Abstract
Dendritic cells (DCs) are professional antigen-presenting cells responsible for the activation of specific T-cell responses and for the development of immune tolerance. Immature DCs reside in peripheral tissues and specialize in antigen capture, whereas mature DCs reside mostly in the secondary lymphoid organs where they act as antigen-presenting cells. The correct localization of DCs is strictly regulated by a large variety of chemotactic and nonchemotactic signals that include bacterial products, DAMPs (danger-associated molecular patterns), complement proteins, lipids, and chemokines. These signals function both individually and in concert, generating a complex regulatory network. This network is regulated at multiple levels through different strategies, such as synergistic interactions, proteolytic processing, and the actions of atypical chemokine receptors. Understanding this complex scenario will help to clarify the role of DCs in different pathological conditions, such as autoimmune diseases and cancers and will uncover new molecular targets for therapeutic interventions.
Introduction
The appropriate localization of dendritic cells (DCs) is a crucial step in the regulation of the immune response and plays a fundamental role in both steady-state and pathological conditions1,2. Based on developmental origin, committing transcription factors, and surface markers, DCs are classified as classical or conventional DCs (cDCs), plasmacytoid DC (pDCs), and monocyte-derived DCs (moDCs)3. DCs are at the interface of innate and acquired immunities since they sense invading pathogens, provide co-stimulatory signals, and trigger specific immune defenses4,5. In homeostatic conditions, a heterogeneous population of immature DCs with sentinel functions resides in the peripheral tissues. Upon early recognition of pathogens or exposure to inflammatory cytokines, DCs induce a tailored activation of innate and adaptive effector cells to face the pathogens. Specific subsets of DCs recruit and activate innate lymphoid cells and natural killer cells through the rapid secretion of cytokines5,6. As potent antigen-presenting cells, DCs also take up antigens and migrate to draining lymph nodes, where they promote T-cell and B-cell responses7–9. Conversely, a constitutive trafficking of DCs from noninflamed tissues to lymph nodes maintains the tolerance against self-antigens10.
DC migration is a tightly regulated process, controlled by a large variety of chemotactic factors, of which chemokines play a fundamental role11,12. Chemokines are small, secreted proteins with conserved sequences and structural features. Chemokines are classified into four families based on the relative position of a conserved cysteine motif, namely, CC, CXC, XC, and CX3C13. Chemokines can also be classified as homeostatic and inflammatory proteins, although some of them (e.g., CCL21 and CXCL12) may have both homeostatic and inflammatory functions14. Chemokines regulate migration, adhesion, phagocytosis, cytokine secretion, proliferation, and apoptosis by activating G-protein-coupled receptors (GPCR)13. In addition to the classic chemokine receptors, there is a subset of chemokine receptors that do not possess canonical signaling and that are endowed with scavenging functions. This subset of receptors is called the atypical chemokine receptors (ACKR). ACKRs are at the forefront of research for their ability to regulate the inflammatory response by different mechanisms13,15–17. This article focuses on chemokines and other chemotactic factors as key molecules for DC migration and function, with a special emphasis on the multiple levels of regulation by the chemokine system.
The chemokine system in DC biology
Most precursors of DCs leave the bone marrow and enter the circulation to localize to lymphoid and nonlymphoid tissues. In both steady-state and inflammatory conditions, resident, peripheral tissue DCs travel via the lymphatic system to draining lymph nodes, where they interact with T lymphocytes4. Human pDCs are usually found only in the circulation and in primary and secondary lymphoid organs where they are likely to localize in a CXCR4-dependent and ChemR23/CMKLR1-dependent manner. Under pathological conditions, pDCs localize to peripheral tissues, including the skin, some tumors, and atherosclerotic aortas by mechanisms that are possibly dependent on CXCR4, CXCR3, and CMKLR1 expression18,19. In mice under both homeostatic and inflammatory conditions, chemokine receptors such as CCR2, CCR5, and CCR9, regulate the migration of pDCs to lymphoid and nonlymphoid organs, such as the small intestine and skin18. To travel such different migratory routes, DCs rapidly change chemokine receptor expression to respond to the chemotactic gradient guiding them to their correct position20. A survey of chemokine receptors and their role in the migration of mouse and human DCs is shown in Tables 1 and 2, respectively.
