Graphical abstract
Arachidonic acid interacts with the surface double lipid bilayer shield of larval, developing and adult schistosomes, leading to its disintegration and eventual parasite attrition.
Keywords: Schistosoma mansoni, Schistosoma haematobium, Rat schistosomiasis, Arachidonic acid, Oil Red O staining, Immunohistochemistry
Abstract
Schistosoma mansoni and Schistosoma haematobium are intravascular, parasitic flatworms that infect >250 million people in 70 developing countries, yet not all people of the same community and household are afflicted. Regarding laboratory rodents, mice but not rats are susceptible to infection with S. mansoni and hamsters but not mice are entirely permissive to infection with S. haematobium. A recent Brazilian publication has demonstrated that resistance of the water-rat, Nectomys squamipes to S. mansoni infection might be ascribed to stores of arachidonic acid (ARA)-rich lipids in liver. Several reports have previously shown that ARA is a safe and effective schistosomicide in vitro, and in vivo in mice, hamsters and in children. Schistosoma haematobium appeared more sensitive than S. mansoni to ARA in in vitro and in vivo experiments. Accordingly, it was proposed that ARA increased levels might be predominantly responsible for natural attrition of S. mansoni and S. haematobium in resistant experimental rodents. Therefore, the levels of ARA in serum, lung, and liver of rats (resistant) and mice (susceptible) at 1, 2, 3, 4 and 6 weeks after infection with S. mansoni cercariae and between mice (semi-permissive) and hamster (susceptible) at 1, 2, 3, 4, and 12 weeks after infection with S. haematobium cercariae were compared and contrasted. Neutral triglycerides and ARA levels were assessed in serum using commercially available assays and in liver and lung sections by transmission electron microscopy, Oil Red O staining, and specific anti-ARA antibody-based immunohistochemistry assays. Significant (P < .05), consistent, and reproducible correlation was recorded between ARA content in serum, lung, and liver and rodent resistance to schistosome infection, thereby implicating ARA as an endoschistosomicide.
Introduction
Schistosoma mansoni and S. haematobium infect >250 million people in 70 developing countries with more than 800 million, namely children, at risk of the infection [1]. Yet, there is no instance where hundred percent or so of individuals are afflicted despite residing in endemic foci, and sharing community, household, and exposure to schistosome-infected water bodies. Indeed, “endemic normals” are repeatedly exposed to viable cercariae of S. mansoni, are seropositive by enzyme-linked immunosorbent assay (ELISA) against crude adult worm antigen and, yet, have no record of previous or current infection when judged by repeated stool examination [2], [3], [4], [5], [6], [7], [8], [9]. The apparent lack of schistosome maturation and egg deposition in “endemic normals” was ascribed to antibody, lymphoproliferative, interferon-gamma (IFN-γ) responses to specific antigens, and/or specific antibody isotypes levels or ratios to soluble egg and worm antigens [2], [3], [4], [5], [6], [7], [8], [9]. However, no consensus or solid explanations were reached.
Rodents too display differential susceptibility to schistosomes. Mice are susceptible to S. mansoni but are hardly semi-permissive to infection with S. haematobium [10], [11], [12]. To our knowledge, no explanation was provided for this phenomenon [13]. Conversely, nearly sterile resistance of laboratory rats (Rattus norvegicus) to S. mansoni infection [14], [15], [16] was attributed to the production of Th2 cytokines, interleukin (IL)-4, IL-5, and IL-13, in response to the invading larvae [17], [18], [19], [20], [21]. A recent study indicated that resistance of the water-rat, Nectomys squamipes to continuous infection with S. mansoni is associated with accumulation of lipids, principally arachidonic acid (ARA), in liver of naïve and naturally-infected animals [22].
Arachidonic acid, an omega-6 polyunsaturated fatty acid, is an essential constituent of biological cell membranes. Free unesterified ARA modulates the function of numerous ion channels, and several receptors and enzymes, via activation as well as inhibition, and readily induces apoptosis of normal and cancer cell lines [23], [24], [25], [26], [27]. It was previously shown that exposure to ARA (10 µM, 30 min) was effective in allowing specific antibody binding to otherwise hidden surface membrane antigens of S. mansoni and S. haematobium lung-stage schistosomula and adult worms [28], [29]. Exposure to 20 µM ARA for 30 min elicited surface membrane disintegration and attrition of the schistosomula, likely as result of excessive ARA activation of the parasite tegument-associated neutral sphingomyelinase (nSMase) [29], [30], [31]. Further studies documented the ARA in vitro and in vivo schistosomicidal action on lung-stage and adult male and female S. mansoni and S. haematobium whereby S. haematobium appeared more sensitive than S. mansoni to ARA in in vitro and in vivo experiments [29], [30], [31], [32], [33], [34]. These findings together prompted examination whether there is a correlation between laboratory rodents' resistance and susceptibility to infection with S. mansoni (rats vs. mice) or S. haematobium (mice vs. hamsters) and ARA levels in serum, lung, and liver in naïve hosts and weekly for 4 weeks after, as well as at the end of the experimental infection.
Experimental
Ethics statement
All animal experiments were performed following the recommendations of the current edition of the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, USA, and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Science, Cairo University (permit number CUIS 36 16).
Animals and parasites
Outbred, female CD-1 mice, albino rats (Rattus norvegicus), and Syrian hamsters (Mesocricetus auratus) were raised at the Schistosome Biological Materials Supply Program, Theodore Bilharz Research Institute (SBSP/TBRI), Giza, Egypt, and at the age of 6 weeks were maintained throughout experimentation at the animal facility of the Zoology Department, Faculty of Science, Cairo University. Cercariae of an Egyptian strain of S. mansoni and S. haematobium were obtained from SBSP/TBRI, and used immediately after shedding from Biomphalaria alexandrina and Bulinus truncatus snails, respectively. Infection of CD-1 mice and rats was with 100 ± 2 cercaria via whole body exposure [21], while hamsters were anesthetized, the abdomen shaved and wetted with sterile deionized water, and then exposed to 100 cercariae in 100 μL deionized water, protected from spilling by a sterile steel ring as described [12].
Experimental design
Experiment 1. A total of 30 rats and 30 mice were randomly assigned to groups of 12 uninfected, naïve hosts and groups of 18 that were exposed to 100 cercariae of S. mansoni. Three naïve and three S. mansoni-infected rats and mice were euthanized on day 7, 14, 21, and 28, and 6 of each infected group on day 40. Individual host serum was used for evaluation of circulating lipid levels. Lung and liver pieces of 2–3 mm3 were immediately fixed in 4% paraformaldehyde (Sigma Chemical Co., St Louis, MO, USA) and destined to transmission electron microscopy, histological examination following haematoxylin and eosin staining of paraffin sections, and Oil Red O staining and immunohistochemistry assays of cryostat sections. Worm burdens as well as liver worm egg load in individual rats and mice (six per group) were evaluated at the last interval (40 days after the challenge infection with S. mansoni cercariae) as described elsewhere [12], [33], [34].
