Abstract
Textbooks teach us that the removal of sensory input to sensory cortex, for example, following arm amputation, results in massive reorganisation in the adult brain. In this opinion article, we critically examine evidence for functional reorganisation of sensory cortical representations, focusing on the sequelae of arm amputation on somatosensory topographies. Based on literature from human and non-human primates, we conclude that the cortical representation of the limb remains remarkably stable despite the loss of its main peripheral input. Furthermore, the purportedly massive reorganisation results primarily from the formation or potentiation of new pathways in subcortical structures and does not produce novel functional sensory representations. We discuss the implications of the stability of sensory representations on the development of upper-limb neuroprostheses.
Plasticity in Sensory Cortical Topographies
One of the key concepts in contemporary neuroscience is that experience shapes the central nervous system throughout life. The ability of the brain to adaptively change how it processes inputs based on new experience is termed ‘plasticity’ and underlies our ability to mature, learn new skills, and recover from injury. Our current understanding of neuroplasticity has been moulded by the work of Hubel and Wiesel [1–3] in the 1960s, who studied the visual cortex of cats following temporary occlusion of visual input from one eye. They found that input loss to one eye in early development drives profound physiological and behavioural changes: neurons in the visual cortex normally devoted to the occluded eye respond to input from the non-occluded eye. Accordingly, when forced to rely on the previously occluded eye, the kittens showed profound visual impairments. This line of research demonstrated the brain’s extraordinary capacity for change: Loss of primary input to a brain area does not lead to the abolishment of processing but rather to a reassignment of processing, resulting in increased functional representation of an alternative input. This process, termed cortical reorganisation, is perhaps the most extreme form of brain plasticity. According to these early studies, however, reorganisation is much more restricted in the adult brain: adult cats subjected to visual occlusion did not exhibit the same deficits and cortical changes as did kittens [3] (see [4] and [5] for related evidence in monkeys and humans; see [6] for current debate on the adult’s visual cortex capacity for reorganisation).
Perhaps the most striking example of the adult brain’s capacity to reorganise comes from electrophysiological studies of primary somatosensory cortex (SI) after the loss of peripheral input (e.g., as a result of limb amputation). A well-known characteristic of SI in intact individuals is the well-defined topographic map of the body – so-called somatotopic organisation – with neighbouring neurons responding to adjacent and overlapping regions of the body [7] (Figure 1A). Removal of input from a body part (due to amputation [8] or nerve transection [9]) results in changes in the somatotopic organisation, such that the representation of cortically adjacent body parts seems to take over the ‘freed up’ brain territory (see [10] for a review of similar results from the barrel cortex of rodents). When input is lost from the entire hand and arm, for example, the cortical territory of the missing hand begins to respond to the lower part of the face, resulting in what appears to be massive reorganisation, sometimes spanning half of the sensory homunculus [11,12] (see [13] for review) (Figure 1B).
Figure 1. Somatosensory Pathways and the Basic Phenomenon of Remapping.
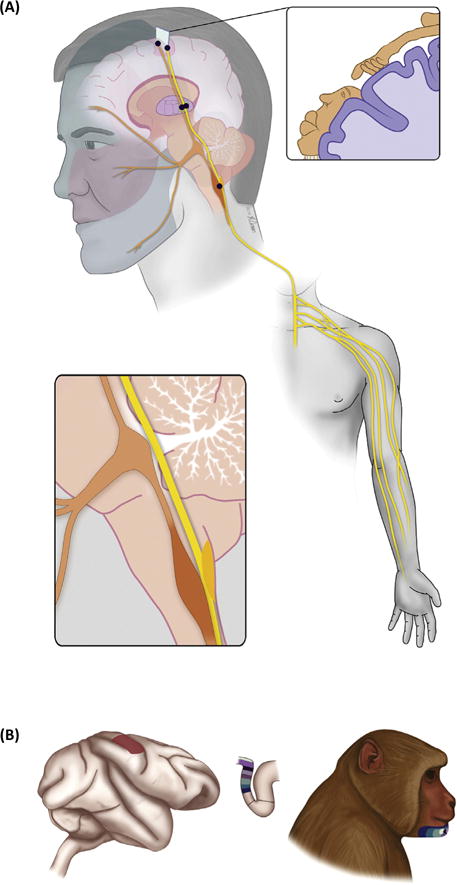
(A) Diagram of the somatosensory pathways from the limb (yellow) and face (orange) to primary somatosensory cortex. The somatosensory nerves from the limb synapse onto the cuneate nucleus, located in the brainstem, which then sends projections to the ventroposterior lateral nucleus of the thalamus, which in turn projects to primary somatosensory cortex. The somatosensory nerves from the face project to the trigeminal nucleus, also in the brainstem, which then projects to the ventroposterior medial nucleus of the thalamus, then to cortex. The primary somatosensory cortex comprises a complete map of the body, where adjacent body parts are represented in adjacent patches of cortex (with some necessary discontinuities, see cartoon in top right inset). In S1 of monkeys, the hand representation borders the lower part of the face. (B) Following arm deafferentation, the cortical territory of the (deafferented) limb becomes responsive to stimulation of the lower face. Adapted, with permission from AAAS, from [11].