Table 1.
The expression and functions of chemokine receptors in mouse DC subtypes
| DC subtypes | Chemokine GPCR | Major functions | References |
|---|---|---|---|
| cDC | CCR1 | Recruitment into the lungs during allergic reactions | 105 |
| CCR2 | Central tolerance | 106 | |
| CCR2, CCR6 | Migration to inflamed tissue (immature) | 107 | |
| CCR2, CX3CR1 | Positioning in the lung | 21 | |
| CCR4 | Emigration of cutaneous DCs to the lymph nodes | 39 | |
| CCR6, CCR1 | Recruitment to Peyer’s patches | 108 | |
| CCR7 | Migration to lymph nodes | 10 | |
| CX3CR1 | Migration to lymph vessels, LEC transmigration | 37 | |
| CXCR4 | Bone marrow retention (DC precursors) | 21 | |
| Cutaneous DC transmigration across LEC | 38 | ||
| CXCR5 | Th2 induction | 109 | |
| Recruitment to Peyer’s patches | 110 | ||
| XCR1 | CD8 + T-cell priming and activation | 111 | |
| Central tolerance induction | 112 | ||
| Intestinal immune homeostasis | 113 | ||
| pDC | CCR2 | Homeostatic trafficking | 114 |
| CCR2, CCR5 | Bone marrow egression | 115 | |
| CCR6, CCR10 | Recruitment to inflamed epithelia | 116 | |
| CCR9 | Homing to the small intestine | 117 | |
| Oral tolerance | 118 | ||
| Central tolerance | 119 | ||
| CXCR4 | Progenitor differentiation in bone marrow niches | 120 | |
| CXCR4, CCR7, and CXCR3 | Spleen- and HEV-mediated lymph node entry | 50,121,122 |
Table 2.
Expression and functions of chemokine receptors in human DC subtypes
| DC subtypes | Chemokine GPCR | Major functions | References |
|---|---|---|---|
| cDC | CCR2, CCR6 | Recruitment to inflamed tissues | 123 |
| 124 | |||
| CCR5 | Recruitment to inflamed tissues | 125 | |
| CXCR4, CCR7 | Recruitment to lymph node and tonsils (in vitro activated) | 47 | |
| 124 | |||
| XCR1 | Antigen cross-presentation, CD8+ T-cell priming | 126 | |
| pDC | CCR6, CCR10 | Recruitment to inflamed epithelia | 116 |
| CXCR3 | Recruitment to diseased tissue | 127 | |
| CXCR4, CCR7 | Recruitment to lymph node (in vitro activated) | 47 | |
| CXCR4 | Homeostatic recruitment to lymph node | 47 | |
| moDC | CCR1, CCR3 | Recruitment to inflamed tissues | 128 |
| 129 | |||
| CCR1, CCR5 | Recruitment to inflamed tissues | 81 |
The identities of the chemokines responsible for the egression of DC precursors from the bone marrow, as well as those that mediate their homeostatic recruitment into nonlymphoid tissues, are poorly characterized. Mice lacking CXCR4 in CD11c+ DC precursors show a significant decrease in bone marrow pre-DCs, suggesting that CXCL12 is a bone marrow retention factor21. Moreover, patients with WHIM syndrome, a genetic alteration characterized by gain-of-function mutations in CXCR4, have a reduced number of circulating DC subsets22. Once in circulation under steady-state conditions, different receptors, including CCR2 and CX3CR1, are responsible for DC localization to the lungs and other peripheral tissues21. In the skin, Langerhans cells are mainly maintained by self-renewal. However, under inflammatory conditions, they can also be replenished by bone-marrow-derived precursors23. Immature resident DCs express several chemokine receptors but it is still unclear if retention in peripheral tissues is an active or passive mechanism.
