Abstract
The kidneys regulate many vital functions that require precise control throughout the day. These functions, such as maintaining sodium balance or regulating arterial pressure, rely on an intrinsic clock mechanism that was commonly believed to be controlled by the central nervous system. Mounting evidence in recent years has unveiled previously underappreciated depth of influence by circadian rhythms and clock genes on renal function, at the molecular and physiological level, independent of other external factors. The impact of circadian rhythms in the kidney also affects individuals from a clinical standpoint, as the loss of rhythmic activity or clock gene expression have been documented in various cardiovascular diseases. Fortunately, the prognostic value of examining circadian rhythms may prove useful in determining the progression of a kidney-related disease, and chronotherapy is a clinical intervention that requires consideration of circadian and diurnal rhythms in the kidney. In this review, we discuss evidence of circadian regulation in the kidney from basic and clinical research in order to provide a foundation on which a great deal of future research is needed to expand our understanding of circadian relevant biology.
Keywords: Clock genes, Blood pressure, Sodium balance, Chronotherapy, Kidney
1. Introduction
Circadian rhythms are defined by Merriam-Webster as “being, having, characterized by, or occurring in approximately 24-h periods or cycles.” These rhythms exist in all mammalian tissues throughout the body with as many as 40% of the protein-coding genes displaying oscillatory expression [1]. We have long known that the kidney functions in a pattern that varies according to the time of day, yet we know very little about the mechanisms that control the various functions that follow a diurnal or circadian pattern. In the early 1950's, Mills and Stanbury published evidence that healthy human subjects excrete water and electrolytes in a diurnal pattern independent of daily habits of eating, sleeping and overall activity [2,3]. Such findings were confirmed by Moore-Ede and colleagues in 1977 using squirrel monkeys where they had more tight control of intake [4]. Unfortunately, these studies generated little enthusiasm from the renal community at large until very recently when several studies provided more mechanistic reasons to explore these mechanisms. Furthermore, recent RNA-seq analysis of mouse tissues revealed that the kidney is second only to the liver in the number of genes expressed in a circadian pattern [1]. The current review will focus on the overall evidence for physiological regulation of specific mechanisms along the nephron in the context of our current understanding of circadian control.
The discovery of the so-called “clock” genes as a series of transcription factors expressed in an oscillating loop provides an opportunity for a tremendous amount of new information related to circadian control systems. In general, the molecular clock mechanism consists of a transcription-translation oscillatory feedback loop that involves products of the core clock genes, Bmal1 (or ARNTL, aryl hydrocarbon receptor nuclear translocator-like protein 1) and Clock, that function as transcription factors to drive gene expression in the nucleus of the nucleus. The formation of a Bmal1-Clock heterodimer undergoes regulation via activation or repression of Bmal1 expression through the retinoic acid-related orphan receptor (ROR) or Rev-ErbA alpha nuclear receptor families, respectively [5]. The Bmal1-Clock heterodimer forms the positive limb of the feedback loop which then binds to the E-box domain of target genes, including core clock genes Period (Per) and Cryptochrome (Cry). Per and Cry then leave the cell to either perform various physiological actions or they can re-enter the nucleus of the cell via phosphorylation by Casein Kinase 1 isoforms δ/ε (CK1δ/ε) [6,7]. Groundbreaking work from the Gumz lab published in the Journal of Clinical Investigation in 2009 has sparked new interest in circadian control of kidney function. These investigators reported that aldosterone, the most well established regulator of renal tubular sodium handling, controls the epithelial sodium channel via the core clock gene, Period 1 [8].
This review is generally divided into three major sections. First, we will discuss what is known about the different sections of the nephron and attempt to provide evidence of a possible circadian intervention, at the physiological level determined by renal function, at the molecular level involving the renal circadian clock, or both. Secondly, we will take an integrated look at how the circadian clock regulates nephron function, and the role hormones and peptides play in that regulation. Finally, after taking a look into some of the pathologies that may be associated with impaired rhythmic activity, we will discuss how circadian rhythms can provide possible solutions to these pathologies.
2. Circadian rhythms along the nephron
2.1. The glomerulus
To be filtered by the kidneys, blood must travel to the glomerulus where the nephron begins. The glomerulus consists of a tortuous bundle of blood capillaries located within the Bowman's capsule. These capillaries receive blood from the afferent arteriole, a unique “high pressure” arteriole that also functions as an endocrine organ through release of renin. The glomerular capillaries are unique in their extreme permeability and very high capillary pressure, thus facilitating the passage of fluid into the proximal tubule to begin the formation of urine [9,10]. Blood that is not filtered by the glomerulus leaves the glomerular capillaries via the efferent arterioles. From there blood passes through the peritubular capillaries and vasa recta, before returning to the systemic vasculature through the renal vein.
Multiple studies have shown evidence of circadian variation in glomerular function (Fig. 1). In normal individuals, glomerular filtration rate (GFR) measured by inulin and creatinine clearance reaches a maximum during the day, peaking around 2–3 p.m., and a minimum in the middle of the night [11–13]. Effective renal plasma flow (ERPF) as measured by p-aminohippurate clearance also shows a circadian rhythm peaking during the day, or active period, although this peak appears to occur later in the afternoon compared to GFR (Fig. 1) [11,12]. As a result, the filtration fraction (GFR/ERPF) also displays circadian rhythmicity. The physiological significance of the adjustments in filtration fraction is unknown, but the rhythm in GFR is presumably commensurate with the need to excrete a larger volume of urine during the active period when consumption of water is also at its highest. It is also relevant to note that the clearance of inulin and creatinine, two important markers used for assessment of GFR, do not have the same level of circadian variation [11] (Fig. 2). This is likely due to the large amount of creatinine secretion that occurs in the proximal tubule and suggests that creatinine clearance is not a reliable way of assessing diurnal variations in GFR.
Fig. 1.
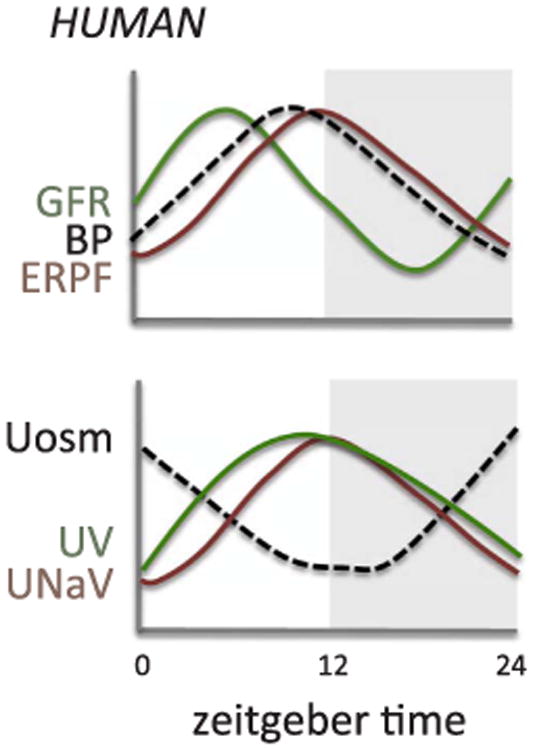
Estimated circadian rhythms for renal functional parameters. GFR, glomerular filtration rate; BP, blood pressure; ERPF, effective renal plasma flow; Uosm, urine osmolality; UV, urine flow rate; UNaV, sodium excretion.
Adapted from Koopman et al. [11]; Koopman et al. [12]; Mills & Stanbury [2]; Graugaard-Jensen et al. [32]; Kamperis et al. [34]; and Perrier et al. [33].
Fig. 2.
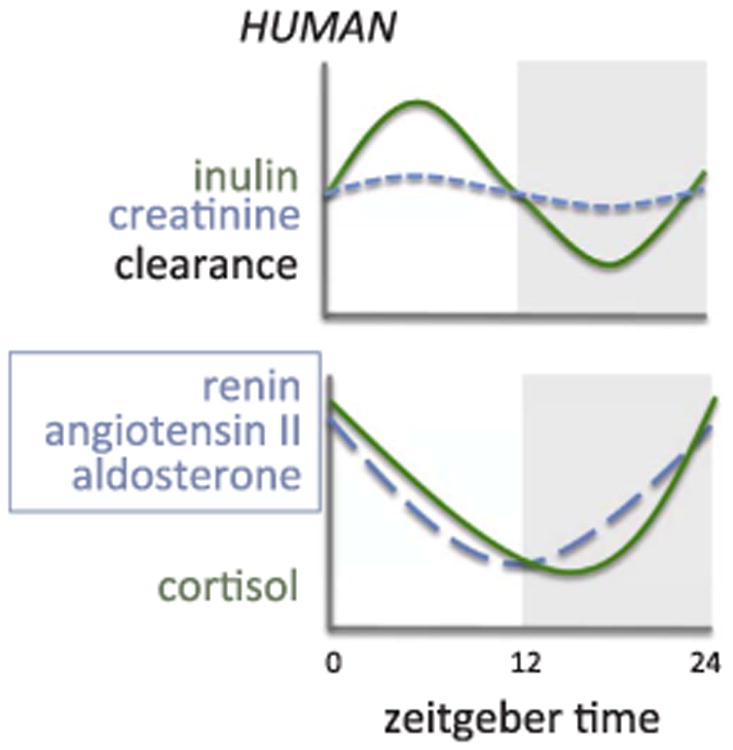
Estimated circadian rhythms for renal clearance of inulin and creatinine in humans (upper panel) and circulating hormones (lower panel).
dapted from Gordon et al. [93]; Kala et al. [103]; Hurwitz et al. [92]; Williams et al. [109]; Guignard et al. [117]; Van Cauter et al. [116].
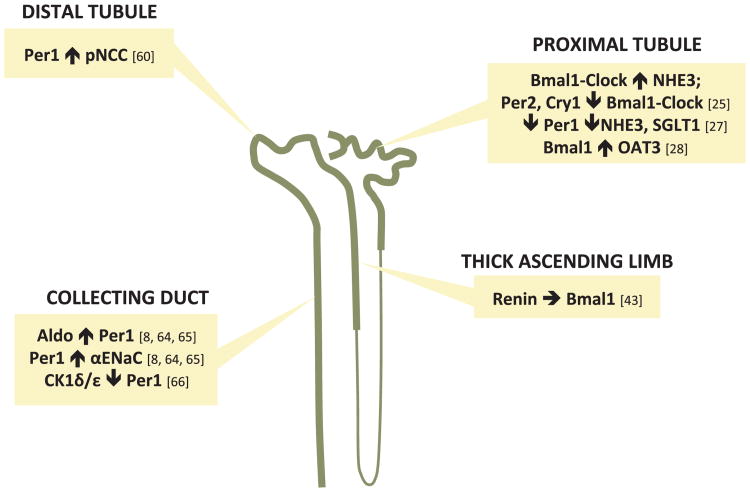
Diurnal variations can also be seen in the filtered load of water and sodium (Fig. 1) [11]. There is also circadian variation seen in urinary albumin and ß2-microglobulin excretion with a phase similar to GFR in normal individuals [11]. Collectively, these observations demonstrate that parameters used as biomarkers for glomerular function such as creatinine could potentially change depending on the time of day the measurements are taken. They also suggest an interaction between the molecular clock mechanism and the glomerulus. A study by Huang et al. noted that in male Wistar rats, the glomerular capillaries express the core clock protein Bmal1 and clock output protein, D site albumin promoter binding protein (Dbp), and that these expression levels change at different times of the day [14]. A list of the expression level acrophases and nadirs of different clock and clock-controlled genes throughout the nephron in whole rodent kidneys is provided in Table 1. Although these results suggest an association between circadian clock proteins and the diurnal variation in renal function, more work is needed to elucidate the localization of other clock proteins within the glomerulus as well as afferent and efferent arterioles to determine what impact each clock gene may have on regulating glomerular function. Fig. 3 summarizes some of what is known about specific clock genes impacting specific transporters along the nephron, although very little is specifically known about these relationships.
Table 1.