These observations have led to the conclusion that even the adult brain has the potential to reorganise under the right circumstances. In this opinion article, we examine the nature of this apparent reorganisation. Do the invading body representations benefit from this additional neuronal territory? What are the perceptual consequences of this reorganisation? What is its neural basis? We bring together behavioural, imaging, and electrophysiological studies investigating the consequences of limb amputation. We highlight evidence showing that the previously observed reorganisation reflects the formation or potentiation of new pathways but that the original pathways are to a large extent spared. We reach the conclusion that the reorganisation in SI does not result in novel functional sensory representations and that the core topography persists despite drastic sensory input loss in adulthood. The stability of sensory topographies has important implications for ongoing efforts to restore somatosensation in upper limb neuroprostheses.
Functional Benefits of Reorganisation?
If deafferented cortex begins to process a new patch of the sensory sheet (on the retina or the skin), one would expect that the additional cortical volume would lead to perceptual gains for this ‘invading’ region (i.e., adaptive plasticity, see [14,15]). For example, SI remapping following digit amputation results in increased representation of the neighbouring digits, which in turn should lead to increased acuity for these digits [8]. Such perceptual gains would imply that signals arising to the reassigned area (e.g., missing digit territory) are processed normally in their new cortical home. However, direct perceptual gains due to input loss have not been conclusively established. For example, finger amputation in humans does not result in lower detection thresholds or improved spatial acuity on the remaining fingers [16]. Earlier reports for increased tactile acuity on the stump of amputees (see [17,18]) have been subsequently challenged (see [16,19]). Other studies showing perceptual gains following temporary experimentally induced input loss emphasise the role of concurrent sensory input from nonaffected body parts (e.g., [20,21]). In other words, previously recorded perceptual gains might be caused by behavioural adaptations and not by deprivation-triggered reorganisation. Similarly, the popular notion that cross-modal reorganisation in the visual cortex of the blind contributes to heightened tactile abilities has been recently challenged (see [71] for review). Instead, enhanced tactile perception in blind individuals can be explained by greater experience with or dependence on touch to guide interactions with objects [22]. Thus, reorganisation in adult SI does not seem to lead to any direct benefits in processing the invading sensory input.
Phantom and Referred Sensations
If remapping in SI does not result in direct perceptual gains, are there any other functional consequences to SI remapping? In other words, are these invading signals behaviourally relevant? The most extensively documented and captivating consequence relates to distorted phantom sensations following amputation. Even decades after injury, amputees report a continued sensation of the limb that is no longer there. These phantom sensations can be as vivid and as natural as the perception of one’s own body and span a range of qualities, including pressure, temperature, tingling, itch, and pain [31]. Phantom sensations can be commonly triggered through stimulation of the stump, which may simply reflect spontaneous peripheral reinnervation (see the following text). However, a more striking phenomenon that implies SI reorganisation is when phantom sensations are evoked through stimulation of the face.
