Abstract
This study aimed to correlate the oxidative stress marker levels in saliva with the clinical stage based on mouth opening, fibrotic bands and histopathological grades of oral submucous fibrosis (OSF) patients. The study included patients clinically diagnosed with OSF (n = 63) and equal number of age and gender matched controls. Patients with OSF were defined by mouth opening stage, fibrotic bands and histopathological grades. Unstimulated saliva from both control and OSF patients were analysed for oxidative markers like lipid peroxides (LPO), non-enzymic antioxidants [reduced glutathione (GSH), vitamin A, vitamin E, vitamin C] and enzymatic antioxidants [glutathione peroxidase (GPx), superoxide dismutase (SOD)] and correlated with different stages and grades. Total salivary protein and LPO were significantly increased in OSF group with no significant change in the levels of GSH compared to controls. In OSF patients, a significant decrease in the levels of vitamins A, C and E was observed. The activities of salivary SOD and GPx were significantly decreased in OSF patients compared to controls. These changes significantly correlated with the increasing and differing grades of OSF that reflects increased oxidative stress with the progress of OSF.
Keywords: Oral submucous fibrosis, Glutathione, Glutathione peroxidase, Superoxide dismutase, Mouth opening, Fibrotic bands
Introduction
Oral submucous fibrosis (OSF) is a chronic debilitating disease that is frequently encountered in the people of South-East Asian origin [1]. It is a connective tissue injury strongly associated with the habit of betel chewing as evidenced by increased incidence and areca nut consumption [2]. The initial risk factor of OSF in betel chewers is correlated with arecoline exposure [3]. Arecoline induces myofibroblast transdifferentiation that has a pathological role in tissue fibrosis [4]. Arecoline inhibits the activity of metalloproteinase (MMP)-2 and also a stimulator for tissue inhibitor of metalloproteinase-1 (TIMP-1) activity in buccal mucosal fibroblasts, which accounts for the excessive accumulation of ECM proteins in OSF [5, 6]. OSF has the highest tendency to undergo malignant transformation among the various potentially malignant disorders of oral cavity [7].
The phenolic compounds in areca-nut and catechu, to which betel-quid chewers are exposed in relatively large quantities are genotoxic at alkaline pH, probably by formation of reactive oxygen species (ROS) [8]. Reactive oxygen species (ROS), and ROS—derived lipid peroxides play an important role in the development and progression of pre-cancerous and cancer conditions. Apart from mitigating ROS induced reactions, antioxidants exert a protective effect [9], like the role of vitamin A in the stabilization of mucous membrane and its deficiency causes loss of mucous secreting cells and epithelial atrophy [10].
Early detection of pathology helps in accurate diagnosis to prompt therapeutic intervention, assess the prognosis, and aids in the follow-up in patients. Diagnosis and monitoring involves painful invasive procedures such as biopsies and serological examination, posing an unpleasant experience. Salivary analysis is a simple, non-invasive procedure and quite confirmatory procedure for diagnosis and prognosis [11]. Saliva as a diagnostic fluid can serve as a potent indicator of local and systemic disorders. Localized biochemical changes and associated tissue changes at cellular level with the progress of OSF are reflected in saliva [11].
Salivary components act as primary defence against free radicals generated during various physiological processes. Alterations in salivary flow rate and its composition reflects the response status and the presence of oral and systemic diseases [12]. The gingival crevicular fluid (GCF) flow increases during gingivitis, with the release of inflammatory mediators into the saliva and thus producing alterations in the antioxidant capacity of saliva [13]. Saliva could thus replace the serum to precisely reflect the systemic redox status [14]. Hence, this study was designed to assess the oxidative status in saliva of OSF patients, as it is a simple, economical diagnostic modality with excellent patient compliance.
Materials and Methods
Study Group
The study group comprised of clinically diagnosed OSF patients (n = 63), and healthy patients without tobacco/areca nut habits as control group in equal numbers, who were age and gender matched reporting to the Outpatient Department of Oral medicine and Radiology, Faculty of Dental Sciences, Sri Ramachandra University, Porur, Chennai. All experiments were performed with the consent of the patients, and institutional ethical committee approval was obtained before the commencement of study (IEC-N1/09/AUG/11/21). Patients with hypertension, asthma, tuberculosis, diabetes mellitus, bleeding disorders, cardiovascular diseases, epilepsy, degenerative joint disease and those undergoing any treatment were excluded from the study. Clinical grading of OSF was based on interincisal distance [15] and the presence of fibrotic bands [16]. Histopathological grading was based on Pindborg and Sirsat [17].
Collection of Saliva
Unstimulated Saliva was allowed to accumulate in the floor of mouth and collected by drooling method in a test tube. Saliva samples were stored at −20 °C until use.
Biochemical Analysis
The protein content in saliva was estimated by the method of Lowry et al. [18]. The levels of lipid peroxides in saliva was measured as thiobarbituric acid reactants (TBARS) [19]. Non-enzymic antioxidants–glutathione [20]; vitamin A [21]; vitamin E [22] and vitamin C [23] were also measured. Enzymic antioxidants–Superoxide dismutase [24] and Glutathione peroxidase [25] were assayed in saliva.