Examples of clock- and clock-controlled genes with cyclic activity in the whole kidneys of rats and mice.
| Mouse Kidney | ||||
|---|---|---|---|---|
|
| ||||
| Gene | Description | Period (hr) | Time of Peaka (ZT) | Time of Nadira (ZT) |
| Arntl | Brain and muscle Arnt-like protein–1 (Bmal1) | 24 | ∼22.5 | ∼10.5 |
| Clock | Circadian Locomotor Output Cycles Kaput (Clock) | 24 | ∼22 | ∼10 |
| Per1 | Period 1 (Per1) | 24 | ∼11 | ∼1.5 |
| Per2 | Period 2 (Per2) | 24 | ∼14 | ∼2 |
| Per3 | Period 3 (Per3) | 24 | ∼11 | ∼23 |
| Cry1 | Cryptochrome 1 (Cry1) | 24 | ∼18 | ∼6 |
| Cry2 | Cryptochrome 2 (Cry2) | 24 | ∼14 | ∼2 |
| Nr1d1 | Rev-ErbA Alpha (Rev-erbα) | 24 | ∼7 | ∼21 |
| Dbp | D site albumin promoter binding protein | 24 | ∼10 | ∼23 |
| Slc9a3 | Solute carrier family 9, member 3 (Na+/H+ exchanger 3; NHE3) | 24 | ∼9 | ∼21 |
| Avpr1a | Arginine vasopressin receptor 1a (V1aR) | 24 | ∼22 | ∼11 |
| Avpr2 | Arginine vasopressin receptor 2 (V2R) | 28 | ∼21 | ∼6-9 |
| Sgk1 | Serum/glucorticord regulated kinase 1 | 24 | ∼15.5 | ∼3 |
| Edn1 | Endothelin–1 (ET–1) | 26 | ∼18 | ∼6 |
| Ednra | Endothelin receptor subtype A | 24 | ∼20 | ∼8 |
| Ednrb | Endothelin receptor subtype B | 23 | ∼7 | ∼18 |
| Cyba | Cytochrome b–245, alpha polypeptide (p22phox) | 32 | ∼14 | ∼0 |
|
| ||||
| Rat Kidney | ||||
|
| ||||
| Gene | Description | Period (hr) | Time of Peak (ZT) | Time of Nadira (ZT) |
|
| ||||
| Arntl | Brain and muscle Arnt-like protein–1 (Bmal1) | 24 | 22:45 | ∼11 |
| Clock | Circadian Locomotor Output Cycles Kaput (Clock) | 24 | 23:21 | ∼11 |
| Per1 | Period 1 (Per1) | 24 | 13:11 | ∼1 |
| Per2 | Period 2 (Per2) | 24 | 14:24 | ∼2 |
| Cry1 | Cryptochrome 1 (Cry1) | 24 | 15:06 | ∼3 |
| Cry2 | Cryptochrome 2 (Cry2) | 24 | 17:53 | ∼6 |
| Scnn1a | Epithelial sodium channel, alpha subunit (αENaC) | 24 | 14:18 | ∼2 |
| Slc9a3 | Solute carrier family 9, member 3 (Na+/H+ exchanger 3; NHE3) | 24 | 14:05 | ∼2 |
| Slc12a3 | Solute carrier family 12, member 3 (Na+-Cl- cotransporter; NCC) | 24 | 13:53 | ∼2 |
| Avpr1a | Arginine vasopressin receptor 1a (V1aR) | 24 | 23:34 | ∼11 |
| Avpr2 | Arginine vasopressin receptor 2 (V2R) | 24 | 20:37 | ∼8 |
Fig. 3.
Nephron sites where direct clock gene interactions have been documented in the literature as noted. Per1, Period 1; pNCC, phosphorylated sodium chloride co-transporter; Aldo, aldosterone; αENaC, alpha subunit of the epithelial sodium channel; CK1δ/ε, casein kinase 1 delta/epsilon; Bmal1, also known as ARNTL, Aryl hydrocarbon receptor nuclear translocator-like protein 1; Per2, Period 2; Cry1, crytochrome 1; NHE3, sodium hydrogen exchanger 3; SGLT1, sodium glucose transporter 1; OAT3, organic anion transporter 3.
2.2. The proximal tubule
The proximal tubule reabsorbs approximately 65% of the glomerular ultrafiltrate, including nearly all of the amino acids and glucose. This fraction stays quite consistent by adjusting proximal reabsorption of solutes and water in response to the variations in GFR in order to keep fractional reabsorption constant; this intrinsic property of the kidney is known as glomerulotubular balance [15–17]. Glomerulotubular balance involves the full range of co-transporters and exchangers of fluids and solutes within this region of the nephron. One of the primary transporters that help to reabsorb Na+ is the sodium-hydrogen exchanger 3 (NHE3) [18–20]. The sodium-glucose co-transporter 2 (SGLT2) in the proximal convoluted tubule and the SGLT1 in the proximal straight tubule are responsible for reabsorbing most of the filtered glucose along with Na+ and can be overwhelmed during hyperglycemia to result is osmotic diuresis during diabetes [21,22]. The proximal tubule contributes to acid-base balance, once again involving the NHE3 [18,20] as it secretes H+ into the tubular fluid. While many of the renal tubular transporters are expressed in a circadian pattern at the whole kidney level, it is not clear whether this is specifically relevant for the proximal tubule. Furthermore, given the circadian changes in GFR, it is not clear whether glomerulotubular balance is maintained throughout a 24-h period or whether glomerulotubular balance also follows a circadian pattern.
The renal excretion of H+ appears to follow a diurnal variation in humans, with urine pH becoming more acidic at night and more alkaline during the day [2,23,24]. Data from animal models is surprisingly not available. Saifur Rohman et al. examined the circadian expression of NHE3 in rats and found that both the protein and mRNA expression of NHE3 reaches peak levels during the dark phase, corresponding to the active phase and the time of greater food consumption [25]. This finding of NHE3 levels peaking in the dark phase of rats was also seen in a study by Zhang et al. [26] Furthermore, the Clock:Bmal1 heterodimer binds directly to the E-Box domain on the NHE3 promoter to induce its expression, and this action can be inhibited by Per2 and Cry1 [25] (Fig. 3). Pharmacological blockade of the circadian clock protein Per1 decreases the expression of both NHE3 and SGLT1 not only in the renal cortex of mice, but in human proximal tubule cells as well [27]. These studies provide a useful foundation for further exploration into the role of the circadian clock in the proximal tubule, especially at the molecular level.
The proximal tubule may also play a significant role in chronopharmacology by way of its ability to control renal tubular handling of drugs through various organic acid and base transporters, which display a circadian pattern of expression that is regulated by clock genes [28]. In the nephron-specific Bmal1 KO mouse, organic anion transporter 3 (OAT3) expression is severely reduced and impairs the natriuretic response to furosemide [28].
2.3. The loop of Henle
The loop of Henle is made of up of three segments that each have unique functional properties: the thin descending limb, thin ascending limb, and thick ascending limb (TAL). The differing structures of each segment have contrasting permeability and transport of water and solutes in the descending and ascending limbs to create a countercurrent mechanism that facilitates concentrating urine and regulating body fluid osmolality [29,30]. The thick ascending limb contains a major co-transporter, the Na+-K+–2Cl- co-transporter (NKCC2), on the apical membrane which actively reabsorbs these ions from the tubular lumen while remaining impermeable to water [31]. This selective permeability allows the loop of Henle to reabsorb about 25% of the filtered load of NaCl while the tubular fluid leaving this segment always becomes hypotonic compared to plasma as it moves into the distal tubule.
Multiple studies in humans demonstrate existence of a diurnal rhythm in urine osmolality, with osmolality being lower (more dilute) during the day and higher (more concentrated) at night [32–35]. A recent study by Hara et al. found that the osmotic pressure in the rat kidney appears to have a diurnal rhythm in the inner medulla but not cortex, peaking during the active phase of the animal and reaching a nadir during the inactive period [36]. This finding mirrors the diurnal rhythm seen in Na+, Cl-, and urea concentration in the inner medulla as well [36]. Unfortunately, the mechanisms regulating this rhythm in urine osmolality are not clear and circadian patterns of loop of Henle function are poorly defined. There is evidence of cyclic activity in NKCC2 gene expression in the mouse kidney [37], but more studies are needed to confirm the result. The expression of NHE3 mRNA in the ascending limbs of the loop of Henle of mice appear to follow circadian oscillations based on PCR and laser capture techniques [38]. These investigators used in situ hybridization to report the surprising finding that loop of Henle structures in the inner strip of the outer medulla had the highest level of NHE3 expression in the kidney, but a direct link between the expression profile of this exchanger and the diurnal rhythm of urine osmolality was not determined. Examination of nuclear receptor expression profiles in the nephron found that estrogen receptor ß (ERß) showed strong expression in the TAL, varying in a circadian manner. Additionally, the expression of ERß appeared to modulate the expression of NKCC2, suggesting a direct regulation of ion transport in the TAL [39]. However, it should be noted that the experiments in this study were performed using kidneys only from male mice. Although protein levels of ERß have been found to be higher in male rats [40], there not does appear to be much prior evidence of their expression at the gene or protein level in mice [41,42]. Therefore an analysis of the circadian variation of nuclear receptors in the TAL of female mice is needed to provide insight into any possible sex differences in the temporal expression of ERß.
Tokonami et al. created a novel mouse model in which Bmal1 expression was knocked out in cells that expressed the renin gene (Bmal1lox/lox/Ren1dCre) [43]. Using these mice, the authors found that Bmal1 gene and protein expression was significantly reduced in the thick ascending limb. They also found that Bmal1lox/lox/Ren1dCre mice had decreased urine osmolality compared to controls, although it is unclear whether this was due to time of day differences since urine was collected in 24-hr intervals [43]. These findings suggest that Bmal1 regulates urine osmolality through actions, at least in part, within the TAL.
2.4. The distal tubule
The distal tubule receives fluid from the TAL that is hypo-osmotic relative to plasma. The initial segment, the distal convoluted tubule (DCT) can be further subdivided into two segments, the early (DCT1) and late (DCT2) distal tubule. The DCT2 is differentiated by the expression of 11ß-hydroxysteroid dehydrogenase in addition to miner-alocorticoid receptors, allowing this segment to respond to aldosterone and not cortisol [44]. The aldosterone-sensitive segment expands through the connecting tubule into the cortical collecting duct, and contains two types of cells that are involved in water and electrolyte transport, principal cells and intercalated cells [45,46]. These types of cells will be discussed further in the collecting duct section. The major ion transporter in the distal convoluted tubule is the thiazide-sensitive NaCl co-transporter (NCC), reabsorbing most of the NaCl in the DCT1 segment [47]. While the NCC also reabsorbs sodium in the DCT2 segment, the amiloride-sensitive epithelial sodium channel (ENaC) reabsorbs Na+ in the DCT2, the connecting tubule (CNT) and collecting duct [48–50]. In the DCT2, NCC co-localizes with ENaC [51–53], and this association can be further enhanced through the actions of aldosterone [54].
An informative microarray study by Zuber et al. in the DCT/CNT and cortical collecting duct (CCD) examined the molecular mechanisms behind the circadian rhythms in these regions of the nephron [55]. Expression of core clock transcription factors, such as Bmal1, Per1/2/3, and Cry1/2, display circadian rhythmicity in the DCT/CNT [55]. In five healthy volunteers, NCC in urinary exosomes was excreted in a diurnal pattern [56]. While these findings provide clear reasons to examine the role of Na+ Cl- co-transporter (NCC) circadian control of renal excretory function, a broader sample size over a longer period of time for sample collection is needed to strengthen the foundation for future studies. In mice, the inactive form of NCC does not appear to show a circadian rhythm [55,57]. However, when examining NCC activation through phosphorylation (pNCC) by the WNK-OSR1/SPAK pathway [58], there appears to be a significant night/day difference in pNCC protein expression, with a peak at the beginning of the active period and a nadir at the start of the inactive period [57]. This night/day difference in pNCC expression was seen in another study as well [59]. Richards et al. showed evidence that Per1 facilitates the expression of NCC through the WNK pathway, as reducing the expression of the clock protein resulted in a reduction of NCC, WNK1 and WNK4 as well [60]. Whether or not other core clock genes or clock-controlled genes have an effect on NCC remains to be elucidated.
2.5. The collecting duct
The collecting duct is made up of three different segments, the cortical collecting duct (CCD), outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD) that are distinguished by varying combinations of principal and intercalated cells as well as variable expression levels of major sodium and water transporter proteins [45,46]. Principal cells reabsorb Na+ from the tubular lumen primarily through the epithelial Na + channel (ENaC; composed of α, β, and γ subunits) and secrete K+ through the renal outer medullary K + (ROMK) channel [61–63]. Water is reabsorbed by principal cells via aquaporin 2 (AQP2) channels [46]. Intercalated cells reabsorb K+ and HCO3- and secrete H+ , making these cells particularly important in regulating acid-base balance [45]. In terms of Na+ conservation, aldosterone is a primary regulator of Na+ and K+ homeostasis by enhancing ENaC and ROMK expression, while arginine vasopressin (AVP; also known as anti-diuretic hormone or ADH) regulates water transport by increasing AQP2 activity [46]. AVP is produced by magnocellular neurosecretory neurons located in the paraventricular nucleus and supraoptic nucleus of the hypothalamus [64]. External cues from the environment such as light enter the retina, leading to downstream signaling in which AVP is an output signal of the master circadian clock of the suprachiasmatic nucleus (SCN), as it has traditionally been viewed [64]. However, it has been suggested recently that AVP may play a more direct role in synchronizing the circadian network [65]. AVP is stored in the posterior pituitary gland until a number of different factors, such as plasma osmolality, hypotension, or hypovolemia, stimulate its release [66]. Plasma osmolality is seen as the most important factor behind AVP release, as miniscule changes in osmolality due to behavioral (increase/decrease in water or Na+ intake) or physiological (increase/decrease in water or Na+ excretion) mechanisms can influence AVP levels [67]. AVP has many different functions, including regulation of water retention in the body, inducing vasoconstriction to increase blood pressure, increasing the release of adrenocorticotropic hormone (ACTH), and influencing learning and memory [66]. There are many other autocrine and paracrine factors that also contribute to the regulation of water and electrolytes in this region, and will be touched on later in this review.