Experiment 2. A total of 30 mice and 30 hamsters were randomly divided into groups of 12 uninfected, naïve hosts and the rest exposed to 100 cercariae of S. haematobium. Three naïve and three S. haematobium-infected mice and hamsters were euthanized on day 7, 14, 21, 28, and 6 of each infected group on day 84, and provided serum and 2–3 mm3 lung and liver pieces, which were processed and examined as mentioned above. Worm burdens as well as liver worm egg load in individual mice and hamsters (six per group) were evaluated at the last interval (84 days after the challenge infection with S. haematobium cercariae) as described elsewhere [12], [33], [34].
Serum lipids levels
Serum samples were assessed on an individual host basis, in duplicates, for enzymatic colorimetric (Multiskan EX, Labsystems, Helsinki, Finland) determination of total cholesterol (Cholesterol-LQ, CHRONOLAB SYSTEMS, S.L., Barcelona, Spain) and triglycerides (Triglycerides, CHRONOLAB) following the manufacturer’s instructions. Levels of circulating unbound, free ARA were evaluated on an individual animal basis, and depending on serum availability in duplicate or quadruplicate wells, by competitive enzyme-linked immunosorbent assays (ELISA) using AA (Arachidonic Acid) ELISA Kit (Elabscience Biotechnology Co., Ltd, WuHan, People Republic of China; catalog No.: E-EL-0051) following the manufacturer’s instructions. Absorbance readings (650 nm) of the ARA standard dilutions were plotted vs. concentration values in ng/mL using scatter graph [35]. For evaluating the concentration of the test samples, absorbance readings (650 nm) were fitted into the following obtained equation Y = −0.0128 X + 1.2044, where Y represents the absorbance values and X the concentration values in ng/ml.
Transmission electron microscopy
Samples were fixed at 4 °C overnight in 4% paraformaldehyde, maintained at 4 °C in Dulbecco's phosphate-buffered saline, pH 7.1 (D-PBS), fixed in 3% glutaraldehyde in sodium cacodylate buffer, pH 7.4 for 2 h and post-fixed in 1% osmium tetroxide (Sigma) for 2 h. Specimens were then dehydrated with ethanol and at critical point dryer were mounted on metal stubs, coated with carbon and gold and examined with a JEOL JEM-1200 EXII electron microscope (Tokyo, Japan) at Central Lab, Faculty of Science, Ein Shams University, Cairo, Egypt.
Cryostat sectioning and Oil Red O staining
Reagents were obtained from Sigma Chemical Co. Lung and liver pieces were fixed at 4 °C overnight in 4% paraformaldehyde, maintained at 4 °C in D-PBS, and soaked in 30% sucrose in D-PBS for 3 days before cryostat (Slee Medical, Mainz, Germany) sectioning at −20 °C. Sections were then rinsed with 60% isopropanol, stained with freshly prepared Oil Red O working solution for 15 min, rinsed with 60% isopropanol before rinsing in distilled water and mounting in glycerol [36], [37], [38], [39].
Immunohistochemistry
Cryostat frozen sections were incubated with 1% bovine serum albumin (BSA, Sigma) for blocking non-specific sites for 30 min and then with 100 μL D-PBS containing 0 (negative controls) or 2 μg rabbit polyclonal antibody to arachidonic acid (MyBioSource, San Diego, CA, USA, catalogue No.: MBS2003715) for 1 h at room temperature. Sections were washed with D-PBS/0.1% BSA and incubated with 2 μg/100 μL goat anti-rabbit immunoglobulins labeled with alkaline phosphatase [Goat F(ab′)2 Anti-Rabbit IgG - H&L (AP), preadsorbed, Abcam, Cambridge, MA, USA] for 1 h at room temperature. After washing, the reaction was visualized with Histomark RED Phosphatase Substrate Kit of Kirkegaard and Perry Laboratories (Gaithersburg, MD, USA), noting that HistoMark RED reagents form a brilliant scarlet reaction product that is stable in organic solvents. Photographs were acquired by light microscopy and visualizer (Olympus Optical Co, LTD, Model BX40F4, Japan).
Statistical analysis
All values were tested for normality. Students' –t- 2-tailed test was used to analyse the statistical significance of differences between selected values and considered significant at P < 0.05.
Results
Parasitological parameters
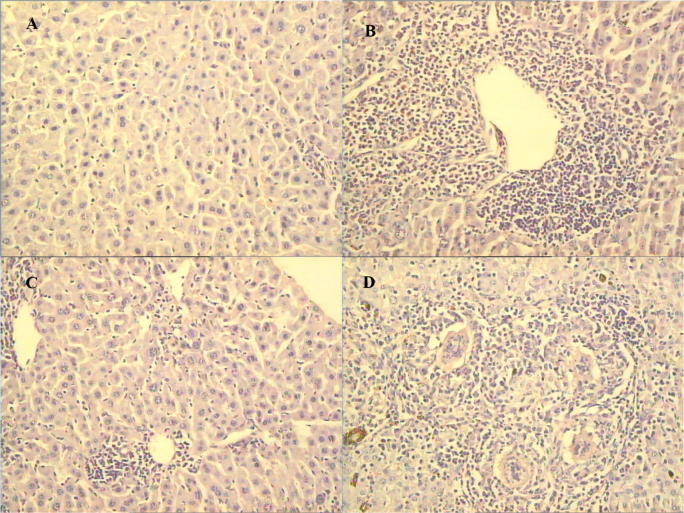
Parasitological parameters recorded at the end of the experiments are shown in Table 1 and Fig. 1, documenting the nearly sterile resistance of rats to S. mansoni and susceptibility and semi-permissiveness of mice to S. mansoni and S. haematobium, respectively.
Table 1.
Rodents' permissiveness to schistosome infection.
| Infection/Host | Total worm |
Male worms |
Female worms |
Total liver eggs |
|---|---|---|---|---|
| Mean counts ± SD | ||||
| S. mansoni | ||||
| Rats | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 50.0 ± 83.6 |
| Mice | 26.3 ± 6.0 | 12.5 ± 3.6 | 13.8 ± 3.0 | 18,062 ± 6366 |
| S. haematobium | ||||
| Mice | 4.2 ± 2.2 | 2.2 ± 0.9 | 2.0 ± 1.4 | 2000 ± 1839 |
| Hamsters | 17.5 ± 1.5 | 9.5 ± 0.8 | 8.0 ± 1.2 | 71,750 ± 12,606 |
Laboratory rodents (number = six/group) were percutaneously exposed to 100 viable cercariae and worm and worm liver egg burdens evaluated 40 and 84 days after infection with S. mansoni and S. haematobium, respectively.