Statistical Analysis
Results are expressed as Mean ± SD. Statistical analysis was performed by χ 2 test, ANOVA and correlation analysis using statistical software SPSS v.23.
Results
In the study group comprising of OSF patients, a detailed history including, duration of habit in years, frequency of chewing per day, number of packets and stacking habit were recorded. Clinical criteria for diagnosis of OSF were applied, which included blanching of oral mucosa, leathery texture, palpable fibrous bands and difficulty in mouth opening. Clinical diagnosis were confirmed with incisional biopsy and graded histopathologically.
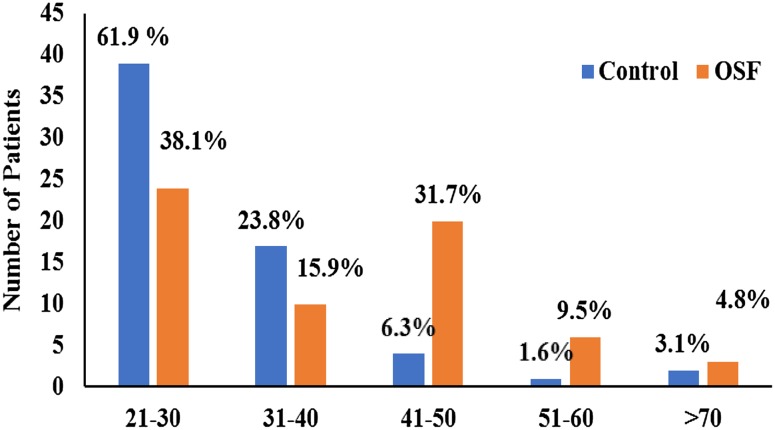
Control group comprised of 61.9% (n = 39) patients between 21 and 30 years; 26.9% (n = 17) between 31 and 40 years; 6.3% (n = 4) between 41 and 50 years; 1.6% (n = 1) between 51 and 60; 3.1% (n = 2) >60 years of age. In experimental group, 38.1% (n = 24) of patients were between 21 and 30 years; 15.9% (n = 10) between 31 and 40 years; 31.7% (n = 20) between 41 and 50 years; 9.5% (n = 6) between 51 and 60; 4.8% (n = 3) >60 years of age. Higher incidence of OSF was recorded in the 21–30 years (Fig. 1).
Fig. 1.
Age distribution in the study group
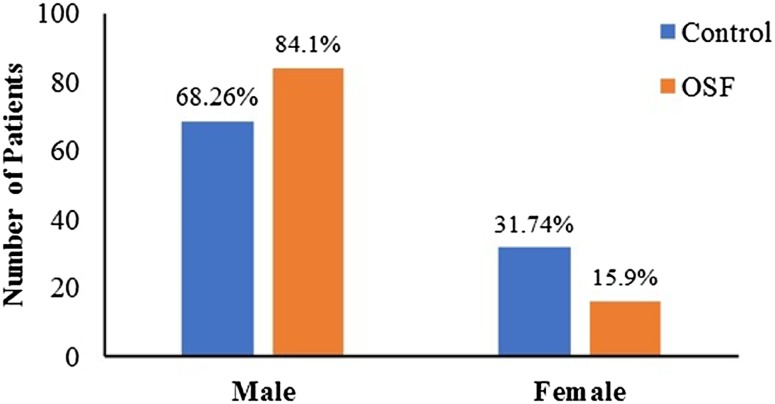
Control group comprised of 68.3% (n = 43) of males and 31.7% (n = 20) of females. Experimental group comprised of 84.1% (n = 53) of males and 15.9% (n = 10) of females (Fig. 2). The incidence of OSF between female: male was in the ratio of 1:5.3.
Fig. 2.
Gender distribution in study group
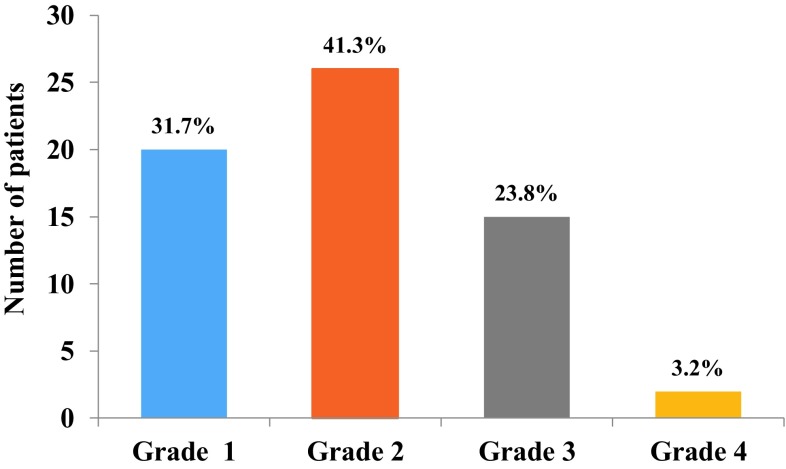
Figure 3 depicts the clinical grading based on mouth opening in OSF group. The study group contained 31.7% (n = 20) under grade I; 41.3% (n = 26) under grade II, 23.8% (n = 15) under Grade III and 3.2% (n = 2) under Grade IV.
Fig. 3.