The CCD shows circadian oscillations in Bmal1, Per1/2/3 and Cry1/2, similar to the DCT and CNT [55]. Significant diurnal rhythms in αENaC were detected in mouse cortex, peaking during the active phase [36]. The diurnal rhythm of AVP in plasma has been previously documented in humans, with levels higher at night than during the day [68]. Expression of AVP mRNA in mice peaks during the inactive period as well [69]. Mice lacking Bmal1 specifically in AVP neurons have reduced mRNA expression of Per1 in the dorsal portion of the SCN, where most of the AVP neurons are located [70]. Unfortunately, the effect of Bmal1 deletion in AVP neurons on urine output or osmolality in these mice was not investigated. Vasopressin receptors V1aR and V2R show diurnal rhythms in the inner medulla, and V1aR in the cortex, of mouse kidney [36]. The urea transporter UT-A2 displays a diurnal rhythm in the inner medulla, peaking during the active period as well [36]. There is evidence of rhythmic expression in αENaC, V1aR and V2R in the rat as well, with mRNA expression for all three genes peaking during the active phase [26]. Per1 was found to positively regulate the expression of αENaC at baseline and after aldosterone stimulation in mouse CCD, OMCD and IMCD cells [8,71]. In the CCD, this regulation occurs through direct binding with an E-box domain on the αENaC promoter along with the mineralocorticoid receptor (MR) in the presence of aldosterone [71,72]. This direct regulation of αENaC by Per1 was also shown to be inhibited by Casein kinase 1δ/ε, a regulator of the core clock mechanism via phosphorylation of Per proteins and inhibition of Per1 nuclear translocation [73].
Tokonami et al. created a cell-specific Bmal1 knockout mouse model using cre-recombinase driven by the renin promoter, which led to reduced expression of Bmal1 in whole kidney samples [43]. After examining Bmal1 levels in each segment of the nephron, reduced Bmal1 mRNA and protein levels were focused in the medullary portion of the TAL, the CCD and OMCD. These reductions were seen with normal blood pressure rhythms although at significantly lower pressures at any one time in the day [43]. The nephron-specific Bmal1 knockout mice also had increased urine volume and what appeared to be a possible phase shift in the circadian rhythm of urinary sodium excretion. The mechanisms for these changes will require further investigation.
The urine concentrating mechanism is dependent upon activity of the Na+K+2Cl- transporter in the TAL, but of course, the activity of ADH to regulate AQP2 activity in the collecting duct is equally important. AQP2 protein levels in healthy children appear to show a day/night difference in expression, with AQP2 expression being higher at night [74]. This night/day difference in AQP2 levels was also correlated with a day/night difference in diuresis, with urine levels higher during the day and lower at night. These investigators collected urine samples in two consecutive 12-h periods with the average age being about 10 years old [74]. A different study did not see a variance in AQP2 levels at different times of the day, but it should be noted that these samples were collected five times over the course of one 12-h period from 9 a.m. to 9 p.m., and the age of the healthy subjects used in this study is unclear [75]. In the inner medulla of mice, a diurnal rhythm in Aqp2 gene expression was observed, peaking at Zeitgeber Time (ZT) 20 [36].
3. Integration of circadian control throughout the nephron
So far, we have discussed our limited knowledge of what is known about how each segment of the nephron individually functions in a diurnal/circadian manner and how that function may be regulated by the molecular clock in the kidney. Next, we will take a look at how these nephron segments act together, either to regulate blood pressure or how hormones and other factors impact the circadian aspects of various kidney functions. Unfortunately, we know very little about how various clock genes function in different parts of the kidney. Speed and colleagues recently reported mRNA expression of the major clock genes in cortex and inner medulla of rat kidneys, which follow similar patterns as reported in other tissues [76] (Fig. 4).
Fig. 4.
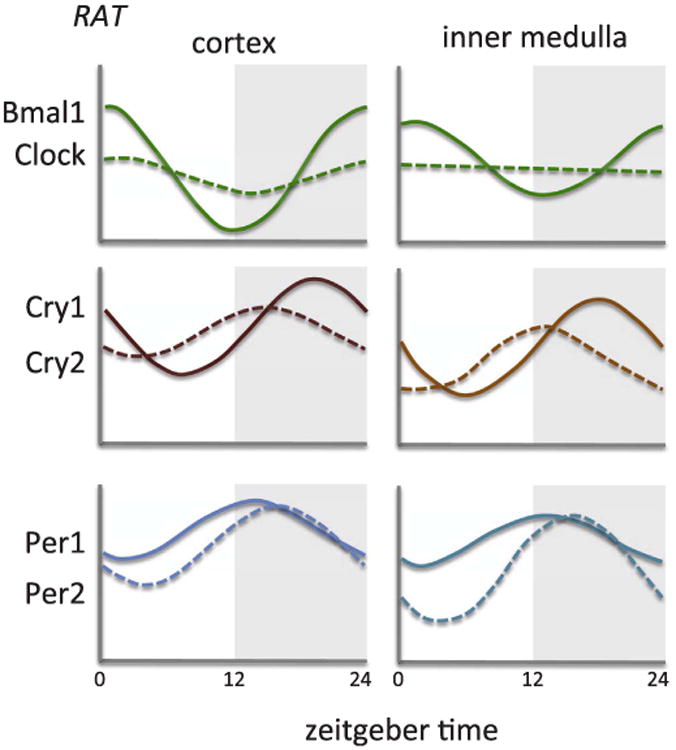
Approximation of mRNA expression from renal cortex or inner medulla from control rats. Bmal1, also known as ARNTL, Aryl hydrocarbon receptor nuclear translocator-like protein 1; Clock, Circadian Locomotor Output Cycles Kaput; Cry, cryptochrome; Per, Period.
Adapted from Speed et al. [76].
Regulating the levels of water and electrolytes throughout the day is one of the major functions of the kidney as it tries to maintain proper balance in the extracellular fluid. This regulation occurs in part through the production and secretion of hormones as well as paracrine/autocrine factors within the kidney. Because these factors typically exhibit circadian variations in expression and activity, it is important to understand how they relate to the molecular clock.
3.1. Impact of the circadian clock on blood pressure
The renal-body fluid system is a powerful mechanism used to control blood pressure (BP) through the regulation of the extracellular fluid volume in the body. As extracellular fluid volume increases in response to increased salt intake, the kidneys excrete the excess fluid and electrolytes in order to return the extracellular fluid volume and BP to normal [77]. BP typically displays a circadian rhythm in healthy individuals, with levels higher during their active/wake cycle and lower during their inactive/rest cycle [78]. However, it has yet to be clarified whether the diurnal variation in blood pressure is a result of changes in kidney function consistent with this body fluid hypothesis.
Individuals that exhibit a ≥ 10% decrease in night-time BP compared to daytime BP are known as “dippers”, while those that show a < 10% decrease in night-time BP appear to have a blunted decline BP and are known as “nondippers” [79,80]. Complications due to BP are thought more likely to occur in the morning, when there is a surge, or rise, in BP [81]. Individuals with impaired BP rhythms, such as non-dippers or those with nocturnal hypertension also have an increased risk of cardiovascular problems. This occurs in disease states such as chronic kidney disease, heart failure, and sleep apnea [82]. The extent of the pathologies caused by impaired rhythms will be discussed later in this review.
The use of mouse models containing whole-body mutations or complete knockout of circadian clock genes have allowed us to gain more knowledge on how each transcription factor contributes to the BP phenotype. The Bmal1 knockout mouse lacks a circadian blood pressure rhythm, but is considered hypotensive since blood pressure is similar to genetic controls during the inactive phase, but fails to increase during the active phase [43,83,84]. Mice with a loss of Bmal1 in the renal tubules maintain a normal circadian rhythm, although with a slight but significantly lower systolic BP [28]. A mouse model where the renin promoter drive Bmal1 gene deletion displays a significantly lower systolic and diastolic BP compared to controls [43]. It is important to note that Bmal1 was deleted not only in the juxtaglomerular cells, but also in a fairly wide range of cell types including the TAL, CD, as well as in the liver [43]. Global Clock knockout mice have significantly lower BP compared to wild type mice, but again, with a maintained rhythm [55]. Per1 knockout mice also display a hypotensive phenotype while maintaining diurnal BP variation. Per2 and Per triple knockout (knockout of Per1, Per2, and Per3), however, do not show any difference in BP under normal conditions compared to controls [85]. Cry-null mice appear to have a unique BP phenotype in that they display a normal blood pressure and rhythm, but develop salt-sensitive hypertension when placed on a high salt diet [86]. This appears due to the overproduction of aldosterone caused by the overexpression of type VI 3ß-hydroxyl-steroid dehydrogenase (Hsd3b6), as demonstrated both at the mRNA and protein levels [86]. All of these studies illustrate the complexities involved in the circadian regulation of blood pressure. It is not clear how the molecular clock mechanism contributes to overall BP rhythms versus the overall 24-h BP. Further, the molecular clock also appears to regulate a variety of BP control mechanisms such as aldosterone, but the nature of this complex relationship is far from evident. It is also worth noting that the zeitgeber for blood pressure rhythm does not appear to be light or the central clock since BP rhythms are maintained in total darkness [85].
3.2. Excretion of metabolic waste products and drugs
The kidney is responsible for eliminating metabolic waste products from the body such as urea and creatinine. It is also important for excreting drugs and other toxins into the urine by secreting them through organic ion transporters, especially in the proximal tubule. Recently, the circadian clock in the renal tubules was shown to play a role in drug and metabolite breakdown. They observed altered metabolism when the Bmal1 gene was deleted specifically throughout the length of the nephron of mice [28]. In addition, they found that the organic anion transporter 3 (OAT3), a transporter important for the secretion of creatinine among other substances [87], had reduced mRNA and protein levels, leading to a reduction in the amount of the loop diuretic furosemide excreted in the urine [28]. This study provides the only evidence to our knowledge that the renal molecular clock can regulate the excretion of drugs and control metabolism, but has important implications for chronotheraputic research. The relation between the circadian clock and metabolism in general has been reviewed elsewhere [88].
3.3. Renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system (RAAS) is designed to regulate long-term arterial pressure in part through its ability to regulate Na+ balance through Na+ conservation [89,90]. By controlling the levels of water and electrolyte reabsorption in the kidney, the RAAS plays a role in maintaining the renal excretion of salt and water throughout the day in response to differing amounts of salt intake (Fig. 2). The kidney is the main source of renin in the circulation, and the expression and secretion of renin are regulated by the juxtaglomerular (JG) cells, which are located in the JG area of the afferent arteriole [91]. The release of renin from these cells are determined by changes in BP and salt intake [91]. In humans, plasma renin activity (PRA) has a diurnal rhythm higher in the morning and lower in the afternoon and evening [92,93]. TGR(mREN-2)27 rats, a transgenic rat model that had the introduced the mouse renin gene (Ren-2) into the rat genome [94], display a hypertensive BP phenotype that has a reversed BP rhythm [95]. A study by Herichová et al. found that the mesor of Bmal1 gene expression was down regulated and Clock was upregulated in the kidneys of these rats, along with different sections in the brain, suggesting an interaction between peripheral and central clocks and the RAAS [96]. Further studies are needed to look at possible effects on the circadian expression of various other components important in regulating BP. Importantly, the functional relevance of changes in renal clock gene expression require further clarification as detailed above.
Angiotensinogen (Agt) is the only known substrate of renin and is cleaved by renin to form angiotensin I [91]. Although the liver is the main source of Agt production in the body, many other organs produce Agt as well, including the brain, heart, lung, adrenal gland and spleen [91]. In the kidney, Agt that ends up in the urine is primarily produced by the proximal tubules, and the amount of Agt in the urine can be modulated by angiotensin II [97,98]. There does not appear to be a circadian rhythm in the levels of Agt in either the plasma or urine of humans [99]. This may not be surprising if one considers that Agt is not considered the rate-limiting step in this pathway. Furthermore, there does not appear to be any studies that have examined the circadian expression of clock genes or other factors in animal models where the Agt gene has been knocked out. Thus, more remains to be elucidated as to whether Agt plays any role in the circadian regulation of renal function.
Angiotensin II (Ang II) performs its physiological actions through its two receptors, angiotensin type 1 (AT1, including AT1a and AT1b) and type 2 (AT2) receptors. The vast majority of the actions of Ang II, which include vasoconstriction and sodium reabsorption [100], are performed through AT1 receptors that are expressed throughout the vascular and tubular segments of the kidney [101]. In the kidney, AT2 receptors are expressed largely in the proximal tubules and generally oppose the actions of the AT1 receptors [101,102].
Circulating levels of Ang II have a diurnal variation in humans, with higher plasma concentrations in the morning [103]. In rats, the AT1 receptor appears to show diurnal variation in protein expression, peaking in the inactive period [104]. A study published by Tsujino et al. examined the circadian expression of clock genes in the heart, aorta, and liver of whole-body AT1a receptor knockout mice (AT1a-KO), and found no significant difference in clock gene expression or oscillation between WT and AT1a-KO mice in these organs [105]. Kidneys were collected from AT1a-KO mice but circadian clock gene expression was not examined, although the focus of this study was to determine whether the AT1a receptor was playing a role in circadian plasminogen activator inhibitor-1 expression, which was similar to wild-type controls [105]. A loss of circadian BP and heart rate rhythms have been reported in AT2 receptor knockout mice that are hypertensive relative to wild-type controls [106]. However, there have been no studies to explore the relationship between the circadian clock and AT2 receptor expression and activity.