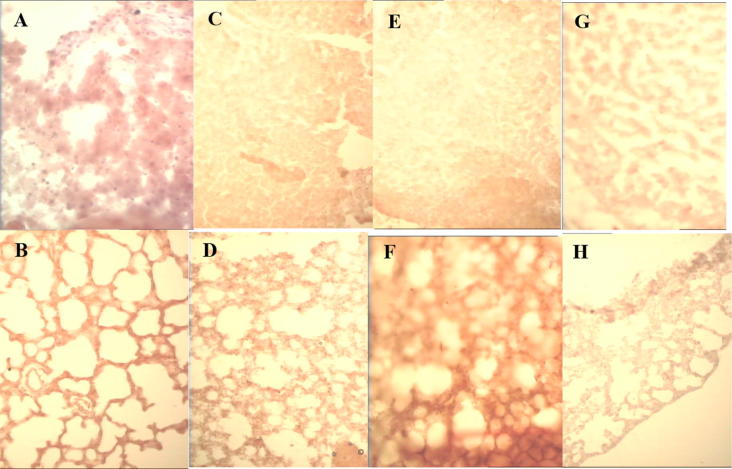
Fig. 1.
Haematoxylin and eosin-stained sections of liver at time of worm perfusion. (A) rat, and (B) mouse, 40 days after S. mansoni infection; (C) mouse, and (D) hamster, 84 days after S. haematobium infection. 200X. Each figure is typical of 6 individual hosts.
Serum lipids levels
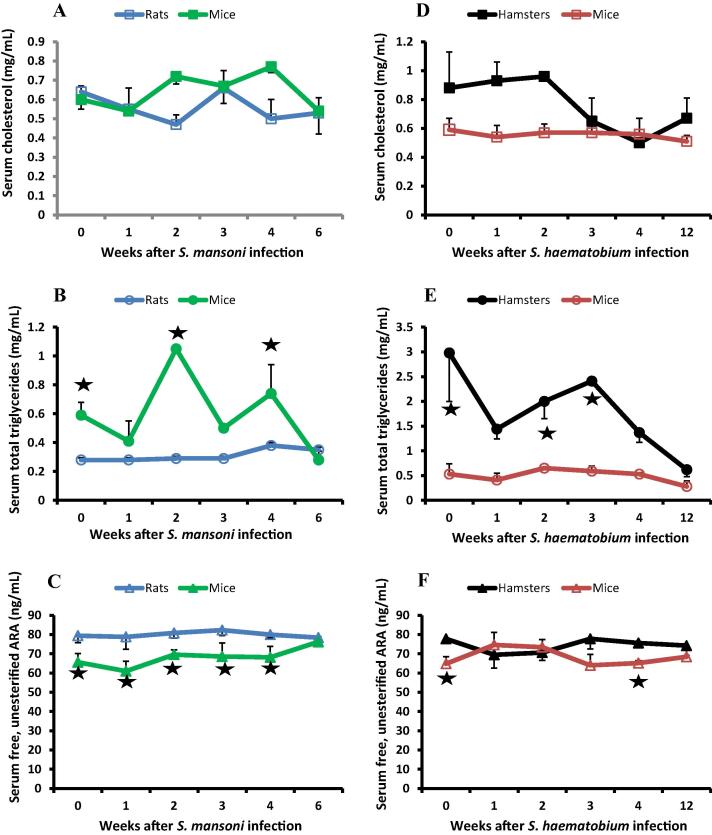
Serum cholesterol levels were identical in naïve rats and mice and at 1, 3 and 6 weeks post S. mansoni infection. Cholesterol levels showed significant decrease in rats at 2 (P < 0.0001), and 4 (P = 0.0042) weeks, and significant (P < 0.05) increase in mice at 2 and 4 weeks after infection as compared to naïve hosts. Yet, cholesterol levels did not show highly significant differences between rats and mice at all test intervals (Fig. 2A). Levels of triglycerides were significantly (P = 0.0003) higher in mice than rats in naïve hosts and at every interval except 6 weeks after infection, the time at which triglycerides levels fell to reach those consistently recorded in rats (Fig. 2B). Conversely, levels of free, unesterified ARA were significantly higher in rats than mice in naïve hosts (P = 0.0006) and at 1 (P = 0.0046), 2 (P < 0.0001), 3 (P = 0.0013) and 4 (P = 0.0018) weeks after S. mansoni infection (Fig. 2C).
Fig. 2.
Serum lipid levels. Each point represents mean of three individual donors tested in duplicate or quadruplicate and vertical bars denote the standard deviation about the mean. Asterisks indicate highly significant (P < .005) differences between schistosome susceptible and resistant hosts.
Serum cholesterol levels were significantly higher in hamsters than mice in naïve hosts (P = 0.0004) and at 1 (P = 0.0002) and 2 (P < 0.0001) weeks after S. haematobium infection then decreased to the levels consistently recorded in mice (Fig. 2D). Levels of triglycerides were significantly (P < 0.0001) higher in hamsters than mice in naïve hosts and at every interval except at 12 weeks after infection, the time at which triglycerides levels in hamsters fell to reach those consistently recorded in mice (Fig. 2E). Levels of free, unesterified ARA were also significantly (P = 0.0002) higher in naïve hamsters than naïve mice (P = 0.0002) and at 3 (P = 0.0043) and 4 (P = 0.0001) weeks post S. haematobium infection. Yet, at 1 and 2 weeks post infection, when the S. haematobium schistosomula are negotiating the lung capillaries, free ARA levels significantly decreased (P < 0.05) in the susceptible hamsters and increased (P < 0.01) in the semi-permissive mice (Fig. 2F) as compared to naïve hosts.
