Graphical abstract
Keywords: Algae, Arachidonic acid, Metabolic engineering, Pathways, Plant, Polyunsaturated fatty acids
Abstract
Some of the essential polyunsaturated fatty acids (PUFAs) as ARA (arachidonic acid, n-6), EPA (eicosapentaenoic acid, n-3) and DHA (Docosahexaenoic acid, n-3) cannot be synthesized by mammals and it must be provided as food supplement. ARA and DHA are the major PUFAs that constitute the brain membrane phospholipid. n-3 PUFAs are contained in fish oil and animal sources, while the n-6 PUFAs are mostly provided by vegetable oils. Inappropriate fatty acids consumption from the n-6 and n-3 families is the major cause of chronic diseases as cancer, cardiovascular diseases and diabetes. The n-6: n-3 ratio (lower than 10) recommended by the WHO can be achieved by consuming certain edible sources rich in n-3 and n-6 in daily food meal. Many researches have been screened for alternative sources of n-3 and n-6 PUFAs of plant origin, microbes, algae, lower and higher plants, which biosynthesize these valuable PUFAs needed for our body health. Biosynthesis of C18 PUFAs, in entire plant kingdom, takes place through certain pathways using elongases and desaturases to synthesize their needs of ARA (C20-PUFAs). This review is an attempt to highlight the importance and function of PUFAs mainly ARA, its occurrence throughout the plant kingdom (and others), its biosynthetic pathways and the enzymes involved. The methods used to enhance ARA productions through environmental factors and metabolic engineering are also presented. It also deals with advising people that healthy life is affected by their dietary intake of both n-3 and n-6 FAs. The review also addresses the scientist to carry on their work to enrich organisms with ARA.
Introduction
Polyunsaturated fatty acids (PUFAs) are represented by two families: n-6 (or ω-6) and n-3 (or ω-3), which are biosynthesized from linoleic acid (LA) and linolenic acid (ALA), respectively. These two fatty acids (FAs) are essential for human fitness. In n-3 PUFAs family, Alfa-linolenic acid (α-ALA, C18:2, n-3), EPA (C20:5, n-3) and DHA (C22:6, n-3) are the main representatives. While n-6 PUFAs include γ- linoleic acid (LA, C18:3, n-6) and ARA (C20:4, n-6). PUFAs especially n-3 series are necessary nutrients for health, growth and development of human and animals [1]. EPA and DHA (n-3) play an important role in the cardiovascular system and treating psychiatric disorders [2]. DHA being an essential FA, it can protect against neuro-generative diseases as Alzheimer and Parkinson as well as multiple sclerosis diseases [3].
There must be an equilibrium between ω-3 and ω-6 fatty acids (FAs) in our daily meals because both work together to promote healthy life. ω-3 FAs exhibits anti-inflammatory and antioxidant activities and prevent breast cancer. On the contrary, ω-6 FAs, precursors of arachidonic acid, promote inflammation, tumor growth [4], [5]. Larger amounts of n-6 over n-3 PUFAs appear to be directly proportional to the increased pathogenesis of acute diseases (as coronary heart disease) [6]. Due to the benefits of PUFAs to human and animals, high amount of PUFAs supplement are needed. But the scarcity of PUFA biological resources always limited their wide application [7], [8].
The objective of this review was to record the importance of the C20 PUFA termed arachidonic acid (C20:4, ɷ6), its different sources, biosynthetic pathways, its derivatives (eicosanoids) and their functions, the balance between ɷ6 and ɷ3 fatty acids to keep healthy life as well as how to increase ARA content either through environmental and growth culture conditions and/or metabolic engineering techniques.
Importance of arachidonic acid
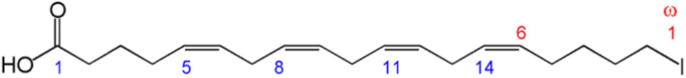
ARA (C20H32O2, C20:4) is a long chain polyunsaturated fatty acid (LC-PUFA) of ω-6 family also known as 5,8,11,14-eicosatetraenoic acid [9] (Fig. 1).
Fig. 1.
Chemical configuration of arachidonic acid. Adapted from Llewellyn [9].
It is considered as an important constituent of the biomembranes, a precursor of prostaglandins and many other eicosanoids. Both ARA and DHA (C22:6, ɷ-3) are the major constituents of the brain phospholipid membrane, can act as an immune-supressant, and induce inflammatory responses, blood clotting and cell signalling [10], [11], [12], [13]. Free ARA and its metabolites are important for the function of skeletal muscle and nervous system as well as the immune system for the resistance to allergies and parasites. Oxidation-independent ARA derivatives are necessary for stress responses, pain and emotion [14]. Their deficiency can cause dramatic problems as hair loss, fatty liver degeneration, anemia and reduced fertility in adults [10]. The insufficient synthesis of ARA in premature infants encourage the Food and Agricultural Organization (FAO)/World Health Organization (WHO) to propose the supplementation of ARA in the neonates’ formula (non-breast feeding) for their best growth and development (central nervous system and retina) [15]. ARA also acts as natural antifreeze compound to arctic animals and reindeer when feed on mosses. Although mosses have low nutritional values, high level of ARA help these animal cells working at low temperatures as an adaptive mechanism [16].
Sources of arachidonic acid
Microbes
Many microbes including fungi, yeast and some bacteria have the ability to synthesize significant amounts of LC-PUFAs, mainly ARA [17], [18], [19], [20], [21], [22], [23]. Psychrophilic bacterium Flavobacterium strain 651 produced 1.4–2.7% ARA [20]. The higher ARA-producers were the non-pathogenic fungi Mortierella spp. from which the species M. alpina 1S-4 and ATCC 32,222 produced ARA up to 70% of lipids [24], [25], [26], [27].
Algae
Cyanobacteria (blue-green algae)
In unicellular, non-heterocystous and heterocystous cyanobacterial species, no ARA was detected but different C18 FAs (C18:1, C18:2, C18:3 (α- and γ-types) as well C18:4 FAs) [28]. According to Pushparaj et al. [29], ARA was only found in cyanobacterium, Phormidium pseudopristleyi strains 79S11 and 64S01 recording 24% and 32% of their total FA contents, respectively.
Microalgae
Porphyridium purpureum is a unicellular red alga that approximately the only microalga reported to produce significant quantity of ARA. Under stress culture conditions (suboptimal light intensity, pH and temperature, increased salinity and limited nutrients), ARA production may reach as much as 40% of the total FAs, while in the favorable growth conditions PUFA largely represented by eicosapentaenoic acid (EPA), as reported by many investigators [30], [31], [32], [33], [34], [35]. Euglena gracilis was recorded to contain ARA which was synthesized from LA (C18:2) [36].
The fresh-water green alga Parietochloris incisa is considered the richest plant source of ARA, which reached 77% of total FAs content [37]. The biosynthetic pathway of this PUFA was known by labeling the algal culture with radioactive precursors (pulse follow labeling with [2-14C]sodium acetate) which was incorporated via new FAs biosynthetic pathway. Through elongation and desaturation, C20 PUFAs were synthesized. The main labeled FAs just after the pulse were 16:0, 16:1 and 18:1, however, all other C18 as well as C20 FAs were already labeled (after short pulse, 0.5 h) [38]. Labeled acetate involved in the new synthesis and elongation of C18 to C20 FAs. Similar phenomena occur in Pavlova lutheri [39]. During the track, ARA became the second most labeled FA after 16:0. The presence of labeled 18:1, 18:2, 18:3n-6 and 20:3n-6 indicated that the biosynthetic pathway leading to ARA is the same as that of Porphyridium cruentum [39]. Labelling of oleic acid ([1-C14] OA) suggested rapid conversion of 18:1 to 18:2, 18:3 to 20: 3n-6 and ARA through the n-6 pathway. Fatty acids shorter than 18:1 were not labeled. Parietochloris incisa, contrary to higher plants, algal triacylglycerols (TAG) contains saturated (SFAs) and monounsaturated fatty acids (MUFAs) accumulate PUFAs within TAG lipids [32].
ARA has been identified in many algal groups which grow photoautotrophically or heterotrophically. The biosynthetic pathway of PUFAs involves elongation of the short chain fatty acid followed by progressive desaturation using desaturases (Des) and elongases (Elo) [36]. Many earlier studies were performed based on screening for PUFAs presence in marine microalgae as well as in different seaweeds belonging to various algal divisions (Phaeophyceae, Rhodophyceae, Dinophyceae, Chlorophyceae) [41], [40], [42]. Screening of ARA presence in green microalgae Myremica incisa [43] and Parietochloris incisa [37], [44] and following the pathway of its biosynthesis by labeled acetate was recorded. Red microalgae are used for testing the different environmental and culture conditions on FA and ARA production using the algal species Porphyridium purpureum, P. cruentum, Ceramium rubrum and Rodomella subfusca where ARA production reached 40–60% of total FAs content [30], [31], [34], [35], [45], [46], [47]. Diatoms were recorded to contain great amount of ARA and C22 FAs. From diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana were selected for genetic manipulation and altering culture requirements for PUFAs biosynthesis [38], [48], [49], [50]. Not only ARA was detected in variable amounts in Chryso, Crypto, Hapto, Dino, Phaeo and Rodophycean species but also C18 and C22 FAs (with 4, 5 and 6 double bounds) [51], [45].
Macroalgae
Marine macroalgae are considered as an excellent wellspring of PUFAs with ω-6 FA: ω-3 FA ratio less than 10 which is largely recommended by the WHO to prevent inflammatory, cardiovascular and neuro-chronic sickness [52]. The red alga Palmaria palmata contains EPA as predominant fatty acid as well as a marginal concentration of ARA and LA. In the red alga Gracilaria sp., ARA can reach 60% of total FAs content [53], [54]. The brown seaweed Sargassum natans have DHA as reported by Van Ginneken et al. [52] who analyzed the fatty acid composition of nine seaweeds (four brown, three red and two green). The investigated green seaweeds (Ulva lactuca, Caulerpa taxifolia) showed no ARA.
