Abstract
Activating transcription factor 4 (ATF4) is a member of the PERK signaling pathway, which directly binds endoplasmic reticulum stress target genes and plays a crucial role in both adaptations to stress and activation of apoptosis. Previous publications demonstrated conflicting evidence on the role of ATF4 in the pathogenesis of neurodegenerative disorders. In this study, we used recombinant adeno-associate virus (rAAV)-mediated gene transfer to investigate if the sustained up-regulation of ATF4 launches a pro-survival or pro-death trend in the dopamine (DA) cells of the substantia nigra pars compacta in a rat model of Parkinson-like neurodegeneration induced by human alpha-synuclein (αS) overexpression. We showed that ATF4 does not protect nigral DA neurons against an αS-induced pathology. Moreover, the rAAV-mediated overexpression of ATF4 resulted in severe nigra-striatal degeneration via activation of caspases 3/7.
Keywords: Parkinson’s disease, ER stress response, ATF4, Alpha-synuclein, AAV
1. Introduction
Parkinson’s disease (PD) is neurodegenerative disorder characterized by a severe loss of DA neurons in the substantia nigra pars compacta (SNc) and by the presence of intracellular inclusions containing mostly misfolded alpha-synuclein (αS). Accumulation of αS on the endoplasmic reticulum (ER) lumen [3] can induce stress, which in turn triggers ER stress response, also known as the unfolded protein response (UPR). UPR is a cellular adaptive mechanism aiming to restore the protein-folding capacity of the ER. However, cells undergo apoptosis if the early UPR is unable to resolve a protein-folding crisis. An activation of UPR markers was previously identified in melanin-containing neurons of the SNc in post-mortem tissues of PD patients [8]. Many other studies have demonstrated the involvement of UPR signaling in the degeneration of DA cells in experimental in vitro and in vivo models of PD caused by human wild-type or mutated αS, 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), or rotenone [13,2,5,19].
The protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway has been identified as one UPR arm that is strongly protective at modest levels of signaling but can contribute signals to cell death pathways [17]. This signaling is known to promote PERK-induced phosphorylation of the eukaryotic initiation factor 2alpha, which results in the inhibition of protein synthesis in a stressed cell. While the level of translational capacity is attempting to restore cellular balance, preferential translation of activating transcription factor 4 (ATF4) mRNA and the induction of the proapoptotic CCAAT-enhancer-binding protein homologous protein (CHOP) protein are known to be induced in the cell. Induction of CHOP strongly depends on ATF4. With this said, the role of ATF4 in triggering the pro-survival [6,15] or pro-death [10] trend is a source of continuous debates, which suggest that ATF4 is an important element in cell fate decision.
ATF4 is a member of the cAMP-responsive element-binding protein family of basic zipper-containing proteins. Analysis of existing literature shows that ATF4 controls the expression of growth factors, cytokines, chemoattractants, and adhesion molecules [11]. It also plays an important role in many biological functions, such as hematopoiesis, skeletal development, learning and memory formation, hypoxia resistance, tumor growth, autophagy, and amino acid deprivation [18]. ATF4 has been found to be up-regulated in many pathological conditions, and this indicates its therapeutic potential in targeting its expression.
Previously, we showed the association between experimental PD progression and ATF4 protein level [5]. We found that the rAAV-mediated overexpression of human αS results in a seven to ninefold induction of the ATF4 protein. We also identified that during aging, which is considered the most well-established predisposing factor for PD, the nigral tissues of old (24 months) rats have a twice higher rate of ATF4 expression than do young (2 months) animals [14]. Moreover, a recent study [15] found that ATF4 levels are increased in neuromelanin-positive neurons in the SNc of a subset of PD patients relative to the controls. This work also demonstrated that silencing of ATF4 in a tissue culture PD model enhances, and conversely, overexpression of ATF4 reduces cell death in response to either 6-OHDA or 1-methyl-4-phenylpyridinium [15].