The migration of DCs to the lymph nodes is a complex process that relies on two main chemokines, namely, CCL19 and CCL21. CCL21 is important for directing DCs toward and along lymphatic vessels while CCL19 is involved, together with CCL21, in DC migration within the lymph nodes. The expression of the cognate receptor, CCR7, is crucial for the correct positioning of DCs and for the initiation of specific immune responses24,25. Under resting conditions, CCL21 is constitutively released at low levels from intracellular granules of lymphatic endothelial cells, whereas following activation, CCL21 is transcriptionally activated26,27. Secreted CCL21 binds the heparan sulfates present in the interstitium, leading to the formation of a haptotactic gradient. Once in the lymphatic system, DCs crawl along a CCL21 gradient until they reach larger vessels, where they are passively transported by the flow of lymph28. The CCL21/CCR7 axis not only promotes chemotaxis but also the arrest of DCs on the lymphatic endothelium29. In addition, DC migration can be amplified by the paracrine and autocrine secretion of inflammatory cytokines, which induce increased expression of CCR7 and its ligand CCL21 on DCs and lymphatic endothelial cells, respectively30,31. A similar mechanism is responsible for the recruitment and retention of mature DCs in Crohn’s disease32.
In lymph node sinuses, CCL19, in addition to CCL21, also contributes to DC migration24,33. In the lymph nodes, the confined expression of ACKR4 to the endothelial cells of the ceiling but not the floor of the sinus contributes to the formation of a CCL21 gradient (see below)34. The CCL21 gradient is crucial for guiding DCs to lymph node T-cell-rich areas35. In addition, CCR7-dependent DC migration coordinates the activation of organ-specific Tregs, thereby promoting peripheral immune tolerance36.
Under inflammatory conditions, CX3CL1 and CXCL12 may also play a role in DC migration. Both cytokines are expressed by activated lymphatic endothelial cells and promote DC transendothelial migration37,38. The migration of cutaneous DCs to the skin-draining lymph nodes is regulated by the inducible chemokine CCL17 and its cognate receptor CCR4, which are involved in the pathogenesis of allergic skin inflammation39. In an experimental model of autoimmune encephalomyelitis, CCL17 modulates DC trafficking in the central nervous system40.
Synergistic interactions of chemotactic factors in DC migration
The concomitant activation of multiple chemokine receptors promotes the synergistic migration of leukocyte subsets, including DCs41–43, in vitro and in vivo. Similarly, cooperative interactions between chemokines and lipid mediators, such as platelet-activating factor (PAF), arachidonic acid, prostaglandin E2 (PGE2), and leukotriene B4 (LTB4) also occur31,44–46. One of the first described cooperative interactions was of human pDCs. Circulating pDCs do not respond to CXCR3 ligands, even though they express CXCR3 at high levels47. However, CXCR3 ligands become chemotactic in the presence of low levels of the homeostatic chemokine CXCL1248. A similar cooperative interaction was observed between CXCL12 and CCR7 in vivo for the constitutive migration of pDCs to the splenic white pulp49,50. In monocyte-derived DCs, synergism between CC and CXC chemokines was described, with CCL3 synergizing with CXCL8 and CXCL12 and CCL2 synergizing with CXCL1242.
A similar cooperation was described between the classical nonchemokine chemotactic receptors and the chemokine receptors. Chemerin, a ligand for CMKLR1, increases the migration of immature DCs to CCL7 and formylated peptides synergized with CCL342. Finally, in a model of 2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity, the recruitment of DCs to the draining lymph nodes was dependent on BLT1 signaling, the high-affinity receptor for LTB4. In vitro, LTB4-stimulated DCs upregulate the expression of CCL19 and CCR7 and exhibit increased migration to CCL19 and CCL2131. The molecular mechanisms underlying these cooperative actions may involve multiple levels of action, including (a) simultaneous activation of multiple intracellular pathways42; (b) agonist heterocomplex formation51; and (c) receptor heterodimerization52. Further studies are needed to fully understand the diverse levels of complexity implicated in this action.