Distribution of subjects based on the clinical staging of mouth opening
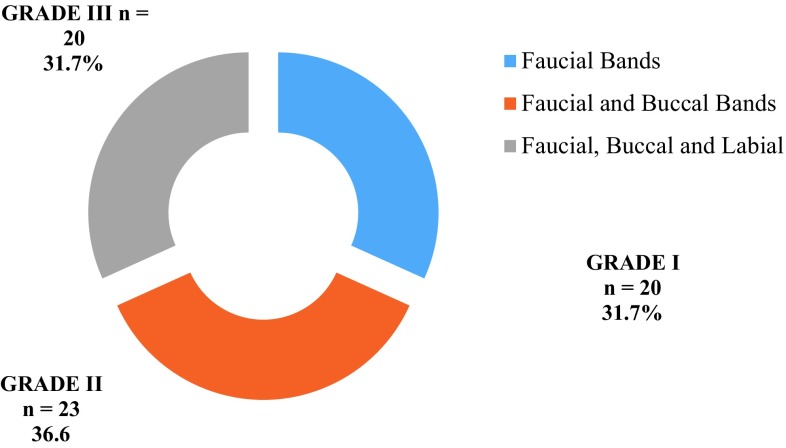
Figure 4 shows the distribution of OSF patients based on the clinical grading of fibrotic bands. The study group contained 31.7% (n = 20) in Grade I; 36.6% (n = 23) in Grade II, and 31.7% (n = 20) in Grade III.
Fig. 4.
Distribution of subjects based on the clinical staging of fibrotic bands
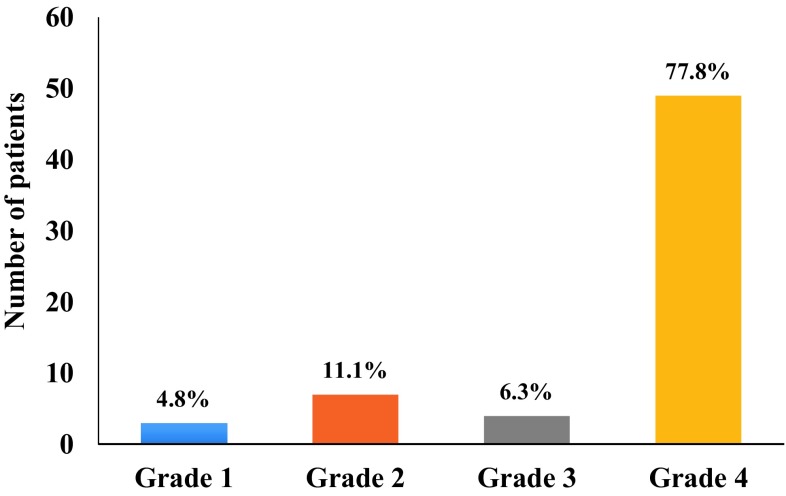
Figure 5 shows distribution of subjects based on histopathological grade. The study group comprised of 4.8% (n = 3) in Grade 1; 11.1% (n = 7) in Grade 2; 6.3% (n = 4) in Grade 3 and 77.8% (n = 49) were in Grade 4.
Fig. 5.
Distribution of subjects based on histopathological grading
Table 1 summarizes the distribution of OSF patients based on mouth opening, fibrotic bands and histological grade. The number of patients defined based on mouth opening stage are: Stage 1 = 20; Stage 2 = 26; Stage 3 = 15 and Stage 4 = 2, whereas based on fibrotic bands are: Grade I = 20; Grade II = 23 and Grade III = 20. Histologically, the number of patients in Stage I = 3; Stage II = 7; Stage III = 4 and Stage IV = 49.
Table 1.
Distribution of OSF patients clinically based on mouth opening grade, fibrotic band and histological grade
| Mouth opening stage | Fibrotic bands | Mouth opening stage | Histopathological grade | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Grade I | Grade II | Grade III | Total | Control | Stage I | Stage II | Stage III | Stage IV | Total | ||
| Control | 63 | 0 | 0 | 0 | 63 | Control | 63 | 0 | 0 | 0 | 0 | 63 |
| 1 | 0 | 19 | 1 | 0 | 20 | 1 | 0 | 3 | 2 | 2 | 13 | 20 |
| 2 | 0 | 1 | 16 | 9 | 26 | 2 | 0 | 0 | 4 | 1 | 21 | 26 |
| 3 | 0 | 0 | 6 | 9 | 15 | 3 | 0 | 0 | 1 | 1 | 13 | 15 |
| 4 | 0 | 0 | 0 | 2 | 2 | 4 | 0 | 0 | 0 | 0 | 2 | 2 |
| 63 | 20 | 23 | 20 | 126 | 63 | 3 | 7 | 4 | 49 | 63 | ||
Table 2 summarizes the levels of protein, lipid peroxides(LPO), glutathione(GSH), vitamins-A, C, E and activities of Superoxide dismutase (SOD) and glutathione peroxidase (GPx) in saliva of experimental and control groups. Total salivary protein was significantly increased in OSF group (p < 0.001) compared to controls. The levels of LPO was significantly increased (p < 0.001) in the OSF group compared to control group. No significant decrease in the levels of GSH was recorded in OSF patients compared to controls. A significant decrease in the levels of vitamins A (p < 0.001), vitamin C (p < 0.001) and vitamin E (p < 0.001) were observed compared to controls. The activities of salivary SOD (p < 0.001) and GPx (p < 0.001) were significantly decreased in OSF patients compared to controls. The results obtained were then correlated to clinical staging based on mouth opening, fibrotic bands and histopathologic grade.