Lung and liver lipids levels
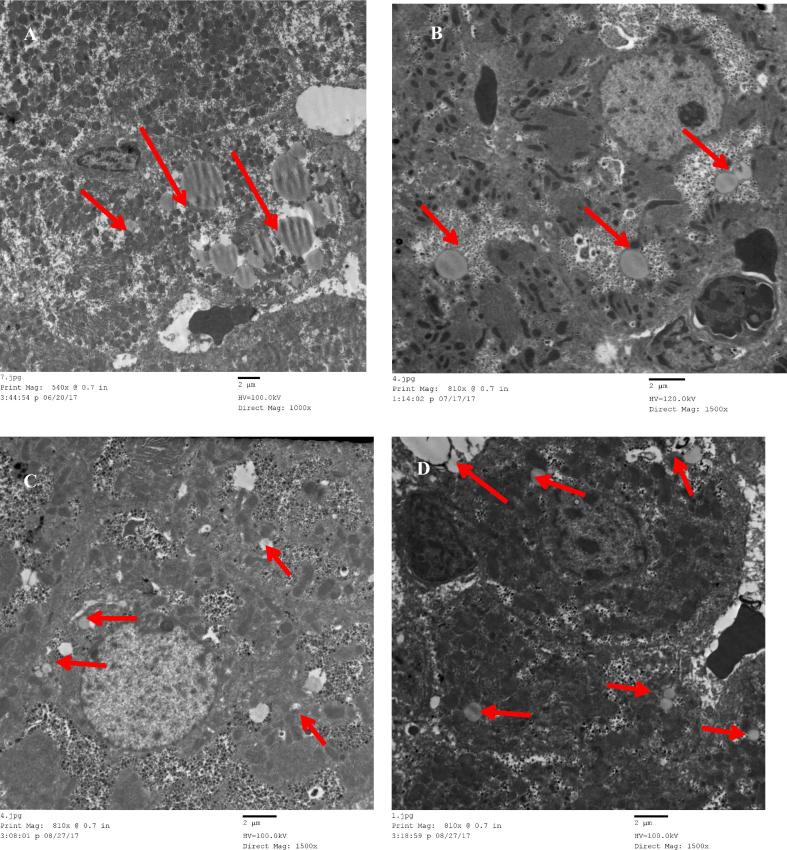
Transmission electron microscopy examination of liver sections revealed that lipid droplets in cytoplasm of the S. mansoni-resistant species, the rat were significantly (P ≤ 0.0001) more numerous and larger than in the susceptible mouse whether naïve (Fig. 3A, B) or 1 or 2 weeks after infection (Table 2). Contrary to the serum triglycerides levels, lipid droplets in cytoplasm of the S. haematobium-semi-permissive mice and susceptible hamster whether naïve and one or two (Table 2, Fig. 3C, D) weeks after infection did not show significant differences in number or size.
Fig. 3.
Transmission electron microscopy examination of liver. Upper panel, typical picture of naïve (A) rat and (B) mice. Lower panel, representative of (C) hamsters and (D) mice, two weeks after S. haematobium infection. The red arrows point to the lipid droplets.
Table 2.
Lipid droplets in liver of schistosome-resistant and permissive rodents.
| Infection/Hosts | Lipid droplets mean ± SD | Lipid droplets mean ± SD |
|---|---|---|
| Naive | After infection | |
| S. mansoni | ||
| Rats | 13.6 ± 1.5 | 10.8 ± 1.1 |
| Mice | ||
| P value | 5.1 + 1.1 | 4.0 ± 2.8 |
| Rats versus mice | <0.0001 | 0.0001 |
| S. haematobium | ||
| Mice | 6.7 ± 2.2 | 6.8 ± 2.0 |
| Hamsters | ||
| P value | 5.5 ± 2.4 | 7.0 ± 3.3 |
| Mice vs. hamsters | NS | NS |
Liver droplets were enumerated in 10 sections (1500×) per group of naïve hosts and one and two weeks after infection to yield the mean and SD.
Liver and lung sections of three naïve and three 1 and 2 week schistosome-infected hosts were stained with Oil Red O for detecting triglycerides and cholesteryl esters but no other lipids or biological membranes. Indeed, Oil Red O stains only the most hydrophobic and neutral lipids (i.e., triglycerides, diacylglycerols, and cholesterol esters), whereas polar lipids (i.e., phospholipids, free ARA, sphingolipids, and ceramides) are not stained [36], [37], [38], [39], [40]. Additionally, the lipid droplets were not easily discerned, likely because the isopropanol used to dissolve the Oil Red O and the glycerol for mounting cause fusion of adjacent lipid droplets [40]. Contrary to the serum levels, triglycerides amounts in liver and lung of rats were higher than for mice in naïve hosts and at 1 and 2 weeks after S. mansoni infection (Fig. 4A–D). At 1 and 2 weeks post S. haematobium infection, the amount of triglycerides in the lung was remarkably higher in the semi-permissive mouse than the susceptible hamster (Fig. 4E–H).
Fig. 4.
Oil Red O-stained liver (upper panel) and lung (lower panel) sections. Typical rat liver (A) and lung (B) and mouse liver (C) and lung (D) one week after S. mansoni infection. Mouse liver (E) and lung (F) and hamster liver (G) and lung (H) two weeks after S. haematobium infection are representative of three hosts per group.
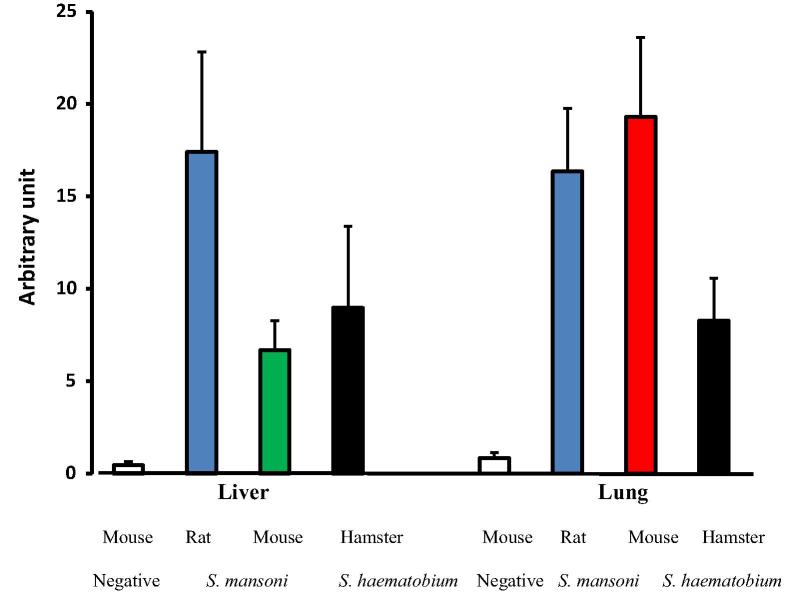
In contrast to transmission electron microscopy and Oil Red O staining, reactivity of liver and lung sections of experimental hosts with anti-ARA antibody in immunohistochemistry assays specifically reveals the content of ARA in a free, triglyceride or phospholipid form [41]. The data of repeat analyses of liver and lung of naïve and one and two week-infected hosts were in line with the levels of serum free, unesterified ARA (Fig. 2C, F), whereby the total ARA content in naïve rat organs was remarkably higher than for naïve mice (Fig. 5A–F) and did not show great variations after S. mansoni infection (Fig. 6). Additionally, the total ARA content was higher in lung of naïve mice than naïve hamsters (Fig. 5E–H), and at one and two weeks after S. haematobium infection (Fig. 6).
