Abstract
Background
A torn rotator cuff tendon will retract over time causing changes in muscle properties and decreasing its extensibility, or deformation. During surgery, large tensile loads are applied to bring the torn tendon to the footprint. Poor muscle extensibility and large tensile stresses at the repair might lead to gap formation or re-tear of the repair. A quantitative evaluation of muscle properties could be used to predict the extensibility of the supraspinatus (SSP) muscle.
Method
Magnetic resonance imaging (MRI)-measured volumetric fat fraction and shear wave elastography (SWE)-measured elastic modulus of the SSP muscle were obtained on seventeen cadaveric shoulders. Experimental extensibility and stiffness were then measured by axially pulling the tendon up-to 60N. Univariate and multivariate analyses were used to determine the correlation and contribution of fat fraction and elastic modulus to experimental outcomes.
Findings
SWE moduli negatively correlated with SSP muscle extensibility (r = 0.54–0.58, P ≤ 0.0259); fat fraction resulted in a positive correlation (r = 0.69, P=0.0021). SWE measurements, solely, explained up to 34% and 33% of the variability in measured extensibility and stiffness, respectively. Fat Fraction, solely, explained 48% of the variability in extensibility and 36% of the variability in stiffness. These methods combined predicted up to 62% of the musculotendinous extensibility.
Interpretation
This study showed a comprehensive quantitative assessment of SSP muscle properties using SWE to estimate stiffness and MRI to measure fatty infiltration. The extensibility of the detached muscle/tendon unit was highly correlated to material properties of the muscle when these methods were used in combination.
Keywords: Rotator cuff tear, Ultrasound, Magnetic resonance imaging, Muscle properties, Tendon retraction
Introduction
Pain, disability, and muscle atrophy are all sequela of rotator cuff tendon and muscle injury (Milgrom et al., 1995; Minagawa et al., 2013; Yamamoto et al., 2010). Patients who fail conservative treatment undergo individualized surgical procedures in order to reduce pain and restore shoulder motion and function (Caldwell et al., 1997; Gazielly et al., 1994; Harryman et al., 1991). Due to the nature of the lesion and muscle dysfunction over time, the detached rotator cuff tendon will retract causing a decrease in the supraspinatus (SSP) cross-sectional area, and changes in intramuscular fat infiltration, muscle stiffness, and compliance. These abnormal changes will lead to a deteriorated length-tension relationship of the muscle, representing the force the muscle will be able to generate at a specific length, and a decrease in its extensibility, or deformation. Consequently, during surgery, the muscle and tendon are subjected to large tensile loads in order to bring the torn tendon to the footprint (Berth et al., 2010; Ward et al., 2006). Poor muscle extensibility and large tensile stresses at the repair might eventually lead to gap formation or re-tear of the repair, as observed in about 17–57% of the surgical repaired cases (Bartl et al., 2012; Iannotti et al., 2013; Zumstein et al., 2008). Furthermore, poor muscle environment attributed to high content of fatty infiltration and characterized by muscle fiber atrophy, fibrosis, loss of muscle strength and poor vascularization, will decrease the regenerative potential of the tissue and repair (Gerber et al., 2007; Gladstone et al., 2007; Goutallier et al., 2003; Gumucio et al., 2014; Lohr and Uhthoff, 1990; Rathbun and Macnab, 1970).
In order to obtain a successful repair of the rotator cuff tear and achieve positive outcomes during the rehabilitation process, in addition to the routine clinical parameters measured using computed tomography (CT) and magnetic resonance imaging (MRI), a personalized quantitative assessment of muscle properties is necessary to better estimate the extensibility of the muscle as well as morphological parameters. Current clinical outcomes include the evaluation of gross muscle size and the presence and size of a tear, which can often be quantified; and a two dimensional (2D) qualitative evaluation of fatty infiltration. However, these methodologies and outcome measurements are mainly 2D, and more importantly, are unable to estimate the extensibility of the muscle-tendon unit and provide a quantitative evaluation of muscle properties.
Shear wave elastography (SWE) has been previously used to estimate the stiffness of skeletal muscles (Bouillard et al., 2011; Eby et al., 2015; Hug et al., 2015). Our group has previously implemented SWE to evaluate the properties of the SSP muscle under various positions and surgical conditions (Hatta et al., 2016a; Hatta et al., 2015b; Hatta et al., 2016b). More importantly, we have previously estimated the extensibility of the SSP musculotendinous unit by quantifying the stiffness (Hatta et al., 2017) and volumetric fat infiltration (Giambini et al., 2017) of the SSP muscle, independently, using SWE and MRI, respectively. In the current study, we hypothesized that a combined evaluation of muscle properties, namely stiffness and fatty infiltration, using SWE and MRI, respectively, could be used to predict the extensibility of the muscle, and obtain quantitative measurements of muscle properties that could be used to aid in pre-operative planning and post-surgical evaluation of rotator cuff tears.