In a famous series of studies [24,25,72], three amputees reported experiencing precise and stable point-to-point correspondence between touch applied on their face and referred sensation perceived on the phantom hand (see [26,27] for similar reports). Importantly, the reported referred sensations from the face to the hand were topographically organised, such that neighbouring sites on the face elicited sensations on neighbouring fingers (Figure 2A). These findings were interpreted as perceptual correlates of the face-driven activity in the limb representation that had been previously observed in electrophysiological experiments with monkeys [11]: If hand neurons in SI now respond to the face (Figure 1B), brain regions receiving input from the SI hand representation will interpret activity in this region as arising from the missing hand, resulting in dual sensations on the face and the phantom hand. Importantly, the phenomenology of face-elicited sensations referred to the missing limb is consistent with the interpretation that reorganisation is taking place in the cortex, since the cortical topography (the proximity of the face and hand representations in SI) is predictive of the perceptual remapping. The hypothesis is that, following elimination of input from the limb, lateral projections from face to limb representations either sprout or become unmasked, leading to the observed reorganisation, as demonstrated with electrophysiology and anatomical tracing [28] (see [29] for the roles of the thalamus and brainstem in driving reorganisation). Furthermore, the mismatch between invading facial inputs and residual representation of the missing hand is thought to result in an ‘error’ signal, interpreted by the brain as pain arising from the missing hand (phantom limb pain [30,23]; see [32] for a critical review).
Figure 2. Referred Sensations.
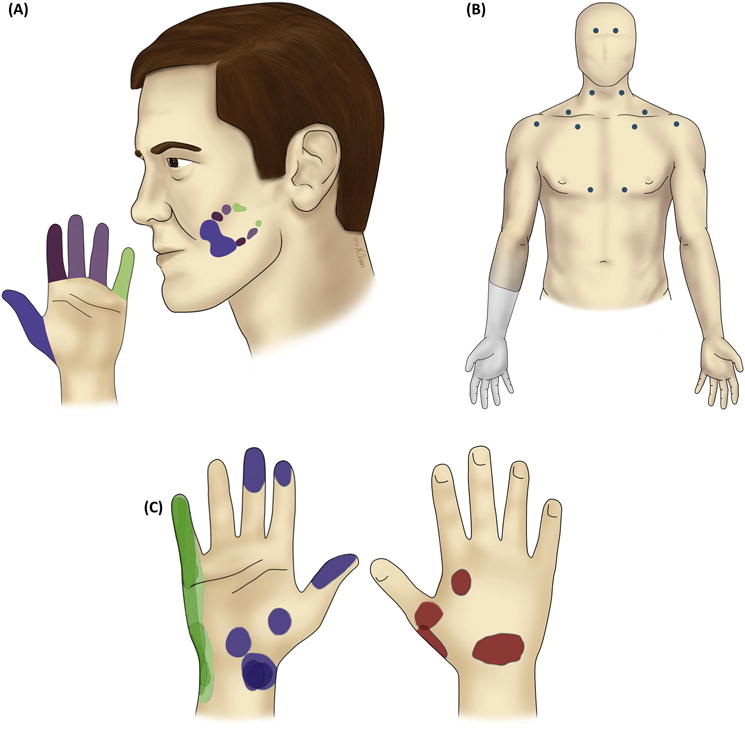
(A) In a case study with human amputees, a systematic mapping was observed between the location of the trigger region on the face and the location of the referred sensation on the left phantom hand. Adapted from [31] with permission from Oxford University Press. (B) In other studies, however, touches on many different parts of the body, many of which were not cortical neighbours of the deafferented limb, were found to evoke referred sensations on the missing limb. The blue dots denote cutaneous trigger points evoking referred sensation on the phantom (right) hand in one example participant. Adapted from [34] with permission from Oxford University Press. (C) In an amputee with a missing right hand, electrical stimulation of the residual somatosensory nerves evokes well-localised and stable percepts on the missing hand. The coloured patches indicate locations of consistent perceived sensations on the phantom hand over the course of 2 months, during stimulation through different electrodes located on the median (blue), ulnar (green), and radial (red) nerves. Adapted, with permission from AAAS, from [42].
Referred sensations following amputations received tremendous attention both in the scientific community and in the popular media [33] but some of the key findings should be interpreted with caution. Indeed, subsequent studies that used more objective approaches to characterise referred sensations found that these could be triggered by touch applied on multiple body parts (e.g., feet, chest, and neck; [34,35]) whose representations are not cortical neighbours of the hand area (Figure 2B). Referred sensations were even reported when touch was applied to body parts contralateral to the missing hand. Moreover, the mapping from the trigger region to the referred region was typically not consistent across amputees. These findings thus generally weakened the hypothesis that referred sensations result from SI reorganisation since referred sensations do not seem to respect cortical topographies.
