
| I have never used them | 27 (13%) |
| Refused | 2 (1%) |
A preliminary analysis of the qualitative interviews with key staff and referring clinicians showed that there was enthusiasm for implementing a digital diabetes prevention program because there was lack of available programs for their patient population. From the qualitative interviews, the clinicians stated they welcomed the opportunity to refer patients to the program. However, we found that only a few were responsible for consistently referring patients. In each of the sites, one or two clinicians referred most of the patients to the program. All referring clinicians who were interviewed indicated that it would have been helpful to receive updates about the progress of their patients in the program. The clinicians were not informed and were unaware of which of their patients enrolled in the trial. Because of the lack of communication between the study staff and referring clinicians, some clinicians may have lost incentive to refer their patients to participate after the initial introduction. Many patients expressed to the study coordinators that they were excited to participate because of the opportunity to better manage their health and receive assistance in adopting lifestyle changes to reduce their risk of progressing to type 2 diabetes. The participants also mentioned to coordinators their appreciation for the flexibility of the online format and the benefit of fitting the intervention into their schedule.
4. Discussion
Previous studies have shown that lifestyle modification is a successful approach to preventing diabetes and reducing the risk of developing diabetes. We found that it is feasible to recruit a safety net population unfamiliar with online or digital health programs to participate in a digitally-delivered diabetes prevention program. When approached, a majority of the patients had access to a computer or a mobile handheld device and were receptive to an online/digital program. We were able to recruit a large and diverse sample of low-income participants who could benefit from a DPP. Very few participants (n = 9) were ineligible due to lack of prediabetes or overweight/obese status; this indicates that EHR data and clinician accuracy for identifying clinically eligible patients for a DPP was reliable.
Our initial assessment of the trial also showed that there were various challenges and barriers at different phases of the intervention related to referrals, enrollment, and data collection.
Referrals. The program managers at each of the three participating health care facilities were responsible for identifying the appropriate clinics and introducing the study to the clinicians and patients. Clinicians at each of the sites were informed of the study and asked to refer their patients to the clinical trial. Introductory study presentations and the sending of reminders to clinicians had a limited effect on increasing referrals. Some clinicians indicated that frequent and regular communication from the study staff about patients' progress in the program would have motivated more referrals. To meet the targeted enrollment numbers, other recruitment strategies had to be implemented, primarily direct patient outreach to patients identified through the EHR. We found that it was important to tailor recruitment strategies to the targeted population and continue to communicate with the site staff to modify strategies. For example, recruitment by handing out information pamphlets at health fairs or talking to patients in clinic waiting rooms was often effective. Dedicated staff at each recruitment site (employed at least half-time on the study) who approached potentially eligible participants by phone or in person at a clinic were responsible for most of the enrollments. In the focus groups, participants mentioned that they were more receptive to being approached by the coordinator to participate in the trial because their doctor had previously discussed it with them.
Enrollment. There were also challenges in completing the requirements for enrollment. Although enrolled subjects rated their comfort with computers on the high end in the self-report assessment, many of the participants demonstrated limited proficiency in using computers or mobile devices when it was time to enroll in the digital program. This discrepancy suggests that some participants were comfortable using certain familiar features of their phones and computers, but when challenged with a new process (e.g., finding and downloading a specific app, or navigating a sign-up form webpage), others may have been less comfortable and less likely to engage in the digital tools. The enrollment coordinators spent time with enrolled subjects to provide technical support. Some participants needed help in completing the online enrollment and baseline surveys, as well as initially completing the application and account creation for the digital program. In particular, Spanish-speakers needed extra help with the initial sign-up process to access the program. It took more time and effort than planned to ensure that the participants completed all the enrollment requirements. However, data elements from the digital program's internet connected devices are still transmitting and collecting data (i.e., the weight scale, the nutrition and activity trackers), so we will have data to demonstrate participants' program engagement and outcomes even if they do not respond to the study coordinators attempts to schedule follow-up visits.