Chemokines as relay signals in DC migration
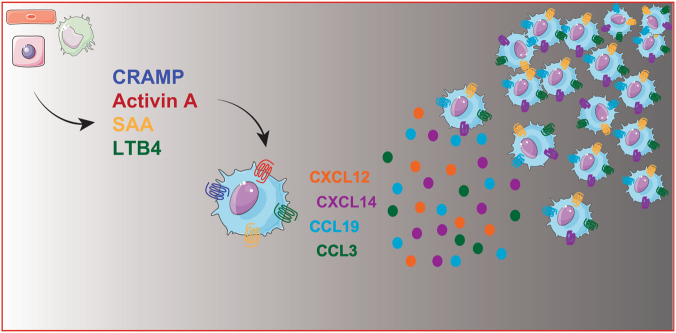
Inflammatory signals play an important role in the amplification and persistence of the inflammatory response45,53,54. Relaying signals ensure that primary chemotactic agents, produced early by the invading pathogen or by damaged cells, act locally on neighboring cells and induce the production of secondary waves of chemoattractants that enhance and promote the recruitment of cells localized far from the damaged site53 (Fig. 1). This phenomenon was initially described for neutrophils and showed that the local production of LTB4 at the injured site functions as a relay signal responsible for the attraction of waves of distant cells to the injury site55,56. Data suggest that this mechanism may be more generally relevant53,54 and several examples of putative relay signals in both in vivo and in vitro models have been proposed to regulate DC migration.
Fig. 1.
Chemokines as relay signals in the migration of DCs. This cartoon depicts the different types of chemotactic signals such as DAMPs (e.g., CRAMP; cathelin-related antimicrobial peptide), acute-phase proteins (e.g., SAA; serum amyloid A), proinflammatory cytokines (e.g., activin-A), or eicosanoids (e.g., LTB4; leukotriene B4), produced by different cell types (macrophages, endothelial cells, and hepatocytes), that, in addition to directly promoting DC migration, activate migrating cells to produce chemokines that will promote a second wave of cell recruitment
In a model of allergic airway inflammation, the migration of DCs to the peribronchiolar areas is mediated by the sequential involvement of the chemotactic receptors CCR2 and CCR7 and the formylpeptide receptor Fpr2. These relaying signals were required for the correct trafficking of DCs to the draining lymph nodes and for the priming of Th2 cells57.
In vitro, the pleiotropic cytokine activin A induces the polarization of immature human DCs and the polarized release, at the front edge, of two CXC chemokines, namely, CXCL12 and CXCL14. The use of blocking antibodies for these two chemokines caused inhibition of activin A-induced chemotaxis58. Similarly, serum amyloid A, an acute-phase protein produced during inflammatory responses, indirectly amplifies the recruitment signal for DCs by the rapid secondary induction of CCL3. CCL3 is responsible for the generation of an optimal chemotactic gradient59 (Fig. 1). Finally, CCL21 by itself may act as a relay signal to promote the CCR7-induced migration of mature DCs to the lymph nodes. There are two forms of CCL21 that differ in their ability to bind heparan sulfate and induce DC migration. They represent two distinct chemotactic signals acting in sequential waves. The full-length heparan sulfate-bound CCL21 provides the first chemotactic signal. The soluble tailless-CCL21, produced during inflammation by endogenous proteases or by activated DCs33, represents a stronger, sustained second chemotactic signal60.
In conclusion, the data suggest the existence of multiple levels of regulation of DC migration. These mechanisms are likely to orchestrate the sequence of signals that finely tune the recruitment of DCs to the peripheral tissues and secondary lymphoid tissues.
Atypical chemokine receptors as regulators of DC migration
Atypical chemokine receptors (ACKRs) represent a small subset of proteins that express a high degree of homology with chemokine receptors. However, ACKRs do not activate G-protein-dependent signaling or chemotactic responses61,62. The ACKR family includes four proteins, namely, ACKR1, ACKR2, ACKR3, and ACKR4 with ackr5 and ackr6 reserved for the receptors CCRL2 and PITPNM3, pending confirmation of their ability to bind chemokines. ACKRs regulate inflammation by acting as scavenger receptors, promoting chemokine transcytosis or regulating the formation of the chemokine gradient15–17,62. ACKRs regulate DC functions. For instance, ACKR2, when expressed by lymphatic endothelial cells, is able to remove inflammatory chemokines and enhances the interaction of CCR7 ligands, selectively expressed by the lymphatic vasculature, with mature DCs that have undergone CCR7 switching63. In addition, in a model of experimental autoimmune encephalomyelitis, ACKR2 deficiency is responsible for the reduced local accumulation of DCs and impaired T-cell priming64,65. As mentioned previously, ACKR4 controls the emigration of DCs from the subcapsular sinus to the lymph node parenchyma, regulating the formation of CCL19 and the CCL21 gradient35. Finally, the expression of ACKR4 by skin stromal cells is involved in the CCR7-dependent migration of DCs, both under steady-state and inflammatory conditions34.