Reorganisation in Humans
Results from neuroimaging studies in human amputees further challenge the view that neighbouring cortical representations invade the deafferented ones. While the lip representation encroaches somewhat on the limb representation following amputation, it does not annex it completely [36–38] in contrast to what is observed in electrophysiological recordings from amputated or deafferented monkeys [11,12]. Rather, the deafferented territory in human somatosensory cortex begins to respond to body regions that the amputees overuse to supplement lost hand function (mainly the intact hand), resulting in a highly idiosyncratic remapping which, again, does not necessarily involve adjacent representations in SI [39,40]. A possible explanation for the difference in reorganisation observed in humans and monkeys is that disabled monkeys (following long-term deafferentation) may use their mouths to compensate for the lost limb function more than humans do. In any case, the evidence suggests that, while cortical neighbours sometimes invade deafferented cortex, this is far from the rule. The most straightforward prediction of the cortical reorganisation hypothesis – that it will be dictated by cortical topographies – is thus violated.
Persistent Representation Despite Input Loss
A further challenge to the notion that reorganisation causes functional consequences is provided by the perceptual correlates of nerve stimulation. Numerous studies have shown that, when the residual (injured) nerve is electrically stimulated, either directly [41,42] or transcutaneously [43,44], individuals experience the evoked somatosensory percepts as vividly and clearly arising from their phantom hand (Figure 2C), and not from other body parts such as the face. In fact, stimulation of the nerve can be used to evoke quasi-naturalistic percepts that are highly localised to spatially restricted regions of the missing hand [42], as one would expect in the absence of any reorganisation. These results suggest that the pathway from somatosensory nerves to their cortical targets seems to be preserved, even years after amputation (cf. [8]). Perhaps the most striking evidence for the immutability of SI topographies despite input loss comes from cortical microstimulation studies in humans. Flesher and colleagues [45] investigated the sensory consequences of intracortical microstimulation of SI in a human tetraplegic patient. Despite the fact that the somatosensory input from the hand had been massively reduced for a decade, induced activity in the hand area resulted in vivid localised sensations on the patient’s insensate hand and never elsewhere. Thus, sensory input loss did not result in replacement of the original representation (see [46] for an analogous result obtained through stimulation of the SI surface).
Neural Basis of Reorganisation
The persistence of sensory experience despite peripheral input loss can be explained in part by nerve regeneration. Indeed, a severed sensory axon typically regrows and spontaneously reinnervates intact skin, for example, on the residual arm (see [47] for physiological review). As a result, touch applied to the reinnervated skin will produce signals that are mislabelled by the central nervous system as arising from the missing hand and result in a sensation projected to the missing hand. As might be expected, then, phantom and referred sensations can be substantially reduced if the peripheral nerve is blocked [48,49]. This phenomenon has been elegantly exploited to redirect cutaneous sensations from the hand to the chest skin of amputees to create an intuitive interface for controlling an artificial limb (targeted reinnervation, [50]). Peripheral nerve regeneration through spontaneous reinnervation but also nerve repair [51] or hand transplantation [52,53] can lead to the restoration of somatosensory input to the deafferented cortical region.
The massive functional cortical reorganisation observed in SI – with the face remapped to the deafferented limb representation – was originally thought to result from widespread sprouting of intracortical connections, as evidenced using electrophysiology and anatomical tracing [11,28]. However, evidence from recent electrophysiological and inactivation studies in monkeys suggests that much of the reorganisation following nerve injury takes place in the brainstem. Indeed, the neural activity in the deafferented limb representation in SI through face stimulation is abolished when the cuneate nucleus is inactivated [54]. This suggests that projections from the trigeminal nucleus – which receives signals from the face – to the cuneate nucleus – which receives signals from the limb – become potentiated or actually sprout after the cuneate nucleus is deafferented [55,56] (Figure 1A; see [56] for evidence of the formation of alternative somatosensory pathways). In fact, there is little anatomical evidence that the face-elicited activity in SI is mediated by the growth of new cortico-cortical projections: Very few axons cross the face–hand boundary in SI of intact animals (see [57] for analogous results in humans revealed with neuroimaging) and deafferentation of the hand region does not result in any measurable increase in these boundary-crossing projections [58]. Furthermore, inactivation of the SI face representation has no measurable effect on face-elicited activity in the deafferented limb representation [54]. These new findings resolve the seeming discrepancy between the classical evidence, showing face-related activity in the missing hand cortex of monkeys, and recent evidence in humans showing little structural and functional change following amputation.