At the time the study was launched, some elements of the online program were not hard coded in both Spanish and English, and required adjusting web browser or operating system (OS) language settings to Spanish on laptops, tablets or smartphones. These browser/OS translations were not as robust, and led to some confusion because of imprecise translations or instructions that were misunderstood upon translation. The digital health company has since invested resources to improve and enhance these elements for a better user experience.
Data Collection. Information was collected from both the participants and the health care facilities. Participant data were collected at baseline, 6-months, and 12-months. Some patients were uncomfortable with the self-administered fingerstick HbA1c home-kit that was required at study enrollment. Many patients wanted to complete the baseline HbA1c in the clinic, and the rate of completing the test at home was lower than expected and required reminders and occasional repeat tests to get an adequate sample. Although we have complete baseline data, contacting participants to schedule follow-up visits has been more challenging than expected. Many calls were unanswered and staff have to make multiple attempts to get a response.
Second, there were also barriers to obtaining historical health record data from the three participating health care facilities. We have been working with the project managers at each of the sites to identify the matched sample for comparison and extract their data on health care utilization. These data were difficult to isolate and extract, and the lack of human resources at those facilities to extract the data proved challenging. We are learning that patient data is often incomplete and the quality of the comparison data may not be optimal, in part due to the electronic health records that were not designed to extract data for research purposes.
In this trial, although there were some challenges, we successfully recruited, enrolled, and engaged a low-income population at risk for developing diabetes to participate in a digital diabetes prevention program. The results of this trial will contribute meaningfully to the field of mobile health interventions in underserved populations. Information gathered from the study participants in focus groups will provide valuable insights into the usefulness of the program adaptations, how the program fit into their lives, and any barriers to success that were encountered throughout the study period. The initial results demonstrating interest and receptivity to the program are encouraging, and work towards dispelling the myth that underserved populations are unwilling and/or unable to successfully engage with health technology. The changes in weight and HbA1c at 6 months and 12 months will provide insight into how well digital diabetes prevention programs work to help underserved populations make lifestyle modifications to reduce risk for diabetes. Additionally, the lessons learned during the implementation of the trial regarding recruitment, enrollment, and data collection should inform future integrations of diabetes prevention program implementation and delivery in health care systems serving lower-income populations.
Conflicts of interest
CCS, EG, and ENM are employed by Omada Health, Inc. and receive salary and stock options.
Authors contributions
EG, MC, and SK contributed to the conception of the study design and methodology.
CCS and ENM coordinated the data collection, program delivery, and monitoring of data quality and participant safety.
BR, JM, DR and WI were the local site Principal Investigators responsible for overseeing site-level recruitment, data collection, and monitoring of data quality and participant safety.
ClinicalTrials.gov Identifier
Funding
The California Health Care Foundation (CHCF Grant # 19966) and Kresge Foundation provided financial support for the adaptation of the program and conduct of the research. The funding sources did not have involvement in the study design, collection, analysis or interpretation of the data, writing of the report, or decision to submit for publication.
Acknowledgements
The authors express gratitude to Monsi Portillo, Stacy Hernandez, Cathy Hagglund, and Telma Menendez for their work on the trial as study coordinators, and to all the participants who contributed their time and efforts to the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.05.007.
Contributor Information
Sue E. Kim, Email: sueekim@usc.edu.
Cynthia M. Castro Sweet, Email: cynthia@omadahealth.com.
Eliza Gibson, Email: eliza.gibson@omadahealth.com.
Erica N. Madero, Email: erica.madero@omadahealth.com.
Barbara Rubino, Email: brubino@dhs.lacounty.gov.
Janina Morrison, Email: janina@thewellnesscenterla.org.
Debra Rosen, Email: DebraRosen@nevhc.org.
Wendy Imberg, Email: wendy.imberg@providence.org.