Methods
Specimen Preparation
Seventeen fresh-frozen cadaveric shoulders (mean age: 70 yrs.; range: 25–94 yrs.; 14 males, 3 females) were obtained after institutional review board approval from our institution. The shoulder specimens contained the scapula, the humerus cut at the mid shaft, muscles, and all other soft tissues including the skin. The specimens were kept frozen at −20°C and thawed overnight at room temperature before imaging and testing.
Quantitative Magnetic Resonance Imaging
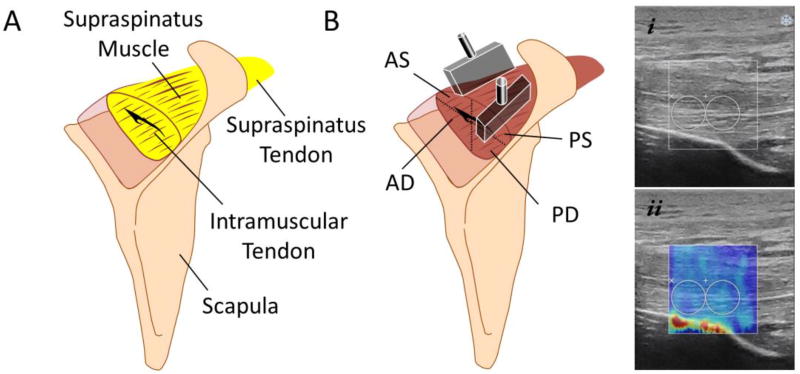
After thawing overnight at room temperature the shoulder specimens were imaged with MRI, and volumetric fat fraction of the whole SSP muscle was quantified as previously described (Giambini et al., 2017). Briefly, scans were performed using a three dimensional (3D), isotropic 2-point Dixon MRI sequence (LAVA FLEX, TR/TE, 3.9/1.2; FOV, 30cm; matrix, 200×200; slice, 1.5mm; flip angle, 10°; bandwidth, 142.86kHz; NEX, 1). Image datasets were imported into MATLAB (Rev. 2015b; The MathWorks, Natick, MA), obtaining real-valued fat and water images as previously described (Rydell et al., 2007). These new water and fat images were then corrected for intensity by the CIIC-method (Andersson et al., 2015), and exported as vtk-files to ITK-SNAP for segmentation of the SSP muscle (Yushkevich et al., 2006). The entire volume of the SSP muscle was manually segmented from both the water and the fat images and the volume masks was then imported back into MATLAB together with the associated water and fat datasets. Mean volumetric intramuscular fat fraction values were obtained based on Equation 1 (Figure 1A).
| (1) |
Fig. 1.
A) Schematic of intramuscular fat infiltration. Fatty infiltration, depicted as yellow, was quantified in the whole supraspinatus (SSP) muscle using magnetic resonance imaging (MRI); B) The ultrasound probes were placed in the SSP muscle according to their muscle fiber orientation and SWE measurements were obtained for the 4 muscle regions: anterior-superficial (AS), posterior-superficial (PS), anterior-deep (AD), and posterior-deep (PD) muscular regions. i) Example of B-mode ultrasound image of the SSP muscle, and ii) SWE image of the SSP muscle with the respective region of interest (ROI).
Shear-wave Elastography Measurements
After imaging the shoulders with MRI, the scapula was disarticulated from the thorax and the humerus was cut at the level of the midshaft. The scapula and a fiberglass rod inserted into the humeral medullary canal were then secured in a custom-built experimental set-up (Hatta et al., 2015a). The scapula was fixed at 0° upward/downward rotation considered as a neutral position based on a recommendation by the International Society of Biomechanics (ISB) (Schwartz et al., 2014; Wu et al., 2005). The experimental set-up, designed to provide 6 degrees-of-freedom motion of the glenohumeral joint in consistent motion paths, was used to place the humerus at 0° abduction for all measurements. All soft tissues including skin, subcutaneous fat, and muscles within the cut level were kept intact during this process.