Aldosterone is a mineralocorticoid produced in the zona glomerulosa of the adrenal cortex. It is primarily responsible for increasing Na+ reabsorption through ENaC and increasing K+ secretion through K+ channels in the distal nephron [107]. Because of these actions, aldosterone stimulation results in sodium retention to conserve Na+ and increase K+ excretion. In healthy individuals, aldosterone displays a circadian rhythm in plasma/serum concentrations, peaking in the early morning and reaching a nadir at night [92,108,109]. In mice, aldosterone levels appear to reach their peak during their active period as well [43,110]. However, Tokonami et al. observed that the rhythm in aldosterone levels in control mice may be biphasic, with a peak also occurring during the inactive period [43]. Cry-null mice produce five times the levels of aldosterone compared to control mice, an effect apparently caused by up regulation of 3ß-hydroxysteroid de-hydrogenase-isomerase (3ß-HSD) enzymatic activity within the zona glomerulosa cells. These mice also exhibit salt-sensitive hypertension that can be reversed with the administration of the aldosterone blocker, eplerenone [86]. In cultured collecting duct cells, aldosterone stimulates Per1 expression, which can then mediate the effect of aldosterone on αENaC in the collecting duct [8,71]. In addition, a reduction in Per1 expression has been shown to reduce plasma and urinary aldosterone and 3ß-HSD mRNA levels, while increasing sodium excretion [110]. These studies suggest that Per1 accounts for the circadian pattern of aldosterone production and action, but the relation with other clock genes and dietary sodium intake need further elucidation, especially as they relate to the many other factors that control sodium balance.
3.4. Glucocorticoids
Glucocorticoids (GC) are produced in the adrenal gland and have a variety of different functions, including energy metabolism and the response to stress [111]. In the kidney, GC helps regulate body fluid homeostasis by improving the diuretic and natriuretic response in the inner medullary-collecting duct of rats under specific conditions [112]. GC can regulate circadian genes in peripheral organs such as the kidney, liver and heart [113,114] and are known to entrain rhythmic activity in peripheral clock tissues as well [115]. In humans, cortisol levels typically peak in the morning hours and reach a nadir overnight [116,117]. In rodents, corticosterone levels typically peak at the beginning of their active phase as well [115]. This regulation of the circadian variation of plasma GC is controlled by the SCN through neural and humoral signals [111], however little is known about the effects of GC on circadian function in the kidney. A study by Ivy et al. found that chronic corticosterone infusion led to increased levels of pNCC, Bmal1 and Per1 during the inactive phase, and a non-dipping BP phenotype [59]. Treatment of these mice with hydrochlorothiazide reversed the non-dipping phenotype back to normal [59]. Furthermore, adrenalectomy resulted in a blunted rhythm of pNCC expression in the kidney. While this study provides valuable information about the influence of GC on circadian components of kidney function, future studies involving GC will be able to provide more insight into the mechanisms behind these functions.
3.5. Endothelin-1
Endothelin-1 (ET-1) is a peptide involved in the maintenance of BP through the regulation of sodium handling by the kidney [118]. The actions of ET-1 are controlled by activation of its two receptors, ETA and ETB, which generally have opposing physiological actions in the body [119]. In the kidney, ETB receptors inhibit renal tubular Na+ re-absorption and promote Na+ excretion in response to increased NaCl intake through its actions on sodium transporters throughout the nephron [120]. There have been a few reports that ETA receptors also promote sodium excretion as well [121,122]. However, because of the very high density of ETB receptors in the collecting duct, ET-1 is typically thought to play an important role in fine-tuning the final amount of sodium that leaves the nephron through ETB receptor activity and inhibition of ENaC activity [123].
In healthy individuals, there is a diurnal difference in the levels of plasma ET-1, with lower levels observed at night [124], but this is less evident in rats [76] (Fig. 5). In kidneys collected from mice and rats (Fig. 5), there appears to be lower levels of ET-1 during the active period [76,125]. A recent study by Johnston et al. demonstrated that rats lacking ETB receptor function have an impaired natriuretic response to an acute salt load [126]. However, the novel aspect of this study was that the degree to which these rats have an impaired ability to facilitate sodium excretion is dependent on the time of day the salt load is given. The findings from this study suggest an interaction between endothelin and the circadian clock mechanism to regulate the renal excretion of excess salt. Speed et al. expanded on this finding, showing that control rats fed a high salt diet for two weeks exhibited a phase delay of about 5½ hours in the acrophase of Bmal1, along with a suppression of Cry1 and Per2, specifically in the inner medulla but not the cortex [76]. In ETB deficient rats, the phase delay in Bmal1 expression was not seen [76], suggesting a possible contribution of clock genes to the delayed natriuretic response seen in the ETB deficient rat model [126]. Several studies have examined the influence of Per1 on the endothelin axis in various tissues including the kidney [125,128]. Stow et al. examined the contributions of Per1 to the control of BP and renal Na+ transport and found that Per1KO mice displayed elevated levels of ET-1 gene expression, suggesting that Per1 negatively regulates ET-1 [125]. In a study by Richards et al., mice heterozygous for Per1 having a 50% reduction in Per1 expression had similar levels of ET receptor expression compared to wild-type controls [128]. This includes tissues taken from the cortex and inner medulla of the kidney, as well as heart, liver and lung at noon and midnight (the middle of the animal's inactive and inactive periods, respectively). Interestingly, ETB receptor expression was significantly higher at midnight in the inner medulla and at noon in the cortex consistent with increased excretion of Na+ during period of higher Na+ intake. ETA receptor expression appeared to be higher at ZT6 in the inner medulla, but this difference was not statistically significant [128]. In the renal cortex, ETA receptor expression does not appear to show a diurnal rhythm in wild type or Per1 heterozygous KO mice. The reduction in Per1 did not appear to significantly alter the expression of either receptor at either time point [128]. It is possible that while a 50% reduction was sufficient to observe an increase in ET-1 peptide levels, a complete knockout of Per1 may be necessary to find differences in ET receptor expression at different times of the day. Studies examining whether Per2 and Per3 regulate ET-1 are lacking and warrant future examination.
Fig. 5.
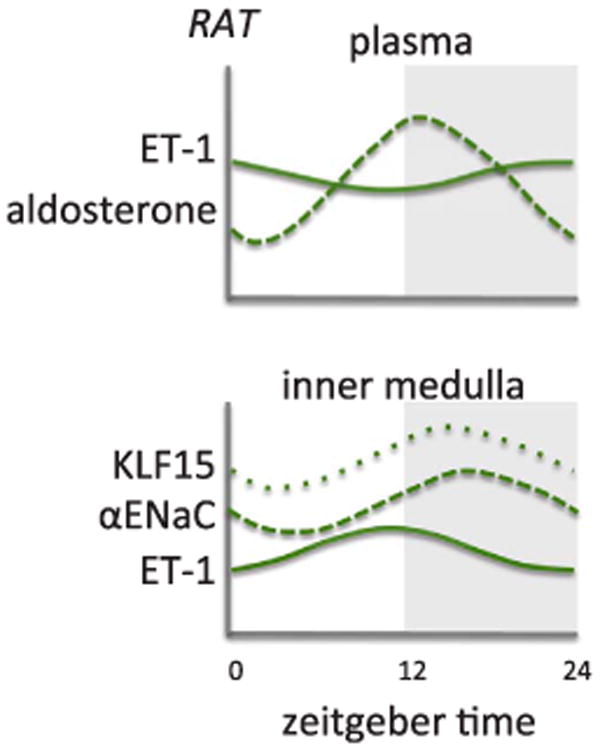
Approximation of plasma ET-1 (endothelin-1) and aldosterone from control rats (upper panel) and mRNA expression in inner medulla from control rats (lower panel). KLF15, Krüppel-like factor 15; αENaC, alpha subunit of the epithelial sodium channel; Adapted from Speed et al. [76].
3.6. Erythropoietin
Erythropoietin (EPO) is a circulating hormone that is mainly responsible for the production of red blood cells, a process known as erythropoiesis. The kidneys are the main organs that produce EPO, stimulating production of the hormone when the renal system senses either a low supply or a high demand of oxygen in the blood [129,130]. In animal models, EPO has been shown to protect against ischemia-reperfusion injury and have anti-apoptotic effects [131,132]. Clinically, EPO is used to treat the anemia associated with end-stage renal failure and chemotherapy [133,134].
In terms of circadian variation of circulating EPO, Wide et al. reported that serum EPO concentrations peak on average around 8 p.m. in hospitalized men and women from Uppsala University Hospital [135]. Despite having various illnesses, these patients had EPO levels within the reference range for healthy individuals at 8 a.m. or 12 p.m. While the study in Uppsala was performed at an elevation of about 15 m above sea level, Cristancho et al. reported EPO rhythms in men and women living at least 3 years in of Bogotá, Columbia, with an elevation more than 2600 m over sea level. EPO peaked in both genders late in the night around 4 a.m. [136]. While these studies provide potential evidence that the rhythm of circulating EPO may change at different elevations, more studies involving different populations at varying elevations are necessary to confirm these findings.
Bozek et al. examined the potential regulation of the EPO gene (Epo) by the circadian clock. Epo appears to have an E-Box binding domain in human neuronal precursor cells, but the presence of Bmal1 and CLOCK had no effect on Epo activity [137]. Per2-null mice had elevated levels of Epo in the liver when measured at ZT1 and ZT13, possibly mitigating the effects of the shortened erythrocyte life span seen in these mice [138]. More work is required to elucidate how the molecular clock may regulate Epo, and to determine how similar this regulation is across different tissues.
3.7. Atrial natriuretic peptide
Atrial natriuretic peptide (ANP), also known as atrial natriuretic factor, is derived from the atrial muscle of the heart and has potent diuretic and natriuretic effects in the kidney [139]. In response to increased atrial stretch or pressure caused by increased central blood volume, ANP inhibits collecting duct Na+ reabsorption through a cGMP-dependent mechanism, but can also cause vasodilation and increase GFR thus reducing renin release and circulating Ang II [140]. ANP has extrarenal effects as well, decreasing aldosterone production in the adrenal gland and acting as an antagonist to vasoconstriction in the vascular smooth muscle [140,141]. Clinically, ANP has been used to treat heart diseases, such as heart failure and acute myocardial infarction [142–145].
Numerous studies that have sought to examine the circadian variation in plasma ANP levels paint a somewhat complicated picture of the rhythm of circulating ANP. In humans, Donckier et al. and Portaluppi et al. both reported that plasma ANP has a diurnal rhythm that peaks at 4 a.m. and troughs in the early evening between 6 and 8 p.m. [146,147]. Rittig et al. noted a diurnal variation in plasma ANP levels as well, however the normal individuals in this study peaked at midnight and showed a dip at 4 a.m. [148]. Leppäluoto and Ruskoaho noted that plasma ANP levels displayed peak concentration levels at midnight and a nadir at 4 a.m., although the levels in this study did not appear to have a strong diurnal rhythm [149]. Richards et al. reported a diurnal change in plasma ANP concentration, with the levels in these subjects peaking in the middle of the afternoon and dipping in the early evening [150]. The study by Hartter et al. showed that in elderly patients below the age of 65, a rhythm in plasma ANP concentration was not seen [151]. Some of these studies may show similar results, but there does not appear to be a clear consensus of the time of day the rhythm of plasma ANP peaks and troughs. The varying populations, ages and genders used in the studies listed above make this hormone's rhythmicity even harder to gauge. In animals, there has been little examination of the circadian variation of ANP. Male Wistar rats display low plasma ANP levels between ZT1-ZT5 and significant peak in plasma ANP at ZT13, possibly as an anticipatory response to increased feeding and activity [152]. In atrial samples collected from C57Bl/6 mice, there does not appear to be a circadian rhythm in ANP mRNA under normal light-dark conditions. Under constant darkness however, there was a significant oscillation, peaking at circadian time (CT) 16 [153]. To our knowledge, there does not appear to be any evidence of the circadian clock regulating ANP levels or vice-versa, prompting the need for further analysis into this hormone in basic research.
4. Pathologies associated with impaired circadian rhythms
At this point, considerable evidence has been provided that different cell types within the kidney exert their physiological functions in a circadian or diurnal manner, but also that the mechanisms behind some of these functions involve the renal molecular clock. Various studies discussed above have provided great insight into the rhythmic activity in renal function under normal conditions. Unfortunately, disrupted rhythmic activity is a phenotype often seen in numerous cardiovascular diseases [82,154], and so it is tempting to speculate that disorders involving the kidney have a similar pathogenesis that may be related to the renal molecular clock (Table 2). These pathologies include obvious problems such as hypertension, but may likely include many others as examined further in the following section.
Table 2.
Summary of circadian impact on kidney-related diseases.
| Pathology | Prevalence | Influence of Circadian Rhythms/Clock Genes |
|---|---|---|
| Hypertension | 1 in 3 in the United States, over 1 billion worldwide [155] | |
| Chronic Kidney Disease (CKD) | ∼1 in 10 people worldwide [160] | |
| Diabetes Mellitus | Over 30 million people in the United States [175] | |
| Monosymptomatic Nocturnal Enuresis | Affects ∼10% of young children and ∼1% of adults [188–190] |
BP: Blood Pressure; 5/6Nx: 5/6 nephrectomy; Dbp: D site albumin promoter binding protein; Na+: sodium; LD: 12-h/12-h light/dark (LD) cycle; DD: constant darkness.