In the current study, we used rAAV vector gene transfer to examine if the sustained overexpression of ATF4 affects nigra-striatal degeneration induced by human αS cytotoxicity.
2. Methods
2.1. rAAV vectors
All vectors have been packaged in rAAV5 capsid and purified as described previously [14]. Titers for all rAAV constructs were equalized to 6 × 1012 vg/ml.
2.2. Intracerebral injection of rAAV vectors
All surgical procedures were performed using aseptic techniques and isofluorene gas anesthesia as previously described [5,14]. The rats were placed in the stereotaxic frame and injected into the SNc (anterior posterior −5.6 mm, lateral −2.4 mm from bregma and dorsoventral −7.2 mm from dura), through a glass micropipette at a rate of 0.5 μl per minute. Animals were injected with a total of 1.5 μl for single gene or 3 μl for gene combination.
2.3. Isolation and processing of tissues
Animals were deeply anesthetized by pentobarbital injection. Brains were removed and divided into two parts by a coronal blade cut at approximately −3.5 mm behind bregma. The caudal part containing the SNc was fixed in the ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The fixed part of brains were stored overnight at 4 °C and then transferred into 30% sucrose in 0.1 M PB for cryoprotection. Forty μm thick coronal sections were cut and processed for immunohistochemistry. The rostral piece of brain tissue was used immediately to dissect the right and left striatum. Tissue samples were frozen separately on dry ice and kept at −80 °C until assayed. All rats from 4 week time point were used to obtain SNc tissue samples for Western blot analysis. Frozen brains were sectioned on a cryostat (Leica Microsystems) into 150 μm slices with SNc tissue subsequently dissected out under a microscope as described previously [14]. Total tissue weight of nigral tissue obtained from 1 animal did not exceed 0.2–0.4 mg.
2.4. Immunohistochemistry
For the bright-field microscopy analysis sections were preincubated first with 1% H2O2–10% methanol for 15 min and then with 5% normal goat serum for 1 h. Sections were incubated overnight at room temperature with anti-TH (1:2000; #MAB318, mouse; Millipore) or anti-α-syn (1:1000; #61-787, mouse; BD Laboratories) antibodies. Incubation with biotynilated secondary anti-mouse antibody was followed by incubation with avidin–biotin–peroxidase complex (ABC; Vector Laboratories, Burlingame, CA, USA). Reactions were visualized using NovaRED Peroxidase (HRP) Substrate Kit (Vector Laboratories, Burlingame, CA, USA).
2.5. Unbiased stereology
The unbiased stereological estimation of the total number of the TH-positive neurons in SNc was performed using the optical fractionator method as described previously [9,5,14]. Sampling of cells to be counted was performed using the MicroBrightfield StereoInvestigator System. The software was used to delineate the transduction area at 5 × on 40 μm sections and generate counting areas of 100 × 100 μm. The estimate of the total number of neurons and coefficient of error due to the estimation was calculated according to the optical fractionator formula [9].
2.6. Stiatal DA measurements
Frozen striatal tissue samples were shipped on dry ice to the Neurochemistry Core Lab at Vanderbilt University Medical Center, Nashville, TN for HPLC analysis of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the striatum. A total of 6–8 animals were analyzed for striatal DA metabolites per group.
2.7. Rotational behavior
Drug-induced rotational behavior was measured following an injection of d-amphetamine sulfate (2.5 mg/kg i.p.; Sigma, St Louis, MO) at 8 weeks after the viral injection. Rotations were measured during a 90-min period, and full 360° turns were counted.