Following deafferentation, then, two pathways can lead to activity in deafferented cortex: one pathway from the residual nerve of the missing limb (which often reinnervates the stump), and the other from the face via newly formed connections between the trigeminal and cuneate nuclei. When these two pathways are engaged simultaneously (in a former amputee with hand transplants), signals from one pathway compete with those from the other: Touching the face abolishes sensations triggered by touching the grafted hand, the sensation of which relies on the original hand pathway [59].
From this perspective on the neural basis of reorganisation, sensations referred to the missing limb by touching parts of the body whose representations are not adjacent in the cortex (Figure 2B) may reflect the somatotopic organisation within upstream structures in the somatosensory pathway, including the spinal cord, brainstem, and thalamus. Careful experimentation will be required to more extensively document this phenomenon and understand its neural basis.
Stability of Sensory Topographies in Adult Cortex
In summary, loss of input from a body region in adulthood leads to the formation or potentiation of lateral connections in the brainstem, which gives rise to a new pathway from periphery to cortex. This new pathway alone can account for the face-elicited activity in monkeys’ hand cortex, and the contribution of cortical reorganisation per se remains unconfirmed. The original pathway seems to be relatively spared as evidenced by the elicitation of sensations evoked on the amputated or insensate limb through stimulation of the peripheral nerve or somatosensory cortex. Furthermore, human imaging studies show that the representation of the missing limb, while noisier than that of an intact limb (as might be expected since it lacks its natural afferent input), is maintained in human amputees decades after amputation [36,60], as evidenced by a canonical functional hand layout [61] (Figure 3). Interestingly, hand topography is also preserved in individuals who have suffered brachial plexus injuries – which result in tearing the nerves – suggesting that the persistence of hand topography is SI does not depend on peripheral inputs. Finally, there is no evidence that the new pathway afforded by brainstem is in any way functional: the increased cortical volume has never been conclusively shown to result in functional benefits. In other words, the remapped activity described in previous studies does not result in a functional sensory representation of the remapped body part. These new pathways can thus lead to activation of deafferented cortex, but do not seem to do so in the way the original pathways did.
Figure 3. Persistent Finger Topography for the Missing Hand.
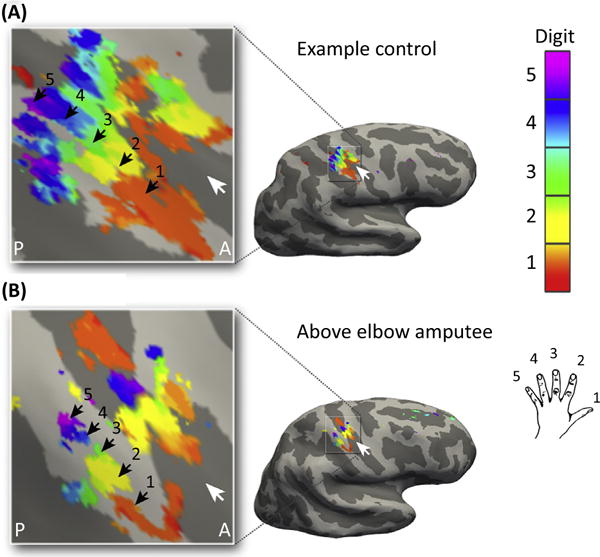
(A) Canonical hand representation in SI of a control subject with an intact hand, showing the distinct, somatotopically organised representation of the five digits. (B) Missing hand representation in an amputee 31 years after amputation, mapped during phantom finger movements. Although reduced, digit selectivity, order, and extent of the missing hand maps were similar to those observed in controls. White arrows indicate the central sulcus. A, anterior; P, posterior. © 2016. Adapted, with permission, from Kikkert, S. et al. (2016). Revealing the neural fingerprints of a missing hand. Elife 5, e15292 Published and distributed under the terms of the Creative Commons Attribution License; https://creativecommons.org/licenses/by/4.0/.