CCRL2 (ackr5) represents a paradigmatic example of regulation of the immune response by atypical chemotactic receptors66. In a model of OVA-induced lung hypersensitivity, CCRL2-deficient mice are defective in the induction of Th2 responses. Defective T-cell priming directly correlates with the impaired migration of antigen-loaded lung DCs to the mediastinal lymph nodes67. In neutrophils, CCRL2 forms heterodimers and regulates CXCR2 signaling68. These data suggest that this mechanism applies to other receptors and that CCRL2 might regulate the function of CCR7 in mature DCs. Moreover, CCRL2 binds to the endothelial cell barrier and presents chemerin, a chemotactic peptide, which promotes the transmigration of DCs across the endothelial cell monolayer69,70.
Nonchemokine chemotactic factors in DC migration
More than just chemokines are involved in DC trafficking. Several nonchemokine agonists, released at inflammatory sites, promote the recruitment of DCs or their precursors11. These chemotactic stimuli include bacterial components, bioactive lipid mediators, and tissue danger signals (Table 3); thus, their actions may temporally precede chemokine production11. For instance, DCs express Fpr1 and Fpr2, two functional receptors for formylated peptides and damage-associated molecular patterns (DAMPs)71. Fpr2 and one of its endogenous ligands, cathelin-related antimicrobial peptide (CRAMP), is involved in DC activation and accumulation during allergic airway inflammation57,72. Another DAMP, the nuclear protein high-mobility group box 1 (HMGB1), regulates DC migration and function by a RAGE-dependent pathway73. Components of the complement cascade, such as C3a, C5a,71,74,75 and C1q76,77 have chemotactic functions for DCs both in vitro and in vivo. Nucleotide sensing by the purinergic receptors P2YR and P2XR regulates the DC chemotactic response78. Degradation of ATP to adenosine by the ectonucleotidase CD39 represents a way for regulatory T cells to induce DC migration79. Finally, components of the coagulation cascade, such as plasmin, regulate DC accumulation in atherosclerotic lesions80.
Table 3.
Nonchemokine chemotactic factors for DC migration
| Nonchemokine agonists | Nonchemokine receptors | References |
|---|---|---|
| Chemerin | ChemR23 | 90 |
| fMLP | FPR | 71 |
| LL37 | FPR2 | 130 |
| F2L | FPR3 | 131 |
| SAA | FPR2 | 59 |
| C5a | C5aR | 74 |
| PAF | PAFR | 81, 82 |
| CRAMP | Fpr2 | 57 |
| C1q | gC1qR, DC-SIGN | 77, 132 |
| PGE2 | EP2, EP4 | 84 |
| Plasmin | Akt2 | 80 |
| LTB4 | BLT1/2 | 31 |
| CysLT | CysLT1 | 83 |
| 7α, 25-OHC | EBI2 | 86 |
| S1P | S1PR1 | 87 |
| Adenosine | P2YR, P2XR | 78 |
| Epac1-Rap1 | 79 | |
| Activin A | ALK4, ActRIIA | 58 |
| HMGB1 | RAGE | 73 |
| IL-18 | IL-18R | 97 |
Several observations underline the importance of lipid mediators in DC migration. Human and mouse DCs express functional receptors for the chemotactic lipid PAF81,82. Cysteinyl leukotrienes promote DC migration to lymph nodes in response to the CCR7 ligands CCL19 and CCL2183. Prostaglandin E2, acting through the EP2 and EP4 receptors, is a general mandatory factor for the development of migratory DCs in humans84. The LTB4/BLT1 axis is crucial in the regulation of DC trafficking and in the induction of adaptive immune responses31. In contrast, the lipid mediator Resolvin E1 inhibits cutaneous DC motility, possibly through the BLT1 receptor85. Some lipid chemotactic signals are implicated in homeostatic DC recruitment. The 7alpha,25-dihydroxycholesterol (7α,25-OHC), an oxysterol that binds the EBI2/GPR183 receptor, is required for the correct localization of a subset of splenic DCs, a necessary process for the activation of immune responses to particular antigens86, whereas sphingosine-1 phosphate (S1P) regulates the localization of a subset of splenic immature DCs87. S1P is also involved in the regulation of DC migration in a model of skin contact hypersensitivity88.