2.8. Western blot analysis
The total protein was extracted via sonication in a protein extraction buffer containing 970 uL of RIPA, 10 uL of 100 mM PMSF, 10 uL of 100 mM EGTA, and a mixture of protease inhibitors (PMSF, TLCK, aprotinin, leupeptin, and pepstatin). The protein concentrations were determined using BioRad Protein Assays and based on the Bradford method of protein quantitation. Next, the proteins (30 ug) were separated in 4–20% Criterion Precast gels and 5% polyacrylamide gels (BioRad), transferred to a polyvinylidene difluoride (PVDF) membrane using the trans-Blot Turbo Transfer System (BioRad), and incubated with primary antibodies overnight at 4° C under agitation. Mouse anti-αS (1:5000; BD Transduction Laboratories, Zymed Laboratories #610787), mouse anti-TH (1:2000; Millipore #MAB318), anti-ATF4 (1:1000; Abcam #ab50546) primary antibodies were used. Goat anti-rabbit (1:10,000, #926-68021), donkey anti-goat (1:10000, #926-32214), and donkey anti-mouse (1:10000, #926-32210) secondary antibodies were used (LI-COR Odyssey). In addition, β-actin was used as the gel loading control, and was detected using an anti-β-actin antibody (1:5000, Sigma-Aldrich, #A1978). Finally, the developed membrane was imaged using the LI-COR Odyssey Quantitative Fluorescence Imaging System.
2.9. Caspase 3/7 activity assay
The detection of caspase 3/7 activity was performed using the Caspase-Glo 3/7 Luciferase assay (#G8091; Promega, Madison, WI) kit in accordance with the manufacturer’s recommendations. Luciferase signal was read by a luminescent plate reader (Infinite m200, Tecan Group Ltd., Meannedorf, Switzerland) and used to compare the activation of caspase 3/7 in nigral tissues from intact un-injected brains as well as from rats injected with control virus and ATF4 expressing virus.
2.10. Statistical analysis
Data were analyzed using unpaired t test and one-way analysis of variance with Tukey post-test using Prism 6 (GraphPad Software, Inc.). Data are presented as mean ± standard error.
3. Results
Adult (200–250 g) female Sprague-Dawley rats were injected with an equal volume and titer of rAAV vectors to express green fluorescent protein (GFP), human wild-type αS, ATF4, or both αS and ATF4 simultaneously in rat SNc. rAAV vector containing a blank expression cassette (BV), which is an αS vector with an early stop codon, was used to maintain a consistent volume and particle number between single and double vector injections. The rAAV-BV does not express any protein and was used in our recent study as a control virus [14]. All viral vectors in this study utilized the rAAV serotype 5 capsid. All genes engineered into the rAAV vectors were expressed from the same chicken β-actin cytomegalovirus hybrid promoter. Viral vectors were administered in one side of the brain, whereas the contralateral side was kept as an un-injected internal control.
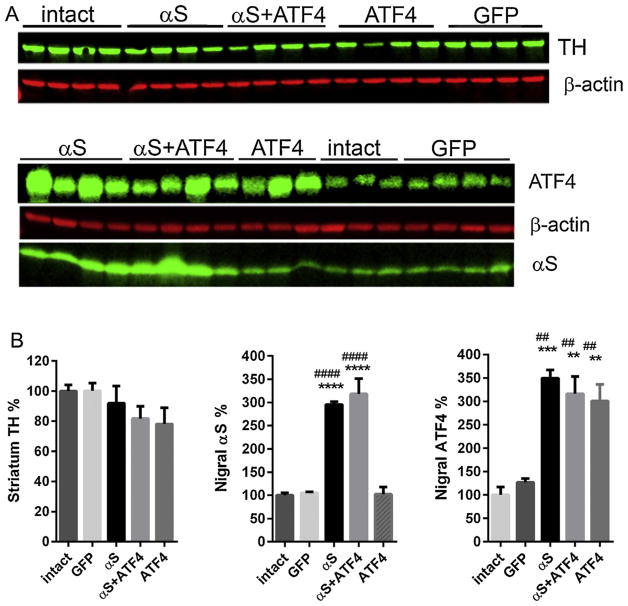
The animals were killed at 4 and 8 weeks after virus administration. The selection of time points was based on our previous studies [5,14], which revealed that the expression of αS bulked up to the maximum at 3–4 weeks after virus injection, and nigrastriatal degeneration became significant after 8 weeks. Dissected striatal and nigral tissues from rats sacrificed at 4 weeks after viral injection were used for Western blot analysis to confirm that rAAV transduction resulted in a sufficient expression level of αS or ATF4 and that co-injection of both viruses did not have a significant effect on the expression level of either protein (Fig. 1A). Tissues from the un-injected side were used as internal intact controls, and data obtained in experimental animals were shown as a percentage of the injected side versus the un-injected side (Fig. 1B). When antibody signals were normalized to housekeeping protein β-actin, quantitative immunoblot analysis showed that the level of striatal TH protein was not changed significantly in hemispheres injected with rAAV-αS compared with the un-injected control side. Given this, we observed some reduction of striatal TH protein in experimental groups injected with rAAV-ATF4 and both rAAV-ATF4 and rAAV-αS simultaneously (by 22 ± 10.8% and 18.3 ± 8.2%, accordingly). However, it did not reach a statistically significant level.