Neural Basis of Stable Cortical Representations
The stability of sensory topographies may be attributable to at least two factors. First, to form a new sensory representation requires reorganisation spanning a wide swath of cortex over which the representation is distributed in a functionally organised way, and the mechanisms of plasticity may not operate on sufficiently large spatial scales in the nervous system to allow for this. In fact, sensory topographies have been shown to be in part determined by genetically controlled patterning mechanisms intrinsic to the dorsal telencephalon, from which cortex develops [62,63]. De novo sensory representations would not have access to these mechanisms. Furthermore, to form a sensory representation, reorganisation must culminate in functional neuronal circuits that extract behaviourally relevant information from patterned input. The output of these circuits must then be appropriately read out by downstream structures. The potentiation of existing connections or even the sprouting of new ones may not systematically produce circuits that meet these requirements. Mechanisms of synaptic plasticity may thus not be well suited to form complex new sensory representations. Second, sensory representations are reciprocally connected to each other and to motor representations: SI is directly interconnected with multiple hubs in the somatosensory system (e.g., secondary somatosensory cortex) and the motor system (e.g., primary motor cortex) [64,65]. Descending input from these other cortical regions may anchor the somatotopic organisation in SI and restrict its reorganisation [66]. Input from motor cortex in particular may serve to maintain somatosensory topographies. Indeed, the evidence for persistent topography described earlier (Figure 3) was obtained by asking amputees to produce individuated finger movements with their phantom hand [60,67], which led to somatotopically appropriate patterns of activity in SI despite the absence of peripheral input [61].
Concluding Remarks and Future Perspectives for Brain Machine Interfaces
The aforementioned reinterpretation of the behavioural, imaging, and neurophysiological results implies a more nuanced view of cortical plasticity: while sensory cortices of adults are endowed with plasticity, this plasticity cannot result in the formation of completely novel representations, even under the extreme circumstance of deafferentation. To establish that aberrant activity in deprived cortex constitutes a new sensory representation of the displaced input requires causal evidence, for example, by showing that disrupting local processing in deprived cortex impairs perception [68] or that artificially activating deprived cortex systematically induces novel sensory experiences referred to the invading body region [69]. Whether large-scale reorganisation in SI can produce functional representations of the invading input remains to be established.
The development of chronically implanted electrodes arrays has opened up the possibility that intracortical microstimulation could be used as a means to restore sensation to patients who have lost it (e.g., due to deafferentation) and for whom more peripheral neural interfaces are not an option. The evidence reviewed earlier for preserved functional layout of somatosensory cortical processing opens up exciting opportunities for restoring tactile feedback following peripheral or spinal cord injury. The most straightforward strategy to restore sensation through intracortical microstimulation is to mimic natural patterns of cortical activity [70]. The idea is that the more the electrically induced neuronal activity resembles its natural counterpart, the more naturalistic the evoked sensations will be. The obvious way to attempt to produce naturalistic patterns of activity is to respect and exploit the native topographies. For example, to signal contact at some location on the body, one would stimulate neurons that responded to that part of the body before the injury. However, if those topographies are completely remapped after injury, as the classical theory of cortical reorganisation suggests, the biomimetic approach would no longer be tenable. From the standpoint of neuroprosthetics, then, the stability of cortical representations implies that exploiting native topographies in sensory cortex is an option. Conversely, reshaping these topographies – which may be necessary if the neural interface is not positioned over the distal digit representation, for example – may be challenging.
Trends.
The reorganisation of primary somatosensory cortex (SI) following arm amputation is considered a prime example of neural plasticity in the adult brain and of its consequences on altered perception.
Recent evidence from human and non-human primates shows that the reorganisation in SI does not result in novel functional sensory representations and that somatotopic organisation persists despite drastic loss of sensory input.
Perceptual reports from human subjects suggest that the loss of sensory input does not result in a replacement of the original representation: activation of the missing hand area evokes sensations referred to the missing (phantom) hand and not to the ‘invading’ body regions (e.g., the face).
The evidence for preserved somatotopy following long-term deafferentation has important implications for providing artificial touch through electrical interfaces with the nervous system.
Outstanding Questions.
The evidence suggests that, after amputation, neurons downstream from SI interpret activity in SI-deprived cortex based on preinjury somatotopic organisation. Are other preinjury receptive field properties of SI neurons – for example their feature preference – also interpreted in a stable manner after deafferentation? Is there another set of circumstances (e.g., complete input loss) that could trigger reassignment of the original functional properties of the deprived cortex in adults?
What is the role of the motor system in restricting reorganisation in SI?
How stable is somatotopic organisation if deafferentation occurs in childhood, during the critical period?
What is the functional significance of activity observed in limb cortex when the natural input is congenitally absent (e.g., in congenital limb absence)?
What factors determine which body part(s) can lead to referred sensations to the missing hand?