4.1. Hypertension
Hypertension, or high BP, affects 1 in 3 adults in the United States and over a billion people worldwide [155]. By definition, hypertension is been typically diagnosed as a higher than normal blood pressure that is measured during a typical office visit during the day. This simple definition is currently being redefined to include forms such as non-dipping blood pressure, sometimes referred to as nocturnal hypertension, where blood pressure remains elevated specifically during the night or inactive period. Since the non-dipping phenotype was first recognized in hypertensive patients nearly 30 years ago [80], studies have consistently reported a greater prevalence for cardiovascular complications in hypertensive individuals that have non-dipping BP compared to those with maintained diurnal BP rhythms [156–158]. Because of this, the use of ambulatory blood pressure monitoring (ABPM) to measure blood pressure throughout the day has proven to be a very useful tool for the prognosis of cardiovascular complications associated with non-dipping BP in hypertension [79,159]. As noted earlier in this review, a molecular link between the circadian clock and hypertension was noted by Doi et al., who found that Cry-null mice display a hypertensive phenotype that is salt-sensitive [86]. However, most of the clock gene knockout mice display a fairly normal day-night difference in blood pressure and primarily lower blood pressures suggesting a fairly high level of redundancy among the genetic controllers of circadian blood pressure rhythms [43,55,125]. The lack of a rhythm in the Bmal1 KO mouse is reflected in a failure of blood pressure to rise during the active phase [83]. Bmal1 contribution to the blood pressure rhythm does not appear to be related to activity within renin-secreting cells or renal tubular cells [28,43], but there are no other clues at this time to explain the loss of BP rhythm in the Bmal1 KO mice.
4.2. Chronic kidney disease
Chronic kidney disease (CKD) affects about 1 in 10 people worldwide [160]. Diabetes and hypertension are the leading causes of CKD, but interestingly, the loss of normal BP rhythms in individuals with CKD has been well documented and may be a contributing factor for CKD progression [124,161,162]. The association and prevalence of non-dipping BP with a decline in renal function has also been previously reported [163–165], suggesting an impairment of the circadian clock mechanism in patients with the disease. Mojón et al. published a multicenter study known as the Hygia project, which examined the prognostic value of ABPM in a Spanish population [166]. They found that a higher percentage of hypertensive patients with CKD had a non-dipping BP compared to hypertensive patients without CKD, and that the prevalence of the ‘riser’ BP pattern (rise in sleep-time BP compared to awake BP) increased from about 8% of patients with stage 1 CKD to nearly 35% of patients with end-stage renal disease (ESRD) [166]. Agarwal et al. highlighted the use of ABPM as a predictor of ESRD compared to clinic BP measurements in patients with CKD [167].
There have been a few animal studies that explore the effects of CKD in relation to the molecular clock outside of the kidney. Using an animal model of CKD produced by 5/6 nephrectomy (5/6Nx), Hamamura et al. recently suggested that expression of clock-controlled gene Dbp might alleviate the aggravation of renal dysfunction in CKD by restoring hepatic function, specifically its ability to metabolize retinol [168]. Hsu et al. found elevated expression levels of Per1 and Per2 mRNA at different times of the day in the hypothalamus of rats that had undergone 5/6Nx along with altered sleep activity patterns [169].
In humans, recent studies have examined the association between sleep quality and renal function in CKD patients. As part of the Chronic Renal Insufficiency Cohort (CRIC) study, Knutson et al. reported worse objective sleep quality was associated with a lower estimated GFR (eGFR) and higher urinary protein to creatinine ratio [170]. In addition, a shorter duration of sleep and later timing of sleep was associated with a lower eGFR Similar studies involving participants from the Chinese Kailuan cohort [171] and Korean Kangbuk Samsung Health Study [172] also found an association between poor sleep quality and reduced renal function. Given that sleep disturbances are a common problem in dialysis patients [173], and that disturbances in circadian timekeeping have been observed in the peripheral tissues of patients on dialysis as well [174], these studies suggest a possible dyssynchrony between central and peripheral clocks in the body and require further analysis of the circadian clock mechanism in CKD patients to reveal new clinical avenues to treat the disease and its symptoms.
4.3. Diabetes mellitus/diabetic nephropathy
Over 30 million people in the United States are believed to have diabetes, and high blood sugar levels can damage the kidneys over time, causing CKD [175]. Proteinuria has long been thought of as a sign of impending cardiovascular mortality in diabetics, but is not always predictive of future outcomes [176]. In recent years, the role of circadian rhythms in this disease has grown in interest. A retrospective study of English patients by Sturrock et al. found a non-dipping BP phenotype was associated with an increased rate of mortality in both type 1 and 2 diabetic patients compared to those with normal circadian variation in BP [177]. Additional studies have shown that impaired circadian rhythms in blood pressure can be seen prior to further renal damage in patients with either type 1 [178,179] or type 2 diabetes [180]. It can be hypothesized that inappropriately timed elevations in arterial pressure can elevate glomerular capillary pressures and increase the likelihood of elevated albumin filtration and barotrauma that would exacerbate the development of albuminuria. Given that albuminuria in diabetes mellitus patients worsens as patients progress to end-stage renal disease, the case has been made for monitoring nocturnal blood pressure using ABPM as a predictor for the progression of albuminuria, or for the risk of cardiovascular events associated with diabetes [181–185].
Using a mouse model of type 1 diabetes induced by streptozotocin (STZ), Oishi et al. found an increase in the temporal levels of Per1 and a decrease in the levels of Per2 in the kidneys of STZ-injected mice [186]. In db/db mice, a model of type 2 diabetes, the expression profiles of Per1, Rev-erbα and Dbp were either increased or decreased compared to controls [187]. Both of these studies provide some insight into which clock genes may be playing a role in regulating kidney function in diabetes. A recent study by Solocinski et al. has shown that Per1 regulates the expression levels of SGLT1, but not SGLT2, in the proximal tubule of 129/sv mice and human proximal tubule (HK-2) cells [27], suggesting that Per1 regulates glucose transport in the kidney. In the diabetic state, the up regulation of Per1 seen in both models of diabetes [186,187] may be contributing to the renal damage at the transcriptional level. The aforementioned studies involved male mice, so it would be important to see if there are any possible sex differences in how the renal clock is disturbed in diabetes.
4.4. Nocturnal enuresis & nocturnal polyuria
Monosymptomatic nocturnal enuresis, or bedwetting, is characterized by involuntary urination at night and affects about 10% of young children and about 1% of adults [188–190]. The main symptom, nocturnal polyuria, is a syndrome that is characterized by an increase in nocturnal urine output and an increase in voiding frequency at night [191], which is not the same as nocturnal enuresis since bedwetting does not always occur. Individuals with nocturnal polyuria often have relatively normal 24-h urine output totals due to decreased urine output during the day. The rhythm of AVP in the plasma is also disrupted in individuals with increased nocturnal urine output, although this effect is more visible in men than in women [191]. Nocturnal polyuria is one of the factors that can increase the prevalence of nocturia, a symptom defined as waking up at night to void, with each voiding period preceded and followed by sleep [192]. The overall risk for this symptom increases with age, however it occurs more in women at a younger age and men at an older age [193,194]. That, in addition to nocturia occurring more frequently in minorities compared to white individuals [194,195], emphasizes the importance for understanding the underlying associations of these risks with impaired rhythms in renal function.
De Gutchenaere et al. noted that children with nocturnal enuresis and nocturnal polyuria have an absent circadian rhythm in GFR, along with abnormal rhythms in sodium excretion and diuresis [196]. Raes et al. reported that loss of diurnal rhythms in urine volume are linked to reduced Na+ excretion due to increased Na+ reabsorption during the daytime in the proximal and distal tubules [197]. A recent study by Dossche et al. proposed that nocturnal polyuria, whether it be in patients with nonmonosymptomatic or monosymptomatic nocturnal enuresis, resulted in diminished circadian rhythms of GFR and solute excretion [198].
Impaired circadian rhythms of renal water and solute handling suggest that the regulatory control by the molecular clock has become disrupted. Noh et al. showed that dual Per1-/-/Per2-/- mice lost their daily variation in urine excreted in light-dark conditions after placing them in constant darkness [199]. In addition these mice excreted more urine over a 24-h period compared to wild-type mice. Zuber et al. found that spot urine samples taken from mice deficient in clock have elevated fractional excretion during the mouse's inactive period (ZT2) compared to a sample taken during the active period (ZT12) [55], a phenotype also seen in children with nocturnal enuresis [197]. When placed in metabolic cages, only 24-h urine samples were collected, but clock-deficient mice excreted more urine during the 24-h period compared to controls [55]. There are other components of the urinary system that have shown evidence of circadian regulation, such as the bladder. The reader is referred to other reviews that delve into how the circadian clock interacts with the lower urinary system in more detail [199,200].
5. Chronotherapy and the kidney
Up to this point in this review we have presented research on how the circadian clock interacts with the renal system, both by examining how it is involved in everyday renal function and by exploring the dysfunction or pathologies that may result when the system has been disrupted. In the latter case, impaired circadian or diurnal rhythms in renal function often lead to more severe cardiovascular risks, which can be costly in terms of treatment. While these diseases are problematic and can impact a large number of individuals around the world, rhythmic physiological activity has also provided great prognostic value in tracking the progression of future cardiovascular events, especially through the use of ABPM methods to monitor BP throughout the day. Being able to identify abnormal rhythms in BP using ABPM is expected to enhance clinical therapy used to treat the diseases, but has not been accepted as standard of care.
Timing the treatment of medications to an individual's biological clock is believed to be very useful for treating hypertension. Administration of just one antihypertensive medication before bedtime as opposed to all medications in the morning has been shown to reduce the prevalence of non-dipping BP in patients with resistant hypertension [201]. Unfortunately, it is not clear whether there was a similar distribution of specific medications in the morning versus bedtime dosing. A study by Ernst et al. went further to show that chlorthalidone, a thiazide diuretic, was more effective in lowering BP compared to another thiazide diuretic, hydrochlorothiazide (HCTZ), due to its ability to lower nighttime BP. This reduction was apparent using ABPM and not clinic BP measurements [202]. Reducing blood pressure at night by taking one antihypertensive medication before bedtime has been shown to reduce cardiovascular risk in patients with uncontrolled hypertension [203] and in patients with CKD [204,205]. In addition to the thiazide diuretics listed above, several other types of BP-lowering drugs were randomized in these studies (angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and other diuretics), emphasizing the importance of using nighttime BP as a target for therapeutic intervention in addition to the type of BP medication. This time of day difference in the efficacy of BP-lowering medications suggests a potential link to the renal circadian clock, but the evidence at this point is scarce. To our knowledge, the only evidence linking the circadian clock in the kidney with drug pharmacokinetics is the study by Nikolaeva et al. in which the effectiveness of the loop diuretic furosemide was examined in mice with a nephron-specific knockout of Bmal1 [28]. In these mice, the furosemide-induced natriuretic response was reduced, along with the urinary excretion of the diuretic [28]. More studies are necessary to further elucidate the link between the renal circadian clock and the drug efficacy at the basic science level, along with chronotherapy studies involving broader populations and demographics, in order to reveal better and more personalized therapeutic targets for patients at the clinical level.
The beneficial effects of nighttime dosing with anti-hypertensive drugs can be a cost-effective way of delaying the onset of further renal damage, but what about those with renal failure? Fortunately, chronotherapy has been shown to play a significant role in treating patients on hemodialysis as well. A group of nephrologists from Humber River Regional Hospital and St. Michael's Hospital within the University of Toronto, along with Toronto General Hospital, have provided a wealth of knowledge comparing the effects of nocturnal hemodialysis (NHD) versus conventional hemodialysis (CHD) using patients recruited from these hospitals [206–210]. NHD provides about eight hours of renal replacement therapy while the patient sleeps, 5–7 times a week. The use of NHD has been shown to reduce BP, total peripheral resistance, plasma norepinephrine and endothelium-dependent vasodilation after two months of treatment [209], and serum phosphate levels after six months of treatment [206]. NHD has even been shown to reduce the frequency of sleep apnea in chronic renal failure patients [207] and improve ejection fraction in patients with coexisting ESRD and congestive heart failure [208]. In addition to improving left ventricular mass, researchers from the University of Calgary, the University of Alberta and the Alberta Kidney Disease Network found that NHD improved patient-reported quality of life in patients recruited from both universities. [211]. Unfortunately, we do not know to what extent circadian factors versus other aspects of NHD, such as duration and frequency of treatment, contribute to the improved outcomes. Furthermore, significant benefits of NHD compared to conventional hemodialysis have not always been observed [212,213]. More work needs to be done to elucidate these disparate findings.
6. Conclusion & perspectives
In the past decade, the topic of circadian rhythms has moved forward from the shadows into the light (no pun intended) as part of the meaningful conversation in both biomedical research and our society. At the basic science level, more researchers realize the importance of accounting for the time of day in which they perform their experiments. This can simply be considering the time of day in which their genes or physiological parameters of interest reach their peak and nadir and applying this concept to their experimental protocols. In the clinic, it has become increasingly important to factor in time of administration of pharmacological intervention to optimize drug effectiveness, or even when best to take certain diagnostic measurements. At the individual level, timing of when to eat a meal could significantly affect the rate at which the meal is absorbed, metabolized and excreted [126,214,215]. As a result, gaining more knowledge about how our biological rhythms play a role in our daily lives can provide clues to help us understand what factors may be at play when our rhythms are disrupted and the health risk associated with those disruptions. With regards to the kidney, understanding the physiological significance of the interaction between the kidney and the circadian clock allows us to expand and redefine what we know about kidney function as well as provide valuable insight into future experimental designs. As we have shown throughout this review, the work performed by many scientists around the world allows us to appreciate the inner workings of the kidney and its effects on other organs. It is clear that circadian physiology will be important for solving the some of the unanswered questions regarding control of renal function and provide a strong foundation for more research in the future.
