Abstract
Purpose:
The current study explored physical activity-induced bone adaptation at different stages of somatic maturity by comparing side-to-side differences in midshaft humerus properties between male throwing athletes and controls. Throwers present an internally controlled model, while inclusion of control subjects removes normal arm dominance influences.
Methods:
Throwing athletes (n=90) and controls (n=51) were categorized into maturity groups (PRE, PERI, POST-EARLY, POST-MID and POST-LATE) based on estimated years from peak height velocity (<−2, −2-to-2, 2-to-4, 4-to-10 and >10 years). Side-to-side percent differences in midshaft humerus cortical volumetric bone mineral density (Ct.vBMD) and bone mineral content (Ct.BMC), total (Tt.Ar), medullary (Me.Ar) and cortical (Ct.Ar) area, average cortical thickness (Ct.Th), and polar Strength Strain Index (SSIP) was assessed.
Results:
Significant interactions between physical activity and maturity on side-to-side differences in Ct.BMC, Tt.Ar, Ct.Ar, Me.Ar, Ct.Th and SSIP resulted from: 1) greater throwing-to-nonthrowing arm differences than dominant-to-nondominant arm differences in controls (all p<0.05), and; 2) throwing-to-nonthrowing arm differences in throwers being progressively greater across maturity groups (all p<0.05). Regional analyses revealed greatest adaptation in medial and lateral sectors, particularly in the three POST maturity groups. Years throwing predicted 59% of the variance of the variance in throwing-to-nonthrowing arm difference in SSIP (p<0.001).
Conclusion:
These data suggest physical activity has skeletal benefits beginning prior to and continuing beyond somatic maturation, and that a longer duration of exposure to physical activity has cumulative skeletal benefits. Thus, physical activity should be encouraged at the earliest age possible and be continued into early adulthood to optimize skeletal benefits.
Keywords: biomechanics, exercise, growth, mechanical loading, osteoporosis, puberty
MINI ABSTRACT:
Physical activity benefits the skeleton, but there is contrasting evidence regarding whether benefits differ at different stages of growth. The current study demonstrates that physical activity should be encouraged at the earliest age possible and be continued into early adulthood to gain most skeletal benefits.
INTRODUCTION
Growth is an important time to take advantage of the ability of the skeleton to respond and adapt to its prevailing mechanical environment. Approximately 25–30% of adult bone mineral is accrued within the 2–3 years around puberty and approximately 95% of adult bone mass has accrued by the end of adolescence [1–3]. As fracture risk during aging doubles for each standard deviation of bone lost from mean peak bone mass [4] and a 10% increase in peak bone mass is predicted to delay the onset of osteoporosis by 13 years [5], physical activity to increase peak bone mass during growth is advocated as a means of offsetting the increase in low trauma fracture risk associated with aging [6–8].
The skeletal advantage of physical activity during specific phases of growth was eloquently shown by Kannus and colleagues [9]. Using racquet sport players as an internally controlled model, they observed players who began racquet sports before puberty had more than two-fold greater differences in bone mass between their playing and nonplaying arms compared to players who began post-puberty. In support of this observation, Heinonen et al. [10] reported high impact exercise increased bone mineral accrual in premenarcheal, but not postmenarcheal girls. Similarly, Ducher et al. [11] observed post-pubertal racquet sport players had equivalent side-to-side differences in dual-energy x-ray absorptiometry (DXA) derived bone mass between their playing and nonplaying arms compared to peri-pubertal players, despite the former playing for longer. These cumulative findings suggest a reduction in skeletal mechanoadaptation following puberty and the presence of a ‘window of opportunity’ during pre- and early-puberty where the skeleton maybe most amenable to the mechanical loading associated with physical activity [12].
Although there is evidence for heightened skeletal benefits of physical activity during early phases of puberty, contrasting findings have more recently been reported. Data from the Bone Mineral Density in Childhood Study indicated that self-reported weight bearing physical activity had a significant longitudinal effect on bone mass accrual, but there was not a differential effect according to maturational stage [13]. Meanwhile, data provided by Rantalainen et al. [14] suggested that the peri- and post-pubertal periods were actually more favourable than pre-puberty to positively modify skeletal traits via physical activity. These contrasting findings regarding the window of opportunity for the skeletal benefits of physical activity may reflect study design differences, but indicate a need for further research into the skeletal benefits of physical activity at different stages of growth.
Further highlighting a need for additional studies into physical activity effects during growth is recent evidence revealing lifelong benefits of physical activity completed when young on bone size and strength, but not mass [15–17]. Comparing the throwing and nonthrowing arms within former professional baseball players, half of the bone size (total cross-sectional area) and one-third of the bone strength (polar moment of inertia) benefits of physical activity when young were maintained lifelong [17]. In contrast, the bone mass benefits of physical activity when young were gradually lost as a result of medullary expansion and cortical trabecularization. These data suggest that physical activity during growth should focus on the optimization of bone size and strength rather than the current paradigm of increasing mass. Few studies have explored the interaction between physical activity and stage of growth on skeletal structural adaptation [10, 11, 14, 18, 19].
The aim of the current study was to explore physical activity-induced bone adaptation at different stages of growth. To address this aim, we cross-sectionally compared side-to-side differences in midshaft humerus properties between male baseball players and controls at different stages of somatic maturity. Baseball players perform unilateral upper extremity physical activity which loads and induces adaptation of the humerus within the throwing arm [17, 20]. The unilateral nature of throwing enables the contralateral nonthrowing arm to minimize selection bias associated with our cross-sectional study design by serving as an internal control site for inherited and other systemic traits. By comparing throwing-to-nonthrowing arm differences in throwing athletes with dominant-to-nondominant arm differences in controls, we also isolated the skeletal benefits of throwing-related physical activity from side-to-side differences due to elevated habitual unilateral loading associated with simple arm dominance.