SWE-measured elastic modulus [kPa] (as a surrogate for stiffness) of the SSP muscle was evaluated using a commercial ultrasound system (Aixplorer; Supersonic Imagine Ltd., Aix-en-Provence, France) with a linear array transducer (2–10 MHz; Supersonic Imagine, Ltd.) as previously described (Hatta et al., 2015b). A built-in-software was used to obtain the modulus for each region. Placement of the ultrasound transducer and measurement regions of the SSP muscle had been previously established (Hatta et al., 2015a; Itoigawa et al., 2015). Briefly, the SSP muscle was divided into 4 regions according to the muscle fiber orientation; anterior deep (AD), anterior superficial (AS), posterior deep (PD), and posterior superficial (PS). Measurements for each region were assessed independently on the plane parallel to the muscle fibers (Figure 1B). SWE measurements were obtained repeatedly 9 times and the mean values were then calculated for each region (Hatta et al., 2015a). This process has previously shown excellent intra- and inter-observer reliability for all regions of the SSP muscle (Hatta et al., 2015a).
Musculotendinous Unit Extensibility Measurement
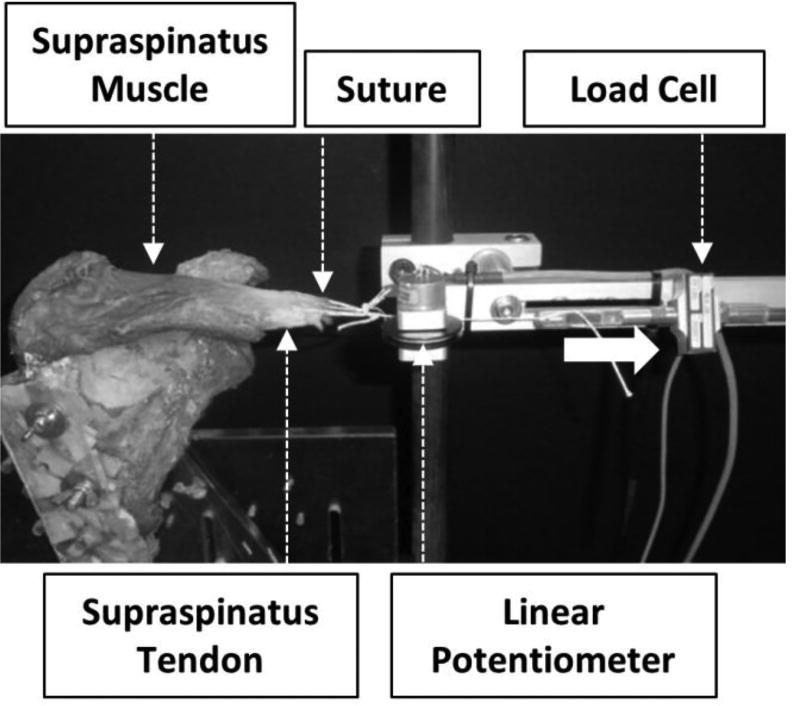
Post ultrasound SWE measurements, all soft tissues and acromion from the shoulder specimens were removed. The presence of a rotator cuff tear, including width and length, were recorded, and the size of the tear was classified into small, medium, large, or massive (Post et al., 1983). The shoulders were then placed in a custom-built experimental set-up allowing for axial deformation of the SSP muscle and load/displacement recordings of the whole muscle-tendon unit (Figure 2). The distal edge of the SSP tendon was cut from the greater tuberosity and the scapulae were rigidly fixed to the fixture. The SSP muscle/tendon junction edge was sutured and this suture was then looped around a linear potentiometer and clamped to a load cell. As the muscle was loaded, the linear potentiometer measured musculotendinous displacement data. To avoid repetitive loading that could damage the muscle fibers and affect the experimental outcomes, each muscle was axially pulled to 60N manually using a pneumatic system for three cycles. This load has been previously implemented as a safe limit to avoid damaging the tendon or the muscle (Meyer et al., 2004; Meyer et al., 2006). The first cycle was used to precondition the muscles and data from the two additional cycles were analyzed. Mean displacement values at 60N were then calculated for all shoulders from the two cycles. Muscle extensibility referred to the mean displacement at a 60N load. Mean stiffness was evaluated from the linear region of the force/displacement curves.
Fig. 2.
Experimental extensibility and stiffness outcomes were obtained using a custom-built experimental set-up that allowed for load and deformation recordings.
Statistical Analysis
JMP version 10.0.0 (SAS Institute Inc., NC) was used for statistical analysis. In all analyses, the outcomes were the measured extensibility and stiffness. Pearson correlation coefficient analysis was used to determine the correlation between fat fraction measured with MRI, or SWE elastic modulus from each muscular region of the SSP muscle, with the extensibility or stiffness outcomes obtained experimentally. For each univariate analysis a coefficient of determination (R2) was calculated. t-test was used to evaluate differences in SWE-measured moduli and fat fraction outcomes between tear and intact specimens (level of significance was set to 0.05).