Pereira et al. [45] investigated seventeen macroalgal species from Chlorophyta, Phaeophyta and Rhodophyta as novel dietary sources of PUFAs. They recorded that the major PUFAs in all phyta were C18 and C20 (LA, ARA and EPA). They reported that Rhodophycean and Phaeophycean investigated species showed higher concentration of PUFAs especially of ω-3 family. Ulva sp. was the only Chlorophyta which presented high concentration of ω-3 PUFA (ALA). Macroalgae can be deeming as a potential source of essential PUFA which may provide human beings with the needed FAs in their diets when it is used as foods or food products.
El-Shoubaky et al. [41] investigated four marine seaweeds (three green; Enteromorpha intestinalis, Ulva rigida, U. fasciata and one red; Hypnea cornuta) for their essential FA contents. They emphasized that the red alga Hypnea cornuta produce ARA and EPA by 1.09 and 6.26%, respectively which disappear from the tested green algal samples. The authors mentioned the presence of Oleic acid (C18:1, ω-9). Omega-9 family is necessary and the body can manufacture the required amount by itself and doesn’t need to be supplemented. Also, the red seaweed Porphyra sp. contains the essential FAs; ALA, ARA and EPA as mentioned by Sánchez-Machado et al. [55].
Barbosa et al. [56] performed a review dealing with oxylipins biosynthesis (oxygenated derivatives of PUFA) in macroalgae and their biological activities. They recorded the marine oxylipins derived from lipoxygenases (LOX) metabolism of PUFA precursors (of C16 to C22) and unsaturation types (ω3, ω6, ω9) [57]. Similar to higher plants, Chlorophyta oxidize C18 substrates, while Rhodophyta exploit C18 and C20 PUFAs for oxylipin production. In algal systems, oxidized FA derivatives may participate in defense mechanisms against pathogenic infection, injuries, metal toxicity or other stresses [53], [54], [58], [59], [60], [61], [62], [63].
Studies concerning macroalgae proposed that metabolic pathway of octadecanoid may be derived from the chloroplast, while eicosanoid pathway may be from ancient eukaryotes. So, microalgae are able to metabolize C18 PUFA at C9, C11 and C15 through 5-, 8-, 12- and 15-lipoxygenases, respectively [64]. Different from macroalgae, Diatoms (microalgae) has no C18 PUFA-derived Lox products [65].
Lichens
ARA was detected in some species of lichens (symbiosis association between fungi and algae). According to Yamamoto and Watanabe [66], small amount of ARA was detected in Cetraria pseudocomplicata (5.2%), Cladonia mitis (2.3%), and Nephroma arcticum (1.7%). Rezanka and Dembitsky [67] found ARA in 8 lichens collected in the Tian Shan mountains of Kirghizstan; 1.47% in Peltigera canina, 1.90% in Xanthoria sp., 2.39% in Acarospora gobiensis, 2.52% in Cladonia furcate, 2.92% in Parmelia tinctina, 3.43% in P. comischadalis, 3.64% in Lecanora fructulosa and 4.17% in Leptogium saturninum. ARA composition of the lichen Ramalina lacera varied from 0.96 to 2.25% according to the type of substrate it grown on [68]. Epiphytic lichens of Collema species (Collema flaccidum and C. fuscovirens) recorded 1.9% and 2.1% ARA [69]. Lichens Cetraria islandica and Xanthoria parietina recorded 2708.8 and 24535.4 pmol/g plant weight, respectively [70].
Plants
All the paragraph will be changed to: ARA was found in lower plant species; Liverworts [70], Mosses [70], [71], [72], [73], [74], [75], Hornworts, Lycophytes and Monilophytes [70]. ARA was also detected in seagrasses [76]. Some higher terrestrial plants have little amounts of ARA [70], [77], [78], [79]. Table 1 summarized amounts of ARA in species of the plant kingdom.
Table 1.
ARA amounts in species of plant kingdom.
| Type | Species | ARA contents* | References |
|---|---|---|---|
| Liverworts | Conocephalum conicum | 1233225.6+ | [70] |
| Marchantia polymorpha | 903496.0+ | ||
| Riccia fluitans | 452189.2+ | ||
| Mosses | Marchantia polymorpha | 92# | [71] |
| Physcomitrella patens | 15.9–18.7 | [72] | |
| Pottia lanceolata | 6–10 | ||
| Atrichum undulatum | |||
| Brachythecium rutabulum | Up to 31 | ||
| Rhynchostegium murale | |||
| Mnium cuspidatum | 30 | [73] | |
| Mnium medium | |||
| Hylocomium splendens | |||
| Pleurozium schreberi | |||
| Mnium hornum | 26.03 | [74] | |
| Mnium hornum | 26.03 | [74] | |
| Leptobryum pyriforme | 20 | [75] | |
| Physcomitrella patens | 2648874.2+ | [70] | |
| Funaria hygrometrica | 898972.3+ | ||
| Polytrichum juniperinum | 35394.6+ | ||
| Hedwigia ciliate | 20046.8+ | ||
| Hylocomium splendens | 86608.3+ | ||
| Hornworts | Anthoceros agrestis | 69691.7+ | [70] |
| Anthoceros punctatus | 24687.6+ | ||
| Phaeoceros laevis | 316375.9+ | ||
| Lycophytes | Huperzia phlegmaria | 83663.7+ | [70] |
| Monilophyte (fern) | Polypodium vulgare | 44425.8+ | [70] |
| Davallia canariensis | 2884.3+ | ||
| Tectaria zeylanica | 3848.3+ | ||
| Polystichum aculeatum | 16165.1+ | ||
| Onoclea sensibilis | 32079.4+ | ||
| Blechnum spicant | 7979.1+ | ||
| Thelypteris palustri | 6753.9+ | ||
| Gymnocarpium robertianum | 12083.0+ | ||
| Asplenium trichomanes | 20835.6+ | ||
| Adiantum venustum | 3516.8+ | ||
| Sphaeropteris cooperi | 75994.9+ | ||
| Salvinia natans | 4784.5+ | ||
| Salvinia molesta | 13175.0+ | ||
| Anemia phyllitidis | 307394.3+ | ||
| Lygodium volubile | 12143.7+ | ||
| Osmunda regalis | 64628.3+ | ||
| Angiopteris evecta | 1386.0+ | ||
| Equisetum trachyodon | 6125.1+ | ||
| Seagrasses | Cymodocea sp. | 0.3–2.3 | [76] |
| Thalassia sp. | |||
| Enhalus sp. | |||
| Halodule sp. | |||
| Higher terrestrial plants | Agathis araucana | 26773.4+ | [70] |
| Beta maritima L. (wild beet) | 0.52 | [77] | |
| Cardaria draba L. (hoary cress) | 0.56 | ||
| Chenopodium album L. (goosefoot) | 1.30 | ||
| Chenopodium murale L. (goosefoot) | 1.01 | ||
| Malva sylvestris L. (common mallow) | 5.30 | ||
| Plantago major L. (plantain) | 1.02 | ||
| Sisymbrium irio L. (hedge mustard) | 0.32 | ||
| Sonchus tenerrimus L. (sow-thistle-of-the-wall) | 1.83 | ||
| Stellaria media Villars (chickweed) | 0.41 | ||
| Verbena offieinalis L. (vervain) | 0.62 | ||
| Araucaria bidwillii | 0.6 | [78] | |
| Araucaria cunninghamii | 4.6 | ||
| Araucaria araucana | 8.7 | ||
| Agathis robusta | 2.00 | ||
| Agathis araucana | 0.5 | ||
| Agathis dammara | 5.2 | ||
| Artemisia armeniaca | 6.47 | [79] | |
| Artemisia incana | 7.79 | ||
| Artemisia tournefortiana | 2.61 | ||
| Artemisia hausknechtii | 7.44 | ||
| Artemisia scoparia | 3.17 | ||
% of total FAs.
mg/L under photomixotrophic conditions.
pmol/g plant weight.
Others
The major supply of ARA is from marine fish oil and animal tissues [80]. In aquaculture and marine ecosystem, ARA, EPA and DHA are the main food constituents of the larvae of many aquatic organisms. Some species of shrimps, bivalves and abalone had intermediate amount of ARA, while sea cucumber, starfish and some species of corals had higher level of ARA (20–30%) [76]. Really, fishes aren’t the real producers of PUFA; fishes only heap them by the intake of PUFA-rich microalgae through food-chain [48]. Mammals including humans cannot synthesize ARA directly due do the genetic absence of some of its biosynthesis enzymes [43]. Therefore, human and animal needs for ARA must require supplementation via dietary intake of its precursors [81].
Biosynthesis of arachidonic acids
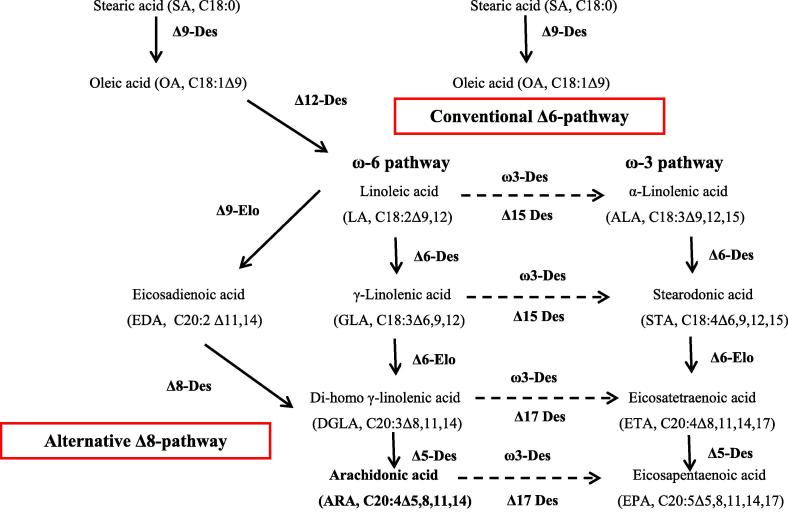
The entire genes involved in LC-PUFAs biosynthesis have been distinguished in animals, plants, mosses, fungi, algae and aquatic organisms. Within these organisms, two different pathways have been identified for the synthesis of ARA (C20:4, ω-6) depends on the action and types of both desaturases (Des) and elongases (Elo) on linoleic acid [82], [83], (Fig. 2). The first pathway is the conventional Δ6-pathway in eukaryotes and the second is the alternative Δ8-pathway in protists and some microalgae [84].