MATERIALS AND METHODS
Participants
Convenience samples of male baseball players (throwers) and controls aged ≥8 years were recruited. Throwers aged ≤23 years were recruited from local regional, high school and collegiate baseball teams, whereas those aged >23 years were recruited from individuals competing in professional baseball at the Minor League Baseball (Triple-A) level. Throwers were included if they: 1) play or practice baseball during the competitive season at least 3 times per week; 2) play or practice baseball at least 6 months per year; 3) begun playing baseball prior to 6 years of age and have been playing for a minimum of 3 years, and; 4) have not had a hiatus from competitive baseball for more than 12 months at any time. Throwers ≤23 years completed a questionnaire to determine their eligibility and estimate weekly playing time and number of throws. Throwers >23 years (professional baseball players) did not complete the throwing questionnaire, with an interview being used in place to determine eligibility. Exclusion criteria for both throwers and controls were: 1) known metabolic bone disease; 2) administration of pharmacological agents known to influence skeletal metabolism; 3) participation more than twice per month for longer than six months in an activity that primarily involves unilateral upper limb use (except baseball in the thrower group); 4) history of a humeral fracture or stress fracture, and; 5) exposure to upper extremity immobilization for more than 2 weeks within the past 2 years. Arm dominance was determined as the arm one does or would throw a ball with. The study was approved by the Institutional Review Board of Indiana University, and written informed assent was obtained from all participants <18 years of age and written informed consent obtained from all participants ≥18 years of age and parent/guardians of participants <18 years of age.
Anthropometry
Standing and sitting height were measured to the nearest 0.1 cm using a wall-mounted digital stadiometer, whereas an electronic balance scale was used to measure weight to the nearest 0.1 kg. Body mass index (BMI, kg/m2) was derived from standing height and weight. Humeral length was measured using a sliding anthropometer to the nearest 0.1 cm as the distance between the lateral border of the acromion and the radiohumeral joint line.
Maturity
Throwers and controls were categorized into groups based on their estimated years from peak height velocity (PHV) (also referred to as ‘maturity offset’). PHV is an accepted indicator of somatic maturity and can be used to account for the range of variability in somatic maturity between individuals of the same chronological age [21]. We did not use Tanner staging of secondary sex characteristics as it more provides an indication of pubertal development (as opposed to somatic development), with substantial variability being observed in the timing of PHV across Tanner stages [22]. Also, Tanner staging is limited in its ability to subcategorize individuals who have completed sexual maturation because of its staging of all post-pubertal individuals in a single category (stage 5).
In participants aged <18 years, maturity offset was calculated from standing height, sitting height, leg length (calculated as standing height minus sitting height), and chronological age using the sex-specific multiple regression equation described by Mirwald et al. [23]. The equation explains 92% of the variance in actual years from longitudinally measured PHV and provides estimate values of age of PHV that differ from actual values by only 0.24 years [23]. In participants aged ≥18 years, maturity offset was estimated as chronological age minus self-reported age at the time of their adolescent growth spurt. Participants estimated to be <−2, −2-to-2, 2-to-4, 4-to-10 and >10 years from their PHV were categorized into PRE, PERI, POST-EARLY, POST-MID and POST-LATE maturity groups, respectively.
Dual-energy x-ray absorptiometry
Whole-body, hip and lumbar spine dual-energy X-ray absorptiometry (DXA) was performed using the manufacturer’s standard protocols on a Hologic Discovery-W machine equipped with Apex v4.0 software (Hologic, Inc., Waltham, MA, USA). Analyses of whole body scans included assessment of whole body areal bone mineral content (BMC, kg), lean mass (kg) and percent fat mass (%). Sub-regional analyses provided dominant and nondominant whole arm lean mass, with the glenohumeral joint line being the landmark for the division of the upper extremity from the trunk.
Peripheral quantitative computed tomography
Peripheral quantitative computed tomography (pQCT) of the midshaft humerus was performed bilaterally using a Stratec XCT 3000 machine (Stratec Medizintechnik GmbH, Pforzheim, Germany) equipped with Stratec software version 6.20C, as previously described [17, 20, 24, 25]. Subjects were positioned in supine with their upper extremity positioned in 90° shoulder abduction. A scout scan was performed to visualize the radiohumeral joint and a reference line placed at the distal edge of the humeral capitulum. A tomographic slice (thickness=2.3 mm; voxel size=300 μm; scan speed=12 mm/s) was taken at 50% of humeral length (midshaft) from the reference line, with humeral length being assessed earlier using a sliding anthropometer.
Tomographic slices were analyzed for cortical bone mineral density, content, structure and estimated strength, and muscle cross-sectional area. Cortical mode 1 (threshold, 710 mg/cm3) was used to obtain cortical volumetric bone mineral density (Ct.vBMD, mg/cm3), cortical BMC (Ct.BMC, mg/mm), and cortical area (Ct.Ar, cm2). Total area (Tt.Ar, cm2) and average cortical thickness (Ct.Th, mm) were obtained by analyzing slices using contour mode 1 (threshold, 710 mg/cm3) to define the outer bone edge and peel mode 2 (threshold, 400 mg/cm3) to separate the cortical and subcortical/medullary compartments. Cortical thickness measures used a circular ring model, and medullary area (Me.Ar, mm2) was derived as total area minus cortical area. Bone strength was estimated by the polar Strength Strain Index (SSIP, mm3) obtained using cortical mode 2 (threshold = 400 mg/cm3). SSIP represents the density-weighted section modulus and predicts over 90% of the variance in ex vivo midshaft humerus mechanical properties [25].