Table 2.
Levels of protein, lipid peroxides (LPO), glutathione (GSH), vitamins-A, C, E and activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in saliva of control and OSF patients
| Parameter | Control (n = 63) | Experimental (n = 63) | F value |
|---|---|---|---|
| Protein (mg/ml) | 2.26 ± 0.66 | 2.77 ± 0.60b | 19.78 |
| LPO (μM of MDA/ml) | 15.86 ± 4.63 | 197.22 ± 64.5c | 494.16 |
| GSH (μM/ml) | 474.6 ± 47.2 | 464.51 ± 84.15NS | 0.68 |
| Vitamin A (µg/ml) | 379.20 ± 99.63 | 229.29 ± 36.31c | 125.88 |
| Vitamin E (µg/ml) | 558.47 ± 88.88 | 403.58 ± 93.44c | 82.77 |
| Vitamin C (µg/ml) | 302.65 ± 95.32 | 226.91 ± 77.34b | 23.98 |
| SOD (U/100 mg protein) | 1.42 ± 0.28 | 0.72 ± 0.22c | 237.47 |
| GPx (mM of GSH reduced/min/mg protein) | 1.41 ± 0.38 | 0.85 ± 0.33c | 75.47 |
Values are expressed as Mean ± SD
Statistical significance is indicated for comparisons between control and SMF with ANOVA as a p < 0.05; b p < 0.01; c p < 0.001; NS non-significant
Table 3 depicts the levels of protein, lipid peroxides, glutathione, vitamins-A, C, E and activities of Superoxide dismutase (SOD) and glutathione peroxidase (GPx) in saliva of OSF patients grouped based on clinical staging of mouth opening. The increase in the levels of salivary protein and lipid peroxides (LPO) were significant (p < 0.001) at all stages compared to control. No significant alterations were noted in salivary GSH levels compared to control. The decrease in salivary vitamin A, vitamin C and vitamin E levels was significantly (p < 0.001) at all stages compared to control. Salivary enzymic antioxidants—SOD and GPx were significantly decreased (p < 0.001) compared to control. The increase in the levels of salivary protein and LPO positively correlated (p < 0.01) with the advancing stages of mouth opening whereas no significant correlation was noted with the GSH levels. The decreases in the levels of salivary vitamins-A, C and E and activities of enzymic antioxidants—SOD, GPx was significant (p < 0.01) and negatively correlated with the advancing stages of mouth opening.
Table 3.
Levels of protein, LPO, GSH, vitamins-A, C, E and activities of SOD and GPx in saliva of control and OSF patients in different clinical stage based mouth opening
| Parameters | Group | F value | R 2 | ||||
|---|---|---|---|---|---|---|---|
| Control (n = 63) | Mouth opening | ||||||
| Stage 1 (n = 20) | Stage II (n = 26) | Stage III (n = 15) | Stage IV (n = 2) | ||||
| Protein (mg/ml) | 2.27 ± 0.66 | 2.69 ± 0.55a | 2.77 ± 0.42c | 2.79 ± 0.90 | 3.45 ± 0.32 | 5.57c | 0.366# |
| LPO (μM MDA/ml) | 15.86 ± 4.63 | 187.19 ± 65.59c | 189.92 ± 52.55c | 220.35 ± 82.54c | 218.88 ± 9.95c | 128.08c | 0.814# |
| GSH (μM/ml) | 474.6 ± 47.2 | 475.73 ± 85.14 | 453.8 ± 90.46 | 469.45 ± 80.48 | 454.69 ± 2.20 | 0.49NS | −0.83NS |
| Vitamin A (µg/ml) | 379.20 ± 99.63 | 230.96 ± 27.2c | 240.67 ± 27.87c | 219.04 ± 41.78c | 141.6 ± 58.83c | 32.54c | −0.647# |
| Vitamin E (µg/ml) | 558.47 ± 88.88 | 441.80 ± 80.58c | 405.38 ± 96.69c | 398.6 ± 89.21c | 239.50 ± 14.84c | 27.24c | −0.662# |
| Vitamin C (µg/ml) | 302.65 ± 95.32 | 259.3 ± 93.51a | 242.29 ± 19.37c | 166.06 ± 83.25c | 152.76 ± 61.25 | 9.91c | −0.484# |
| SOD (U/mg protein | 1.42 ± 0.28 | 0.81 ± 0.19c | 0.70 ± 0.20c | 0.66 ± 0.27c | 0.479 ± 0.005c | 61.8c | −0.757# |
| GPx (mM of GSH reduced/min/mg protein) | 1.41 ± 0.38 | 1.007 ± 0.31c | 0.88 ± 0.35c | 0.69 ± 0.17c | 0.29 ± 0.016c | 23.37c | −0.647# |
Values are expressed as Mean ± SD
Statistical comparison by Dunnett’s post hoc test are expressed as a p < 0.05; b p < 0.01; c p < 0.001; NS non-significant for comparisons: control versus stage I, stage II, stage III and stage IV
For correlation analysis, as; # p < 0.01; NS non-significant
Table 4 shows the levels of salivary protein, LPO, vitamins A, C, E and the activities of SOD and GPx in OSF patients grouped on the basis of fibrotic bands. Salivary protein and lipid peroxide levels were significantly increased (p < 0.001) in all grades compared to control. No significant changes were noted in salivary GSH levels at all grades compared to control. Salivary vitamin A, vitamin C and vitamin E levels were significantly decreased (p < 0.001) at all grades compared to control. Salivary enzymic antioxidants—SOD and GPx activities were significantly decreased (p < 0.001) at all stages compared to control. Correlation analysis showed a significant positive correlation between the increases in salivary protein and LPO and increasing grades of fibrotic bands, whereas the decreases in salivary vitamin A, vitamin C, vitamin E and enzymic antioxidants—SOD, GPx negatively correlated (p < 0.01) with the increasing grades of fibrotic bands.