Fig. 2.
Conventional and alternative pathways for the biosynthesis of ARA after Venegas-Caleron et al. [82] and Ruiz-Lopez et al. [83]. Des, desaturase; Elo, elongase.
In plants, LC-PUFAs syntheses start in plastids with the formation of FAs using fatty acid synthase (FAS) complex. Stearic acid (SA, C18:0) is desaturated to Oleic acid (OA, C18:1Δ9) by Δ9-Des. Some terrestrial plants, cyanobacteria and microbes have Δ12-Des which convert OA to linoleic acid (LA, C18:2Δ9,12, ω-6).
Human and animals have lost their ability to synthesize LC-PUFAs due to the absence of Δ12-Des gene and consequently cannot produce LA from OA [85], but have restricted potential to synthesize ARA [86]. Most of the synthesized ARA is provided by β-oxidation of small portion of the dietary LA [81].
In the conventional pathway, the Δ6-Des converted LA (n-6) to gamma-linolenic acid (GLA, C18:3Δ6,9,12), which in turn yielded dihomo-γ-linolenic acid (DGLA, C20:3Δ8,11,14) by Δ6-Elo. Finally, Δ5-Des produces ARA (C20:4Δ5,8,11,14, n-6).
In alternative Δ8-pathway, the Δ9-Elo converts LA to form eicosadienoic acid (EDA, C20:2Δ11,14) which in turn with the help of Δ8-Des generates DGLA, then to ARA by Δ5-Des.
Arachidonic acid and other FA metabolism in algae
Biosynthesis of PUFAs by algae can progressively desaturate monoenoic acids yielding di- and poly-enoic acids. Nichols and Wood [87] examined FA metabolism in the chloroplast of many algae. He showed that, cyanobacteria and green algae incorporate radioactive acetate efficiently into the FAs of their polar lipids with no differences in the rate of labeling in different lipids.
Nichols and Appleby [36] reported that Ochromonas danica and Porphyridium cruentum (Rhodophyceae) synthesized ARA (C20:4) through a pathway involving γ-linolenic acid (C18:3). Whereas Euglena gracilis (Euglenophyceae) was incapable of converting γ-linoleic acid to C20:2 ɷ-6 then to ARA (but use α-linoleic acid, C18:2, Δ9, 12). TAG are indigent in PUFAs and are composed of saturated (SFAs) and monounsaturated fatty acids (MUFAs) will be: composed of SFAs and MUFAs. TAG of only few algae have PUFAs as EPA and ARA in P. cruentum [31] and EPA in Ectocarpus fasciculatus [88]. In P. cruentum, C18:1 is stepwise desaturated to C18:2 and C18:3 ɷ-6 before it is elongated to C20:3 ɷ-6 and then (by Δ5) desaturased to C20:4 ɷ-6 (ARA) as demonstrated by Khozin et al. [89].
The biosynthesis of LC-PUFAs in microalgae was understood by using several inhibitors as (SHAM): 4-chloro-5(dimethylamino)-2-phenyl-3(2H) pyridazinone and SAN 9785, BASF13-338, which are selective inhibitors of the ω-3 chloroplastic desaturase [90]. SAN9785 was shown to inhibit the assembly of TAG [91], while SHAM (Salicyl hydroxamic acid) was proved to affect both Δ12 and Δ15 microsomal Des in root of wheat seedlings and in cotyledons of linseed [92]. SHAM was recently shown to inhibit the Δ6 desaturation of LA in P. cruentum. SHAM or SAN 9785 can hinder either ARA production or TAG accumulation in P. incisa. Labeling investigations indicated that ARA accumulated in TAG could be transported to polar lipids as a response to low temperature stress in the experimental alga [32], [93].
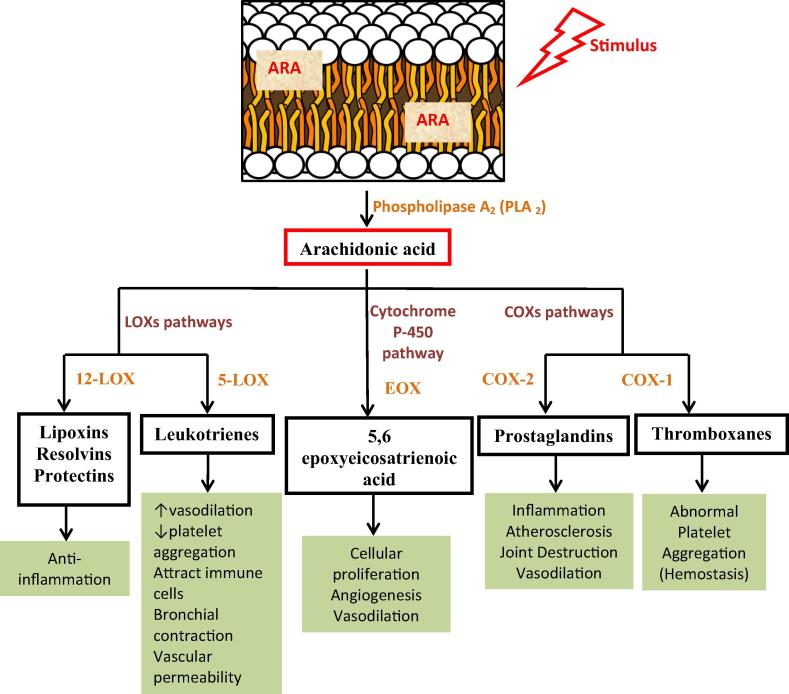
Arachidonic acids avalanche and eicosanoids
ARA is localized in the sn-2 position of phospholipid in membranes. Firstly, ARA is released from the membranes phospholipids by phospholipase A2 (PLA2). It is the precursor of C20 PUFAs known as eicosanoids which is formed through ARA cascade via three different pathways (Fig. 3): cyclooxygenase (COX), cytochrome P-450 (cyt P-50) or lipoxygenase (LOX). Many eicosanoids exhibit biological and pharmaceutical activities which may have physiological or pathological values [12], [13]; ω-6 ARA produces powerful inflammatory, immune-active and pro-aggregatory eicosanoids, while those derived from ω-3 FAs are anti-inflammatory and modulate plaque aggregation and immune-reactivity [94], [95].
Fig. 3.
Production of eicosanoids from arachidonic acid and their harmful effects. Adapted after Neitzel [12] and Pratt and Brown [13]. PLA2, phospholipase A2; COX, cyclooxygenase; LOX, lipoxygenase; EOX, epoxygenase.
Factors promoting arachidonic acid biosynthesis
Environmental and growth culture conditions
High yield of ARA always achieved in unfavorable conditions which reduced cell growth. Both high algal biomass and ARA content were stimulated by the addition of small amount of the phytohormone 5-aminolevulinic acid (20 mg/l) to the algal culture medium of the red microalga Porphyridium purpureum. Studies pivot on green algae as Parietochloris incisa and Myrmecia incisa for the improvement of ARA synthesis through the optimization of growth culture conditions [44], [96]. Environmental factors (light, temperature, pH, …) and culture conditions (chemical composition of media, stress, …) may affect lipid profile and PUFA proportion but have no direct effect on ARA production.
Metabolic engineering of arachidonic acids
Genetically modified crops and microalgae emanate as divergent source of PUFAs [97], [98]. Significant improvement has been made to identify the genes implicated in LC-PUFAs biosynthesis of numerous organisms [81], [99], [100], [101] and utilize them for the formation of transgenic plants, microbes and algae with novel FAs as ARA or over-expressing its amounts in the naturally producing tissues. Plants possess the ability to be green factories for the yield of non-native important compounds via metabolic engineering [102], [103], [104]. The main goal of the metabolic transgenic plants is the accumulation of high levels of LC-PUFAs especially ARA, which would provide a novel and cost-effective spring of these FAs [105], [106].
Transgenic with Bryophyte genes
The Bryophyte Marchantia polymorpha L. produces ARA from linoleic acid by a successive reactions catalyzed by Δ6-desaturase, Δ6-elongase, and Δ5-desaturase genes [107].
Kajikawa et al. [108] separated a β-ketoacyl CoA synthase (KCS) gene, MpFAE2 from liverwort M. polymorpha, and distinguished its substrate peculiarity using dsRNA-mediated gene silencing (MpFAE2-dsRNA) technique as well as studying its overexpression (MpFAE2-Overexpression). Transgenic Marchantia plants with MpFAE2-dsRNA accumulated about 1.3–1.6 folds of ARA as compared with the amount present in thalli of wild type (2.7% of total FAs), while the transgenic ones overexpressing the MpFAE2 gene produce an amount nearly similar to the wild type (2.6–3.2% of total FAs).
Kajikawa et al. [109] isolated and characterized the three cDNAs coding for 6-desaturase (MpDES6), 6-elongase (MpELO1), and 5-desaturase (MpDES5) from M. polymorpha. The presence of LA and ALA in the wild-type yeast Pichia pastoris encouraged Kajikawa and his co-authors to co-express these genes in this yeast. The metabolic engineered yeast could accumulate ARA (0.1% of the total lipid). They referred the increase in ARA yield to MpDES6 which use LA in both glycerolipids and acyl-CoA pool so, facilitate substrate supply to MpELO1.