Michael R. Cousineau, Email: cousinea@med.usc.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Knowler W.C., Barrett-Connor E., Fowler S.E. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research G, Knowler W.C., Fowler S.E. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications: the DPP outcomes study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)%2000291%5f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albright A.L., Gregg E.W. Preventing type 2 diabetes in communities across the U.S.: the national diabetes prevention program. Am. J. Prev. Med. 2013;44(4 Suppl 4):S346–S351. doi: 10.1016/j.amepre.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Office of the Actuary . In: Certification of Medicare Diabetes Prevention Program. C.f.M.a.M. Services, editor. Center for Medicare and Medicaid Services, U.S. Department of Health and Human Services; Baltimore, MD: 2016. [Google Scholar]
- 6.Ely E.K., Gruss S.M., Luman E.T. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC's national diabetes prevention program. Diabetes Care. 2017 doi: 10.2337/dc16-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkley A.J., Bodicoat D.H., Greaves C.J. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 8.Bian R.R., Piatt G.A., Sen A. The effect of technology-mediated diabetes prevention interventions on weight: a meta-analysis. J. Med. Internet Res. 2017;19(3) doi: 10.2196/jmir.4709. e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali M.K., Echouffo-Tcheugui J., Williamson D.F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y., You W., Almeida F., Estabrooks P., Davy B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: a systematic review and meta-analysis. J. Acad. Nutr. Diet. 2017;117(3):404–421. doi: 10.1016/j.jand.2016.11.016. e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckles G.L., Chou C. Disparities in the prevalence of diagnosed Diabetes - United States, 1999-2002 and 2011-2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:1265–1269. doi: 10.15585/mmwr.mm6545a4. [DOI] [PubMed] [Google Scholar]
- 12.Levine J.A. Poverty and obesity in the U.S. Diabetes. 2011;60(11):2667–2668. doi: 10.2337/db11-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ockene I.S., Tellez T.L., Rosal M.C. Outcomes of a latino community-based intervention for the prevention of diabetes: the lawrence latino diabetes prevention project. Am. J. Publ. Health. 2012;102(2):336–342. doi: 10.2105/ajph.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman K.J., Ocana L.L., Walker C. Outcomes from a culturally tailored diabetes prevention program in Hispanic families from a low-income school: horton Hawks Stay Healthy (HHSH) Diabetes Educat. 2010;36(5):784–792. doi: 10.1177/0145721710377360. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz C.R., Eckhardt S., Talavera S., Goytia C., Lorig K. Effectively translating diabetes prevention: a successful model in a historically underserved community. Transl. Behav. Med. 2011;1(3):443–452. doi: 10.1007/s13142-011-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millard A.V., Graham M.A., Wang X. Pilot of a diabetes primary prevention program in a hard-to-reach, low-income, immigrant Hispanic population. J. Immigr. Minority Health. 2011;13(5):906–913. doi: 10.1007/s10903-010-9412-y. [DOI] [PubMed] [Google Scholar]
- 17.Rosal M.C., Haughton C.F., Estabrook B.B. Fresh Start, a postpartum weight loss intervention for diverse low-income women: design and methods for a randomized clinical trial. BMC Publ. Health. 2016;16:953. doi: 10.1186/s12889-016-3520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mensa-Wilmot Y., Bowen S.A., Rutledge S. Early results of states' efforts to support, scale, and sustain the national diabetes prevention program. Prev. Chronic Dis. 2017;7(14):E130. doi: 10.5888/pcd14.170478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L., Manson S., Dill E.J. Participant and site characteristics related to participant retention in a diabetes prevention translational project. Prev. Sci. 2015;16(1):41–52. doi: 10.1007/s1121-013-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cene C., Haymore L., Ellis D. Implementation of the power to prevent diabetes prevention educational curriculum into rural African American communities: a feasibility study. Diabetes Educat. 