Table 4.
Levels of protein, LPO, GSH, vitamins-A, C, E and activities of SOD and GPx in saliva of control and OSF patients in different clinical stage based on fibrotic bands
| Parameters | Group | F value | R 2 | |||
|---|---|---|---|---|---|---|
| Control (n = 63) | Fibrotic bands | |||||
| Grade I (n = 20) | Grade II (n = 23) | Grade III (n = 20) | ||||
| Protein (mg/ml) | 2.27 ± 0.0.34 | 2.76 ± 0.52b | 2.70 ± 0.56b | 2.85 ± 0.74b | 6.78c | 0.343# |
| LPO (μM MDA/ml) | 15.86 ± 4.63 | 195.08 ± 70.77c | 210.97 ± 66.93c | 183.54 ± 54.64 | 168.65c | 0.763# |
| GSH (μM/ml) | 474.6 ± 47.2 | 478.02 ± 87.65NS | 471.65 ± 87.64NS | 442.81 ± 76.04NS | 1.26NS | −0.136NS |
| Vitamin A (µg/ml) | 379.20 ± 99.63 | 228.56 ± 26.17c | 235.06 ± 32.02c | 223.40 ± 48.52c | 41.45c | −0.625# |
| Vitamin E (µg/ml) | 558.47 ± 88.88 | 429.25 ± 83.64c | 427.08 ± 79.69c | 371.30 ± 110.71c | 30.14c | −0.441# |
| Vitamin C (µg/ml) | 302.65 ± 95.32 | 255.87 ± 92.22 | 229.96 ± 57.74c | 194.42 ± 71.76c | 9.93c | −0.658# |
| SOD (U/mg protein | 1.42 ± 0.28 | 0.79 ± 0.18c | 0.72 ± 0.21c | 0.64 ± 0.26c | 81.24c | −0.754# |
| GPx (mM of GSH reduced/min/mg protein) | 1.41 ± 0.38 | 1.01 ± 0.32c | 0.81 ± 0.28c | 0.75 ± 0.37c | 27.84c | −0.661# |
Values are expressed as Mean ± SD
Statistical comparison by Dunnett’s post hoc test are expressed as a p < 0.05; b p < 0.01; c p < 0.001; NS non-significant for comparisons: control versus grade I, grade II and grade III
For correlation analysis, as # p < 0.01; NS non-significant
Table 5 shows the levels of protein, LPO, vitamins A, C, E and activities of SOD and GPx in saliva of patients grouped on the basis of histological grades. The levels of salivary protein (p < 0.01) and LPO (p < 0.001) were significantly increased in all histological grades compared to control. Salivary GSH levels showed no significant alterations compared to control at all stages compared to control. Salivary vitamin A, vitamin C and vitamin E levels were significantly decreased (p < 0.001) at all grades compared to control. The activities of salivary SOD (p < 0.001) and GPx (p < 0.001) were decreased compared to control. Correlation analysis of salivary protein, LPO levels with histological grades showed a positive correlation (p < 0.01), whereas the decreases in the levels salivary vitamin A, vitamin C, vitamin E and enzymic antioxidants—SOD and GPx negatively correlated with all histological grades.
Table 5.