Few years later, Kajikawa et al. [110] overexpressed these native three genes in the same liverwort, while newly introduced and co-expressed them in both Nicotiana tabacum cv. Petit Havana SR1 and Glycine max cv. Jack plants. Transgenic M. polymorpha plants yield an improvement of ARA 3-folds more than the wild type. The production of ARA in transgenic tobacco plants were up to 15.5% of the total FAs in the leaves and 19.5% of the total FAs in the seeds of transgenic soybean plants. These results proposed that M. polymorpha can provide genes critical for ARA-engineering in plants.
Transgenics with fungal genes
Many studies describing efforts to perform transgenes carrying genes encoding for desaturase and elongase isolated from the fungus Mortierella alpina. Parker-Barnes et al. [99] demonstrated that the coexpression of elongase and Δ5-desaturase genes from M. alpina in yeast could produce 1.32 μg endogenous ARA. Seed-specific expression of Δ6, Δ5 desaturase and GLELO elongase genes from M. alpina combined with the endogenous Δ15-desaturase in soybean plant led to the production of 2.1%, 0.8% and 0.5% ARA in transgenic embryos, T1 and T2 seeds, respectively [111].
Transgenics with algal genes
Transgenic production of ARA in oilseeds was performed using Des and Elo originated from marine microalgae. Petrie et al. [112] focused on constructing a microalgal Δ9-elongase pathway in oilseeds. They found that the seed-specific expression of a Δ 9-elongase of the alga Isochrysis galbana and Δ8- and Δ5-desaturases of the alga Pavlova salina in Arabidopsis thaliana plant produced 20% ARA in seed oil, while their expressions in Brassica napus plant yielded 10% ARA in seed oil. They found that the bulk of ARA was naturally improved at sn-2 position in triacylglycerol.
Transgenics with heterogenous genes
Several reports were conducted to produce and increase the yield of ARA in transgenics using the suitable diverges of sources and combinations of genes encoding from ARA-producing organisms. Metabolic engineering using the fatty acids front-end Des from the marine diatom Phaeodactylum tricornutum was firstly recorded by Domergue et al. [113]. The genes encoding for Δ5- and Δ6-desaturases (PtD5 and PtD6) were expressed in the yeast Saccharomyces cervisiae to determine their role in EPA biosynthesis and no ARA was recorded in this case. While co-expressing both PtD5 and PtD6 desaturases with Δ6-elongase from the moss P. patens (PSE1) in yeast induced 0.17% ARA of the total FAs in the presence of 250 μM FA (C18:2Δ9,12) in the culture medium. They mentioned that these reconstructs showed similar function of both Des in the ω3 and ω6 pathways present in this unicellular diatom.
Abbadi et al. [106] selected genes encoding for desaturases (Δ6 and Δ5) and a Δ6-elongase from Mortierella alpina (fungi), Phaeodactylum tricornutum (diatom, algae), Physcomitrella patens (mosses), Borago officinalis (plant) and Caenorhabditis elegans (lower animals). They found that genes encoding for Δ6- and Δ5-desaturases from diatom Phaeodactylum tricornutum and Δ6-elongase from the moss Physcomitrella patens were the useful combination for ARA productions. Seed-specific expression of those genes in linseed (Linum usitatissimum) and tobacco (Nicotiana tabacum) plants able them to produce non-native ARA (absent in wild-types) recording 1% and 1.5% of the total seed FAs, respectively. They refer the low yield of ARA in these transgenes due to substrate incompatibility produced by the enzymes of the two organisms as diatom Δ6-desaturase uses acyl groups in the glycerolipid pool, while moss Δ6- elongase uses the acyl-CoA pool. The movement of FAs by lysophosphatidyl acyltransferase activity between these pools is slow in higher plants causing an inadequate feeding of substrate to Δ6- elongase.
Similarly, Kinney et al. [114] expressed genes encoding the Δ6-desaturase pathway in seeds and somatic embryos of soybean plant using Δ6-desaturase from the fungus Saprolegnia diclina or M. alpina in addition to Δ5-desaturase and Δ6-elongase from M. alpina. They found that the transgenic somatic embryos produced twice the yield of ARA compared to transgenic seeds. By adding an Arabidopsis FAD3 gene and a S. diclina Δ17-desaturase to the previous construct, almost no ARA was detected.
In order to compass this problem and accumulate higher amount of ARA, Qi et al. [105] transformed A. thaliana plant with genes encoding for Δ9-elongase from alga Isochrysis galbana, Δ8-desaturase from alga Euglena gracilis and Δ5-desaturase from the fungus Mortierella alpina. The leaves of transgenic A. thaliana plants accumulated ARA of about 6% of the total FAs. This alternative pathway permit the Δ9-elongated FAs to traffic efficiently from the acyl-CoA to glycerolipid pool to be used as substrates by both Δ8- and Δ5-desaturases leading to a high conversion rate.
Using a similar approach, Wu et al. [115] studied the production of ARA in transgenic Brassica juncea plants (breeding line 1424) by the stepwise addition of gene(s) from the LC-PUFA pathway to the construct binary vector. The first construct contained Δ5-desaturase from the fungus Thraustochytrium sp., a Δ6-desaturase from the fungus Pythium irregulare, and a Δ6-elongase from the moss Physcomitrella patens producing 7.3% ARA. While the addition of Δ12-desaturase of the plant Calendula officinalis to the construct achieving high production of ARA (12% of total seed FAs). Addition of Δ6/ Δ5-elongase of Thraustochytrium sp to the transgenic B. juncea plant achieved a small significant increment of ARA reaching 13.7% of total seed FAs. While by adding ω3/Δ17-desaturase of fungus Phytophthora infestans to the construct a decrease in ARA amount were recorded. Moreover, further introduction of Δ6/Δ5-elongase from the fish Oncorhynchus mykiss as well as Δ4-desaturase and a lysophophatidic acid acyl transferase of fungus Thraustochytrium sp. improves the movement of LC-PUFAs between the acyl-CoA and glycerolipid pools producing 9.6% of C20-C22 n-3 FAs, but only 4% ARA of total seed FAs.
Avoiding the “elongation bottleneck”, Robert et al. [116] use group of genes encoding elongation and desaturation for LC-PUFA to be expressed in the model plant A. thaliana. Δ5/Δ6 desaturase from the zebrafish Danio rerio (D5/D6Des) in combination with Δ6-elongase from the nematode Caenorhabditis elegans (D6Elo) were introduced in Arabidopsis recording 0.2–1.4% ARA in seeds. Transgenic plant with a second construct bearing genes encoding for Δ4-desaturase (D4Des) and Δ5-elongase (D5Elo) from the microalga Pavlova salina detected lower ARA in seeds. Employing the acyl-CoA dependant desaturase (Δ5/Δ6) revealed high production of C20 PUFA than the acyl-PC pathway.
Due to the similarity between the acyl-CoA-dependent Δ6-pathway and the alternative Δ8-pathway through LA-CoA and ALA-CoA, Sayanova et al. [117] isolated a gene coding for C20 Δ8-desaturase from soil amoeba, Acanthamoeba castellanii. This amoeba has the capability of synthesis and accumulation of ARA through the alternative Δ9 elongation/Δ8 desaturation pathway. Successive expression of Δ8- and Δ5-desaturation from A. castellanii in the yeast Saccharomyces cerevisiae strain W303-1A revealed the formation of small amounts of ARA in their transgenic cells. Similar unpredicted yield of C20 FAs (ARA) in acyl-CoA pool was reported in the leaf tissues of the transgenic Arabidopsis plants coexpressing both Δ8-desaturase of the amoeba A. castellani and Δ9-elongase of alga Isochrysis galbana.
Hoffmann et al. [118] isolated genes encoding for acyl-CoA-dependent EPA biosynthesis Δ6- and Δ5-desaturases from both microalgae Mantoniella squamata (MsΔ6, MsΔ5) and Ostreococcus tauri (OtΔ6, OtΔ5) and the moss P. patens (PtΔ6, PtΔ5). All these genes were successfully established in seeds of A. thaliana plants under the control of a seed-specific promoter Δ6-elongase PSE1 from the moss P. patens. Transformed Arabidopsis signed as triple-Ms. plants (MsΔ6, MsΔ5, PSE1), triple-Ot (OtΔ6, OtΔ5, PSE1) and triple-Pt plants (PtΔ6, PtΔ5, PSE1) were constructed to avoid the bottleneck described by Abbadi et al. [106]. The FAs analysis of T2 seeds of transgenic plants showed the induction of new FAs and denoting that triple-Ms. plants has an established ω3 pathway, while triple-Ot and triple-Pt plants has both the ω6 and ω3 pathways so, this indicate that the modified pathway enhance the flux during LC-PUFA biosynthesis. They also reported the formation of non-native ARA in transgenic plants showing its highest yield in triple-Ms plants (>0.8%) followed by triple-Ot plants (0.8%) and finally triple-Pt plants (<0.4%). Their results supported the possibility of using acyl-CoA-dependent EPA biosynthesis to solve the problem of substrate dichotomy.
Savchenko et al. [119] compared two techniques for producing transgenic Arabidopsis lines using Δ8-desaturation pathway. The first technique is to sequential introduction the genes of Δ9-elongase from alga Isochrysis galbana, Δ8-desaturase from the alga Euglena gracilis and Δ5-desaturase from the fungus Mortierella alpina in Arabidopsis (EP1) according to Qi et al. [105]. The second one is to introduce them together in Arabidopsis plant (EP2). The analysis of FA composition of the transgenic leaves revealed that EP1 contained more ARA (0.42%) than EP2 (0.25%).