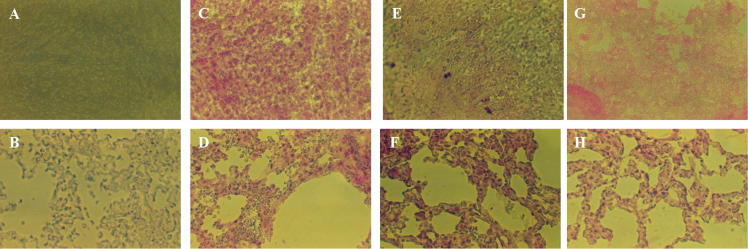
Fig. 5.
Typical organ reactivity of naïve hosts with anti-ARA antibody in immunohistochemistry assays. Cryostat sections of liver (upper panel) and lung (lower panel) sections of naïve rat (A–D), mouse (E, F), and hamster (G, H) were reacted with D-PBS containing 0 (A, B) or 2 (C–H) μg rabbit antibody to ARA, then alkaline phosphatase-labeled antibody to rabbit immunoglobulins, and the reaction visualized with Histomark RED Phosphatase Substrate Kit of Kirkegaard and Perry Laboratories. X200.
Fig. 6.
Reactivity to anti-ARA antibody of liver and lung at 2 weeks after schistosome infection in immunohistochemistry assays. A semi-quantitative analysis and pixel processing of digital images was performed using IMAGEJ software. The intensity of the antibody staining of tissue sections was assessed and scored using RGB (Red/Green/Blue) vector of color deconvolution plugin for red color separation. Threshold was manually optimized and acquired values from images were analyzed using Microsoft excel for mean (represented by columns) and standard deviation (denoted by vertical bars) calculations.
Discussion
Schistosomiasis is a severe parasitic disease. Symptoms and sequelae are essentially not due to the cercariae that invade the epidermis and therein transform into schistosomula. The larvae penetrate the dermis, enter into the blood capillaries en route to the lung and liver and then to the inferior mesenteric veins (S. mansoni) or the peri-vesical venous plexus (S. haematobium) residence, within less than 6 and more than 10 weeks, respectively. Schistosomiasis major clinical manifestations are due to the host immunological inflammatory responses to the soluble antigens derived from the parasite eggs that fail to exit via stool (S. mansoni) or urine (S. haematobium) and are trapped in the liver, intestine, and urinary bladder [42], [43], [44]. In rodent experimental S. haematobium infection, a large proportion of parasite eggs are drifted towards the liver, distal colon, and rectum rather than to the lower urinary tract as in humans [42], [43]. In support, numerous and large granulomas were readily observed in liver of mice and hamsters harbouring mature S. mansoni and S. haematobium, respectively. Despite that laboratory mice are readily susceptible to S. mansoni, they appeared to allow development of a small number of adult S. haematobium worms and eggs, confirming their semi-permissiveness to S. haematobium infection the reason(s) of which remain obscure. Mature worms were not detected in rats infected in parallel with mice with S. mansoni cercariae and the liver appeared normal, in accordance with numerous reports documenting the near sterilizing resistance of the laboratory [14], [15], [16], [17], [18], [19], [20], [21] and the water- [22] rat to schistosome infection. A breakthrough in natural schistosomiasis was recently reported whereby resistance of the water-rat to continuous natural infection with S. mansoni was strongly correlated with the occurrence of numerous, ARA-rich fat depots in the liver of naïve and infected animals [22]. Since ARA is a documented schistosomicide [33], [34], [45] it was important to examine whether ARA levels in serum and organs of laboratory rodents correlate with, and are thus likely instrumental to, resistance and susceptibility to schistosome infection.
Serum cholesterol levels did not greatly differ in naïve and at several intervals after schistosome infection between the resistant and susceptible hosts. These findings concord with the statistical analysis that indicated there was no correlation between rodent worm count or tissue egg load and circulating cholesterol levels [46], [47], [48], [49], [50]. No perceptible changes in serum cholesterol levels were noted during the experiment in the rat-S. mansoni and mouse-S. haematobium models. Conversely, serum cholesterol levels declined in the hosts with patent infection, an observation previously ascribed to the schistosome modulating influence on host lipid metabolism [46], [47], [48], [49], [50].
Levels of serum total triglycerides were significantly (P < 0.005) higher in naïve and at one through four weeks after infection in susceptible than in non-permissive hosts, but declined thereafter. In contrast to the susceptible hosts, the serum triglyceride levels remained constant, without any decrease, at every test interval in the hosts that resisted the schistosome infection. The results collectively point to the likely impact of developed schistosomes on host lipid metabolism and the ability of schistosomes to act as lipid-lowering agents [46], [47], [48], [49], [50], but fail to suggest relationship between host serum triglyceride levels and parasite survival or infection outcome [47].
The levels of serum free ARA were significantly (P < 0.005) higher in rats than in mice in naïve hosts and at one through four weeks after infection with S. mansoni, thus revealing correlation between relatively high levels of circulating free ARA and prevention of parasite survival, growth, and maturation. Despite that esterified ARA was reported to be significantly reduced in plasma phospholipids and cholesterol esters of S. mansoni-infected mice [51], the influence of S. mansoni parasitization on ARA metabolism in mice appeared to be lower than for cholesterol and triglycerides (Fig. 2A–C). Conversely, S. haematobium invasion in mice had no effect on serum cholesterol or total triglyceride, but elicited significant (P < 0.01) increase in serum free ARA levels at one and two weeks after infection. Increased free ARA levels appear, thus, implicated in attrition of the juvenile parasite, at the most vulnerable stage of its development, migration from the dermal vessels and residence in the lung capillaries. In hamsters, infection with S. haematobium is associated with strong decline in serum cholesterol and triglyceride serum levels rather late in the course of the infection. Curiously enough, S. haematobium infection elicited significant (P < 0.05) decrease in ARA levels uniquely at one and two weeks post-infection (Fig. 2D–F). When most vulnerable, schistosomula developing in hamsters were, thus, protected from exposure to elevated ARA concentration.
Because of the presence of albumin, free, unbound ARA in human serum concentration is kept below 0.1 μM = 30 ng/mL [24]. The assay herein used measures uniquely the ARA free from albumin binding, and revealed that the levels of free, unesterified and albumin-unbound ARA in the rodents assayed ranged between 60 and 80 ng/mL, which is equivalent to 0.2–0.3 μM. Free ARA may interact with parasite surface membrane lipids as it was demonstrated that ARA was readily incorporated by adult S. mansoni while no prostaglandin biosynthesis was detected [52]. Intercalation of ARA in the outer lipid bilayer would lead to physical or electrostatic-mediated modulation of the activity of voltage-gated ion channels [[27], [53] and references therein]. It may, like praziquantel, interacts with surface membrane Ca++ channels and interferes with the worm calcium homeostasis [54], [55]. Yet, ARA severely impairs the integrity of the surface membrane architecture but does not lead to the immediate spastic paralysis praziquantel elicits [33], [54], [55], [56]. Free ARA may readily engage the parasite surface membrane nSMase [32]. Excessive nSMase activation leads to apical lipid layer sphingomyelin (SM) hydrolysis and disruption of the worm SM-based protective hydrogen bond network [30], [31], [32], [33], [34]. Subsequently, surface membrane antigens become accessible to binding host specific antibodies and the worm susceptible to the diverse host humoral and cell-mediated immune effectors. Additionally, SM hydrolysis generates in the parasite accumulation of ceramide, an important inducer of apoptosis [57]. Indeed, ARA was shown to be a physiological mediator of cell death via apoptosis [58]. Of interest, apoptosis was readily detected in 14 > 23 days-old Schistosoma japonicum and, furthermore, the sterile resistance of the reed vole, Microtus fortis to infection with S. japonicum was significantly associated with apoptosis in the worm [59], [60].