Muscle cross-sectional area (cm2) was assessed by using contour mode 3 (threshold, −100 mg/cm3) to locate the skin surface and peel mode 2 (threshold, 40 mg/cm3) to locate the subcutaneous fat-muscle boundary. A 3×3 kernel filter to filter all voxels between −500 and 500 mg/cm3 followed by a 5×5 kernel filter to filter all voxels between −500 and 300 mg/cm3 (F03F05 filter) was used to remove noise. Short-term precision for the pQCT scanning procedure on 30 healthy individuals scanned six times with interim repositioning showed root mean square coefficients of variation (RMS-CVs) of <1.5% for bone density, mass, structure, and estimated strength measures, and <2% for mCSA [25].
To explore regional bone geometry adaptation to throwing, polar pericortical and endocortical radii were obtained for the throwing and nonthrowing arms in throwers and dominant and nondominant arms in controls. Stratec pQCT image files and data were opened in ImageJ (v1.45s; National Institutes of Health) and analyzed using the BoneJ plugin [26], as previously described [27]. Images were rotated to align the bones according to the IMAX and IMIN axes, and nonthrowing/nondominant arm images were flipped to superimpose throwing/dominant arm images. Using a threshold value of 350 mg/cm3 to locate bone tissue, the distance of the endocortical and pericortical surfaces from the centroid of the medullary cavity were measured in 10° polar sectors. Ct.Th within each sector was calculated as the pericortical minus endocortical radius.
Statistical analyses
Two-tailed analyses with α = 0.05 were performed with IBM SPSS Statistics (v23; SPSS Inc., Chicago, IL), unless otherwise specified. Participant characteristics within each maturity group were compared between throwers and controls using independent sample t-tests. Throwing demographics between maturity groups in throwers were compared using a chi-square analysis or one-way analyses of variance (ANOVA) followed by Tukey pairwise comparisons. Side-to-side differences between the throwing and nonthrowing arms in throwers were assessed by calculating mean percent differences ([throwing–nonthrowing]/nonthrowing x 100%) and their 95% confidence interval (CI). 95% CIs not crossing zero were considered statistically significant, as determined by single sample t-tests with a population mean of 0%. Similar analyses were performed to determine side-to-side differences between the dominant and nondominant arms in controls.
The effects of physical activity (throwers vs. controls) and maturity (PRE vs. PERI vs. POST-EARLY vs. POST-MID vs. POST-LATE) on side-to-side percent differences were determined using two-way factorial ANOVAs. In the advent of a non-significant ANOVA interaction, main effects for each independent variable were explored. Significant ANOVA interactions were explored using simple effects tests to assess for the effect of physical activity within each maturity group (independent sample t-tests) and maturity within each physical activity group (one-way ANOVA followed by Tukey pairwise comparisons), with a false discovery rate threshold set at q = 0.05 used to correct for multiple comparisons [28].
To explore the regional-specificity of bone geometry adaptation associated with throwing within each maturity group, polar pericortical and endocortical radii and polar Ct.Th data were assessed in throwers using two-way repeated measures ANOVA, with arm (throwing vs. nonthrowing) and sector (1 through 36) as within-subject variables. Data in each sector were corrected a priori for dominant-to-nondominant arm differences observed in controls to remove any regional side-to-side differences attributable to simple arm dominance. In the presence of a significant arm x sector interaction, post-hoc paired t-tests were used to compare throwing vs. nonthrowing arm differences within each individual sector, with a false discovery rate threshold set at q = 0.05 used to correct for multiple comparisons [28].
Linear regression analysis was used in the thrower group to assess whether specific demographic characteristics (years throwing, estimated playing time per week and estimated throws per week) and dominant-to-nondominant differences in upper extremity lean measures (whole arm lean mass and upper arm muscle CSA) predicted throwing-to-nonthrowing differences in midshaft humerus estimated strength (SSIP). The fit of each univariate model assessed using the coefficient of determination (R2).
RESULTS
Participant characteristics
A total of 90 throwers and 51 controls were categorized into the 5 maturity groups (Table 1). Thrower and control groups within each maturity group were comparable for age, maturity offset and estimated age of PHV (all p > 0.05). Whole-body and regional anthropometry was generally well-matched between throwers and controls within each maturity group; however, throwers in the POST-MID group had greater spine and hip aBMD than their controls, while throwers in the POST-LATE group had greater total mass, lean mass, and hip aBMD than their controls (all p < 0.05). In throwers, there were no differences between maturity groups in terms of age started throwing, estimated playing time per week or estimated throws per week (all p = 0.13 to 0.69); however, each maturity group had progressively more years throwing (all p < 0.001).