In an endeavor to identify the optimal combination between host plant species (Brassica carinata and B. juncea), genes and promoters for the accumulation of high levels of PUFAs, Cheng et al. [120] constructed three, four and five gene- constructs signed as Napin-3, Napin-4 and Napin-5. Napin-3 construct was made by inserting a Δ6-desaturase gene from fungus P. irregulare (PiΔ6), Δ5-desaturase gene from the fungus Thraustochytrium sp. ATCC 26,185 (TcΔ5) and an elongase gene from the diatom Thalassiosira pseudonana (TpElo) into this cassette. Napin-4 contained the desaturase gene CpDesX from the fungus Claviceps purpurea and five-gene construct Napin-5, contained the ω3 desaturase gene (Pir-ω3) from P. irregular. Total FA composition of oilseeds revealed that all transgenic plants produced non-native ARA recording 4.3% in zero-erucic B. juncea line 1424, 2.8% in higher erucic line C90-1163 and 5.7% in zero-erucic line10H3 of B. carinata.
Ruiz-Lopez et al. [121] constructed three different constructs (JB7, JB352 and JB289) for Arabidopsis transformation via floral dip to study the non-native heterogenous transgenic activities of Des and Elo to accumulate high level of LCPUFAs (as ARA). The JB7 consists of Δ6-desaturase from the fungus Pythium irregular (PiD6), Δ5-desaturase from the fungus Thraustochytrium sp. (TcD5), Δ6-elongase from the moss Physcomitrella patens (PSE1) and Δ15-desaturase gene from the plant Linum usitatissimum (LuD15) under the control of conlinin promoter (Cnl) as reported previously by Cheng et al. [120]. The JB352 comprises six gene cassettes, two for each vector [pENTRY-A vector has Δ12/15 bi-functional desaturase gene from the amoeba Acanthamoeba castellanii (Ac Δ12/15) in cassette 1 and x3- desaturase gene from the fungus Phytophthora infestans (Piω3) in cassette 2; pENTR-B vector consisted of Δ6-elongase gene from the diatom Thalassiosira pseudonana (TpElo6) in cassette 1 and Δ5-desaturase from the fungus Thraustochytrium sp. (TcD5) in cassette 2; pENTRY C vector contained Δ6- desaturase from the fungus P. irregulare (PiD6) in cassette 1 and Δ6-elongase from P. patens (PSE1)]. The construct JB289 comprises two vectors, each with two cassettes [pENTRY-A2 construct contained Δ12-desaturase gene from the fungus Phytophthora sojae (PsD12) in cassette 1 and the Δ6-desaturase Ostreococcus tauri (OtD6); The pENTRY-B2 construct incorporates the Δ6-elongase gene from T. pseudonana (TpElo6) in cassette 1 and Δ5-desaturase from the fungus Thraustochytrium sp. (TcD5) in cassette 2]. They found that the seeds of Arabidopsis transgenic lines expressing these constructs (JB7, JB352 and JB289) accumulated significant levels of the non-native ARA, recording 5.2 mol% in JB7, 2.8 mol% in JB289 and 1 mol% in JB352.
A year later, Ruiz-Lopez et al. [122] sequentially estimate the efficiency of 12 combinations of 13 diverse genes in two different host genetic backgrounds for their ability of accumulating non-native ARA in A. thaliana (Columbia plant ecotype) transgenic lines. They perform a core construct of three expression cassettes (A3.1), which contain Δ6-desaturase gene from the alga O. tauri (OtΔ6) in the first one, Δ6-elongase from the moss Physcomitrella patens (PSE1) in the second and a Δ5-desaturase from Thraustochytrium sp. (TcΔ5). Then they built 4, 5 and 6-gene constructs designed as A4, A5 and A6. The A4 constructs were built by adding three different ω3 desaturases [Δ15-desaturase gene from the cyanobacterium Microcoleus chthonoplastes (McΔ15), Δ15-desaturase gene from the higher plant Perilla fruticosa (PerfΔ15) and Hp-ω3 gene from the fungus Hyaloperonospora parasitica) to A3.1 core forming A4.1, A4.2 and A4.3, respectively. The A5.1 construct was formed from A3.1 core in addition to Δ12-desaturase gene from the fungus Phytophthora sojae (PsΔ12) and ω3 desaturase gene from the fungus Phytophthora infestans (Piω3). The six-gene constructs, A6.1 and A6.2, were designed by incorporating FAD3 genes (McΔ15 and PerfΔ15) into A5.1, respectively. They reported that the analysis of total fatty acid methyl-esters (FAMEs) indicated that the transgenic Arabidopsis T2 lines carrying the A3.1 construct accumulated 0.4 to 6.4% ARA in their seeds. The Fatty acid analysis of T2 seeds of the three constructs, containing FAD3-like sequences, A4.1, A4.2 and A4.3 revealed that average levels of ARA in A4.1 (with McΔ15) were increased from 2.2% to 4.6% and were reduced to 1.5% in A4.2 (with PerfΔ15), while no significant decrease in ARA were recorded for A4.3 (with Hp-ω3). They revealed that the expression of cyanobacterial McΔ15 might be the cause of the extra-plastidial lipid enrichment in transgenic seeds while, the expression of PerfΔ15 microsomal desaturase shifted the pathway streaming in the transgenic seeds from n-6 to n-3. They also demonstrated that the mature seeds of A5.1, A6.1 and A6.2 transgenic plants expressed low amount of ARA, ranging from 0.4% to 1.8% in A5.1, 0.7% to 2.8% in A6.1 and 0.3% to 1.4% in A6.2.
To summarize the main requirement for metabolic engineering, Ruiz-Lopez et al. [83] revealed that stable transformation with multiple genes (sources and combinations) required their coordination of expressions with a least three successive non-native genes from PUFAs pathways.
Conclusions
The higher ARA-producers fungi were the non-pathogenic Mortierella spp. which produces ARA up to 70% of total FAs. Algal species belonging to different divisions were recorded either to have lower ARA content or C18, C20 and C22 FAs. Certain algal species were reported to contain naturally higher ARA content which may reach 77% of total FAs as in green microalga Parietochloris incise, 40% of total FAs in the red alga Porphyridium purpureum and 20–30% in diatoms as Phaeodactylum tricornutum and Thalassiosira pseudonona. Lower plants (mosses and ferns) have higher amounts of ARA than seagrasses and terrestrial higher plants. Environmental factors and chemical composition of media have no direct effect on ARA production. Transgenic techniques using types of Des and Elo genes from different sources were isolated and co-expressed in different plants with non-native ARA. This technique led to increase ARA production ranges from 10 to 20% total FAs.
Future perspectives
There is an urgent demand for searching of more candidates in the plant kingdom which could naturally provide valuable amounts of PUFAs or could be stably genetically modified for higher PUFAs content, especially ARA. Large scale production of the selected algal species that biosynthesize the needed PUFAs (ARA, EPA, DHA) and maximize their production through the abiotic stress factors and/or the metabolic engineering that must be applied worldwide to satisfy the humanity’s need of these valuable PUFAs. Major advances should focus more on green biotechnology to ameliorate PUFAs profile in metabolic engineering plants (native and non-native ARA producers), taking in consideration the sources, combinations and promoters of the constructed genes vectors.
Attentions must be made to all peoples to avoid excess consumption of ω-6 PUFA and to keep balance between ω-3 and ω-6 PUFAs ingested in dietary sources to keep healthy life and avoid dangerous diseases caused by this unbalanced intake. Serious Awareness must be addressed to vegetarian peoples to add n-6 oil supplements to their diet to have equipoise between n-3 and n-6 PUFA to become healthier. This must be achieved by informing peoples that ω6-FAs are not interconvertible to ω3-FAs due to the absence of the some specific enzymes so, the balance of ω-3 and ω-6 PUFAs can be easily influenced by food.
More researches must be performed to assure the beneficial or harmful effects of the metabolically engineered ARA on human especially those incorporated in food and pharmaceuticals. So, consumers will accept dealing with these products without fear.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Sanaa M.M. Shanab is a Professor of Phycology in Botany and Microbiology Department, Faculty of Science, Cairo University, Egypt. She had her Doctorat d’etat from France. She published over 50 papers in different areas of Phycology. She supervised on 17 M.Sc. and Ph.D. theses. She reviewed numerous online articles and 25 M.Sc. and Ph.D. theses to most of the Egyptian Universities. She attended 14 training and educational courses as well as 23 scientific conferences. She is an active member in 8 regional and international scientific journals and a member of the scientific editorial board of Baghdad Journal of Science.

Rehab M. Hafez is a Lecturer of Plant Cytogenetics from Botany and Microbiology Department, Faculty of Science, Cairo University, Egypt. Her research interests lie in the area of Cytogenetics in relation with tissue culture, mutation, transformation, biotechnology, phytoremediation and nanotechnology. She published 3 papers and 6 abstract in conferences. She supervises on 4 M.Sc. and one Ph.D. theses. She also attended 8 scientific training courses, 16 educational courses, 4 training courses in quality assurance of education and 6international conferences. She is the director of questionnaires unit and an internal auditing for quality management systems in her Faculty.

Ahmed S. Fouad is a Lecturer of Plant Cytology and Genetics from Botany and Microbiology Department, Faculty of Science, Cairo University, Egypt. His research interests lie in the area of Genetics, Cytology, Plant Tissue Culture, Transformation, Molecular Biology, Bioinformatics and Bio-Nanotechnology. He attended 4 international conferences, 5 scientific workshops, 16 educational courses, 6 workshops in quality assurance of education and has 6 ISO certifications. He has 7 publications (paper and sequences in the Gene Bank). He is an internal auditing for quality management systems in his Faculty.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Schmidt E.B., Christensen J.H., Aardestrup I., Madsen T., Riahi S., Hansen V.E. Marine n-3 fatty acids: basic features and background. Lipids. 2001;36:S65–S68. doi: 10.1007/s11745-001-0684-x. [DOI] [PubMed] [Google Scholar]
- 2.Hallahan B., Garland M.R. Essential fatty acids and mental health. Br J Psychiatry. 2005;186:275–277. doi: 10.1192/bjp.186.4.275. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela R., Sanhueza J., Valenzuela A. Docosahexaenoic Acid (DHA), an important fatty acid in aging and the protection of neurodegenerative diseases. J Nutr Ther. 2012;1:63–72. [Google Scholar]
- 4.Okuyama H, Kobayashi T, Watanabe S. Carcinogenesis and metastasis are affected by dietary n-6/n-3 fatty acids. In: Ohigashi H, Osawa T, Terao J, Watanabe S, Yoshikawa T, editors. Food factors for cancer prevention. Tokyo: Springer-Verlag; 1997. p. 677.