Multivariate analyses were performed by combining fat fraction and SWE outcomes to explain the measured extensibility or stiffness. For each multivariate analysis an adjusted R2 was determined. Four combinations were evaluated to correlate and predict extensibility: SWE-PD and fat fraction, SWE-PS and fat fraction, SWE-AD and fat fraction, and SWE-AS and fat fraction. Similar analyses were performed to predict the stiffness using the two imaging techniques as explanatory variables. The variance of inflation factor (VIF) was estimated to quantify the severity of multicollinearity. All possible interactions were ignored and the level of significance was set to 0.05.
Results
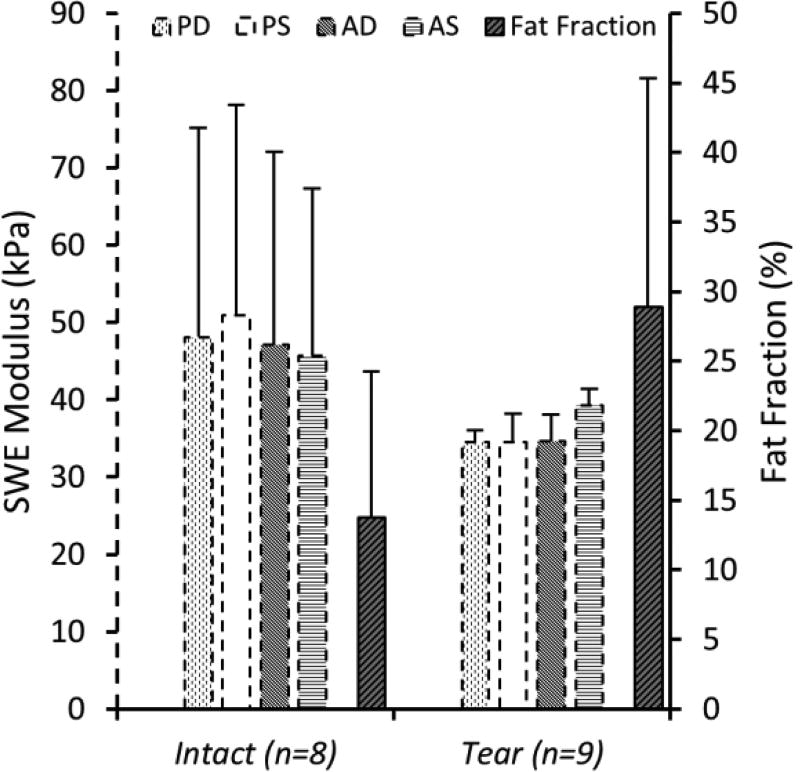
The specimens used for this study consisted of 8 intact shoulders and 9 shoulders with rotator cuff tears. The tear group included 3 small, 1 medium, 3 large, and 2 massive tears based on a tear size classification system. Outcomes from SWE, for the 4 regions of the SSP muscle, fat fraction values from quantitative MRI, and extensibility and stiffness outcomes were successfully obtained in all 17 specimens (Table 1). SWE-measured moduli and MRI-measured fat fraction for the intact and tear specimens are shown in Figure 3. Tear specimens were combined into one group. Although not statistically significant, intact shoulder specimens presented higher SWE moduli in all 4 regions compared to the tear group. On the other hand, the tear group showed higher amounts of fatty infiltration compared to the intact shoulders.
Table 1.
Specimen demographics. Number of specimens, sex and age information along with experimentally measured extensibility, stiffness, SWE-measured moduli, and MRI-fat fraction for intact and tear groups. Tear group includes data from all tear sizes. Values are presented as mean ± SD.
| Intact | Tear | |
|---|---|---|
| Number of specimens | 9 (all men) | 8 (3 females) |
| Age (years) | 59 ± 26 | 80 ± 13 |
| SWE measurements (kPa)* | 47.98 ± 24.53 | 34.30 ± 8.38 |
| Fat fraction (%) | 13.79 ± 0.105 | 28.9 ± 0.164 |
| Extensibility (mm) | 13.88 ± 1.94 | 15.88 ± 2.30 |
| Stiffness (N/mm) | 5.77 ± 0.69 | 4.83 ± 0.80 |
Includes the average values of the 4 muscle regions
The tear group includes all tear sizes from small to massive
Data is presented as Mean (SD)
Fig. 3.
A quantitative description of SWE-measured moduli outcomes (primary y-axis – dashed bars) shows intact shoulder specimens to present higher values compared to the tear group in all 4 muscle regions (not statistically significant). Fat fraction (secondary y-axis – solid bars) measured with MRI was higher in the tear group than in the intact group (P = 0.04).