2013;39(6):776–785. doi: 10.1177/0145721713507114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontos E., Blake K.D., Chou W.Y., Prestin A. Predictors of eHealth usage: insights on the digital divide from the health information national trends survey 2012. J. Med. Internet Res. 2014;16(7) doi: 10.2196/jmir.3117. e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pew Research Center Digital divide persists even as lower-income Americans make gains in tech adoption. http://www.pewresearch.org/fact-tank/2017/03/22/digital-divide-persists-even-as-lower-income-americans-make-gains-in-tech-adoption/
- 23.Schickedanz A., Huang D., Lopez A. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J. Gen. Intern. Med. 2013;28(7):914–920. doi: 10.1007/s11606-012-2329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athavale P., Thomas M., Delgadillo-Duenas A.T. Linking high risk postpartum women with a technology enabled health coaching program to reduce diabetes risk and improve wellbeing: program description, case studies, and recommendations for community health coaching programs. J. Diabetes Res. 2016;2016:4353956. doi: 10.1155/2016/4353956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reininger B., Mecca L.P., Stine K.M. A type 2 diabetes prevention website for african americans, Caucasians, and mexican americans: formative evaluation. JMIR Res. Protoc. 2013;2(2) doi: 10.2196/resprot.2573. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontil V., McDermott K., Tieu L. Adaptation and feasibility study of a digital health program to prevent diabetes among low-income patients: results from a partnership between a digital health company and an academic research team. J. Diabetes Res. 2016;2016:10. doi: 10.1155/2016/8472391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart E.A., Rubin D.B. Best practices in quasi-experimental designs: matching methods for causal inference. In: Osborne J., editor. Best Practices in Quantiative Methods. SAGE Publications, Inc; Thousand Oaks, California: 2008. pp. 155–176. [Google Scholar]
- 28.Alere . Alere; Waltham, MA: 2013. Performance Evaluations on the Alere Cholestech LDX System. [Google Scholar]
- 29.DeSalvo K.B., Fan V.S., McDonell M.B., Fihn S.D. Predicting mortality and healthcare utilization with a single question. Health Serv. Res. 2005;40(4):1234–1246. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambino C.P., Acosta Y.D., Grieco E.M. English-speaking ability of the foreign-born population in the United States. Washington, D.C. 2014. https://www.census.gov/prod/2014pubs/acs-26.pdf
- 31.Napoles-Springer AM, Stewart AL. Overview of qualitative methods in research with diverse populations: making research reflect the population. Med. Care 44(11 (Suppl 3)) S5–S9. 10.1097/01.mlr.0000245252.14302.f4. [DOI] [PubMed]
- 32.Chew L.D., Griffin J.M., Partin M.R. Validation of screening questions for limited health literacy in a large VA outpatient population. J. Gen. Intern. Med. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorlie P.D., Avilés-Santa L.M., Wassertheil-Smoller S. Design and implementation of the hispanic community health study/study of Latinos. Ann. Epidemiol. 2010;20(8):629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez A, Alos VA, Scanlan A, et al. The rationale, design, and baseline characteristics of PREVENT-DM: a community-based comparative effectiveness trial of lifestyle intervention and metformin among Latinas with prediabetes. Contemp. Clin. Trials 45 320–327. 10.1016/j.cct.2015.10.011. [DOI] [PMC free article] [PubMed]
- 35.Sommers B.D., Rosenbaum S. Issues in health reform: how changes in eligibility may move millions back and forth between Medicaid and insurance exchanges. Health Aff. 2011;30(2):228–236. doi: 10.1377/hlthaff.2010.1000. [DOI] [PubMed] [Google Scholar]
- 36.Sommers B.D., Gourevitch R., Maylone B., Blendon R.J., Epstein A.M. Insurance churning rates for low-income adults under health reform: lower than expected but still harmful for many. Health Aff. 2016;35(10):1816–1824. doi: 10.1377/hlthaff.2016.0455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.