Levels of protein, LPO, GSH, vitamins-A, C, E and activities of SOD and GPx in saliva of control and OSF patients in different histological grades
| Parameters | Group | F value | R 2 | ||||
|---|---|---|---|---|---|---|---|
| Control (n = 63) | Histological grade | ||||||
| Grade I (n = 3) | Grade II (n = 7) | Grade III (n = 4) | Grade IV (n = 49) | ||||
| Protein (mg/ml) | 2.27 ± 0.66 | 2.89 ± 0.16a | 2.47 ± 0.41NS | 2.77 ± 0.70NS | 2.80 ± 0.63c | 5.35c | 0.37# |
| LPO (μM MDA/ml) | 15.86 ± 4.63 | 185.94 ± 72.83c | 155.44 ± 40.20c | 228.64 ± 89.85c | 201.31 ± 64.02c | 131.35c | 0.869# |
| GSH (μM/ml) | 474.6 ± 47.2 | 481.95 ± 117.1NS | 419.2 ± 61.07 | 438.02 ± 132.93NS | 472.08 ± 81.05 | 1.31NS | −0.034NS |
| Vitamin A (µg/ml) | 379.20 ± 99.63 | 216.26 ± 15.01c | 241.14 ± 31.62c | 240.60 ± 29.11c | 227.47 ± 38.35c | 30.9c | −0.675# |
| Vitamin E (µg/ml) | 558.47 ± 88.88 | 480.00 ± 54.8NS | 373.85 ± 87.87b | 462.50 ± 90.3NS | 406.67 ± 95.45c | 21.97c | −0.425# |
| Vitamin C (µg/ml) | 302.65 ± −95.32 | 266.76 ± 89.92a | 276.72 ± 90.36NS | 217.38 ± 122.73NS | 218.13 ± 69.86c | 6.89c | −0.633# |
| SOD (U/mg protein | 1.42 ± 0.28 | 0.77 ± 0.23a | 0.64 ± 0.23b | 0.85 ± 0.20a | 0.71 ± 0.22c | 59.29c | −0.767# |
| GPx (mM of GSH reduced/min/mg protein) | 1.41 ± 0.38 | 1.15 ± 0.077b | 0.863 ± 0.269b | 1.04 ± 0.37a | 0.82 ± 0.345c | 19.85c | −0.614# |
Values are expressed as Mean ± SD
Statistical comparison by Dunnett’s post hoc test are expressed as a p < 0.05; b p < 0.01; c p < 0.001; NS non-significant for comparisons: control versus grade I, grade II, grade III and grade IV
For correlation analysis, as # p < 0.01 NS non-significant
Discussion
Oral submucous fibrosis (OSF) clinically presents as an inflammation, blanching of the oral mucosa, with fibrotic bands, leading to trismus [26]. The stacking of areca nut quid mixture contacts the oral tissues and causes its constant irritation by alkaloids in areca nut, including arecoline, arecaidine, guvacine and guvacoline. The components of areca nut autoxidize in alkaline condition and produce ROS [27]. The local injury caused by areca nut chewing results in chronic inflammation, subsequent release of inflammatory mediators, ROS and cytokines. Chronic ROS mediated injury to the cells results in precancerous changes in the oral mucosa and subsequently its malignant transformation [8]. Despite proper counselling and motivation to quit chewing pan/betel nut/gutka by health care providers, incidence of OSF is high and its potential to undergo malignant transformation necessitates curbing the disease at the grass root level. The present study analysed the enzymatic and non-enzymatic antioxidants in saliva of patients with OSF to assess the changes in oxidative status with the progress of this potentially malignant disorder condition.
Salivary proteins such as α-amylase, proline-rich proteins, histatins are synthesised within the salivary glands, whereas salivary albumin is a derivative of serum [28]. Salivary protein composition reflects the cellular signal interactions resulting from stress as well as various environmental influences [29]. In cancer and pre-cancer patients, the salivary protein concentration increases [30]. In the present study, salivary proteins increased in OSF group and the increase positively correlated with progressive clinical grades based on mouth opening, fibrotic bands and histological grades.
Free radicals and antioxidants play a significant role in oral cancer and carcinogenesis. Lower levels of ROS are involved in cell growth, however, higher levels produce damage to various components of the cell at the RNA, DNA, protein and cell membrane levels to induce cytotoxicity and ultimately resulting in cell death [31]. Salivary lipid peroxides reflect the local oral oxidative stress and is increased in OSF, leukoplakia and cancer [32–35]. In the present study, a significant increase in salivary lipid peroxide levels were observed in OSF group compared to control group. The increases in salivary lipid peroxides correlated with severity of mouth opening stage, fibrotic grades and histologic grades of OSF. Increased LPO is attributed to high copper levels in saliva of patients chewing arecanut [36].
The inherent antioxidants in saliva include uric acid, ascorbate, reduced glutathione (GSH) and α-tocopherol [37]. In the presence of ascorbic acid or thiols, urate scavenges the free radicals [38]. Albumin, catalase-positive oral commensals along with fresh blood from injured capillaries and enzymatic antioxidants like SOD, GPx and catalase also serve as antioxidants [39]. Decrease of GSH leads to cell cycle arrest, cytotoxicity and concomitant epithelial atrophy in OSF [40]. In the present study, absence of an increase in the salivary GSH levels could be due to the repression of GSH synthesising enzymes. Absence of an increase in GSH levels is not only from repression of synthesising enzymes, but also from increased conjugation with arecoline [41].
Superoxide dismutase reduces superoxide (O2 −) anion generated in cells and acts as a pro-oxidant by producing H2O2, which requires other antioxidant systems, such as CAT and GPx enzymes to detoxify H2O2 [42]. An imbalance in the ratio of SOD to CAT/GPx is involved in the incidence of many disease [43]. In the oral cavity, radiation induced fibrosis is restored by SOD and thence the normal physiological functions are retained [44]. In the present study, however the activity of SOD was decreased in OSF, and could have resulted in low levels of H2O2. Detoxification of H2O2 requires GPx and CAT. Detoxification of H2O2 by GPx occurs at lower concentration of H2O2, whereas CAT is effective when GPx pathway reaches saturation with substrate and at higher concentration of H2O2. In the present study, lower activity of SOD could have resulted in higher levels of superoxide anion and resulted in inhibition of CAT [45]. Lower activity of GPx could be a consequence of suboptimal generation of H2O2. Further GPx, is also involved in the maintenance of GSH in reduced form [46].