To what extent is aberrant activation of the missing limb area by touching another body part determined by the usage of that body part?
Acknowledgments
The authors thank Jon Kaas, Murray Sherman, Jeffrey Yau, Patrick Haggard, and Jeremy Winberry for helpful comments on a previous version of this manuscript. T.R.M. was supported by a Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant No. 104128/Z/14/Z). S.J.B. was supported by NINDS grantsR01 NS095251 and NS 095162.
References
- 1.Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- 2.Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 3.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 4.Smirnakis SM, et al. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- 5.Baseler HA, et al. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci. 2011;14:649–655. doi: 10.1038/nn.2793. [DOI] [PubMed] [Google Scholar]
- 6.Haak KV, et al. Plasticity, and its limits, in adult human primary visual cortex. Multisens Res. 2015;28:297–307. doi: 10.1163/22134808-00002496. [DOI] [PubMed] [Google Scholar]
- 7.Kaas JH, et al. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204:521–523. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- 8.Merzenich MM, et al. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 9.Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 10.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 11.Pons TP, et al. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 12.Jain N, et al. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaas JH, et al. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nava E, Röder B. Adaptation and maladaptation insights from brain plasticity. Prog Brain Res. 2011;191:177–194. doi: 10.1016/B978-0-444-53752-2.00005-9. [DOI] [PubMed] [Google Scholar]
- 15.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2009;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega-Bermudez F, Johnson KO. Spatial acuity after digit amputation. Brain. 2002;125:1256–1264. doi: 10.1093/brain/awf129. [DOI] [PubMed] [Google Scholar]
- 17.Teuber H, et al. Reorganization of sensory function in amputation stumps: two-point discrimination. Fed Proc. 1949;8:156. [Google Scholar]
- 18.Haber WB. Reactions to loss of limb: physiological and psychological aspects. Ann N Y Acad Sci. 1958;74:14–24. doi: 10.1111/j.1749-6632.1958.tb39524.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Boyle DJ, et al. Human locognosic acuity on the arm varies with explicit and implicit manipulations of attention: implications for interpreting elevated tactile acuity on an amputation stump. Neurosci Lett. 2001;305:37–40. doi: 10.1016/s0304-3940(01)01805-5. [DOI] [PubMed] [Google Scholar]
- 20.Weiss T, et al. Deafferentation of the affected arm: a method to improve rehabilitation? Stroke. 2011;42:1363–1370. doi: 10.1161/STROKEAHA.110.601138. [DOI] [PubMed] [Google Scholar]
- 21.Werhahn KJ, et al. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- 22.Grant AC, et al. Tactile perception in blind Braille readers: a psychophysical study of acuity and hyperacuity using gratings and dot patterns. Percept Psychophys. 2000;62:301–312. doi: 10.3758/bf03205550. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc Natl Acad Sci U S A. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran VS. Perceptual correlates of massive cortical reorganization. Neuroreport. 1992;3:583–586. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Halligan PW, et al. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. Neuroreport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Halligan PW, et al. Sensory disorganization and perceptual plasticity after limb amputation: a follow-up study. Neuroreport. 1994;5:1341–1345. [PubMed] [Google Scholar]
- 28.Florence SL, et al. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 29.Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 30.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran VS, Hirstein W. The perception of phantom limbs. The DO Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 32.Mezue M, Makin TR. Immutable body representations: lessons from phantoms in amputees. In: De Vignemont F, Alsmith A, editors. The Subject’s Matter. MIT Press; 2017. [Google Scholar]
- 33.Ramachandran VS, Blakeslee S. Phantoms in the Brain. Harpercollins; 2005. [Google Scholar]
- 34.Knecht S, et al. Reorganizational and perceptional changes after amputation. Brain. 1996;119:1213–1219. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 35.Grüsser SM, et al. Remote activation of referred phantom sensation and cortical reorganization in human upper extremity amputees. Exp Brain Res. 2004;154:97–102. doi: 10.1007/s00221-003-1649-4. [DOI] [PubMed] [Google Scholar]