SWE and MRI outcomes were successfully used to predict extensibility and stiffness. Statistical summary data for the univariate analyses is presented in Table 2. The PD, PS and AD regions of the SSP muscle showed a significant negative correlation between the SWE-measured elastic modulus and muscle extensibility under 60N load (r = 0.54–0.58, P ≤ 0.0259). Although not significant, the elastic modulus of the anterior superficial (AS) muscle region also showed a negative correlation with extensibility (r = −0.4, P ≤ 0.1062). SWE elastic modulus on the PS region showed the highest negative correlation with extensibility under 60N (r = −0.58, P = 0.0142). A significant positive correlation was found between stiffness and SWE elastic modulus outcomes of the PS and AD regions (r = 0.56–0.57, P ≤ 0.0203). Although not statistically significant in the PD and AS muscle compartments, this positive correlation was observed on all muscular regions. SWE measurements from ultrasound, solely, explained up to 34% and 33% of the variability in measured extensibility and stiffness, respectively.
Table 2.
Univariate linear regression analyses. SWE-measured moduli or MRI-derived fat fraction were used, separately, to predict (A) experimentally measured extensibility or (B) Stiffness.
| (A) Extensibility Analyses | |||
|---|---|---|---|
|
| |||
| p-value | R2 | r | |
| Ultrasound (kPa) | |||
| SWE_PD | 0.0259* | 0.29 | −0.54 |
| SWE_PS | 0.0142* | 0.34 | −0.58 |
| SWE_AD | 0.0199* | 0.31 | −0.56 |
| SWE_AS | 0.1062 | 0.16 | −0.40 |
|
| |||
| qMRI (%) | |||
| Fat Fraction | 0.0021* | 0.48 | 0.69 |
| (B) Stiffness Analyses | |||
|---|---|---|---|
| Ultrasound (kPa) | |||
| SWE_PD | 0.0907 | 0.18 | 0.42 |
| SWE_PS | 0.0203* | 0.31 | 0.56 |
| SWE_AD | 0.0157* | 0.33 | 0.57 |
| SWE_AS | 0.1738 | 0.12 | 0.35 |
|
| |||
| qMRI (%) | |||
| Fat Fraction | 0.0113* | 0.36 | −0.60 |
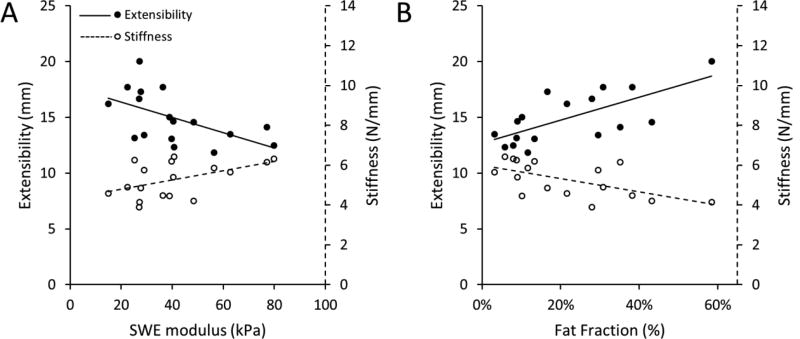
MRI-measured volumetric fat fraction of the SSP muscle was also successfully used to predict both experimentally measured extensibility and stiffness (previous published data (Giambini et al., 2017). Quantitative volumetric fat fraction of the SSP muscle resulted in a significant and positive correlation with SSP musculotendinous extensibility (r = 0.69, P=0.0021, Table 2). A significant negative correlation was found between fat fraction and experimental stiffness (r = −0.60, P=0.0113). Volumetric fat fraction solely explained 48% of the variability in extensibility and 36% of the variability in stiffness. Figure 4 shows the univariate results for the experimental extensibility and stiffness with SWE elastic modulus and fat infiltration.
Fig. 4.
A) There was a significant positive and negative correlation between stiffness and extensibility of the SSP muscle, respectively, with increasing SWE elastic modulus (average SWE outcome from the 4 muscle regions is shown). B) Experimental stiffness negatively correlated with fat fraction outcomes, while musculotendinous extensibility positively correlated.
Finally, SWE-measured moduli and MRI-measured fat fraction outcomes were combined to predict extensibility and stiffness. Table 3 shows the results of the multivariate analyses implemented to determine if a combination of the imaging outcomes, as explanatory variables, best accounted for the estimation of extensibility and stiffness. In all four analyses, fat fraction resulted in a significant variable in predicting extensibility (P ≤ 0.0043). With fat fraction as an explanatory variable, the PD, PS, and AD muscle regions remained significant variables (P ≤ 0.0361), while the AS regions showed no statistical significance. More importantly, when fat fraction was combined with SWE measurements, these combinations accounted for an increase in the predictive power of the extensibility of the SSP muscle (R2 = 0.51–0.62). A combination of the measurements from these two imaging modalities, as explanatory variables, explained up to 62% of the variability in extensibility; an increase of 14% when compared to just the evaluation of fat fraction.