Fig. 1.
Expression of the human wild-type αS and ATF4 4 weeks after rAAV injections in rat SNc. (A) Striatal (top image) and nigral (lower image) tissues from 4-week time point animals were extracted and then immunoblotted sequentially with antibody to TH, αS, ATF4, or β-actin. Antibody signals were measured by fluorescent scanning, normalized to β-actin. (B) Graphs showing the expression level of TH, αS, and ATF4 proteins illustrated in A. The percentage of protein expression was calculated by comparison with the un-injected tissue. One-way group ANOVA statistics. Tukey’s multiple comparison test is indicated as ** (##), *** and **** (####) = P < 0.01, 0.001, and 0.0001, respectively, vs. intact (*) and GFP (#); n = 3–4. The data are presented as mean ± SE.
Quantitative immunoblots of the nigral tissues showed an increase in the total αS protein level in rats injected with rAAV-αS and with rAAV-αS + rAAV-ATF4 (2.95 ± 0.1 and 3.2 ± 0.3-fold, respectively) over the endogenous rat αS at 4 weeks after injection, when normalized to the total protein. As we showed in our previous study, the antibody that was used binds to human and rat α-syn with the same affinity [5]. Western blot analysis of nigral ATF4 expression indicated the analogous up-regulation of the protein in animals injected with rAAV-ATF4 or rAAV-ATF4 + rAAV-αS (3.0 ± 0.4 and 3.1 ± 0.4-fold, respectively) compared with intact nigral tissue. However, the ATF4 protein level in nigras overexpressing only exogenous αS was similar to that in the above experimental groups (3.5 ± 0.4-fold). This result confirms our previous findings that the up-regulation of ATF4 protein is a consequence of human αS overexpression in rat SNc [5]. Additional control experiments were done with mixtures of viruses carrying green fluorescent protein (GFP) and rAAV-ATF4, as well as either GFP or ATF4 viruses in addition to a “blank” virus to maintain an equal injection volume. These experiments proved that no inhibition of GFP or ATF4 expression that might be due to viral competition or protein–protein interaction by co-infection with two viral vectors without a significant interference between the vectors was seen (Suplementary Fig. 1).