Table 1:
Demographic and anthropometric characteristics of throwers and their controls a
| Characteristic | PRE |
PERI |
POST-EARLY |
POST-MID |
POST-LATE |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Throwers | Controls | Throwers | Controls | Throwers | Controls | Throwers | Controls | Throwers | |
| n | 10 | 22 | 9 | 16 | 9 | 14 | 13 | 14 | 10 | 24 |
| Demographics | ||||||||||
| Age (yr) | 10.5 ± 1.6 | 10.0 ± 1.1 | 14.2 ± 1.0 | 13.8 ± 1.5 | 17.5 ± 1.1 | 17.3 ± 1.4 | 20.1 ± 2.0 | 19.6 ± 2.1 | 27.5 ± 3.4 | 28.1 ± 2.3 |
| Maturity offset (yr) | −2.9 ± 0.9 | −3.1 ± 0.7 | 0.5 ± 0.8 | 0.6 ± 1.1 | 3.6 ± 0.7 | 3.2 ± 0.7 | 6.2 ± 1.7 | 6.0 ± 2.0 | 13.9 ± 3.2 | 14.5 ± 2.3 |
| Estimated age of peak height velocity (yr) | 13.4 ± 0.9 | 13.1 ± 0.5 | 13.8 ± 0.5 | 13.4 ± 0.8 | 13.9 ± 1.1 | 14.1 ± 1.0 | 13.9 ± 2.0 | 13.6 ± 1.1 | 13.6 ± 1.1 | 13.6 ± 1.2 |
| Age started throwing (yr) | — | 4.6 ± 0.7 | — | 4.8 ± 0.9 | — | 5.3 ± 0.9 | — | 5.5 ± 1.0 | — | 5.1 ± 1.2 |
| Playing time (hrs/wk) | — | 5.1 ± 3.1 | — | 5.0 ± 2.4 | — | 6.7 ± 1.9 | — | 6.7 ± 1.8 | — | —b |
| Throws per week (#/wk) | — | 245 ± 180 | — | 245 ± 180 | — | 322 ± 176 | — | 302 ± 161 | — | —b |
| Years throwing (yr) | — | 5.2 ± 1.3† | — | 8.1 ± 1.9† | — | 12.0 ± 1.5† | — | 14.1 ± 2.4† | — | 22.9 ± 2.7† |
| Whole-body anthropometry | ||||||||||
| Height (m) | 1.45 ± 0.13 | 1.41 ± 0.06 | 1.68 ± 0.06 | 1.59 ± 0.10* | 1.77 ± 0.09 | 1.79 ± 0.05 | 1.81 ± 0.07 | 1.78 ± 0.07 | 1.86 ± 0.07 | 1.86 ± 0.07 |
| Mass (kg) | 39.8 ± 9.5 | 39.4 ± 13.3 | 57.5 ± 12.5 | 51.8 ± 13.9 | 70.8 ± 11.7 | 75.6 ± 11.0 | 69.0 ± 9.5 | 76.1 ± 9.9 | 88.4 ± 17.0 | 97.9 ± 9.0* |
| BMI (kg/m2) | 18.7 ± 2.4 | 19.6 ± 6.3 | 20.1 ± 2.9 | 20.2 ± 3.9 | 23.4 ± 3.4 | 22.6 ± 3.7 | 21.6 ± 2.2 | 23.2 ± 2.3 | 25.7 ± 4.7 | 28.3 ± 2.6* |
| BMC (kg)c,d | 0.90 ± 0.19 | 0.92 ± 0.14 | 1.55 ± 0.21 | 1.56 ± 0.38 | 2.51 ± 0.43 | 2.57 ± 0.47 | 2.79 ± 0.46 | 2.88 ± 0.47 | 3.35 ± 0.54 | 3.63 ± 0.43 |
| Lean mass (kg)c | 23.7 ± 6.6 | 22.2 ± 3.4 | 35.0 ± 7.3 | 33.5 ± 10.4 | 50.3 ± 7.9 | 53.6 ± 7.3 | 53.8 ± 7.9 | 58.7 ± 5.7 | 55.7 ± 19.4 | 69.6 ± 6.6* |
| Fat mass (%)c | 29.2 ± 8.7 | 28.2 ± 5.6 | 22.7 ± 7.6 | 25.9 ± 11.3 | 19.3 ± 6.0 | 19.2 ± 6.2 | 15.3 ± 4.6 | 15.7 ± 5.0 | 24.2 ± 7.3 | 22.4 ± 3.8 |
| Regional anthropometry | ||||||||||
| Spine aBMD (g/cm2)c,e | 0.66 ± 0.06 | 0.65 ± 0.07 | 0.81 ± 0.07 | 0.81 ± 0.17 | 1.04 ± 0.16 | 1.07 ± 0.12 | 1.06 ± 0.11 | 1.17 ± 0.14* | 1.17 ± 0.11 | 1.23 ± 0.11 |
| Hip aBMD (g/cm2)c,e | 0.78 ± 0.07 | 0.79 ± 0.07 | 0.96 ± 0.08 | 0.97 ± 0.18 | 1.14 ± 0.21 | 1.16 ± 0.11 | 1.07 ± 0.10 | 1.23 ± 0.14* | 1.13 ± 0.11 | 1.31 ± 0.11* |
Data are mean ± SD (except for frequencies)
POST-LATE consisted of professional baseball players. They did not complete the throwing questionnaire
Obtained via dual-exergy x-ray absorptimometry
Values corrected for height
Values corrected for whole-body lean mass
p < 0.05, as determined by independent sample t-test (controls vs. throwers within maturity group)
p < 0.05 vs. all other maturity groups, as determined by one-way ANOVA followed by Tukey pairwise comparisons
Effect of throwing on midshaft humerus bone properties
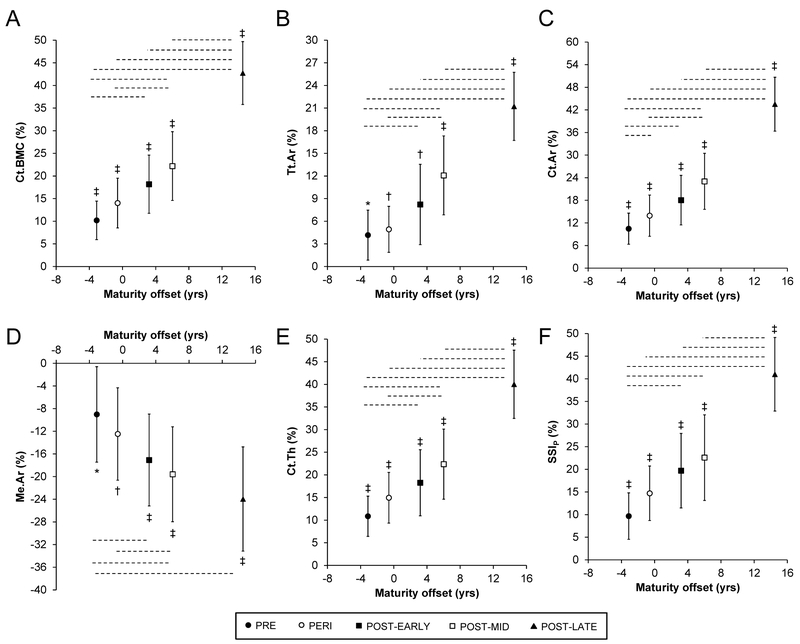
Controls and throwers in each maturity group exhibited side-to-side differences in bone properties generally in favor of the dominant and throwing arm, respectively (Supplementary table 1). There were significant interactions between physical activity and maturity on side-to-side differences in Ct.BMC, Tt.Ar, Ct.Ar, Me.Ar, Ct.Th and SSIP (all p < 0.01), indicating that the physical activity effect differed according to maturity group. Throwers within each maturity group had greater throwing-to-nonthrowing arm differences for Ct.BMC, Tt.Ar, Ct.Ar, Ct.Th and SSIP and smaller throwing-to-nonthrowing differences for Me.Ar than dominant-to-nondominant arm differences in controls (all p < 0.05; Fig. 1). The significant interactions between physical activity and maturity resulted from throwing-to-nonthrowing arm differences in throwers being progressively greater across maturity groups from PRE to POST-LATE (all p < 0.05; Fig. 1). There was no interaction between physical activity and maturity or main effects for either independent variable on Ct.vBMD (all p > 0.25; data not shown).