Acknowledgments
The authors have been supported by National Heart, Lung, and Blood Institute grants P01 HL069999, P01 HL95499, P01 HL136267, and American Heart Association grant 15SFRN2390002 to DMP. A National Institute of General Medicine Sciences T32 (GM008111) and an AHA pre-doctoral fellowship (15PRE25560074) have supported JGJ.
Abbreviations
- 3ß-HSD
3ß-hydroxysteroid dehydrogenase-isomerase
- ABPM
ambulatory blood pressure monitoring
- Ang II
angiotensin II
- ARB
angiotensin receptor blocker
- AT1
angiotensin receptor type 1
- AT2
angiotensin receptor type 2
- ACE
angiotensin-converting enzyme
- Agt
angiotensinogen
- ADH
anti-diuretic hormone
- AQP2
aquaporin 2
- AVP
arginine vasopressin
- ARNTL or Bmal1
aryl hydrocarbon receptor nuclear translocator-like protein 1
- BP
blood pressure
- CCB
calcium channel blocker
- CK1δ/ε
casein kinase 1 isoforms δ/ε
- CKD
chronic kidney disease
- CRIC
chronic renal insufficiency cohort
- CNT
connecting tubule
- CHD
conventional hemodialysis
- CCD
cortical collecting duct
- Cry
cryptochrome
- DCT
distal convoluted tubule
- ERPF
effective renal plasma flow
- ESRD
end-stage renal disease
- ET-1
endothelin-1
- ENaC
epithelial sodium channel
- EPO
erythropoietin
- ERß
estrogen receptor ß
- GFR
glomerular filtration rate
- GC
glucocorticoids
- HK-2 cells
human kidney-2/human proximal tubule
- HCTZ
hydrochlorothiazide
- IMCD
inner medullary collecting duct
- NKCC2
Na+-K+–2Cl- co-transporter
- NCC
Na+Cl- co-transporter
- NHD
nocturnal hemodialysis
- OAT3
organic anion transporter 3
- OMCD
outer medullary collecting duct
- Per
period
- PRA
plasma renin activity
- ROMK
renal outer medullary K+
- Ren-2
renin gene
- ROR
retinoic acid-related orphan receptor, renin-angiotensin-aldosterone system (RAAS)
- SGLT2
sodium-glucose co-transporter 2
- NHE3
sodium-hydrogen exchanger 3
- STZ
streptozotocin
- SCN
suprachiasmatic nucleus
- TAL
thick ascending limb
- ZT
zeitgeber time
Footnotes
Conflicts of interest: The authors declare no conflicts of interest, financial or otherwise
References
- 1.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills JN, Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117(1):22–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Mills JN, Stanbury SW. Intrinsic diurnal rhythm in urinary electrolyte output. J Physiol. 1951;115(1):18–19. [PubMed] [Google Scholar]
- 4.Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: independence from feeding and activity patterns. Am J Physiol. 1977;232(2):F128–F135. doi: 10.1152/ajprenal.1977.232.2.F128. [DOI] [PubMed] [Google Scholar]
- 5.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20(5):391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 6.Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA. 2011;108(39):16451–16456. doi: 10.1073/pnas.1107178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, Kiuchi R, Ishida M, Ukai-Tadenuma M, Minami Y, Kito R, Nakao K, Kishimoto W, Yoo SH, Shimomura K, Takao T, Takano A, Kojima T, Nagai K, Sakaki Y, Takahashi JS, Ueda HR. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106(37):15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Investig. 2009;119(8):2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltra-tion in the rat. J Clin Investig. 1971;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak MR, Quaggin SE, Hoenig MP, Dworkin LD. The glomerulus: the sphere of influence. Clin J Am Soc Nephrol. 2014;9(8):1461–1469. doi: 10.2215/CJN.09400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci. 1989;77(1):105–111. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 12.Koopman MG, Koomen GC, van Acker BA, Arisz L. Urinary sodium excretion in patients with nephrotic syndrome, and its circadian variation. Q J Med. 1994;87(2):109–117. [PubMed] [Google Scholar]
- 13.Koopman MG, Koomen GC, van Acker BA, Arisz L. Circadian rhythm in glomerular transport of macromolecules through large pores and shunt pathway. Kidney Int. 1996;49(5):1242–1249. doi: 10.1038/ki.1996.178. [DOI] [PubMed] [Google Scholar]
- 14.Huang XM, Chen WL, Yuan JP, Yang YH, Mei QH, Huang LX. Altered diurnal variation and localization of clock proteins in the remnant kidney of 5/6 nephrectomy rats. Nephrology. 2013;18(8):555–562. doi: 10.1111/nep.12111. [DOI] [PubMed] [Google Scholar]
- 15.Tucker BJ, Blantz RC. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978;235(2):F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- 16.Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol. 2008;19(12):2272–2275. doi: 10.1681/ASN.2007121326. [DOI] [PubMed] [Google Scholar]
- 17.Haberle DA, von Baeyer H. Characteristics of glomerulotubular balance. Am J Physiol. 1983;244(4):F355–F366. doi: 10.1152/ajprenal.1983.244.4.F355. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol. 1999;277(2 Pt 2):F298–F302. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 19.Alexander RT, Dimke H, Cordat E. Proximal tubular NHEs: sodium, protons and calcium? Am J Physiol Ren Physiol. 2013;305(3):F229–F236. doi: 10.1152/ajprenal.00065.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Ren Physiol. 2001;281(4):F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 21.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal re-absorptive mechanism for D-glucose. J Clin Investig. 1994;93(1):397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose co-transporter. Re-evaluation of function and distribution of expression. J Biol Chem. 1994;269(16):12032–12039. [PubMed] [Google Scholar]
- 23.Steinmetz PR, Eisinger RP. Influence of posture and diurnal rhythm on the renal excretion of acid: observations in normal and adrenalectomized subjects. Metabolism. 1966;15(1):76–87. doi: 10.1016/0026-0495(66)90012-6. [DOI] [PubMed] [Google Scholar]
- 24.Stanbury SW, Thomson AE. Diurnal variation in electrolyte excretion. Clin Sci. 1951;10(3):267–293. [PubMed] [Google Scholar]
- 25.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na+/H+ exchanger NHE3 in the kidney. Kidney Int. 2005;67(4):1410–1419. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang RY, Mou LJ, Li XM, Li XW, Qin Y. Temporally relationship between renal local clock system and circadian rhythm of the water electrolyte excretion. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37(6):698–704. doi: 10.3881/j.issn.1000-503X.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Ren Physiol. 2015;309(11):F933–F942. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, Tokonami N, Firsov D. Nephron-specific deletion of circadian clock gene bmal1 alters the plasma and renal metabolome and impairs drug disposition. J Am Soc Nephrol. 2016;27(10):2997–3004. doi: 10.1681/ASN.2015091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokko JP. Sodium chloride and water transport in the descending limb of Henle. J Clin Investig. 1970;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959;196(4):927–936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- 31.Burg MB. Tubular chloride transport and the mode of action of some diuretics. Kidney Int. 1976;9(2):189–197. doi: 10.1038/ki.1976.20. [DOI] [PubMed] [Google Scholar]
- 32.Graugaard-Jensen C, Hvistendahl GM, Frokiaer J, Bie P, Djurhuus JC. The influence of high and low levels of estrogen on diurnal urine regulation in young women. BMC Urol. 2008;8:16. doi: 10.1186/1471-2490-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrier E, Demazieres A, Girard N, Pross N, Osbild D, Metzger D, Guelinckx I, Klein A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol. 2013;113(8):2143–2151. doi: 10.1007/s00421-013-2649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamperis K, Hansen MN, Hagstroem S, Hvistendahl G, Djurhuus JC, Rittig S. The circadian rhythm of urine production, and urinary vasopressin and prostaglandin E2 excretion in healthy children. J Urol. 2004;171(6 Pt 2):2571–2575. doi: 10.1097/01.ju.0000110421.71910.c0. [DOI] [PubMed] [Google Scholar]
- 35.Mills JN. Diurnal rhythm in urine flow. J Physiol. 1951;113(4):528–536. doi: 10.1113/jphysiol.1951.sp004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara M, Minami Y, Ohashi M, Tsuchiya Y, Kusaba T, Tamagaki K, Koike N, Umemura Y, Inokawa H, Yagita K. Robust circadian clock oscillation and osmotic rhythms in inner medulla reflecting cortico-medullary osmotic gradient rhythm in rodent kidney. Sci Rep. 2017;7(1):7306. doi: 10.1038/s41598-017-07767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41(Database issue):D1009–D1013. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishinaga H, Komatsu R, Doi M, Fustin JM, Yamada H, Okura R, Yamaguchi Y, Matsuo M, Emoto N, Okamura H. Circadian expression of the Na +/H+ exchanger NHE3 in the mouse renal medulla. Biomed Res. 2009;30(2):87–93. doi: 10.2220/biomedres.30.87. [DOI] [PubMed] [Google Scholar]
- 39.Krid H, Dorison A, Salhi A, Cheval L, Crambert G. Expression profile of nuclear receptors along male mouse nephron segments reveals a link between ERRbeta and thick ascending limb function. PLoS One. 2012;7(3):e34223. doi: 10.1371/journal.pone.0034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R794–R799. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 41.Sharma PK, Thakur MK. Estrogen receptor alpha expression in mice kidney shows sex differences during aging. Biogerontology. 2004;5(6):375–381. doi: 10.1007/s10522-004-3191-6. [DOI] [PubMed] [Google Scholar]
- 42.Sabolic I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflug Arch. 2007;455(3):397–429. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 43.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, Firsov D. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol. 2014;25(7):1430–1439. doi: 10.1681/ASN.2013060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol. 1998;9(8):1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 45.Roy A, Al-bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10(2):305–324. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol. 2015;10(1):135–146. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellison DH, Velazquez H, Wright FS. Thiazide-sensitive sodium chloride co-transport in early distal tubule. Am J Physiol. 1987;253(3 Pt 2):F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 48.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 49.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC) Pflug Arch. 2009;458(1):111–135. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 50.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frokiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Ren Physiol. 2001;280(6):F1093–F1106. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- 51.Mistry AC, Wynne BM, Yu L, Tomilin V, Yue Q, Zhou Y, Al-Khalili O, Mallick R, Cai H, Alli AA, Ko B, Mattheyses A, Bao HF, Pochynyuk O, Theilig F, Eaton DC, Hoover RS. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem J. 2016;473(19):3237–3252. doi: 10.1042/BCJ20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13(4):836–847. doi: 10.1681/ASN.V134836. [DOI] [PubMed] [Google Scholar]
- 53.Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Ren Physiol. 2003;284(4):F628–F643. doi: 10.1152/ajprenal.00217.2002. [DOI] [PubMed] [Google Scholar]
- 54.Wynne BM, Mistry AC, Al-Khalili O, Mallick R, Theilig F, Eaton DC, Hoover RS. Aldosterone modulates the association between NCC and ENaC. Sci Rep. 2017;7(1):4149. doi: 10.1038/s41598-017-03510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA. 2009;106(38):16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castagna A, Pizzolo F, Chiecchi L, Morandini F, Channavajjhala SK, Guarini P, Salvagno G, Olivieri O. Circadian exosomal expression of renal thiazide-sensitive NaCl cotransporter (NCC) and prostasin in healthy individuals. Proteom Clin Appl. 2015;9(5–6):623–629. doi: 10.1002/prca.201400198. [DOI] [PubMed] [Google Scholar]
- 57.Susa K, Sohara E, Isobe K, Chiga M, Rai T, Sasaki S, Uchida S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem Biophys Res Commun. 2012;427(4):743–747. doi: 10.1016/j.bbrc.2012.09.130. [DOI] [PubMed] [Google Scholar]
- 58.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9(12):2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivy JR, Oosthuyzen W, Peltz TS, Howarth AR, Hunter RW, Dhaun N, Al-Dujaili EA, Webb DJ, Dear JW, Flatman PW, Bailey MA. Glucocorticoids induce nondipping blood pressure by activating the thiazide-sensitive cotransporter. Hypertension. 2016;67(5):1029–1037. doi: 10.1161/HYPERTENSIONAHA.115.06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem. 2014;289(17):11791–11806. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garty H, Palmer LG. Epithelial sodium channels: function. Struct Regul, Physiol Rev. 1997;77(2):359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 62.Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflug Arch. 2009;458(1):157–168. doi: 10.1007/s00424-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossier BC. 1996 Homer Smith Award Lecture. Cum grano salis: the epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8(6):980–992. doi: 10.1681/ASN.V86980. [DOI] [PubMed] [Google Scholar]
- 64.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28(3):145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Evans JA. Collective timekeeping among cells of the master circadian clock. J Endocrinol. 2016;230(1):R27–R49. doi: 10.1530/JOE-16-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Ren Physiol. 2015;309(4):F280–F299. doi: 10.1152/ajprenal.00093.2015. [DOI] [PubMed] [Google Scholar]
- 67.Voisin DL, Bourque CW. Integration of sodium and osmosensory signals in vasopressin neurons. Trends Neurosci. 2002;25(4):199–205. doi: 10.1016/s0166-2236(02)02142-2. [DOI] [PubMed] [Google Scholar]
- 68.George CP, Messerli FH, Genest J, Nowaczynski W, Boucher R, Kuchel Orofo-Oftega M. Diurnal variation of plasma vasopressin in man. J Clin Endocrinol Metab. 1975;41(2):332–338. doi: 10.1210/jcem-41-2-332. [DOI] [PubMed] [Google Scholar]