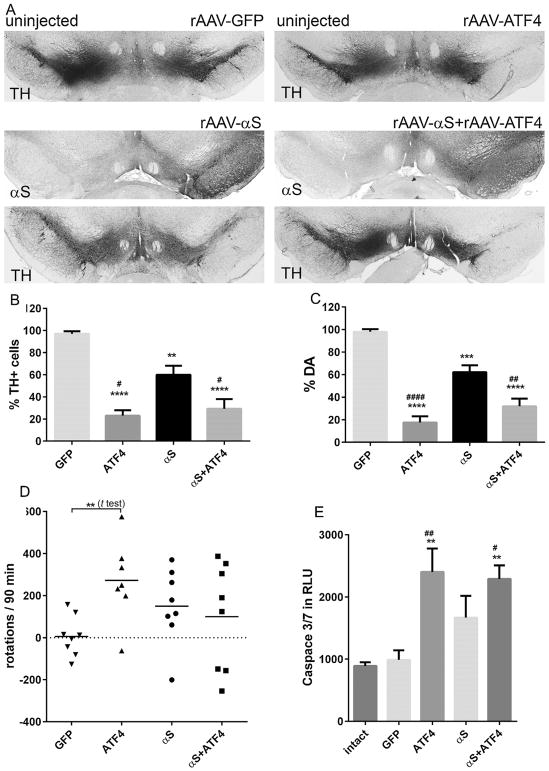
As expected, the unbiased estimation of TH-positive cells in the SNc at 8 weeks (Fig. 2A,B) showed a statistically significant neuronal loss in animals that were injected with rAAV-αS compared with the GFP-injected rats (60.1 ± 8.2% vs. 99.1 ± 3.3%, both n = 5; P < 0.01). We also found that in hemispheres injected with rAAV-αS, the level of striatal DA (Fig. 2C) was reduced significantly compared with that in the control animals (62.2 ± 7.0%, n = 8 vs. 99.7 ± 2.5%, n = 7; P < 0.001). Thus, we confirmed our previous results [5] indicating that human αS induces significant neuronal death at 8 weeks after virus delivery.
Fig. 2.
Overexpression of ATF4 alone or in combination with human αS in SNc-induced neurodegeneration 8 weeks after virus delivery in the SNc. (A) Bright-field photomicrographs showing the remaining TH-positive cells and human αS immunostaining in the SNc of representative animals from different experimental groups 8 weeks after rAAV delivery. Whereas the expression of GFP protein did not alter the number of TH-positive neurons, the expression of human αS, ATF4, or both proteins simultaneously led to the loss of TH-positive cells in the SNc compared with the contralateral intact side. (B–C) Graphs showing the results of the unbiased stereology count of remaining TH-positive neurons in the SNc and striatal DA assay obtained from different experimental groups illustrated in (A). The number of TH-positive neurons counted in the αS-injected SNc was significantly reduced compared with the control GFP rats. Co-expression of αS and ATF4 proteins did not rescue TH-positive cells and striatal DA level. Moreover, both overexpression of ATF4 alone or in combination with αS dramatically aggravated nigra-striatal impairment. The loss of TH-positive cells and striatal DA was significant not only compared with the control GFP animals but also with the rats overexpressing human αS alone. One-way group ANOVA statistics. Tukey’s multiple comparison test is indicated as #, **, ***, and **** (####) = P < 0.05, P < 0.01, P < 0.001, and 0.0001, respectively, vs. GFP (*) and αS (#); n = 5 (all groups in graph C), n = 7–8 (graph D) per group. (D) Amphetamine-induced rotation test performed at 8 weeks post-injection. Tukey’s multiple comparison test did not show a significant number of rotations toward the injected side (positive, ipsilateral rotation) in any experimental group. However, unpaired t test showed a significant increase of ipsilateral rotations in rats overexpressing ATF4 alone compared with the GFP control group. Unpaired t test is indicated as ** = P < 0.01, n = 7–8. (E) Nigral cell loss in rats overexpressing ATF4 alone or in combination with αS was associated with a highly activated caspase-3/7 compared with both the control GFP and un-injected intact animals. One-way group ANOVA statistics. Tukey’s multiple comparison test is indicated as # and ** (##) = P < 0.05 and 0.01, respectively, vs. intact (*) and GFP (#); n = 4. The data are presented as mean ± SE.
In an attempt to evaluate if a sustained increase in ATF4 can potentially protect nigral DA cells against αS-induced neurodegeneration, in PC12 cells treated with the dopaminergic neuronal toxins [15], we found that the rAAV-mediated overexpression of ATF4 has an adverse effect. Both experimental animal groups expressing exogenous ATF4 or ATF4 + αS displayed severe nigral TH-positive cell loss (23.0 ± 4.9% or 29.4 ± 6.7%, both n = 5) and reduction of striatal DA (17.2 ± 5.4% or 32.6 ± 7.0%, both n = 8), which were significant not only compared with the control group injected with the GFP virus (P < 0.0001 for all comparisons) but also with the rats overexpressing human αS alone (P < 0.01 or P < 0.05 for TH-positive cell count and P < 0.0001 or P < 0.01 for striatal DA assay) (Fig. 2A–C).