The circulating ARA levels recorded are considerably lower than the concentration effective for parasite attrition in vitro (20 μM and up to 2.5 mM). Yet, in vitro worm exposure to ARA was for a limited period of time (1/2 h to a maximum of 5 h) and in the entire absence of host immune effectors [28], [29], [30], [31], [32], [33], [34]. It may be assumed that continuous, uninterrupted exposure to ARA levels of 0.2 μM may mediate elimination of the most vulnerable worms, which are unable to synthesize adequate amounts of SM for repair of the affected sites in the surface double lipid bilayer shield. This suggestion would explain the hitherto not adequately explained limited recovery of schistosomes even in entirely susceptible hosts (Table 1). Continuous, uninterrupted exposure to 0.3 μM ARA in the circulation would prevent survival and development of even the most robust worms as in the rat/S. mansoni model. If S. haematobium infection failed to modulate ARA metabolism and the levels of serum ARA during the first two weeks after infection of mice and hamsters, the outcome of the infection would be entirely different. It is necessitated to examine the effect of extended exposure of juvenile and adult schistosomes to ARA concentration of <1.0 μM, and more importantly to investigate whether any relationship exists between circulating ARA levels and resistance to schistosome infection in children residing in endemic areas.
Circulating triglycerides and free fatty acids are readily replenished from serum albumin and lipid depots in cells [61]. Transmission electron microscopy revealed that largest and most numerous fat droplets were consistently found in rat liver, and that S. mansoni or S. haematobium one and two week-infection failed to appreciably alter fat droplet content in rat, mice, and hamsters liver. These findings support data regarding timing in decline of serum cholesterol and total triglycerides levels, whereby developed schistosomes appeared to impact lipid metabolism at 4 weeks and later after infection with S. mansoni or S. haematobium. Data obtained using Oil Red O staining were not different except for additionally disclosing that the highest and lowest content in neutral triglycerides was recorded in lung of mouse and hamster, respectively, at one and two weeks after S. haematobium infection. These data indicate that S. haematobium infection modulates lipid metabolism in lung of semi-permissive and permissive hosts, probably because of the extended residence of the parasite in the lungs [62]. The immunohistochemistry assays targeting ARA in free or esterified form revealed that its highest content is consistently observed in rat liver and lung before and after S. mansoni infection. The data fully confirm the findings recently reported for the naïve and S. mansoni-infected water-rat, which liver displays numerous ARA-rich fat droplets [22]. High ARA content was also recorded in lung of naïve and S. haematobium-infected mice. The relatively high ARA concentration in serum and lung of mice during the extended period of the schistosomula pulmonary residence may explain the poor survival of S. haematobium, which has consistently shown higher susceptibility than S. mansoni to ARA in vitro and in vivo [28], [29], [30], [31], [32], [33], [34]. Indeed, 12 week-old S. haematobium worms incubated in the presence of 50% and 100% fetal calf serum and 10 mM ARA were essentially all dead after 3 h incubation while attrition of 6 week-old S. mansoni required 5 h exposure [33]. Percentage reduction in worm burden following ARA in vivo treatment was consistently higher for S. haematobium than S. mansoni in mice [33] and hamsters [34]. Studies using quasi-elastic neutron scattering for characterizing the diffusion of larval and adult Schistosoma mansoni and adult Schistosoma haematobium in the surrounding medium revealed that S. mansoni worms create a stronger hydrogen-bonded barrier with the medium in comparison to that created by S. haematobium worms. These findings were construed to provide support for the high sensitivity of S. haematobium to ARA, a documented nSMase activator and hydrogen bond network disruptor, and an explanation for S. haematobium requirement of ARA lower concentrations and shorter exposure time than S. mansoni for eventual attrition [31], [63], [64].
Conclusions
The data reported in the present study reveal correlation between levels of the schistosomicide ARA in serum, lung, and liver, and recovery of S. mansoni and S. haematobium in rodents, thus suggesting ARA is an endoschistosomicide. Differential vulnerability of developing schistosomes to different levels of circulating ARA may provide an explanation for the limited and inconsistent recovery of experimental schistosome infection in susceptible rodents, and poor or lack of parasite survival in semi-permissive and resistant hosts.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
The research was funded by the Science and Technology Development Fund (STDF) grant ID. 13874 to R. El Ridi and H. Tallima.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016. Wkly Epidemiol Rec 2017; 92(49): p. 749–60. [PubMed]
- 2.Correa-Oliveira R., Pearce E.J., Oliveira G.C., Golgher D.B., Katz N., Bahia L.G. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans R Soc Trop Med Hyg. 1989;83(6):798–804. doi: 10.1016/0035-9203(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 3.Gazzinelli G., Viana I.R., Bahia-Oliveira L.M., Silveira A.M., Queiroz C.C., Carvalho Odos S. Immunological profiles of patients from endemic areas infected with Schistosoma mansoni. Mem Inst Oswaldo Cruz. 1992;87(Suppl 4):139–142. doi: 10.1590/s0074-02761992000800020. [DOI] [PubMed] [Google Scholar]
- 4.Viana I.R., Sher A., Carvalho O.S., Massara C.L., Eloi-Santos S.M., Pearce E.J. Interferon-gamma production by peripheral blood mononuclear cells from residents of an area endemic for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1994;88(4):466–470. doi: 10.1016/0035-9203(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 5.Viana I.R., Correa-Oliveira R., Carvalho Odos S., Massara C.L., Colosimo E., Colley D.G. Comparison of antibody isotype responses to Schistosoma mansoni antigens by infected and putative resistant individuals living in an endemic area. Parasite Immunol. 1995;17(6):297–304. doi: 10.1111/j.1365-3024.1995.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 6.El Ridi R., Farouk F., Sherif M., Al-Sherbiny M., Osman A., El Gengehi N. T and B cell reactivity to a 42-kDa protein is associated with human resistance to both schistosomiasis mansoni and haematobium. J Infect Dis. 1998;177(5):1364–1372. doi: 10.1086/515274. [DOI] [PubMed] [Google Scholar]