Table 3.
Multivariate linear regression analyses. SWE-measured moduli and MRI-derived fat fraction were used, simultaneously, to predict (A) experimentally measured extensibility or (B) Stiffness.
| (A) Extensibility Analyses | |||
|---|---|---|---|
|
| |||
| p-value (SWE) | p-value (Fat Fraction) | R2 | |
| Ultrasound + MRI | |||
| SWE_PD + Fat Fraction | 0.0148* | 0.0015* | 0.62 |
| SWE_PS + Fat Fraction | 0.0220* | 0.0036* | 0.60 |
| SWE_AD + Fat Fraction | 0.0361* | 0.0043* | 0.57 |
| SWE_AS + Fat Fraction | 0.1024 | 0.0026* | 0.51 |
| (B) Stiffness Analyses | |||
|---|---|---|---|
| Ultrasound + MRI | |||
| SWE_PD + Fat Fraction | 0.1134 | 0.0158* | 0.39 |
| SWE_PS + Fat Fraction | 0.0430* | 0.0245* | 0.46 |
| SWE_AD + Fat Fraction | 0.0346* | 0.0251* | 0.47 |
| SWE_AS + Fat Fraction | 0.2212 | 0.0165* | 0.34 |
All significant variables from the univariate analysis remained significant during the multivariate analysis of stiffness. Fat fraction remained a significant variable along with the SWE measurements from the PS and AD muscle regions (P ≤ 0.0430). As with the previous analyses, when fat fraction was combined with SWE measurements, these combinations explained up to 47% of the variability in experimental stiffness; with 11% attributed to SWE measurements.
The variance of inflation factor (VIF) was estimated and found to be very small (VIF ≤ 1.084), confirming no noteworthy confounding variables in the statistical models (Kutner et al., 2004).
Discussion
The current study illustrates the unique ability of a combined method using shear wave elastography (SWE) and quantitative magnetic resonance imaging (MRI) to 1) estimate the extensibility, or deformation, of the supraspinatus (SSP) muscle and 2) provide a more robust quantitative measurement of muscle properties. In other words, the use of SWE to measure muscle stiffness, combined with MRI to obtain a quantitative volumetric measurement of intramuscular fat infiltration, was found to be a more effective process for estimating SSP extensibility compared to implementing the methods individually.
Currently, there are no clinical tools available for estimating the extensibility of the SSP muscle or that provide quantitative measurements of the properties of rotator cuff muscles. While classification systems have been developed to qualitatively evaluate intramuscular fat, namely the Goutallier (Goutallier et al., 1994) and Fuchs (Fuchs et al., 1999) classifications, these have several shortcomings. First, they provide a 2D evaluation of fatty infiltration; second, they are qualitative in nature and are mainly implemented to estimate the regenerative capacity of the muscle for healing after repair of the tear; finally, these methods are unable to provide a quantitative measurement of muscle properties, and more important, they do not provide an estimate of muscle extensibility. The current study used two different modalities (SWE and MRI) to quantify stiffness and fatty infiltration of the SSP muscle. SWE-measured moduli and MRI-measured fat fraction, combined together, were able to explain up to 62% of the variability in extensibility.
Intact shoulders tended to present higher SWE-measured mean moduli when compared to rotator cuff tear specimens. This aligns well with previous work (Gerber et al., 2007; Hersche and Gerber, 1998) that showed a decrease in the elasticity of rotator cuff muscles with chronic changes. This suggests that SWE can potentially be employed to assess muscle injuries. More importantly, fatty infiltration showed a positive correlation with tear size (from intact to massive tears) and the extensibility of the supraspinatus muscle. In a cohort of 262 patients, fatty infiltration in the supraspinatus has been previously associated with increase in tear size and location (Kim et al., 2010). Similarly, chronic tears have been shown to produce fatty build up in the muscle and this build up was associated to the size of the tear (Kim et al., 2012). Irreversible changes result from fatty infiltration, as a consequence of musculotendinous retraction after a rotator cuff tear, even after a successful repair is performed (Gerber et al., 2004); with a failed repair resulting in a significant progression of fatty build up and muscle degeneration (Gladstone et al., 2007). Quantification of fat fraction using MRI to assess the quality of the muscle is an important prognostic factor of functional results and may provide useful information in advance of surgery and after rotator cuff surgery. This is important because bringing the torn tendon and retracted muscle back to the greater tuberosity during surgery, and determining the regenerative capacity of the repaired tear, dictated by the muscle and tendon environment, can be challenging. With increasing tear size, age, and time following tendon rupture, these degenerative changes magnify, and quantification of fat fraction could help intervene before degeneration becomes severe resulting in irreversible functional loss.