- 5.Tokudome S., Nagaya T., Okuyama H., Tokudome Y., Imaeda N., Kitagawa I. Japanese versus Mediterranean diets and cancer. Asian Pacific J Cancer Prev. 2000;1:61–66. [PubMed] [Google Scholar]
- 6.Martins D.A., Custodio L., Barreira L., Pereira H., Ben-Hamadou R., Varela J. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs. 2013;11:2259–2281. doi: 10.3390/md11072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brett M., Müller-Navarra D. The role of highly unsaturated fatty acids in aquatic food-web processes. Freshw Biol. 1997;38:483–499. [Google Scholar]
- 8.Tocher D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 2003;11:107–184. [Google Scholar]
- 9.Llewellyn W. Arachidonic acid: The new Mass Builder!; 2008 [2008 Feb 19]. Available from: <https://www.bodybuilding.com/fun/llewellyn2.htm>.
- 10.Kinsella J.E., Lokesh B., Broughton S., Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990;6:24–44. [PubMed] [Google Scholar]
- 11.Hansen J., Schade D., Harris C., Merkel K., Adamkin D., Hall R. Docosahexaenoic acid plus arachidonic acid enhance preterm infant growth. Prostaglandins Leukot Essent Fatty Acids. 1997;57:196. [Google Scholar]
- 12.Neitzel J.J. Fatty acid molecules: fundamentals and role in signaling. Nat Edu. 2010;3(9):57. [Google Scholar]
- 13.Pratt C.L., Brown C.R. The role of eicosanoids in experimental Lyme arthritis. Front Cell Infect Microbiol. 2014;4(69):1–6. doi: 10.3389/fcimb.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallima H., El Ridi R. Arachidonic acid: Physiological roles and potential health benefits – a review. J Adv Res. 2018;11:33–41. doi: 10.1016/j.jare.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO and FAO joint consultation Fats and oils in human nutrition. Nutr Rev. 1995;53(7):202–205. doi: 10.1111/j.1753-4887.1995.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 16.Australian Plant Information. Antifreeze, Food and Shelter. In: Information about Australia’s Flora. The Plant Underworld, Australian National Botanic Gardens and Centre for Australian National Biodiversity Research, Canberra; 2012 [last update: 2015 Dec 24], available from: <https://www.anbg.gov.au/cryptogams/underworld/panel-13/index.html>.
- 17.Jacq E., Prieur D., Nichols P., White D.C., Porter T., Geesey G.G. Microscopic examination and fatty acid characterisation of filamentous bacteria colonizing substrata around subtidal hydrothermal vents. Arch Microbiol. 1989;152:64–71. [Google Scholar]
- 18.Gandhi S.R., Weete J.D. Production of the polyunsaturated fatty acids arachidonic acid and eicosapentaenoic acid by the fungus Pythium uftimum. J Gen Microbiol. 1991;137:1825–1830. doi: 10.1099/00221287-137-8-1825. [DOI] [PubMed] [Google Scholar]
- 19.Iwanami H., Yamaguchi T., Takeuchi M. Fatty acid metabolism in bacteria that produce eicosapentaenoic acid isolated from sea urchin Strongylocentrotus nudus. Nippon Suis Gakk. 1995;61:205–210. [Google Scholar]
- 20.Nichols D.S., Brown J.L., Nichols P.D., McMeekin T.A. Production of eicosapentaenoic and arachidonic acids by an Antarctic bacterium: response to growth temperature. FEMS Microbiol Lett. 1997;152:349–354. [Google Scholar]
- 21.Lewis T.E., Nichols P.D., McMeekin T.A. The biotechnological potential of Thraustochytrids. Mar Biotechnol. 1999;1:580–587. doi: 10.1007/pl00011813. [DOI] [PubMed] [Google Scholar]
- 22.Domergue F., Abbadi A., Heinz E. Relief for fish stocks: oceanic fatty acids in transgenic oilseeds. Trends Plant Sci. 2005;10:112–116. doi: 10.1016/j.tplants.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Ells R., Kock L.F.J., Albertyn J., Poh H.C. Arachidonic acid metabolites in pathogenic yeasts. Lipids Health Dis. 2012;11:100. doi: 10.1186/1476-511X-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eroshin V.K., Satroutdinov A.D., Dedyukhina E.G., Chistyakova T.I. Arachidonic acid production by Mortierella alpina with growth-coupled lipid synthesis. Process Biochem. 2000;35:1171–1175. [Google Scholar]
- 25.Dyal D., Narine S.S. Implications for the use of Mortierella fungi in the industrial production of essential fatty acids. Food Res Int. 2005;38:445–467. [Google Scholar]
- 26.Ward O., Singh A. Omega-3/6 fatty acids: alternative sources of production. Process Biochem. 2005;40:3627–3652. [Google Scholar]
- 27.Dedyukhina E.G., Chistyakova T.I., Vainshtein M.B. Biosynthesis of arachidonic acid by Micromycetes (Review) Appl Biochem Microbiol. 2011;47:109–117. [PubMed] [Google Scholar]
- 28.Holten R.W., Blecker H.H., Stevens T.S. Fatty acids in blue green algae, possible relationship to phelogenetic position. Science NY. 1968;160:545–547. doi: 10.1126/science.160.3827.545. [DOI] [PubMed] [Google Scholar]
- 29.Pushparaj B., Buccioni A., Paperi R., Piccardi R., Ena A., Carlozzi P., Sili C. Fatty acid composition of Antarctic cyanobacteria. Phycologia. 2008;47(4):430–434. [Google Scholar]
- 30.Cohen Z., Vonshak A., Richmond A. Effect of environmental conditions on fatty acid composition of the red alga Porphyridium cruentum: correlation to growth rate. J Phycol. 1988;24:328–332. [Google Scholar]
- 31.Cohen Z. The production potential of eicosapentaenoic and arachidonic acids by the red alga Porphyridium cruentum. J Am Oil Chem Soc. 1990;67:916–920. [Google Scholar]
- 32.Bigogno C., Khozin-Goldberg I., Cohen Z. Accumulation of Arachidonic acid-rich triacylglycerols in the microalga Parietochloris incise (Trebuxiophyceae, Chlorophyta) Phytochemistry. 2002;60:135–143. doi: 10.1016/s0031-9422(02)00037-7. [DOI] [PubMed] [Google Scholar]
- 33.Bigogno C., Khozin-Goldberg I., Boussiba S., Vonshak A., Cohen Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry. 2002;60:497–503. doi: 10.1016/s0031-9422(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 34.Su G., Jiao K., Chang J., Li Z., Guo X., Sun Y. Enhancing total fatty acids and arachidonic acid production by the red microalgae Porphyridium purpureum. Bioresour Bioprocess. 2016;3:1–9. doi: 10.1007/s00449-016-1589-6. [DOI] [PubMed] [Google Scholar]
- 35.Su G., Jiao K., Zheng L., Guo X., Chang J., Ndikubwimana T. Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng. 2016;39:1–8. doi: 10.1007/s00449-016-1589-6. [DOI] [PubMed] [Google Scholar]
- 36.Nichols B.W., Appleby R.S. The distribution of arachidonic acid in algae. Phytochem. 1969;8:1907–1915. [Google Scholar]
- 37.Bigogno C., Khozin-Goldberg I., Adlerstein D., Cohen Z. Biosynthesis of arachidonic acid in the oleaginous microalga Parietochloris incisa (Chloropyceae): Radiolabeling studies. Lipids. 2002;37:209–216. doi: 10.1007/s11745-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton M.L., Haslam R.P., Napier J.A., Sayanova O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab Eng. 2014;22:3–9. doi: 10.1016/j.ymben.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichenberger W., Gribi C. Lipids of Pavlova lutheri: cellular site and metabolic role of DGCC. Phytochem. 1997;45:1561–1567. [Google Scholar]
- 40.Al-Hasan R.H., Hantash F.M., Radwan S.S. Enriching marine macroalgae with eicosatetraenoic (arachidonic) and eicosapentaenoic acids by chilling. Appl Microbiol Biotechnol. 1991;35:530–535. [Google Scholar]
- 41.El-Shoubaky G.A., Moustafa A.M.Y., Salem E.A.E. Comparative phytochemical investigation of beneficial essential fatty acids on a variety of marine seaweeds algae. Res J Phytochem. 2008;2:18–26. [Google Scholar]
- 42.Widjaja-Adhi A.M.K., Naoya S., Sayaka I., Nobuko B., Masayuki A., Masashi H. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J Agric Food Chem. 2011;59:4156–4163. doi: 10.1021/jf104643b. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang L.L., Chen S.H., Li Y., Zhou Z.G. Transcriptome analysis reveals unique C4-like photosynthesis and oil body formation in an arachidonic acid-rich microalga Myrmecia incisa Reisigl H4301. BMC Genomics. 2013;14:1–13. doi: 10.1186/1471-2164-14-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solovchenko A.E., Khozin-Goldberg I., Didi-Cohen S., Cohen Z., Merzlyak M.N. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol. 2008;20:245–251. [Google Scholar]
- 45.Pereira H., Barreira L., Figueiredo F., Custódio L., Vizetto-Duarte C., Polo C. Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar Drugs. 2012;10:1920–1935. doi: 10.3390/md10091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao K., Chang J., Zeng X., Ng I.-S., Xiao Z., Sun Y. 5-Aminolevulinic acid promotes arachidonic acid biosynthesis in the red microalga Porphyridium purpureum. Biotechnol Biofuels. 2017;10:168–177. doi: 10.1186/s13068-017-0855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahern T.J., Katoh S., Sada E. Arachidonic acid production by the red alga Porphyridium cruentum. Biotechnol Bioeng. 1983;225:1057–1070. doi: 10.1002/bit.260250414. [DOI] [PubMed] [Google Scholar]
- 48.Sayanova O., Napier J.A. Transgenic oilseed crops as an alternative to fish oils. Prostaglandins Leukot Essent Fatty Acids. 2011;85:253–260. doi: 10.1016/j.plefa.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Xue J., Niu Y.-F., Huang T., Yang W.-D., Liu J.-S., Li H.-Y. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab Eng. 2015;27:1–9. doi: 10.1016/j.ymben.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Wu Z., Zhu Y., Huang W., Zhang C., Li T., Zhang Y. Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour Technol. 2012;110:496–502. doi: 10.1016/j.biortech.2012.01.101. [DOI] [PubMed] [Google Scholar]
- 51.Wood BJB. Fatty acids and saponifiable lipids, In: Stewart WDP. editor. Algal physiology and biochemistry, Blackwell Scientific Publications: Oxford 1974. p. 236–265 [chapter 8].