- 7.Caldas I.R., Correa-Oliveira R., Colosimo E., Carvalho O.S., Massara C.L., Colley D.G. Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. Am J Trop Med Hyg. 2000;62(1):57–64. doi: 10.4269/ajtmh.2000.62.57. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo J.P., Oliveira R.R., Cardoso L.S., Barnes K.C., Grant A.V., Carvalho E.M. Adult worm-specific IgE/IgG4 balance is associated with low infection levels of Schistosoma mansoni in an endemic area. Parasite Immunol. 2012;34(12):604–610. doi: 10.1111/pim.12001. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira R.R., Figueiredo J.P., Cardoso L.S., Jabar R.L., Souza R.P., Wells M.T. Factors associated with resistance to Schistosoma mansoni infection in an endemic area of Bahia, Brazil. Am J Trop Med Hyg. 2012;86(2):296–305. doi: 10.4269/ajtmh.2012.11-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgi J.R., Wade S.E., Dean D.A. Attrition and temporal distribution of Schistosoma mansoni and S. haematobium schistosomula in laboratory mice. Parasitology. 1986;93(Pt 1):55–70. doi: 10.1017/s0031182000049829. [DOI] [PubMed] [Google Scholar]
- 11.Dean D.A., Mangold B.L., Harrison R.A., Ricciardone M.D. Homologous and heterologous protective immunity to Egyptian strains of Schistosoma mansoni and S. haematobium induced by ultraviolet-irradiated cercariae. Parasite Immunol. 1996;18(8):403–410. doi: 10.1046/j.1365-3024.1996.d01-129.x. [DOI] [PubMed] [Google Scholar]
- 12.Tallima H., Dalton J.P., El Ridi R. Induction of protective immune responses against Schistosomiasis haematobium in hamsters and mice using cysteine peptidase-based vaccine. Front Immunol. 2015;6:130. doi: 10.3389/fimmu.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson R.A. The saga of schistosome migration and attrition. Parasitology. 2009;136(12):1581–1592. doi: 10.1017/S0031182009005708. [DOI] [PubMed] [Google Scholar]
- 14.Clegg J.A., Smithers S.R. Death of schistosome cercariae during penetration of the skin. II. Penetration of mammalian skin by Schistosoma mansoni. Parasitology. 1968;58(1):111–128. doi: 10.1017/s0031182000073479. [DOI] [PubMed] [Google Scholar]
- 15.Knopf P.M., Mangold B.L., Makari G.J. Recovery of parasites at different stages of migration following infection of rats with Schistosoma mansoni. Parasitology. 1983;86(Pt 1):37–49. doi: 10.1017/s0031182000057152. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Leitão F.W., Biolchini C.L., Neves R.H., Machado-Silva J.R. Development of Schistosoma mansoni in the laboratory rat analyzed by light and confocal laser scanning microscopy. Exp Parasitol. 2009;123(4):292–295. doi: 10.1016/j.exppara.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Cêtre C., Cocude C., Pierrot C., Godin C., Capron A., Capron M. In vivo expression of cytokine mRNA in rats infected with Schistosoma mansoni. Parasite Immunol. 1998;20(3):135–142. doi: 10.1046/j.1365-3024.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 18.Cêtre C., Pierrot C., Cocude C., Lafitte S., Capron A., Capron M. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67(6):2713–2719. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cêtre C., Pierrot C., Maire E., Capron M., Capron A., Khalife J. Interleukin-13 and IgE production in rat experimental schistosomiasis. Eur Cytokine Netw. 2000;11(2):241–249. [PubMed] [Google Scholar]
- 20.Khalife J., Cêtre C., Pierrot C., Capron M. Mechanisms of resistance to S. mansoni infection: the rat model. Parasitol Int. 2000;49(4):339–345. doi: 10.1016/s1383-5769(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 21.Badr A.M., Al-Halbosiy M.M.F., El Ridi R. Differential immune responses to excretory–secretory antigens of lung-stage larvae of Schistosoma mansoni in mice and rats. J Basic Appl Zool. 2015;69:26–33. [Google Scholar]
- 22.Amaral K.B., Silva T.P., Malta K.K., Carmo L.A., Dias F.F., Almeida M.R. Natural Schistosoma mansoni infection in the wild reservoir Nectomys squamipes leads to excessive lipid droplet accumulation in hepatocytes in the absence of liver functional impairment. PLoS ONE. 2016;11(11):e0166979. doi: 10.1371/journal.pone.0166979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin S.A., Brash A.R., Murphy R.C. The discovery and early structural studies of arachidonic acid. J Lipid Res. 2016;57(7):1126–1132. doi: 10.1194/jlr.R068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brash A.R. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107(11):1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng H., Shen Y., Shen J., Zhou F., Shen S., Das U.N. Effect of n-3 and n-6 unsaturated fatty acids on prostate cancer (PC-3) and prostate epithelial (RWPE-1) cells in vitro. Lipids Health Dis. 2013;12:160. doi: 10.1186/1476-511X-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiktorowska-Owczarek A., Berezińska M., Nowak J.Z. PUFAs: structures, metabolism and functions. Adv Clin Exp Med. 2015;24(6):931–941. doi: 10.17219/acem/31243. [DOI] [PubMed] [Google Scholar]
- 27.Elinder F., Liin S.I. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front Physiol. 2017;8:43. doi: 10.3389/fphys.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Ridi R., Mohamed S.H., Tallima H. Incubation of Schistosoma mansoni lung-stage schistosomula in corn oil exposes their surface membrane antigenic specificities. J Parasitol. 2003;89(5):1064–1067. doi: 10.1645/GE-3122RN. [DOI] [PubMed] [Google Scholar]
- 29.Tallima H., Salah M., El-Ridi R. In vitro and in vivo effects of unsaturated fatty acids on Schistosoma mansoni and S. haematobium lung-stage larvae. J Parasitol. 2005;91(5):1094–1102. doi: 10.1645/GE-514R.1. [DOI] [PubMed] [Google Scholar]
- 30.El Ridi R., Tallima H. Equilibrium in lung schistosomula sphingomyelin breakdown and biosynthesis allows very small molecules, but not antibody, to access proteins at the host-parasite interface. J Parasitol. 2006;92(4):730–737. doi: 10.1645/GE-745R1.1. [DOI] [PubMed] [Google Scholar]
- 31.El Ridi R., Tallima H., Migliardo F. Biochemical and biophysical methodologies open the road for effective schistosomiasis therapy and vaccination. BBA. 2017;1861(1 Pt B):3613–3620. doi: 10.1016/j.bbagen.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 32.Tallima H., Al-Halbosiy M.F., El Ridi R. Enzymatic activity and immunolocalization of Schistosoma mansoni and Schistosoma haematobium neutral sphingomyelinase. Mol Biochem Parasitol. 2011;178(1–2):23–28. doi: 10.1016/j.molbiopara.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 33.El Ridi R., Aboueldahab M., Tallima H., Salah M., Mahana N., Fawzi S. In vitro and in vivo activities of arachidonic acid against Schistosoma mansoni and Schistosoma haematobium. Antimicrob Agents Chemother. 2010;54(8):3383–3389. doi: 10.1128/AAC.00173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Ridi R., Tallima H., Salah M., Aboueldahab M., Fahmy O.M., Al-Halbosiy M.F. Efficacy and mechanism of action of arachidonic acid in the treatment of hamsters infected with Schistosoma mansoni or Schistosoma haematobium. Int J Antimicrob Agents. 2012;39(3):232–239. doi: 10.1016/j.ijantimicag.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 35.https://chemistry.sciences.ncsu.edu/resource/excel/excel.html. A tutorial. Tutorial Created by: Eric Wiebe Mathematics, Science, and Technology Education NC State University.