Fig. 1.
Throwers within each maturity group had more mass (cortical bone mineral content, Ct.BMC [A]), a larger size (total area, Tt.Ar [B]), enhanced structure (larger cortical area, Ct.Ar [C]; smaller medullary area, Me.Ar [D], and; greater cortical thickness, Ct.Th [E]) and greater estimated strength (polar Strength Strain Index; SSIP [F]) at the midshaft humerus in their throwing arm relative to their non-throwing arm, and relative to normal arm dominance effects (i.e. dominant-to-nondominant arm differences observed in controls). Data show the mean percent difference and 95% CI between the throwing-to-nonthrowing arms in throwers corrected for dominant-to-nondominant arm differences in controls (*p < 0.05, †p < 0.01, ‡p < 0.001, independent samples t-test comparing throwers vs. controls). The magnitude of midshaft humerus adaptation to unilateral physical activity within throwers (i.e. throwing-to-nonthrowing arm difference) was progressively larger within each successive maturity group, with broken horizontal lines indicating significant (p ≤ 0.05) differences between pairwise maturity groups (one-way ANOVA followed by Tukey pairwise comparisons, with a false discovery rate threshold used to correct for multiple comparisons).
Effect of throwing on regional geometry of the midshaft humerus
Following a priori correction for dominant-to-nondominant arm differences observed in controls, there were significant arm x sector interactions for polar pericortical and endocortical radii, and polar Ct.Th within each maturity group in throwers (all p < 0.05; Fig. 2). Post-hoc analyses revealed PRE and PERI throwers had throwing-to-nonthrowing arm differences for pericortical radii predominantly in anterior and anterior/lateral sectors, respectively (all p < 0.05; Figs. 2A,B). Both the POST-EARLY and -MID groups exhibited throwing-to-nonthrowing arm differences for pericortical radii mostly in medial and lateral sectors (all p < 0.05; Figs. 2C,D). The throwing arm in the POST-LATE group exhibited greater pericortical radii in all sectors when compared to the nonthrowing arm (all p < 0.05); however, largest throwing-to-nonthrowing arm differences were observed in medial and lateral sectors (Fig. 2E).
Fig. 2.
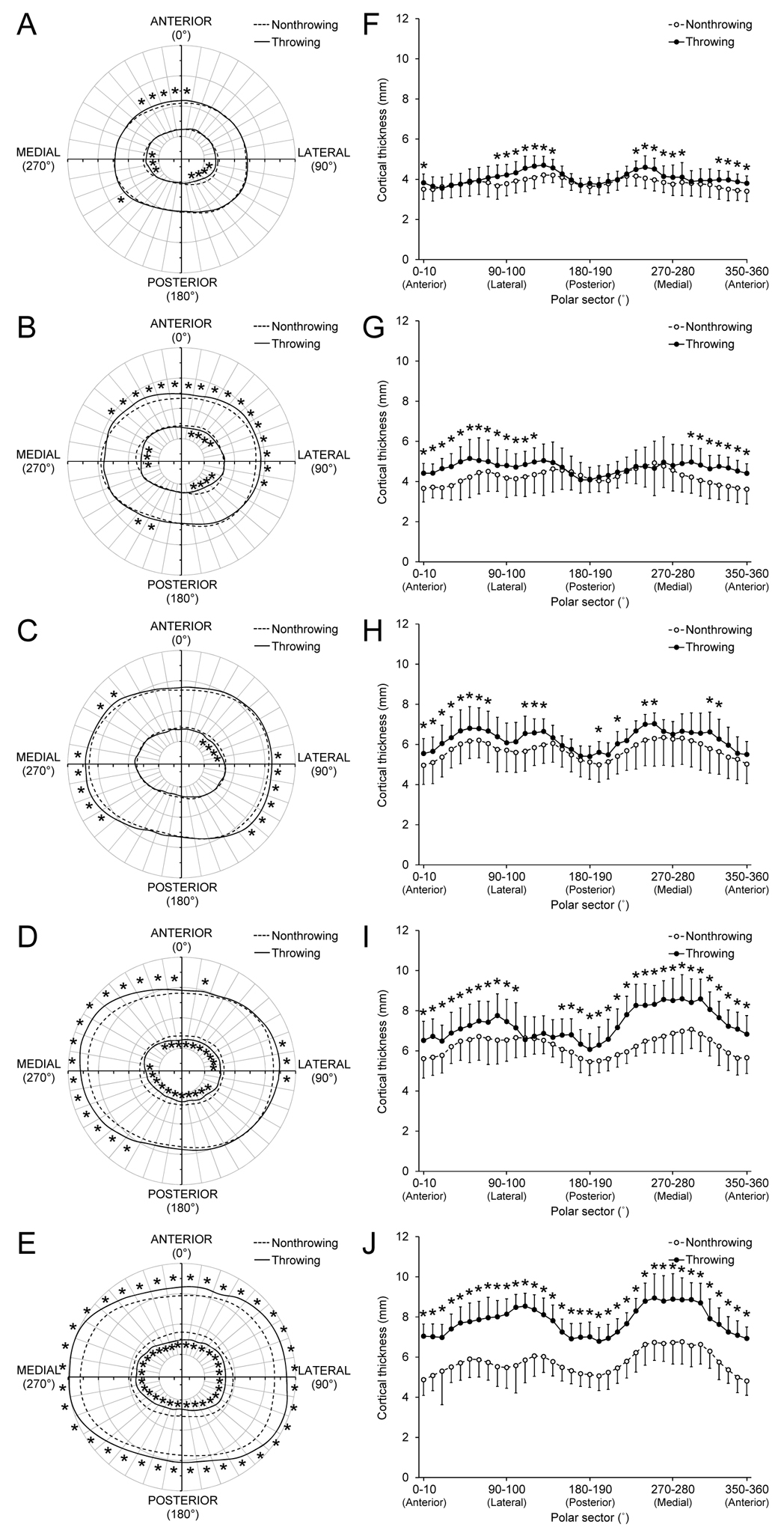
Overhead throwing induced regional bone geometry adaptation at the midshaft humerus. Maps of average pericortical and endocortical radii (A-E) and average ± SD cortical thickness (F-J) in 10˚ polar sectors in the throwing (solid lines) and nonthrowing (broken lines) arms of throwers in the PRE- (A, F), PERI- (B, G), POST-EARLY (C, H), POST-MID (D, I), and POST-LATE (E, J) maturity groups. *indicates throwing vs. nonthrowing arm difference within individual sector (p < 0.05), as determined by post-hoc post-hoc paired t-tests with a false discovery rate threshold used to correct for multiple comparisons. Data in each sector were corrected a priori for dominant-to-nondominant arm differences observed in controls to remove any regional side-to-side differences attributable to simple arm dominance.