Salivary vitamin A levels positively correlate to serum vitamin A levels and the levels are lowered with advancing stages of OSF [47]. In the present study, salivary vitamin A levels decreased significantly with increasing grades and stages of OSF. These results confirm the role of vitamin A deficiency in fibrosis [48] and epithelial atrophy [9]. Ascorbic acid is an important free radical scavenger antioxidant and its levels decrease with increased utilization in collagen synthesis with increased lipid peroxidation caused by the stimulation of fibroblasts [49]. The increase in lipid peroxidation products in the present study could be a contributing factor to the associated severity of disease presentation.
In summary, the action of GSH, vitamin E and vitamin C are synergistic, with vitamin E on the lipophilic domain and vitamin C on the hydrophilic domain of the membrane. Vitamin C and vitamin E together prevent the oxidation of GSH and GSH is required in the regeneration of both vitamin E and vitamin C [50]. The absence of any further increase in the levels of GSH in saliva could have resulted inlow levels of vitamin E and vitamin C in saliva of OSF patients and thus increased the peroxidative damage in oral cavity and favoured the progress of disease.
Conclusion
Salivary diagnostics has a phenomenal application in various basic medical and clinical research, however its application in clinical use is limited. The current results show that assessment of salivary oxidants and antioxidants can be useful in monitoring the progress of OSF and also aid in modifying the therapeutic strategy.
Acknowledgements
The study was funded by Young Faculty Research Grant of Sri Ramachandra University (2010–2011).
Contributor Information
C. V. Divyambika, Email: divyambika@rediffmail.com
S. Sathasivasubramanian, Email: dr_sathasivam@yahoo.co.in
G. Vani, Email: vaniganpat@gmail.com
A. J. Vanishree, Email: vanielango@gmail.com
N. Malathi, Email: narasimhan.malathi@gmail.com
References
- 1.Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003;7(1):1–4. doi: 10.4103/0973-029X.86678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Hazare VK, Goel RR, Gupta PC. Oral submucous fibrosis, areca nut and pan masala use: a case-control study. Natl Med J India. 1998;11(6):299. [PubMed] [Google Scholar]
- 3.Amarasena N, Ekanayaka AN, Herath L, Miyazaki H. Association between smoking, betel chewing and gingival bleeding in rural Sri Lanka. J Clin Periodontol. 2003;30(5):403–408. doi: 10.1034/j.1600-051X.2003.20010.x. [DOI] [PubMed] [Google Scholar]
- 4.Angadi PV, Kale AD, Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: correlation with disease severity. J Oral Pathol Med. 2011;40(3):208–213. doi: 10.1111/j.1600-0714.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang YC, Yang SF, Tai KW, Chou MY, Hsieh YS. Increased tissue inhibitor of metalloproteinase-1 expression and inhibition of gelatinase A activity in buccal mucosal fibroblasts by arecoline as possible mechanisms for oral submucous fibrosis. Oral Oncol. 2002;38(2):195–200. doi: 10.1016/S1368-8375(01)00045-8. [DOI] [PubMed] [Google Scholar]
- 6.Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis: a collagen metabolic disorder. J Oral Pathol Med. 2005;34(6):321–328. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 7.Ekanayaka RPTW. Oral submucous fibrosis: review on mechanisms of pathogenesis and malignant transformation. J Carcinog Mutagene. 2013;S5:002. [Google Scholar]
- 8.Stich HF, Anders F. The involvement of reactive oxygen species in oral cancers of betel quid/tobacco chewers. Mutat Res. 1989;214(1):47–61. doi: 10.1016/0027-5107(89)90197-8. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-D. [DOI] [PubMed] [Google Scholar]
- 10.Bourne GH, Kidder GW. Biochemistry and physiology of nutrition. Cambridge: Academic Press; 1953. [Google Scholar]
- 11.Malamud D. Saliva as a diagnostic fluid. Dent Clin N Am. 2011;55(1):159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miricescu D, Greabu M, Totan A, Didilescu A, Radulescu R. The antioxidant potential of saliva: clinical significance in oral diseases. Ther Pharmacol Clin Toxicol. 2011;2:139–143. [Google Scholar]
- 13.Miricescu D, Totan A, Calenic B, Mocanu B, Didilescu A, Mohora M, et al. Salivary biomarkers: relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand. 2014;72(1):42–47. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rawi NH. Diabetes, oxidative stress, antioxidants and saliva: a review. Rijeka: INTECH Open Access Publisher; 2012. [Google Scholar]
- 15.Khanna JN, Andrade NN. Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases. Int J Oral Maxillofac Surg. 1995;24(6):433–439. doi: 10.1016/S0901-5027(05)80473-4. [DOI] [PubMed] [Google Scholar]
- 16.Haider SM, Merchant AT, Fikree FF, Rahbar MH. Clinical and functional staging of oral submucous fibrosis. Br J Oral Maxillofac Surg. 2000;38(1):12–15. doi: 10.1054/bjom.1999.0062. [DOI] [PubMed] [Google Scholar]