- 36.Ashburn L.L. Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer’s technique. Arch Pathol. 1943;36:432–440. [Google Scholar]
- 37.Fowler S.D., Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 38.Ramírez-Zacarías J.L., Castro-Muñozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 39.Mehlem A., Hagberg C.E., Muhl L., Eriksson U., Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8(6):1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 40.Fukumoto S., Fujimoto T. Deformation of lipid droplets in fixed samples. Histochem Cell Biol. 2002;118(5):423–428. doi: 10.1007/s00418-002-0462-7. [DOI] [PubMed] [Google Scholar]
- 41.Wijendran V., Huang M.C., Diau G.Y., Boehm G., Nathanielsz P.W., Brenna J.T. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr Res. 2002;51(3):265–272. doi: 10.1203/00006450-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Barsoum R.S., Esmat G., El-Baz T. Human schistosomiasis: clinical perspective: review. J Adv Res. 2013;4(5):433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barsoum R.S. Urinary schistosomiasis: review. J Adv Res. 2013;4(5):453–459. doi: 10.1016/j.jare.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amaral K.B., Silva T.P., Dias F.F., Malta K.K., Rosa F.M., Costa-Neto S.F. Histological assessment of granulomas in natural and experimental Schistosoma mansoni infections using whole slide imaging. PLoS ONE. 2017;12(9):e0184696. doi: 10.1371/journal.pone.0184696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergquist R., Utzinger J., Keiser J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect Dis Poverty. 2017;6(1):74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.el-Marzouki Z.M., Amin A.M. Changes in serum lipids of mice experimentally infected with Schistosoma mansoni. J Egypt Soc Parasitol. 1997;27(2):419–429. [PubMed] [Google Scholar]
- 47.Doenhoff M.J., Stanley R.G., Griffiths K., Jackson C.L. An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology. 2002;125(Pt 5):415–421. doi: 10.1017/s0031182002002275. [DOI] [PubMed] [Google Scholar]
- 48.Stanley R.G., Jackson C.L., Griffiths K., Doenhoff M.J. Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis. 2009;207(1):131–138. doi: 10.1016/j.atherosclerosis.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y.F., Shen L. Effect of Schistosoma japonicum infection on serum lipid status in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2014;32(1):12–16. [PubMed] [Google Scholar]
- 50.Martins da Fonseca C.S., Pimenta Filho A.A., dos Santos B.S., da Silva C.A., Domingues A.L., Owen J.S. Human plasma lipid modulation in schistosomiasis mansoni depends on apolipoprotein E polymorphism. PLoS ONE. 2014;9(7):e101964. doi: 10.1371/journal.pone.0101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumheuer M.E., Zerfass M., Ruppel A., Leichsenring M. The fatty acid composition of plasma and erythrocytes in Schistosoma mansoni-infected mice. Trop Med Parasitol. 1994;45(1):5–8. [PubMed] [Google Scholar]
- 52.Rumjanek F.D., Simpson A.J. The incorporation and utilization of radiolabelled lipids by adult Schistosoma mansoni in vitro. Mol Biochem Parasitol. 1980;1(1):31–44. doi: 10.1016/0166-6851(80)90039-0. [DOI] [PubMed] [Google Scholar]
- 53.Tallima H., El Ridi R. Arachidonic acid: physiological roles and potential health benefits. A Review. J Adv Res. 2018;11:33–41. doi: 10.1016/j.jare.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenberg R.M. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35(1):1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Jeziorski M.C., Greenberg R.M. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36(6):625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavasse C.J., Brown M.C., Bell D.R. Schistosoma mansoni: activity responses in vitro to praziquantel. Z Parasitenkd. 1979;58(2):169–174. doi: 10.1007/BF01951341. [DOI] [PubMed] [Google Scholar]
- 57.Hannun Y.A. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. doi: 10.1126/science.274.5294.1855. Review. [DOI] [PubMed] [Google Scholar]
- 58.Pompeia C, Lima T, Curi R. Arachidonic acid cytotoxicity: can arachidonic acid be a physiological mediator of cell death? Cell Biochem Funct 2003; 21(2): p. 97–104 (Review). [DOI] [PubMed]
- 59.Han H., Peng J., Gobert G.N., Hong Y., Zhang M., Han Y. Apoptosis phenomenon in the schistosomulum and adult worm life cycle stages of Schistosoma japonicum. Parasitol Int. 2013;62(2):100–108. doi: 10.1016/j.parint.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Peng J., Gobert G.N., Hong Y., Jiang W., Han H., McManus D.P. Apoptosis governs the elimination of Schistosoma japonicum from the non-permissive host Microtus fortis. PLoS ONE. 2011;6(6):e21109. doi: 10.1371/journal.pone.0021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Figard P.H., Hejlik D.P., Kaduce T.L., Stoll L.L., Spector A.A. Free fatty acid release from endothelial cells. J Lipid Res. 1986;27(7):771–780. [PubMed] [Google Scholar]
- 62.Rheinberg C.E., Moné H., Caffrey C.R., Imbert-Establet D., Jourdane J., Ruppel A. Schistosoma haematobium, S. intercalatum, S. japonicum, S. mansoni, and S. rodhaini in mice: relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol Res. 1998;84(4):338–342. doi: 10.1007/s004360050407. [DOI] [PubMed] [Google Scholar]
- 63.Migliardo F., Tallima H., El Ridi R. Is there a sphingomyelin-based hydrogen bond barrier at the mammalian host-schistosome parasite interface? Cell Biochem Biophys. 2014;68(2):359–367. doi: 10.1007/s12013-013-9716-3. [DOI] [PubMed] [Google Scholar]
- 64.Migliardo F., Tallima H., El Ridi R. Rigidity and resistance of larval- and adult schistosomes-medium interface. Biochem Biophys Res Commun. 2014;446(1):255–260. doi: 10.1016/j.bbrc.2014.02.100. [DOI] [PubMed] [Google Scholar]