Each maturity group had varying regions and numbers of sectors exhibiting significantly smaller endocortical radii in their throwing arm (all p < 0.05; Figs. 2A-E). POST-MID and -LATE throwers had throwing-to-nonthrowing arm differences for endocortical radii in most and all sectors, respectively (all p < 0.05; Figs. 2D,E). The combination of larger pericortical and smaller endocortical radii in the throwing arm contributed to greater polar Ct.Th in varying sectors within each maturity group (all p < 0.05; Figs. 2F-J). In general, each maturity group had increased throwing arm Ct.Th in medial, lateral and anterior sectors. PRE and PERI throwers did not have throwing-to-nonthrowing arm differences for Ct.Th in posterior radii (all p > 0.05; Fig. 2F,G). POST-LATE throwers had throwing-to-nonthrowing arm differences for Ct.Th in all sectors (all p < 0.05); however, differences were greatest in medial and lateral sectors, consistent with the larger pericortical radii differences in these regions (Fig. 2J).
Predictors of throwing-to-nonthrowing arm differences in bone strength
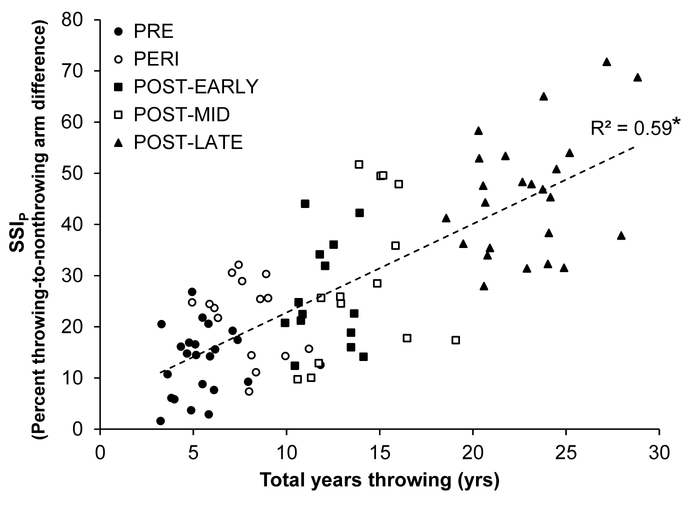
Years throwing predicted throwing-to-nonthrowing arm differences in SSIP explaining 59% of the variance in the latter (p < 0.001; Fig. 3). Estimated playing time per week and estimated throws per week did not predict throwing-to-nonthrowing arm differences in SSIP (R2 = 0.02 to 0.05, p = 0.11 to 0.33) (data not shown). Similarly, throwing-to-nonthrowing arm differences in upper extremity lean measures (whole arm lean mass and upper arm muscle CSA) did not predict throwing-to-nonthrowing arm differences in SSIP (all R2 < 0.01, p = 0.61 to 0.66) (data not shown).
Fig. 3.
Relationship between years throwing and percent throwing-to-nonthrowing arm difference in midshaft humerus estimated strength (polar Strength Strain Index; SSIP). The percent throwing-to-nonthrowing arm difference represents the skeletal gain due to throwing (i.e. unilateral physical activity) over that due to normal growth (i.e. observed contralaterally within the non-throwing arm). *Indicates p < 0.001, as determined linear regression analysis.
DISCUSSION
The principal finding of this cross-sectional study is that individuals at increasingly advanced stages of somatic maturity exhibited progressively greater physical activity-induced skeletal adaptation. This observation suggests that the mechanical loading associated with physical activity has skeletal benefits across all stages of maturation and that benefits are not isolated to a single ‘window of opportunity’ during pre- and early-puberty.