- 52.Van Ginneken V.J.T., Helsper J.P.F.G., de Visser W., van Keulen H., Brandenburg W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011;10:104–112. doi: 10.1186/1476-511X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar M., Kumari P., Gupta V., Reddy C.R.K., Jha B. Biochemical responses of red algae Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J Exp Mar Biol Ecol. 2010;391:27–34. [Google Scholar]
- 54.Kumar M., Gupta V., Trivedi N., Kumari P., Bijo A.J., Reddy C.R.K. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta) Environ Exp Bot. 2011;72:194–201. [Google Scholar]
- 55.Sanchez-Machado D.I., Lopez-Cervantes J., Lopez-Hernandez J., Paseiro-Losada P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004;85:439–444. [Google Scholar]
- 56.Barbosa M., Valentão P., Andrade P.B. Review biologically active oxylipins from enzymatic and nonenzymatic routes in macroalgae. Mar Drugs. 2016;14:23–49. doi: 10.3390/md14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feussner I., Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 58.Howe G.A., Schilmiller A.L. Oxylipin metabolism in response to stress. Curr Opin Plant Biol. 2002;5:230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- 59.Bouarab K., Adas F., Gaquerel E., Kloareg B., Salaün J., Potin P. The innate immunity of a marine red alga involves oxylipins from both the eicosanoid and octadecanoid pathways. Plant Physiol. 2004;135:1838–1848. doi: 10.1104/pp.103.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar M., Kumari P., Gupta V., Anisha P.A., Reddy C.R.K., Jha B. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta) Biometals. 2010;23:315–325. doi: 10.1007/s10534-010-9290-8. [DOI] [PubMed] [Google Scholar]
- 61.Kumar M., Trivedi N., Reddy C.R.K., Jha B. Toxic effects of imidazolium ionic liquids on the green seaweed Ulva lactuca: oxidative stress and DNA damage. Chem Res Toxicol. 2011;24:1882–1890. doi: 10.1021/tx200228c. [DOI] [PubMed] [Google Scholar]
- 62.Weinberger F., Lion U., Delage L., Kloareg B., Potin P., Beltrán J. Up-regulation of lipoxygenase, phospholipase, and oxylipin-production in the induced chemical defense of the red alga Gracilaria chilensis against epiphytes. J Chem Ecol. 2011;37:677–686. doi: 10.1007/s10886-011-9981-9. [DOI] [PubMed] [Google Scholar]
- 63.Kumari P., Kumar M., Reddy C.R.K., Jha B. Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta) Pant Cell Physiol. 2014;55:52–63. doi: 10.1093/pcp/pct156. [DOI] [PubMed] [Google Scholar]
- 64.Mosblech A., Feussner I., Heilmann I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem. 2009;47:511–517. doi: 10.1016/j.plaphy.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Andreou A., Brodhun F., Feussner I. Biosynthesis of oxylipins in non-mammals. Prog Lipid Res. 2009;48:148–170. doi: 10.1016/j.plipres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto Y., Watanabe A. Fatty acid their composition of lichens phyco- and mycobionts. J Gen Appl Microbiol. 1974;20:83–86. [Google Scholar]
- 67.Rezanka T., Dembitsky V.M. Fatty Acids of Lichen Species from Tian Shan Mountains. Folia Microbiol. 1999;44(6):643–646. [Google Scholar]
- 68.Hanusa L.O., Temina M., Dembitsky V. Biodiversity of the chemical constituents in the epiphytic lichenized Ascomycete Ramalina Lacera grown on difference substrates Crataegus Sinaicus, Pinus Halepensis and Quercus Calliprinos. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152(2):203–208. doi: 10.5507/bp.2008.031. [DOI] [PubMed] [Google Scholar]
- 69.Temina M., Levitsky D.O., Dembitsky V.M. Chemical constituents of the epiphytic and lithophilic lichens of the genus Collema. Rec Nat Prod. 2010;4(1):79–86. [Google Scholar]
- 70.Shinmen Y., Katoh K., Shimizu S., Jareonkitmongkol S., Yamada H. Production of arachidonic acid and eicosapentaenoic acids by Marchantia polymorpha in cell culture. Phytochem. 1991;30(10):3255–3260. [Google Scholar]
- 71.Beike A.K., Jaeger C., Zink F., Decker E.L., Reski R. High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 2014;33:245–254. doi: 10.1007/s00299-013-1525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gellerman J.L., Anderson W.H., Richardson D.G., Schlenk H. Distribution of arachidonic and eicosapentaenoic acids in the lipids of mosses Biochimica et Biophysica Acta (BBA) – lipids and lipid. Metabolism. 1975;388(2):277–290. doi: 10.1016/0005-2760(75)90133-2. [DOI] [PubMed] [Google Scholar]
- 73.Pejin B., Vujisic L.J., Sabovljevic M., Tesevic V., Vajs V. The moss Mnium hornum, a promising source of arachidonic acid. Chem Nat Compd. 2012;1:1. [Google Scholar]
- 74.Hartmann E., Beutelmann P., Vandekerkhove O., Euler R., Kohn G. Moss cell cultures as suurces of arachidonic and eicosapentaenoic acids. FEBS. 1986;198(1):51–55. [Google Scholar]
- 75.Gachet M.S., Schubert A., Calarco S., Boccard J., Gertsch J. Targeted metabolomics shows plasticity in the evolution of signaling lipids and uncovers old and new endocannabinoids in the plant kingdom. Sci Rep. 2017;7(41177):1–15. doi: 10.1038/srep41177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suloma A, Ogata HY, Furuita H, Garibay ES, Chavez DR. Arachidonic acid distribution in seaweed, seagrass, invertebrates and dugong in coral reef areas in the Philippines In K Nakamura (Ed), Sustainable Production Systems of Aquatic Animals in Brackish Mangrove Areas 2007; JIRCAS Working Report No 56. p. 107–111.