- 36.Makin TR, et al. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun. 2013;4:1570. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makin TR, et al. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015;138:2140–2146. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raffin E, et al. Primary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limb. NeuroImage. 2016;130:134–144. doi: 10.1016/j.neuroimage.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 39.Makin TR, et al. Deprivation-related and use-dependent plasticity go hand in hand. Elife. 2013;2:e01273. doi: 10.7554/eLife.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philip BA, Frey SH. Compensatory changes accompanying chronic forced use of the nondominant hand by unilateral amputees. J Neurosci. 2014;34:3622–3631. doi: 10.1523/JNEUROSCI.3770-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhillon GS, et al. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J Hand Surg Am. 2004;29:605–615. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Tan DW, et al. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014;6:257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anani A, Körner L. Discrimination of phantom hand sensations elicited by afferent electrical nerve stimulation in below-elbow amputees. Med Prog Technol. 1979;6:131–135. [PubMed] [Google Scholar]
- 44.Ehrsson HH, et al. Upper limb amputees can be induced to experience a rubber hand as their own. Brain. 2008;131:3443–3452. doi: 10.1093/brain/awn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flesher SN, et al. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- 46.Ojemann JG, Silbergeld DL. Cortical stimulation mapping of phantom limb rolandic cortex. Case report. J Neurosurg. 1995;82:641–644. doi: 10.3171/jns.1995.82.4.0641. [DOI] [PubMed] [Google Scholar]
- 47.Devor M, et al. Phantom pain as an expression of referred and neuropathic pain. In: Sherman RA, editor. Phantom Pain. Springer; 1996. pp. 36–57. [Google Scholar]
- 48.Borghi B, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth Analg. 2010;111:1308–1315. doi: 10.1213/ANE.0b013e3181f4e848. [DOI] [PubMed] [Google Scholar]
- 49.Vaso A, et al. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155:1384–1391. doi: 10.1016/j.pain.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 50.Kuiken TA, et al. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci U S A. 2007;104:20061–20066. doi: 10.1073/pnas.0706525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wall JT, et al. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationships to sensory recovery in humans. J Neurosci. 1986;6:218–233. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giraux P, et al. Cortical reorganization in motor cortex after graft of both hands. Nat Neurosci. 2001;4:691–692. doi: 10.1038/89472. [DOI] [PubMed] [Google Scholar]
- 53.Frey SH, et al. Chronically deafferented sensory cortex recovers a grossly typical organization after allogenic hand transplantation. Curr Biol. 2008;18:1530–1534. doi: 10.1016/j.cub.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kambi N, et al. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5:3602. doi: 10.1038/ncomms4602. [DOI] [PubMed] [Google Scholar]
- 55.Jain N, et al. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci U S A. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao CC, et al. Intracortical connections are altered after long-standing deprivation of dorsal column inputs in the hand region of area 3b in squirrel monkeys. J Comp Neurol. 2016;524:1494–1526. doi: 10.1002/cne.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chand P, Jain N. Intracortical and thalamocortical connections of the hand and face representations in somatosensory area 3b of macaque monkeys and effects of chronic spinal cord injuries. J Neurosci. 2015;35:13475–13486. doi: 10.1523/JNEUROSCI.2069-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farnè A, et al. Face or hand, not both: perceptual correlates of reafferentation in a former amputee. Curr Biol. 2002;12:1342–1346. doi: 10.1016/s0960-9822(02)01018-7. [DOI] [PubMed] [Google Scholar]
- 60.Raffin E, et al. Disentangling motor execution from motor imagery with the phantom limb. Brain. 2012;135:582–595. doi: 10.1093/brain/awr337. [DOI] [PubMed] [Google Scholar]
- 61.Kikkert S, et al. Revealing the neural fingerprints of a missing hand. Elife. 2016;5:e15292. doi: 10.7554/eLife.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubenstein JLR. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- 63.Miyashita-Lin EM. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- 64.Jones EG, et al. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol. 1978;181:291–347. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- 65.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci (Regul Ed) 2011;15:97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reilly KT, et al. Persistent hand motor commands in the amputees’ brain. Brain. 2006;129:2211–2223. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- 68.Amedi A, et al. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat Neurosci. 2004;7:1266–1270. doi: 10.1038/nn1328. [DOI] [PubMed] [Google Scholar]
- 69.Stoeckel MC, et al. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc Natl Acad Sci U S A. 2009;106:2395–2400. doi: 10.1073/pnas.0803733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bensmaia SJ. Biological and bionic hands: natural neural coding and artificial perception. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140209. doi: 10.1098/rstb.2014.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Ramachandran VS, et al. Perceptual correlates of massive cortical reorganization. Science. 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]