The observation of greater throwing-to-nonthrowing arm differences with advancing years following somatic maturation contrast those of Bass and colleagues [11, 18] who used a similar internally controlled model (i.e. racquet sport players) and cross-sectional study design to investigate skeletal mechanoadaptation according to pubertal stage. They reported post-pubertal girls and boys had similar magnitudes of racquet-to-nonracquet arm differences to those who were peri-pubertal, despite the former having a longer playing history [11, 18]. These data suggested a decline in skeletal mechanosensitivity and adaptation once growth slowed.
A possible reason for our contrasting data to Bass and colleagues [11, 18], despite their similar cross-sectional study design, include the study of a larger number of post-pubertal/maturation throwers (n=52) who spanned a broader post-maturation range, including a group of individuals who were 14.5 years beyond their estimated PHV (average age = 28.1 years). Bass and colleagues [11] included only a small number of post-pubertal individuals (n=9) in their study of male racquet-sport players who were all within the early stage post-puberty (average age = 17.1 years). Our inclusion of a greater number of subjects who were more years beyond their estimated age of somatic maturation allowed for a greater effect size and enhanced statistical power to identify ongoing skeletal benefits of physical activity beyond somatic maturation. Other possible contributing factors to our observation of ongoing skeletal benefits of physical activity beyond maturation include our inclusion of control subjects to account for side-to-side differences due to habitual loading associated with simple limb dominance and the study of baseball players, with the latter possibly introducing more progressive or specific humeral loading across somatic maturation than occurs in racquet-sport players.
Our findings suggest physical activity has skeletal benefits beginning prior to and continuing beyond somatic maturation, and that a longer duration of exposure to physical activity has cumulative skeletal benefits. The latter was supported by a strong linear association between total years throwing and estimated bone strength. The identification of skeletal benefits of physical activity from a young age fits with the larger body of evidence, while the linear association between duration of physical activity exposure and bone adaptation is consistent with recent longitudinal data suggesting maturational stage does not have a differential effect on physical activity-induced bone mass accrual [13]. Although, the latter study did not assess bone structural outcomes and was limited by the use of participant self-report of physical activity levels which can be inaccurate.
Although we identified a linear association between years throwing and the magnitude of bone adaptation, the true relationship is likely curvilinear plateauing with increasing years of exposure. We know from preclinical studies that skeletal adaptation to repetitive mechanical loading wanes and exhibits a logarithmic pattern [29–31]. This occurs due to cellular accommodation whereby mechanosensitive cells become accustomed to habitual loads [32, 33]. Adaptation alters the local environment around mechanosensitive cells making it progressively more difficult to introduce a large differential between usual and novel loads. Thus, we would predict that it is more difficult to induce adaptation with increasing years throwing resulting in a plateauing in adaptation with advancing somatic maturation. Ducher et al. [34] partially demonstrated this phenomenon in their longitudinal study of racquet-sports players by showing that girls with the largest side-to-side differences at baseline exhibited the least physical activity-induced adaptation over the succeeding 12 months.
Our use of a cross-sectional study design likely explains the observed linear, as opposed to expected curvilinear, relationship between adaptation and duration of physical activity exposure. Individuals within each advancing maturity group were at increasingly higher levels of baseball competition, with those in the POST-EARLY, -MID and -LATE groups competing in high school, collegiate and professional baseball, respectively. As each progressive level of competition is increasingly more selective with only the best athletes continuing to the next level, some of the observed progressive adaptation could be accounted for by the inclusion of athletes who had the greatest physical activity exposure and, thus, adaptation during their preceding stage/s of somatic maturation.
The pattern of midshaft humerus adaptation observed in the current study is consistent with our previous work detailing loading and adaptation of the humeral diaphysis in baseball players [17, 20]. The throwing arm in throwers had a larger midshaft humerus (Tt.Ar) with more mass (Ct.BMC), a contracted medullary cavity (Me.Ar) and thicker cortex (Ct.Th) than the contralateral nonthrowing arm, independent of stage of somatic maturity and normal arm dominance. These mass and structural adaptations contributed to increased bone strength (SSIP) in each maturity group, with SSIP predicting over 90% of the variance in ex vivo midshaft humerus mechanical properties [25]. These data suggest that physical activity before, during and after somatic maturation increased bone accrual on the periosteal surface, and either increased accrual or decreased resorption on the endocortical surface.
Bone adaptive responses to physical activity-related mechanical stimuli are highly site-specific. Adaptation is not only localized to the bones that are loaded, but also to the specific regions within those bones. To provide an indication of the regional pattern of midshaft humerus adaptation to throwing, we assessed polar pericortical and endocortical radii, and polar Ct.Th. Data revealed greatest throwing-to-nonthrowing arm differences in medial and lateral sectors, particularly in the three POST maturity groups. This suggests overhand throwing predominantly loads the midshaft humerus in a mediolateral direction, which we partly modelled using a subject-specific musculoskeletal model and CT-based finite-element model of the humerus during a fastball pitch in a professional baseball player [17]. However, the greater pericortical radii in all polar segments observed in the POST-LATE group suggests that overhand throwing increases midshaft humerus bone strength in all loading directions.
The increase in overall bone cross-sectional area (i.e. size) induced when young is functionally important. Adding a small amount of mass to the outer surface of a bone results in a disproportionate increase in bone mechanical properties as stiffness is proportional to fourth power of the distance from the neutral axis [35, 36]. The evolutionary advantage is the creation of a strong, yet lightweight skeleton required by humans for endurance tasks [37]. However, more importantly an increase in bone size by pericortical expansion induced when young has been shown to persist lifelong even in the absence of continued heightened physical activity and independent of bone mass [17]. The current data suggest that to optimize bone size and, consequently lifelong bone health, physical activity should be commenced at the earliest age possible and be continued at least into early adulthood.