- 77.Guil J.L., Torija M.E., Gimenez J.J., Rodriguez I. Identification of fatty acids in edible wild plants by gas chromatography. J Chromatogr. 1996;A719:229–235. doi: 10.1016/0021-9673(95)00414-9. [DOI] [PubMed] [Google Scholar]
- 78.Wolff R.L., Christie W.W., Aitzetmüller K., Pasquier E., Pedrono F., Destaillats F. Marpeau AM Arachidonic and eicosapentaenoic acids in Araucariaceae, a unique feature among seed plants. Oléagineux, Corps Gras, Lipides. 2000;7(1):113–117. [Google Scholar]
- 79.Kaya Z., Akbuga K., Aribas A., Can I. Is it a typical crosstalk: need for reimplantation? J Arrhythm. 2015;31(2):116–117. doi: 10.1016/j.joa.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gill I., Valivety R. Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol. 1997;15:401–409. doi: 10.1016/S0167-7799(97)01076-7. [DOI] [PubMed] [Google Scholar]
- 81.Huang Y.-S., Pereira S.L., Leonard A.E. Enzymes for transgenic biosynthesis of long-chain polyunsaturated fatty acids. Biochimie. 2004;86:793–798. doi: 10.1016/j.biochi.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 82.Venegas-Caleron M., Sayanova O., Napier J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog Lipid Res. 2010;49:108–119. doi: 10.1016/j.plipres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Ruiz-Lopez N., Usher S., Sayanova O.V., Napier J.A., Haslam R.P. Modifying the lipid content and composition of plant seeds: engineering the production Of LC-PUFA. Mini-review. Appl Microbiol Biotechnol. 2015;99:143–154. doi: 10.1007/s00253-014-6217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallis J.G., Browse J. The Δ8-desaturase of Euglena gracilis: an alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch Biochem Biophys. 1999;365:307–316. doi: 10.1006/abbi.1999.1167. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura M.T., Nara T.Y. Structure, junction and dietary regulation of delta 6, delta 5 and delta 9 desaturases. Ann Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura M.T., Nara T.Y. Essential fatty acid synthesis and its regulation in mammals. Prostagland Leukot Essent Fatty Acids. 2003;68:145–150. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 87.Nichols B.W., Wood B.J.B. The occurrence and biosynthesis of γ-linolenic acid in a blue-green alga Spirulina platensis. Lipids. 1968;3:46–50. doi: 10.1007/BF02530968. [DOI] [PubMed] [Google Scholar]
- 88.Makewicz A., Gribi C., Eichenberger W. Lipids of Ectocarpus fasciculatus (Phaeophyceae). Incorporation of [1-14C]oleate and the role of TAG and MGDG in lipid metabolism. Plant Cell Physiol. 1997;38:952–960. [Google Scholar]
- 89.Khozin I., Adlerstein D., Bigogno C., Heimer Y.M., Cohen Z. Elucidation of the biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum. II. Studies with radiolabeled precursors. Plant Physiol. 1997;114:223–230. doi: 10.1104/pp.114.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Norman H.A. St. John, JB. Differential effects of a substituted pyridazinone, BASF 13–338 on pathways of mono-galactosyldiacylglycerol synthesis in Arabidopsis. Plant Physiol. 1987;85:684–688. doi: 10.1104/pp.85.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hanggi N.S., Eichenberger W. Effect of the substituted pyridazinone SAN 9785 on the lipid and fatty acid biosynthesis in Pavlova lutheri. In: Sanchez J., Cerda-Olmedo E., Martinez-Force E., editors. Advances in plant lipid research. Universidad de Sevilla; Seville: 1998. pp. 259–261. [Google Scholar]
- 92.Banas A., Stenlid G., Lenman M., Sitbon F., Stymne S. Inhibition of polyunsaturated fatty acid synthesis by salicylic acid and salicylhydroxamic acid and their mode of action. In: Williams J.P., Mobashsher U.K., Nora W.L., editors. Physiology, biochemistry and molecular biology of plant lipids. Kluwer Academic Publishers; Dordrecht: 1997. pp. 230–232. [Google Scholar]
- 93.Khozin-Goldberg I., Bigogno C., Cohen Z. Salicylhydroxamic acid inhibits delta-6 desaturation in the microalga Porphyridium cruentum. Biochim Biophys Acta. 1999;1439(3):384–394. doi: 10.1016/s1388-1981(99)00107-9. [DOI] [PubMed] [Google Scholar]
- 94.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 95.Tapiero H., Ba G.N., Couvreur P., Tew K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacoth. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 96.Ouyang L.L., Li H., Liu F., Tong M., Yu S.Y., Zhou Z.G. Accumulation of arachidonic acid in a green microalga, Myrmecia Incisa H4301, enhanced by nitrogen starvation and its molecular regulation mechanisms. In: Dumancas G.G., Murdianti B.S., Lucas E.A., editors. Arachidonic acid: dietary sources and general functions. NOVA Science Publishers, Inc; New York: 2013. pp. 1–20. [Google Scholar]
- 97.Ryckebosch E., Bruneel C., Muylaert K., Foubert I. Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol. 2012;24:128–130. doi: 10.1016/j.foodchem.2014.03.087. [DOI] [PubMed] [Google Scholar]
- 98.Wu X., Ouyang H., Duan B., Pang D., Zhang L., Yuan T. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res. 2012;21:537–543. doi: 10.1007/s11248-011-9554-2. [DOI] [PubMed] [Google Scholar]
- 99.Parker-Barnes J.M., Das T., Bobik E., Leonard A.E., Thurmond J.M., Chaung L.-T. Identification and characterization of an enzyme involved in the elongation of n-6 and n-3 polyunsaturated fatty acids. Proc Natl Acad Sci USA. 2000;97:8284–8289. doi: 10.1073/pnas.97.15.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zank T.K., Zahringer U., Beckmann C., Pohnert G., Boland W., Holtorf H. Cloning and functional characterization of an enzyme involved in the elongation of Δ6-polyunsaturated fatty acids from the moss Physcomitrella Patens. The Plant J. 2002;31:255–268. doi: 10.1046/j.1365-313x.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 101.Kang D.H., Anbu P., Jeong Y.S., Chaulagain B.P., Seo J.W., Hur B.-K. Identification and characterization of a novel enzyme related to the synthesis of PUFAs derived from Thraustochytrium aureum ATCC 34304. Biotechnol Bioprocess Eng. 2010;15:261–272. [Google Scholar]
- 102.Slater S., Mitsky T., Houmiel K.L., Hao M., Reiser S.E., Taylor N.B. Metabolic engineering of Arabidopsis and Brassica for Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Copolymer Production. Nat Biotechnol. 1999;17:1011–1016. doi: 10.1038/13711. [DOI] [PubMed] [Google Scholar]
- 103.Ye X., Al-Babili S., Klöti A., Zhang J., Lucca P., Beyer P. Engineering the provitamin A (Beta-Carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 104.Thelen J.J., Ohlrogge J.B. Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng. 2002;4:12–21. doi: 10.1006/mben.2001.0204. [DOI] [PubMed] [Google Scholar]
- 105.Qi B., Fraser T., Mugford S., Dobson G., Sayanova O., Butler J. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol. 2004;22:739–745. doi: 10.1038/nbt972. [DOI] [PubMed] [Google Scholar]
- 106.Abbadi A., Domergue F., Bauer J., Napier J.A., Welti R., Zähringer U. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell. 2004;16:2734–2748. doi: 10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dembitsky V.M. Lipids of bryophytes. Prog Lipid Res. 1993;32:281–356. doi: 10.1016/0163-7827(93)90010-t. [DOI] [PubMed] [Google Scholar]
- 108.Kajikawa M., Yamaoka S., Yamato K.T., Kanamaru H., Sakuradani E., Shimizu S. Functional analysis of β-ketoacyl-COA synthase gene, MPFAE2, by gene silencing in the liverwort Marchantia Polymorpha L. Biosci Biotechnol Biochem. 2003;67:605–612. doi: 10.1271/bbb.67.605. [DOI] [PubMed] [Google Scholar]
- 109.Kajikawa M., Yamato K.T., Kohzu Y., Nojiri M., Sakuradani E., Shimizu S. Isolation and characterization of Δ6- Desaturase, an ELO-Like Enzyme and Δ5-Desaturase from the liverwort Marchantia Polymorpha and production of arachidonic and eicosapentaenoic acids in the methylotrophic yeast Pichia Pastoris. Plant Mol Biol. 2004;54:335–352. doi: 10.1023/B:PLAN.0000036366.57794.ee. [DOI] [PubMed] [Google Scholar]
- 110.Kajikawa M., Matsui K., Ochiai M., Tanaka Y., Kita Y., Ishimoto M. Production of arachidonic and eicosapentaenoic acids in plants using Bryophyte fatty acid Δ6-Desaturase, Δ6-Elongase, and Δ5-Desaturase genes. Biosci Biotechnol Biochem. 2008;72(2):435–444. doi: 10.1271/bbb.70549. [DOI] [PubMed] [Google Scholar]
- 111.Chen R., Matsui K., Ogawa M., Oe M., Ochiai M., Kawashima H. Expression of Δ6, Δ5 Desaturase and GLELO elongase genes from Mortierella Alpina for production of arachidonic acid in soybean [Glycine Max (L.) Merrill] seeds. Plant Sci. 2005;170:399–406. [Google Scholar]
- 112.Petrie J.R., Shrestha P., Zhou X.R., Mansour M.P., Liu Q., Belide S. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE. 2012;7(11):E49165. doi: 10.1371/journal.pone.0049165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Domergue F., Lerchl J., Zähringer U., Heinz E. Cloning and functional characterization of Phaeodactylum tricornutumfront-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur J Biochem. 2002;269:4105–4113. doi: 10.1046/j.1432-1033.2002.03104.x. [DOI] [PubMed] [Google Scholar]
- 114.Kinney AJ, Cahoon EB, Damude HG, Hitz WD, Kolar CW, Liu ZB. Production of very long chain polyunsaturated fatty acids in oilseed plants. World Patent Appl; 2004. No. WO 2004/071467 [2004 Aug 26].
- 115.Wu G., Truksa M., Datla N., Vrinten P., Bauer J., Zank T. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol. 2005;23:1013–1017. doi: 10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- 116.Robert S., Singh S.P., Zhou X.R., Petrie J.R., Blackburn S.I., Mansour P.M. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Funct Plant Biol. 2005;32:473–479. doi: 10.1071/FP05084. [DOI] [PubMed] [Google Scholar]
- 117.Sayanova O., Haslam R., Qi B., Lazarus C.M., Napier J.A. The alternative pathway C20 Δ8-desaturase from the non-photosynthetic organism Acanthamoeba castellanii is an atypical cytochrome B5-fusion Desaturase. FEBS Lett. 2006;580:1946–1952. doi: 10.1016/j.febslet.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 118.Hoffmann M., Wagner M., Abbadi A., Fulda M., Feussner I. Metabolic engineering of ω3-very long chain polyunsaturated fatty acid production by an exclusively acyl-COA-dependent pathway. J Biol Chem. 2008;283(33):22352–22362. doi: 10.1074/jbc.M802377200. [DOI] [PubMed] [Google Scholar]
- 119.Savchenko T., Walley J.W., Chehab E.W., Xiao Y., Kaspi R., Pye M.F. Arachidonic acid: an evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell. 2010;22:3193–3205. doi: 10.1105/tpc.110.073858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng B.F., Wu G.H., Vrinten P., Falk K., Bauer J., Qiu X. Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res. 2010;19:221–229. doi: 10.1007/s11248-009-9302-z. [DOI] [PubMed] [Google Scholar]
- 121.Ruiz-Lopez N., Haslam R.P., Venegas-Caleron M., Li T., Bauer J., Napier J.A. Enhancing the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Arabidopsis thaliana via iterative metabolic engineering and genetic crossing. Transgenic Res. 2012;21(6):1233–1243. doi: 10.1007/s11248-012-9596-0. [DOI] [PubMed] [Google Scholar]
- 122.Ruiz-Lopez N., Haslam R.P., Usher S.L., Napier J.A., Sayanova O. Reconstitution of EPA and DHA biosynthesis in Arabidopsis: iterative metabolic engineering for the synthesis of n-3 LCPUFAs in transgenic plants. Metab Eng. 2013;17:30–41. doi: 10.1016/j.ymben.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]