Despite severe loss of nigral TH-positive neurons and the striatal DA level, a multiple comparison test with all experimental groups did not detect any statistical significance in the amphetamine induced-rotation. However, an unpaired t test revealed a significantly higher number of rotations in rAAV-ATF4 injected rats compared to control GFP expressing animals (Fig. 2D).
To elucidate if apoptosis is involved in ATF4-induced nigrostriatal degeneration, we performed caspase 3/7 luciferase assay of protein extracts obtained from nigral tissues at 4 weeks after rAAV injections (Fig. 2E). We found that the rAAV-mediated up-regulation of ATF4 expression induced a twice higher level of caspace 3/7 compared with both GFP-expressing nigras or the un-injected control (P < 0.05 or P < 0.01, respectively; n = 4–8).
4. Discussion
The results obtained in this study showed that the sustained over-expression of ATF4 in the SNc leads to DA neuronal cell death presumably through apoptosis. Our results demonstrate that the rAAV-mediated expression of human αS alone or in combination with ATF4, as well as the viral-mediated expression of ATF4 alone, resulted in a relatively similar ATF4 protein expression level, which may be caused by the cellular capacity to handle a certain protein level. With this, attention is drawn to the fact that about the same high ATF4 production as a consequence of both human αS expression or rAAV-mediated ATF4 gene transfer led to a significantly different severity of nigral degeneration (Fig. 2). We speculate that the direct up-regulation of the ATF4 protein via rAAV-mediated gene transfer misses some steps in the UPR, which has been shown to be activated by human αS expression and can hypothetically delay neurodegeneration. In our previous publication and in the works of others, αS overexpression has been shown to activate all UPR branches: PERK, activating transcriptional factor 6 (ATF6), and inositol requiring kinase 1 (IRE1) [1,2,5,12]. Therefore, we assume that the IRE1 pathway [16], or upstream components of PERK signaling [2] can delay nigral degeneration induced by overexpressing αS. Precise mechanisms require further comparative studies.
A previous study conducted by Sun, Liu et al. [15] proposed a cytoprotective role for ATF4 during the treatment of PC12 cells with 6-OHDA. Conversely, we proposed a cytotoxic role of sustained overexpressed ATF4 in animals manifesting PD-like pathology. While our results agree with the published works of others [10] revealing that ATF4 could be toxic, the previous mentioned study assigned an opposite role for ATF4 and contradicted our findings. Perhaps, the major discrepancy between these studies is in the fact that in vivo and in vitro systems might respond ambiguously to the same stress and might reflect the difference between young proliferating and adult non-dividing neuronal cells. In addition, the stress induced in these studies might be activated or maintained at either different powers, durations, or phases of activation, such as acute versus adaptive UPR.
In our study, we found that nigral DA neurons die seemingly through apoptosis, and caspase-3/7 activity is increased in response to ATF4 elevation. A recent study [7] has demonstrated that the ATF4 during ER stress may increase the degradation of the X-linked inhibitor of apoptosis protein (XIAP), which is known to bind to caspase-3 and −7 and inactivate them [4]. Perhaps, the increase in caspase-3/7 activity in ATF4 overexpressed nigral neurons can be due to an enhanced degradation of and impeded control by cytoprotective XIAP. However, the precise cellular signaling activated in response to ATF4 overexpression in these cells needs to be clarified.
In summary, we propose that the elevation of ATF4 detected in an experimental animal model and postmortem melanin containing neurons of PD patients may play a crucial role in neuronal cell death. Therefore, maintaining the ATF4 level seems to be important for the propagation of PD symptoms. Taking into account our previous findings on the up-regulated ATF4 level in aged SNc, we believe that ATF4 could be an attractive therapeutic target for PD intervention.