The current study possesses numerous strengths, including the use of an internally controlled model to minimize the impact of selection bias, inclusion of control subjects to remove side-to-side differences due to normal arm dominance, and the study of bone structural adaptation. The study also used a validated estimator of somatic maturity (i.e. years from PHV [maturity offset]), which has recently been revalidated [38], rather than relying on self-reported stage of sexual maturation. However, the study also possesses limitations. Beyond the aforementioned limitation of being a cross-sectional study, the study focused on the midshaft humerus which is principally a cortical bone site that is not prone to osteoporotic fracture. It is possible that alternative findings and conclusions may be drawn from the study of an osteoporotic-prone corticocancellous site, such as the proximal femur. We did not quantify differences in throwing variables between maturity groups (beyond estimated playing time and throws per week), with other variables such as throwing speed, training intensity, and throwing-to-nonthrowing arm differences in muscle strength potentially contributing to the linear relationship between years throwing and throwing-to-nonthrowing arm differences in estimated bone strength. Also, we studied males only and the findings may not be representative of females.
Within the acknowledged limitation of a cross-sectional study design, our data suggest that physical activity commenced when very young (prior to somatic maturation) induces structural adaptation and that activity continued across and beyond somatic maturation has ongoing structural benefits. These data indicate that physical activity should be encouraged at the earliest age possible and be continued into early adulthood to optimize structural benefits. The current study did not explore the benefits of physical activity continued into mid- and late-adulthood; however, our previous work identified that ongoing activity slows age-related loss of bone mass from the endocortical surface to maintain some of the mass benefits and enhance the strength benefits of activity performed from young and into young adulthood [17].
Supplementary Material
ACKNOWLEDGEMENTS
The present work was partially supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR057740 [to S.J.W.]).
Footnotes
Stuart Warden, Alysa Weatherholt, Andrew Gudeman, Drew Mitchell, William Thompson, and Robyn Fuchs declare that they have no conflict of interest.
REFERENCES
- 1.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9. [DOI] [PubMed] [Google Scholar]
- 2.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–39. [DOI] [PubMed] [Google Scholar]
- 3.Slemenda CW, Reister TK, Hui SL, Miller JZ, Christian JC, Johnston CC Jr., Influences on skeletal mineralization in children and adolescents: evidence for varying effects of sexual maturation and physical activity. J Pediatr. 1994;125:201–7. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–7. [DOI] [PubMed] [Google Scholar]
- 6.Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, et al. The determinants of peak bone mass. J Pediatr. 2017;180:261–9. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannus P, Haapasalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. [DOI] [PubMed] [Google Scholar]
- 10.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos Int. 2000;11:1010–7. [DOI] [PubMed] [Google Scholar]
- 11.Ducher G, Daly R, Bass S. The effects of repetitive loading on bone mass and geometry in young male tennis players: a quantitative study using magnetic resonance imaging. J Bone Miner Res. 2009;24:1686–92. [DOI] [PubMed] [Google Scholar]
- 12.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lappe JM, Watson P, Gilsanz V, Hangartner T, Kalkwarf HJ, Oberfield S, et al. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J Bone Miner Res. 2015;30:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rantalainen T, Weeks BK, Nogueira RC, Beck BR. Effects of bone-specific physical activity, gender and maturity on tibial cross-sectional bone material distribution: a cross-sectional pQCT comparison of children and young adults aged 5–29 years. Bone. 2015;72:101–8. [DOI] [PubMed] [Google Scholar]
- 15.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22:251–9. [DOI] [PubMed] [Google Scholar]
- 16.Warden SJ, Galley MR, Hurd AL, Richard JS, George LA, Guildenbecher EA, et al. Cortical and trabecular bone benefits of mechanical loading are maintained long-term in mice independent of ovariectomy. J Bone Miner Res. 2014;29:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111:5337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–80. [DOI] [PubMed] [Google Scholar]
- 19.Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18:352–9. [DOI] [PubMed] [Google Scholar]
- 20.Warden SJ, Bogenschutz ED, Smith HD, Gutierrez AR. Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone. 2009;45:931–41. [DOI] [PubMed] [Google Scholar]
- 21.Malina RM, Bouchard C. Growth, Maturation, and Physical Activity. 2nd ed. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 22.Granados A, Gebremariam A, Lee JM. Relationship between timing of peak height velocity and pubertal staging in boys and girls. J Clin Res Pediatr Endocrinol. 2015;7:235–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–94. [DOI] [PubMed] [Google Scholar]
- 24.Bogenschutz ED, Smith HD, Warden SJ. Mid-humerus adaptation in fast pitch softballers and the impact of throwing mechanics. Med Sci Sports Exerc. 2011;43:1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weatherholt AM, Avin KG, Hurd AL, Cox JL, Marberry ST, Santoni BG, et al. Peripheral quantitative computed tomography (pQCT) predicts humeral diaphysis torsional mechanical properties with good short-term precision. J Clin Densitom. 2015;18:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rantalainen T, Nikander R, Heinonen A, Daly R, Sievänen H. An open source approach for regional cortical bone mineral density analysis. J Musculoskelet Neuronal Interact. 2011;11:243–8. [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 29.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389–99. [DOI] [PubMed] [Google Scholar]
- 30.Rubin CT, Laynon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg. 1984;66A:397–402. [PubMed] [Google Scholar]
- 31.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12:1480–5. [DOI] [PubMed] [Google Scholar]
- 32.Turner CH. Toward a mathematical description of bone biology: the principal of cellular accommodation. Calcif Tissue Int. 1999;65:466–71. [DOI] [PubMed] [Google Scholar]
- 33.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accomodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. [DOI] [PubMed] [Google Scholar]
- 34.Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res. 2011;26:1321–9. [DOI] [PubMed] [Google Scholar]
- 35.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54. [DOI] [PubMed] [Google Scholar]
- 36.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20:809–16. [DOI] [PubMed] [Google Scholar]
- 37.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–52. [DOI] [PubMed] [Google Scholar]
- 38.Moore SA, McKay HA, Macdonald H, Nettlefold L, Baxter-Jones AD, Cameron N, et al. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47:1755–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.