- 69.Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124(2):143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, Honma S, Sakurai T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015;85(5):1103–1116. doi: 10.1016/j.neuron.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaENaC expression by the circadian clock protein period 1 in mpkCCD(c14) cells. Biochim Biophys Acta. 2010;1799(9):622–629. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richards J, Jeffers LA, All SC, Cheng KY, Gumz ML. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of alphaENaC in renal cortical collecting duct cells. Front Physiol. 2013;4:253. doi: 10.3389/fphys.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1delta/epsilon. Am J Physiol Ren Physiol. 2012;303(7):F918–F927. doi: 10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valenti G, Laera A, Pace G, Aceto G, Lospalluti ML, Penza R, Selvaggi FP, Chiozza ML, Svelto M. Urinary aquaporin 2 and calciuria correlate with the severity of enuresis in children. J Am Soc Nephrol. 2000;11(10):1873–1881. doi: 10.1681/ASN.V11101873. [DOI] [PubMed] [Google Scholar]
- 75.Sasaki S, Ohmoto Y, Mori T, Iwata F, Muraguchi M. Daily variance of urinary excretion of AQP2 determined by sandwich ELISA method. Clin Exp Nephrol. 2012;16(3):406–410. doi: 10.1007/s10157-011-0574-2. [DOI] [PubMed] [Google Scholar]
- 76.Speed JS, Hyndman KA, Roth KJ, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, Pollock DM. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol. 2018;314(1):F89–F98. doi: 10.1152/ajprenal.00028.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in longterm regulation and in hypertension. Am J Med. 1972;52(5):584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 78.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1(8068):795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 79.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 80.O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet. 1988;2(8607):397. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 81.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens. 2010;19(1):51–58. doi: 10.1097/MNH.0b013e3283336ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104(9):3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Investig. 2015;125(1):324–336. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pati P, Fulton DJ, Bagi Z, Chen F, Wang Y, Kitchens J, Cassis LA, Stepp DW, Rudic RD. Low-salt diet and circadian dysfunction synergize to induce angiotensin II-dependent. Hypertens Mice Hypertens. 2016;67(3):661–668. doi: 10.1161/HYPERTENSIONAHA.115.06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 87.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice, American journal of physiology. Ren Physiol. 2012;302(10):F1293–F1299. doi: 10.1152/ajprenal.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guyton AC, Coleman TG, Young DB, Lohmeier TE, DeClue JW. Salt balance and long-term blood pressure control. Annu Rev Med. 1980;31:15–27. doi: 10.1146/annurev.me.31.020180.000311. [DOI] [PubMed] [Google Scholar]
- 90.Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239(3):F271–F280. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- 91.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hurwitz S, Cohen RJ, Williams GH. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol. 2004;96(4):1406–1414. doi: 10.1152/japplphysiol.00611.2003. [DOI] [PubMed] [Google Scholar]
- 93.Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Investig. 1966;45(10):1587–1592. doi: 10.1172/JCI105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344(6266):541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 95.Lemmer B, Mattes A, Bohm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension. 1993;22(1):97–101. doi: 10.1161/01.hyp.22.1.97. [DOI] [PubMed] [Google Scholar]
- 96.Herichova I, Mravec B, Stebelova K, Krizanova O, Jurkovicova D, Kvetnansky R, Zeman M. Rhythmic clock gene expression in heart, kidney and some brain nuclei involved in blood pressure control in hypertensive TGR(mREN-2)27 rats. Mol Cell Biochem. 2007;296(1–2):25–34. doi: 10.1007/s11010-006-9294-4. [DOI] [PubMed] [Google Scholar]
- 97.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultra-structural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46(6):1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 98.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61(2):579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishijima Y, Kobori H, Kaifu K, Mizushige T, Hara T, Nishiyama A, Kohno M. Circadian rhythm of plasma and urinary angiotensinogen in healthy volunteers and in patients with chronic kidney disease. J Renin Angiotensin Aldosterone Syst. 2014;15(4):505–508. doi: 10.1177/1470320314557584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 101.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277(3 Pt 2):F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 102.Carey RM. Newly discovered components and actions of the renin-angiotensin system. Hypertension. 2013;62(5):818–822. doi: 10.1161/HYPERTENSIONAHA.113.01111. [DOI] [PubMed] [Google Scholar]
- 103.Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Investig. 1973;31(4):363–365. doi: 10.3109/00365517309084318. [DOI] [PubMed] [Google Scholar]
- 104.Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res. 2017;40(5):413–422. doi: 10.1038/hr.2016.166. [DOI] [PubMed] [Google Scholar]
- 105.Tsujino T, Naito Y, Kawasaki D, Okuda S, Sakoda T, Fujioka Y, Sugaya T, Ohyanagi M. Circadian expression of plasminogen activator inhibitor-1 in angiotensin II type 1a receptor knockout mice. Clin Exp Hypertens. 2005;27(2–3):159–168. [PubMed] [Google Scholar]
- 106.Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18(7):955–961. doi: 10.1097/00004872-200018070-00018. [DOI] [PubMed] [Google Scholar]
- 107.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na + transport. J Membr Biol. 2001;184(3):313–319. doi: 10.1007/s00232-001-0098-x. [DOI] [PubMed] [Google Scholar]
- 108.Rittig S, Matthiesen TB, Pedersen EB, Djurhuus JC. Circadian variation of angiotensin II and aldosterone in nocturnal enuresis: relationship to arterial blood pressure and urine output. J Urol. 2006;176(2):774–780. doi: 10.1016/S0022-5347(06)00594-5. [DOI] [PubMed] [Google Scholar]
- 109.Williams GH, Cain JP, Dluhy RG, Underwood RH. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Investig. 1972;51(7):1731–1742. doi: 10.1172/JCI106974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Ren Physiol. 2013;305(12):F1697–F1704. doi: 10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu C, Chen Y, Kang Y, Ni Z, Xiu H, Guan J, Liu K. Glucocorticoids improve renal responsiveness to atrial natriuretic peptide by up-regulating natriuretic peptide receptor-A expression in the renal inner medullary collecting duct in de-compensated heart failure. J Pharmacol Exp Ther. 2011;339(1):203–209. doi: 10.1124/jpet.111.184796. [DOI] [PubMed] [Google Scholar]
- 113.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 114.Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, Ishida N. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12(3):191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 115.Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC. Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1031–R1047. doi: 10.1152/ajpregu.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 117.Guignard MM, Pesquies PC, Serrurier BD, Merino DB, Reinberg AE. Circadian rhythms in plasma levels of cortisol, dehydroepiandrosterone, delta 4-androstenedione, testosterone and dihydrotestosterone of healthy young men. Acta Endocrinol. 1980;94(4):536–545. doi: 10.1530/acta.0.0940536. [DOI] [PubMed] [Google Scholar]
- 118.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91(1):1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maguire JJ, Davenport AP. Endothelin receptors and their antagonists. Semin Nephrol. 2015;35(2):125–136. doi: 10.1016/j.semnephrol.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Speed JS, Fox BM, Johnston JG, Pollock DM. Endothelin and renal ion and water transport. Semin Nephrol. 2015;35(2):137–144. doi: 10.1016/j.semnephrol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension. 2009;53(2):324–330. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ET(A) and ET(B) receptors in the mouse cortical collecting duct. Am J Physiol Ren Physiol. 2013;305(4):F568–F573. doi: 10.1152/ajprenal.00613.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Unique endothelin receptor binding in kidneys of ETB receptor deficient rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R674–R681. doi: 10.1152/ajpregu.00589.2002. [DOI] [PubMed] [Google Scholar]
- 124.Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, Kimmitt RA, Brown KE, Kennedy ED, Goddard J, Webb DJ. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension. 2014;64(2):296–304. doi: 10.1161/HYPERTENSIONAHA.114.03533. [DOI] [PubMed] [Google Scholar]
- 125.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59(6):1151–1156. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnston JG, Speed JS, Jin C, Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Ren Physiol. 2016;311(5):F991–F998. doi: 10.1152/ajprenal.00103.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Richards J, Welch AK, Barilovits SJ, All S, Cheng KY, Wingo CS, Cain BD, Gumz ML. Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sci. 2014;118(2):255–262. doi: 10.1016/j.lfs.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacobson LO, Goldwasser E, Fried W, Plzak L. Role of the kidney in erythropoiesis. Nature. 1957;179(4560):633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- 130.Adamson JW, Eschbach J, Finch CA. The kidney and erythropoiesis. Am J Med. 1968;44(5):725–733. doi: 10.1016/0002-9343(68)90254-4. [DOI] [PubMed] [Google Scholar]
- 131.Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18(6):1497–1506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- 132.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA. 2003;100(8):4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2(8517):1175–1178. doi: 10.1016/s0140-6736(86)92192-6. [DOI] [PubMed] [Google Scholar]
- 134.Rizzo JD, Lichtin AE, Woolf SH, Seidenfeld J, Bennett CL, Cella D, Djulbegovic B, Goode MJ, Jakubowski AA, Lee SJ, Miller CB, Rarick MU, Regan DH, Browman GP, Gordon MS. H. American Society of Clinical Oncology, American society of, use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American society of clinical oncology and the american society of hematology. J Clin Oncol. 2002;20(19):4083–4107. doi: 10.1200/JCO.2002.07.177. [DOI] [PubMed] [Google Scholar]
- 135.Wide L, Bengtsson C, Birgegard G. Circadian rhythm of erythropoietin in human serum. Br J Haematol. 1989;72(1):85–90. doi: 10.1111/j.1365-2141.1989.tb07657.x. [DOI] [PubMed] [Google Scholar]
- 136.Cristancho E, Riveros A, Sanchez A, Penuela O, Boning D. Diurnal changes of arterial oxygen saturation and erythropoietin concentration in male and female highlanders. Physiol Rep. 2016;4(17) doi: 10.14814/phy2.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bozek K, Relogio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of clock-controlled genes in mammals. PLoS One. 2009;4(3):e4882. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun Q, Zhao Y, Yang Y, Yang X, Li M, Xu X, Wen D, Wang J, Zhang J. Loss of the clock protein PER2 shortens the erythrocyte life span in mice. J Biol Chem. 2017;292(30):12679–12690. doi: 10.1074/jbc.M117.783985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 140.Maack T. Role of atrial natriuretic factor in volume control. Kidney Int. 1996;49(6):1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 141.Maack T, Marion DN, Camargo MJ, Kleinert HD, Laragh JH, Vaughan ED, Jr, Atlas SA. Effects of auriculin (atrial natriuretic factor) on blood pressure, renal function, and the renin-aldosterone system in dogs. Am J Med. 1984;77(6):1069–1075. doi: 10.1016/0002-9343(84)90190-6. [DOI] [PubMed] [Google Scholar]
- 142.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, Horie H, Ohnishi M, Kinoshita M. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2001;37(7):1820–1826. doi: 10.1016/s0735-1097(01)01233-5. [DOI] [PubMed] [Google Scholar]
- 143.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S J.W. investigators. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370(9597):1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 144.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J. 2005;69(3):283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 145.Nomura F, Kurobe N, Mori Y, Hikita A, Kawai M, Suwa M, Okutani Y. Multicenter prospective investigation on efficacy and safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: carperitide effects observed through monitoring dyspnea in acute de-compensated heart failure Study. Circ J. 2008;72(11):1777–1786. doi: 10.1253/circj.cj-07-0760. [DOI] [PubMed] [Google Scholar]
- 146.Donckier J, Anderson JV, Yeo T, Bloom SR. Diurnal rhythm in the plasma concentration of atrial natriuretic peptide. N Engl J Med. 1986;315(11):710–711. doi: 10.1056/NEJM198609113151114. [DOI] [PubMed] [Google Scholar]
- 147.Portaluppi F, Bagni B, degli Uberti E, Montanari L, Cavallini R, Trasforini G, Margutti A, Ferlini M, Zanella M, Parti M. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. J Hypertens. 1990;8(1):85–95. doi: 10.1097/00004872-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 148.Rittig S, Knudsen UB, Norgaard JP, Gregersen H, Pedersen EB, Djurhuus JC. Diurnal variation of plasma atrial natriuretic peptide in normals and patients with enuresis nocturna. Scand J Clin Lab Invest. 1991;51(2):209–217. doi: 10.1080/00365519109091109. [DOI] [PubMed] [Google Scholar]