Supplementary Material
HIGHLIGHTS.
ATF4 does not protect nigral dopamine neurons against α-synuclein induced pathology.
Up-regulation of the ATF4 in nigral neurons increases caspase 3/7 activity.
Overexpression of the ATF4 leads to severe nigra-striatal degeneration.
Acknowledgments
This work was supported by the M. J. Fox Foundation grant (to O.S.G.) and the VSRC Core Grant P30 EY003039.
Abbreviations
- ATF4
activating transcription factor 4
- αS
alpha-synuclein
- DA
dopamine
- PD
Parkinson’s disease
- TH
tyrosine hydroxylase
- SNc
substantia nigra pars compacta
- rAAV
recombinant Adeno-associated virus
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2016.05.039.
References
- 1.Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S. Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease. J Neurochem. 2011;116:588–605. doi: 10.1111/j.1471-4159.2010.07143.x. [DOI] [PubMed] [Google Scholar]
- 2.Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK. Endoplasmic reticulum stress Is important for the manifestations of α-synucleinopathy in vivo. J Neurosci. 2012;32(10):3306–3320. doi: 10.1523/JNEUROSCI.5367-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J Neurosci. 2012;32(10):3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases? Nature. 1997;388(6639):300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 5.Gorbatyuk MS, Shabashvili A, Chen W, Meyers C, Sullivan LF, Salganik M, Lin JH, Lewin AS, Muzyczka N, Gorbatyuk OS. Glucose regulated protein 78 diminishes [alpha]-synuclein neurotoxicity in a rat model of Parkinson disease. Mol Ther. 2012;20(7):1327–1337. doi: 10.1038/mt.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease? Nat Rev Drug Discov. 2013;12(9):703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu N, Messah C, Han J, LaVail MM, Kaufman RJ, Lin JH. Translational and posttranslational regulation of XIAP by eIF2α and ATF4 promotes ER stress–induced cell death during the unfolded protein response. Mol Biol Cell. 2014;25(9):1411–1420. doi: 10.1091/mbc.E13-11-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Björklund A. Parkinson-like neurodegeneration induced by targeted overexpression of α-synuclein in the nigrostriatal system? J Neurosci. 2002;22(7):2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange PS, Chavez JC, Pinto JT, Coppola G, Sun C-W, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress–inducible: prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205(5):1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malabanan KP, Khachigian LM. Activation transcription factor-4 and the acute vascular response to injury? J Mol Med (Berl) 2010;88(6):545–552. doi: 10.1007/s00109-010-0615-4. [DOI] [PubMed] [Google Scholar]
- 12.Mercado G, Castillo V, Soto P, Sidhu A. ER stress and Parkinson disease: pathological inputs that converge into the secretory pathway. Brain Res. 2016 doi: 10.1016/j.brainres.2016.04.042. [Epub ahead of print] [DOI] [PubMed]
- 13.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salganik M, Sergeyev VG, Shinde V, Meyers CA, Gorbatyuk MS, Lin JH, Zolotukhin S, Gorbatyuk OS. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (α-syn) toxicity to rat nigral neurons. Neurobiol Aging. 2015;36(6):2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Liu J, Crary JF, Malagelada C, Sulzer D, Greene LA, Levy OA. ATF4 Protects Against Neuronal Death in Cellular Parkinson’s Disease Models by Maintaining Levels of Parkin. J Neurosci. 2013;33(6):2398–2407. doi: 10.1523/JNEUROSCI.2292-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdés P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1? Proc Nat Acad Sci. 2014;111(18):6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Guo F. Effects of activating transcription factor 4 deficiency on carbohydrate and lipid metabolism in mammals. IUBMB Life. 2012;64(3):226–230. doi: 10.1002/iub.605. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Tian YY, Shi JP, Xie W, Shi JQ, Lu J, Zhang YD. Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effects of candesartan cilexitil in the rotenone rat model of Parkinson’s disease. Neurosci Lett. 2013;548:50–55. doi: 10.1016/j.neulet.2013.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.