This study has several limitations. The SSP muscle property outcomes were obtained on cadaveric specimens. It should be noted that the imaging results, as well as the extensibility outcomes, might differ between the isolated SSP muscle and those obtained when superficial tissues are included, and from those observed in in-vivo subjects. This difference deserves further investigation. On another note, the sample size used was small especially when considering the inclusion of intact and tear (small, medium, large and massive) specimens. Although the AS region of the SSP muscle did not reach statistical significance, the negative correlation with extensibility could have been strengthened by increasing the sample number. The intact specimen group consisted of shoulders of younger age when compared to the tear group, emphasizing the importance of age in rotator cuff injuries. These observations suggest that age can be considered as an additional explanatory variable along with SWE-measured moduli and MRI-measured fat fraction to increase the explanatory power of the statistical model predicting extensibility. Finally, it is important to note that the applied load was not prescribed, rather, manually ramped using the pneumatic system.
Using these two methodologies combined, future studies should be conducted to 1) obtain muscle property estimates (stiffness and fat fraction) from in-vivo subjects; 2) obtain relationships between intact and tear specimens on a larger population; and 3) correlate in-vivo muscle properties findings with SSP extensibility. Lastly, only the SSP muscle was evaluated, and a torn tendon might affect more than just one muscle. Additional muscles such as the infraspinatus or teres should be investigated.
In conclusion, this study showed a comprehensive quantitative assessment of SSP muscle properties using SWE to measure elastic modulus, as a surrogate for stiffness, and quantitative MRI to measure volumetric fat fraction; and demonstrated that the experimentally-measured extensibility of the detached muscle-tendon unit was highly correlated to these properties when these methods were used in combination. Patient-specific determination of extensibility using these techniques (SWE and quantitative MRI) could offer a non-invasive approach to determine the optimal treatment, evaluate whether the torn tendon edge could be successfully pulled onto its original insertion or if a partial defect would still remain after repair, and whether other treatments (patch augmentation, tendon transfer, etc.) should be considered preoperatively. The ultrasound equipment is an FDA approved and safe technique with no known or foreseeable risks for the subjects, and its benefits in the clinic may outweigh the potential costs associated with the equipment.
Highlights.
Shoulders with rotator cuff tears showed high fat fraction outcomes.
Negative correlation between extensibility and Shear Wave Elastography-measured moduli.
Extensibility of the muscle was correlated to quantitative muscle quality metrics.
Elastography and Magnetic Resonance Imaging outcomes could help prevent repair re-tear.
Acknowledgments
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21 AR065550, and an internal award from the Mayo Clinic.
References
- 1.Andersson T, Romu T, Karlsson A, Noren B, Forsgren MF, Smedby O, Kechagias S, Almer S, Lundberg P, Borga M, Leinhard OD. Consistent intensity inhomogeneity correction in water-fat MRI. J Magn Reson Imaging. 2015;42:468–476. doi: 10.1002/jmri.24778. [DOI] [PubMed] [Google Scholar]
- 2.Bartl C, Kouloumentas P, Holzapfel K, Eichhorn S, Wortler K, Imhoff A, Salzmann GM. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. International journal of shoulder surgery. 2012;6:1–8. doi: 10.4103/0973-6042.94304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11:13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillard K, Nordez A, Hug F. Estimation of individual muscle force using elastography. PLoS One. 2011;6:e29261. doi: 10.1371/journal.pone.0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell GL, Warner JP, Miller MD, Boardman D, Towers J, Debski R. Strength of fixation with transosseous sutures in rotator cuff repair. J Bone Joint Surg Am. 1997;79:1064–1068. doi: 10.2106/00004623-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Eby SF, Cloud BA, Brandenburg JE, Giambini H, Song P, Chen S, LeBrasseur NK, An KN. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 2015;30:22–27. doi: 10.1016/j.clinbiomech.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 8.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994:43–53. [PubMed] [Google Scholar]