- 149.Leppaluoto J, Ruskoaho H. Atrial natriuretic peptide, renin activity, aldosterone, urine volume and electrolytes during a 24-h sleep-wake cycle in man. Acta Physiol Scand. 1990;139(1):47–53. doi: 10.1111/j.1748-1716.1990.tb08896.x. [DOI] [PubMed] [Google Scholar]
- 150.Richards AM, Tonolo G, Fraser R, Morton JJ, Leckie BJ, Ball SG, Robertson JI. Diurnal change in plasma atrial natriuretic peptide concentrations. Clin Sci. 1987;73(5):489–495. doi: 10.1042/cs0730489. [DOI] [PubMed] [Google Scholar]
- 151.Hartter E, Kurz R, Woloszczuk W, Petzl DH. Circadian variation and age dependence of human atrial natriuretic peptide levels in hospitalized patients. Horm Metab Res. 1987;19(10):490–492. doi: 10.1055/s-2007-1011860. [DOI] [PubMed] [Google Scholar]
- 152.Oliveira MH, Antunes-Rodrigues J, Leal AM, Elias LL, Moreira AC. Circadian variations of plasma atrial natriuretic peptide and corticosterone in rats with continuous or restricted access to food. Life Sci. 1993;53(24):1795–1801. doi: 10.1016/0024-3205(93)90487-n. [DOI] [PubMed] [Google Scholar]
- 153.Goetze JP, Georg B, Jorgensen HL, Fahrenkrug J. Chamber-dependent circa-dian expression of cardiac natriuretic peptides. Regul Pept. 2010;160(1–3):140–145. doi: 10.1016/j.regpep.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18(3):563–569. doi: 10.1093/ndt/18.3.563. [DOI] [PubMed] [Google Scholar]
- 155.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yano Y, Bakris GL, Matsushita K, Hoshide S, Shimada K, Kario K. Both chronic kidney disease and nocturnal blood pressure associate with strokes in the elderly. Am J Nephrol. 2013;38(3):195–203. doi: 10.1159/000354232. [DOI] [PubMed] [Google Scholar]
- 157.Ayala DE, Hermida RC, Chayan L, Mojon A, Fontao MJ, Fernandez JR. Circadian pattern of ambulatory blood pressure in untreated hypertensive patients with and without metabolic syndrome. Chronobiol Int. 2009;26(6):1189–1205. doi: 10.3109/07420520903206294. [DOI] [PubMed] [Google Scholar]
- 158.Hermida RC, Chayan L, Ayala DE, Mojon A, Dominguez MJ, Fontao MJ, Soler R, Alonso I, Fernandez JR. Association of metabolic syndrome and blood pressure nondipping profile in untreated hypertension. Am J Hypertens. 2009;22(3):307–313. doi: 10.1038/ajh.2008.358. [DOI] [PubMed] [Google Scholar]
- 159.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 160.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 161.Portaluppi F, Montanari L, Massari M, Di Chiara V, Capanna M. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens. 1991;4(1 Pt 1):20–26. doi: 10.1093/ajh/4.1.20. [DOI] [PubMed] [Google Scholar]
- 162.Portaluppi F, Montanari L, Ferlini M, Gilli P. Altered circadian rhythms of blood pressure and heart rate in non-hemodialysis chronic renal failure. Chronobiol Int. 1990;7(4):321–327. [PubMed] [Google Scholar]
- 163.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166(8):846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 164.Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12(11):2301–2307. doi: 10.1093/ndt/12.11.2301. [DOI] [PubMed] [Google Scholar]
- 165.Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant. 2009;24(8):2400–2406. doi: 10.1093/ndt/gfp074. [DOI] [PubMed] [Google Scholar]
- 166.Mojon A, Ayala DE, Pineiro L, Otero A, Crespo JJ, Moya A, Boveda J, de Lis JP, Fernandez JR, Hermida RC, Hygia Project I. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1–2):145–158. doi: 10.3109/07420528.2012.703083. [DOI] [PubMed] [Google Scholar]
- 167.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 168.Hamamura K, Matsunaga N, Ikeda E, Kondo H, Ikeyama H, Tokushige K, Itcho K, Furuichi Y, Yoshida Y, Matsuda M, Yasuda K, Doi A, Yokota Y, Amamoto T, Aramaki H, Irino Y, Koyanagi S, Ohdo S. Alterations of Hepatic Metabolism in Chronic Kidney Disease via D-box-binding Protein Aggravate the Renal Dysfunction. J Biol Chem. 2016;291(10):4913–4927. doi: 10.1074/jbc.M115.696930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hsu CY, Chang FC, Ng HY, Kuo CC, Lee YT, Lu CY, Lee CT. Disrupted circadian rhythm in rats with nephrectomy-induced chronic kidney disease. Life Sci. 2012;91(3–4):127–131. doi: 10.1016/j.lfs.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 170.Knutson KL, Lash J, Ricardo AC, Herdegen J, Thornton JD, Rahman M, Turek N, Cohan J, Appel LJ, Bazzano LA, Tamura MK, Steigerwalt SP, Weir MR, Van Cauter E, Cric Study I. Habitual sleep and kidney function in chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Sleep Res. 2017 doi: 10.1111/jsr.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, Curhan G, Wu S, Gao X. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol. 2017;12(6):885–892. doi: 10.2215/CJN.09270816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim CW, Chang Y, Sung E, Yun KE, Jung HS, Ko BJ, Kwon MJ, Hyun YY, Lee KB, Kim H, Shin H, Ryu S. Sleep duration and quality in relation to chronic kidney disease and glomerular hyperfiltration in healthy men and women. PLoS One. 2017;12(4):e0175298. doi: 10.1371/journal.pone.0175298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Parker KP. Sleep disturbances in dialysis patients. Sleep Med Rev. 2003;7(2):131–143. doi: 10.1053/smrv.2001.0240. [DOI] [PubMed] [Google Scholar]
- 174.Russcher M, Chaves I, Lech K, Koch BC, Nagtegaal JE, Dorsman KF, Jong A, Kayser M, van Faassen HM, Kema IP, van der Horst GT, Gaillard CA. An observational study on disturbed peripheral circadian rhythms in hemodialysis patients. Chronobiol Int. 2015;32(6):848–857. doi: 10.3109/07420528.2015.1048868. [DOI] [PubMed] [Google Scholar]
- 175.CDC. National Diabetes Statistics Report, 2017. [Accessed 20 September 2017];2017 https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. 2017.
- 176.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294(6588):1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17(5):360–364. doi: 10.1046/j.1464-5491.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 178.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347(11):797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 179.Voros P, Lengyel Z, Nagy V, Nemeth C, Rosivall L, Kammerer L. Diurnal blood pressure variation and albuminuria in normotensive patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13(9):2257–2260. doi: 10.1093/ndt/13.9.2257. [DOI] [PubMed] [Google Scholar]
- 180.Felicio JS, de Souza AC, Kohlmann N, Kohlmann O, Jr, Ribeiro AB, Zanella MT. Nocturnal blood pressure fall as predictor of diabetic nephropathy in hypertensive patients with type 2 diabetes. Cardiovasc Diabetol. 2010;9:36. doi: 10.1186/1475-2840-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Palmas W, Pickering T, Teresi J, Schwartz JE, Eguchi K, Field L, Weinstock RS, Shea S. Nocturnal blood pressure elevation predicts progression of albuminuria in elderly people with type 2 diabetes. J Clin Hypertens. 2008;10(1):12–20. doi: 10.1111/j.1524-6175.2007.07170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lurbe E, Redon J, Pascual JM, Tacons J, Alvarez V. The spectrum of circadian blood pressure changes in type I diabetic patients. J Hypertens. 2001;19(8):1421–1428. doi: 10.1097/00004872-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 183.Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47(9):1501–1506. doi: 10.2337/diabetes.47.9.1501. [DOI] [PubMed] [Google Scholar]
- 184.Knudsen ST, Laugesen E, Hansen KW, Bek T, Mogensen CE, Poulsen PL. Ambulatory pulse pressure, decreased nocturnal blood pressure reduction and progression of nephropathy in type 2 diabetic patients. Diabetologia. 2009;52(4):698–704. doi: 10.1007/s00125-009-1262-6. [DOI] [PubMed] [Google Scholar]
- 185.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21(4):443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oishi K, Kasamatsu M, Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun. 2004;317(2):330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 187.Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2012;302(3):H621–H633. doi: 10.1152/ajpheart.00825.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeung CK, Sihoe JD, Sit FK, Bower W, Sreedhar B, Lau J. Characteristics of primary nocturnal enuresis in adults: an epidemiological study. BJU Int. 2004;93(3):341–345. doi: 10.1111/j.1464-410x.2003.04612.x. [DOI] [PubMed] [Google Scholar]
- 189.Yeung CK, Sreedhar B, Sihoe JD, Sit FK, Lau J. Differences in characteristics of nocturnal enuresis between children and adolescents: a critical appraisal from a large epidemiological study. BJU Int. 2006;97(5):1069–1073. doi: 10.1111/j.1464-410X.2006.06074.x. [DOI] [PubMed] [Google Scholar]
- 190.Butler RJ, Golding J, Northstone K, Team AS. Nocturnal enuresis at 7.5 years old: prevalence and analysis of clinical signs. BJU Int. 2005;96(3):404–410. doi: 10.1111/j.1464-410X.2005.05640.x. [DOI] [PubMed] [Google Scholar]
- 191.Asplund R. The nocturnal polyuria syndrome (NPS) Gen Pharmacol. 1995;26(6):1203–1209. doi: 10.1016/0306-3623(94)00310-j. [DOI] [PubMed] [Google Scholar]
- 192.Johnson TM. Nocturia: Clinical presentation, evaluation, and management in adults. 2017 https://www.uptodate.com/
- 193.Tikkinen KA, Tammela TL, Huhtala H, Auvinen A. Is nocturia equally common among men and women? A population based study in Finland. J Urol. 2006;175(2):596–600. doi: 10.1016/S0022-5347(05)00245-4. [DOI] [PubMed] [Google Scholar]
- 194.Fitzgerald MP, Litman HJ, Link CL, McKinlay JB, Investigators BS. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. 2007;177(4):1385–1389. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 195.Markland AD, Vaughan CP, Johnson TM, 2nd, Goode PS, Redden DT, Burgio KL. Prevalence of nocturia in United States men: results from the National Health and Nutrition Examination Survey. J Urol. 2011;185(3):998–1002. doi: 10.1016/j.juro.2010.10.083. [DOI] [PubMed] [Google Scholar]
- 196.De Guchtenaere A, Vande Walle C, Van Sintjan P, Raes A, Donckerwolcke R, Van Laecke E, Hoebeke P, Vande Walle J. Nocturnal polyuria is related to absent circadian rhythm of glomerular filtration rate. J Urol. 2007;178(6):2626–2629. doi: 10.1016/j.juro.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 197.Raes A, Dehoorne J, Hoebeke P, Van Laecke E, Donckerwolcke R, Vande Walle J. Abnormal circadian rhythm of diuresis or nocturnal polyuria in a subgroup of children with enuresis and hypercalciuria is related to increased sodium retention during daytime. J Urol. 2006;176(3):1147–1151. doi: 10.1016/j.juro.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 198.Dossche L, Raes A, Hoebeke P, De Bruyne P, Vande Walle J. Circadian rhythm of glomerular filtration and solute handling related to nocturnal enuresis. J Urol. 2016;195(1):162–167. doi: 10.1016/j.juro.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 199.Noh JY, Han DH, Yoon JA, Kim MH, Kim SE, Ko IG, Kim KH, Kim CJ, Cho S. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J. 2011;15(2):64–73. doi: 10.5213/inj.2011.15.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Negoro H, Kanematsu A, Yoshimura K, Ogawa O. Chronobiology of micturition: putative role of the circadian clock. J Urol. 2013;190(3):843–849. doi: 10.1016/j.juro.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 201.Hermida RC, Ayala DE, Fernandez JR, Calvo C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension. 2008;51(1):69–76. doi: 10.1161/HYPERTENSIONAHA.107.096933. [DOI] [PubMed] [Google Scholar]
- 202.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47(3):352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 203.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58(11):1165–1173. doi: 10.1016/j.jacc.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 204.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of anti-hypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22(12):2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Minutolo R, Gabbai FB, Borrelli S, Scigliano R, Trucillo P, Baldanza D, Laurino S, Mascia S, Conte G, De Nicola L. Changing the timing of anti-hypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis. 2007;50(6):908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 206.Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53(5):1399–1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 207.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 208.Chan C, Floras JS, Miller JA, Pierratos A. Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant. 2002;17(8):1518–1521. doi: 10.1093/ndt/17.8.1518. [DOI] [PubMed] [Google Scholar]
- 209.Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension. 2003;42(5):925–931. doi: 10.1161/01.HYP.0000097605.35343.64. [DOI] [PubMed] [Google Scholar]
- 210.McCormick BB, Pierratos A, Fenton S, Jain V, Zaltzman J, Chan CT. Review of clinical outcomes in nocturnal haemodialysis patients after renal transplantation. Nephrol Dial Transplant. 2004;19(3):714–719. doi: 10.1093/ndt/gfg582. [DOI] [PubMed] [Google Scholar]
- 211.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. J Am Med Assoc. 2007;298(11):1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 212.Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, Klarenbach S N. Alberta Kidney Disease. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int. 2009;75(5):542–549. doi: 10.1038/ki.2008.639. [DOI] [PubMed] [Google Scholar]
- 213.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, G. Frequent Hemodialysis Network Trial. Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, Mc Grath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network Nocturnal trial. Kidney Int. 2011;80(10):1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161(1):84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 215.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under Caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 2017;26(1):267–277 (e2). doi: 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]