- 17.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22(6):764–779. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 21.Bessey OA, Lowry OH, et al. The determination of vitamin A and carotene in small quantities of blood serum. J Biol Chem. 1946;166(1):177–188. [PubMed] [Google Scholar]
- 22.Emmerie A, Engel C. Colorimetric determination of tocopherol (Vitamin E): III. Estimation of tocopherol in blood-serum. Recl Trav Chim Pays Bas. 1939;58(10):895–902. doi: 10.1002/recl.19390581007. [DOI] [Google Scholar]
- 23.Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem. 1943;147(2):399–407. [Google Scholar]
- 24.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 25.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Paissat DK. Oral submucous fibrosis. Int J Oral Surg. 1981;10(5):307–312. doi: 10.1016/S0300-9785(81)80026-9. [DOI] [PubMed] [Google Scholar]
- 27.Nair UJ, Floyd RA, Nair J, Bussachini V, Friesen M, Bartsch H. Formation of reactive oxygen species and of 8-hydroxydeoxyguanosine in DNA in vitro with betel quid ingredients. Chem Biol Interact. 1987;63(2):157–169. doi: 10.1016/0009-2797(87)90095-0. [DOI] [PubMed] [Google Scholar]
- 28.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Ball WD, Hand AR, Johnson AO. Secretory proteins as markers for cellular phenotypes in rat salivary glands. Dev Biol. 1988;125(2):265–279. doi: 10.1016/0012-1606(88)90210-2. [DOI] [PubMed] [Google Scholar]
- 30.Krasteva AAE, Ivanova A, Altankova I, Bocheva T, Stanimirov P, Bobeva A, Janev N, Kisselova A. Salivary components of treated cancer patients and patients with precancerous lesions. J IMAB. 2008;14(2):41. [Google Scholar]
- 31.Dayem AA, Choi HY, Kim JH, Cho SG. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers. 2010;2(2):859–884. doi: 10.3390/cancers2020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52(2):251–256. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- 33.Metkari S, Tupkari J, Barpande S. An estimation of serum malondialdehyde, superoxide dismutase and vitamin A in oral submucous fibrosis and its clinicopathologic correlation. J Oral Maxillofac Pathol. 2007;11(1):23–27. doi: 10.4103/0973-029X.33960. [DOI] [Google Scholar]
- 34.Shetty SR, Babu SG, Kumari S, Rao V, Vijay R, Karikal A. Malondialdehyde levels in oral sub mucous fibrosis: a clinicopathological and biochemical study. N Am J Med Sci. 2012;4(3):125–128. doi: 10.4103/1947-2714.93887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Reddy MV, Harinath BC. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J Clin Biochem. 2004;19(1):138–141. doi: 10.1007/BF02872409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanna SS, Karjodkar FR. Circulating immune complexes and trace elements (copper, iron and selenium) as markers in oral precancer and cancer: a randomised, controlled clinical trial. Head Face Med. 2006;2:33. doi: 10.1186/1746-160X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med. 2002;32(3):268–277. doi: 10.1016/S0891-5849(01)00806-1. [DOI] [PubMed] [Google Scholar]
- 38.Amerongen AV, Veerman EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 39.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29(3):189–194. doi: 10.1034/j.1600-051X.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 40.Wong DY, Hsiao YL, Poon CK, Kwan PC, Chao SY, Chou ST, et al. Glutathione concentration in oral cancer tissues. Cancer Lett. 1994;81(2):111–116. doi: 10.1016/0304-3835(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 41.Chang YC, Huang FM, Tai KW, Yang LC, Chou MY. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodontal Res. 2002;37(4):279–285. doi: 10.1034/j.1600-0765.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 42.Nordmann R, Ribière C. Superoxydedismutases: role biologique; espoir thérapeutique? Cah Nutr Diét. 1991;26(6):398–402. [Google Scholar]
- 43.Perluigi M, Butterfield DA. Oxidative stress and down syndrome: a route toward Alzheimer-like dementia. Curr Gerontol Geriatr Res. 2012;2012:724904. doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epperly MW, Carpenter M, Agarwal A, Mitra P, Nie S, Greenberger JS. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo. 2004;18(4):401–410. [PubMed] [Google Scholar]
- 45.Laszlo A, Matkovics B, Varge SI, Wittman T, Fazekas T. Changes in lipid peroxidation and antioxidant enzyme activity of human red blood cells after myocardial infarction. Clin Chim Acta. 1991;203(2–3):413–415. doi: 10.1016/0009-8981(91)90319-8. [DOI] [PubMed] [Google Scholar]
- 46.Flohe L. The impact of thiol peroxidases on redox regulation. Free Radic Res. 2015;50(2):1–17. [PubMed] [Google Scholar]
- 47.Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(2):207–213. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Heber D, Bowerman S. Nutrition and cancer treatment. Handbook of nutrition and food. Boca Raton: CRC Press; 2001. [Google Scholar]
- 49.Geesin JC, Hendricks LJ, Falkenstein PA, Gordon JS, Berg RA. Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991;290(1):127–132. doi: 10.1016/0003-9861(91)90598-D. [DOI] [PubMed] [Google Scholar]
- 50.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]