- 9.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16:691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 11.Giambini H, Hatta T, Krzysztof GR, Widholm P, Karlsson A, Leinhard OD, Adkins MC, Zhao C, An KN. Intramuscular Fat Infiltration Evaluated by Magnetic Resonance Imaging Predicts the Extensibility of the Supraspinatus Muscle. Muscle Nerve. 2017 doi: 10.1002/mus.25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 13.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 14.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 15.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, Lynch EB, Claflin DR, Bedi A, Mendias CL. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. Journal of shoulder and elbow surgery. 2014;23:99–108. doi: 10.1016/j.jse.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed] [Google Scholar]
- 17.Hatta T, Giambini H, Hooke AW, Zhao C, Sperling JW, Steinmann SP, Yamamoto N, Itoi E, An KN. Comparison of Passive Stiffness Changes in the Supraspinatus Muscle After Double-Row and Knotless Transosseous-Equivalent Rotator Cuff Repair Techniques: A Cadaveric Study. Arthroscopy. 2016a;32:1973–1981. doi: 10.1016/j.arthro.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatta T, Giambini H, Itoigawa Y, Hooke AW, Sperling JW, Steinmann SP, Itoi E, An KN. Quantifying extensibility of rotator cuff muscle with tendon rupture using shear wave elastography: A cadaveric study. J Biomech. 2017 doi: 10.1016/j.jbiomech.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatta T, Giambini H, Uehara K, Okamoto S, Chen S, Sperling JW, Itoi E, An KN. Quantitative assessment of rotator cuff muscle elasticity: Reliability and feasibility of shear wave elastography. Journal of biomechanics. 2015a;48:3853–3858. doi: 10.1016/j.jbiomech.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatta T, Giambini H, Uehara K, Okamoto S, Chen S, Sperling JW, Itoi E, An KN. Quantitative assessment of rotator cuff muscle elasticity: Reliability and feasibility of shear wave elastography. J Biomech. 2015b;48:3853–3858. doi: 10.1016/j.jbiomech.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatta T, Giambini H, Zhao C, Sperling JW, Steinmann SP, Itoi E, An KN. Biomechanical Effect of Margin Convergence Techniques: Quantitative Assessment of Supraspinatus Muscle Stiffness. PLoS One. 2016b;11:e0162110. doi: 10.1371/journal.pone.0162110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7:393–396. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 23.Hug F, Tucker K, Gennisson JL, Tanter M, Nordez A. Elastography for Muscle Biomechanics: Toward the Estimation of Individual Muscle Force. Exerc Sport Sci Rev. 2015;43:125–133. doi: 10.1249/JES.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 24.Iannotti JP, Deutsch A, Green A, Rudicel S, Christensen J, Marraffino S, Rodeo S. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95:965–971. doi: 10.2106/JBJS.L.00708. [DOI] [PubMed] [Google Scholar]
- 25.Itoigawa Y, Sperling JW, Steinmann SP, Chen Q, Song P, Chen S, Itoi E, Hatta T, An KN. Feasibility assessment of shear wave elastography to rotator cuff muscle. Clinical anatomy. 2015;28:213–218. doi: 10.1002/ca.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am. 2010;92:829–839. doi: 10.2106/JBJS.H.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21:847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. McGraw-Hill/Irwin; 2004. [Google Scholar]
- 29.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990:35–38. [PubMed] [Google Scholar]
- 30.Meyer DC, Jacob HA, Nyffeler RW, Gerber C. In vivo tendon force measurement of 2-week duration in sheep. J Biomech. 2004;37:135–140. doi: 10.1016/s0021-9290(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 31.Meyer DC, Lajtai G, von Rechenberg B, Pfirrmann CW, Gerber C. Tendon retracts more than muscle in experimental chronic tears of the rotator cuff. J Bone Joint Surg Br. 2006;88:1533–1538. doi: 10.1302/0301-620X.88B11.17791. [DOI] [PubMed] [Google Scholar]
- 32.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296–298. [PubMed] [Google Scholar]
- 33.Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, Kijima H, Itoi E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. Journal of orthopaedics. 2013;10:8–12. doi: 10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Post M, Silver R, Singh M. Rotator cuff tear. Diagnosis and treatment. Clin Orthop Relat Res. 1983:78–91. [PubMed] [Google Scholar]
- 35.Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg Br. 1970;52:540–553. [PubMed] [Google Scholar]
- 36.Rydell J, Knutsson H, Pettersson J, Johansson A, Farneback G, Dahlqvist O, Lundberg P, Nystrom F, Borga M. Phase sensitive reconstruction for water/fat separation in MR imaging using inverse gradient. Med Image Comput Comput Assist Interv. 2007;10:210–218. doi: 10.1007/978-3-540-75757-3_26. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz C, Croisier JL, Rigaux E, Denoel V, Bruls O, Forthomme B. Dominance effect on scapula 3-dimensional posture and kinematics in healthy male and female populations. J Shoulder Elbow Surg. 2014;23:873–881. doi: 10.1016/j.jse.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B, International Society of, B ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]