The role of several nonchemokine chemotactic proteins has been investigated. Chemerin, an antimicrobial peptide produced by epithelial cells and stromal cells, induces the in vitro and in vivo migration of DCs through the activation of the chemotactic receptor CMKLR189. Chemerin production was detected in the skin biopsies obtained from patients with autoimmune diseases such as systemic lupus erythematosus, lichen planus, and psoriasis and was correlated with myeloid and plasmacytoid DC tissue infiltration90–94.
Some pleiotropic cytokines also regulate DC migration. Activin A, a member of the TGF-β family, induces the directional migration of immature DCs through the secondary release of chemokines, namely, CXCL12 and CXCL1458,95. In chronic diseases such as psoriasis and inflammatory bowel disease, the chemotactic activity of the proinflammatory cytokine IL-18 for human DC subsets was proposed as an additional mechanism for recruiting DCs to inflammatory areas characterized by a Th1 signature96,97.
Concluding remarks
DCs are professional antigen-presenting cells that bridge the innate and adaptive immune responses. After antigen capture, DCs leave peripheral tissues, enter the lymphatic system, and migrate to lymph nodes to localize in T-cell-rich areas. In the lymph nodes, DCs initiate adaptive responses by presenting antigens to specific T cells1,2,4,5. Although DCs specialize in the recognition and presentation of microbial-derived antigens, they are also activated by DAMPs, such as self-nucleic acids and present self-antigens. Therefore, DCs represent a key element in the activation of immunity versus tolerance. For this reason, DCs are implicated in a large variety of pathological conditions, including autoimmune diseases and cancers, and represent a valuable therapeutic target. Several DC-based antitumor vaccines are being tested on solid and hematological malignancies in clinical trials, and there are a number of studies focused on modulating DC migration to improve therapeutic responsiveness98–100. Blocking DC migration via the lymphatic system is under investigation as a therapeutic strategy for preventing transplant rejections98,101,102.
The correct tissue localization is crucial for DC function. In PI3Kγ-deficient mice, defective signaling of the chemotactic receptors impairs specific immunity18,103. Therefore, chemokines and chemokine receptors represent a promising target for new therapeutic strategies focused on controlling DC activation. So far, only two drugs, acting as chemokine receptor antagonists, have reached the market. Maraviroc targets CCR5 for its role as a co-receptor for the cellular entry of the human immunodeficiency virus (HIV) and plerixafor targets CXCR4 for stem cell mobilization; a second CXCR4 receptor antagonist (X4P-001) is in phase II/III clinical trials61. No chemokine-targeting drug is currently available for inflammatory or autoimmune conditions.
Inflammation involves several amplification mechanisms that sustain the strength, the persistence, and the propagation of the response. Migrating leukocytes are simultaneously exposed to numerous stimuli and their response is the result of the integration of multiple pieces of information. These signals can converge to potentiate the response of already-migrating cells, or alternatively, the release of secondary chemokines that function as a signal relay mechanism.
The understanding of the mechanisms involved in the fine-tuning of DC migration, together with the new findings on the biology and functions of the emerging variety of DC subsets104, are likely to uncover new potential pharmacological targets and represent a major future challenge.
Acknowledgements
This work was supported by AIRC (Associazione Italiana Ricerca sul Cancro); IAP (Interuniversity Attraction Poles) 7–40 program; COST action BM1404 Mye-EUNITER; CARIPLO; and Ministero Salute.
Competing interests
The authors declare no competing interests.
References
- 1.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TL, et al. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 2016;34:93–119. doi: 10.1146/annurev-immunol-032713-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 5.Durai V, Murphy KM. Functions of murine dendritic cells. Immunity. 2016;45:719–736. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briseno CG, Murphy TL, Murphy KM. Complementary diversification of dendritic cells and innate lymphoid cells. Curr. Opin. Immunol. 2014;29:69–78. doi: 10.1016/j.coi.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 9.Del Prete A, et al. Migration of dendritic cells across blood and lymphatic endothelial barriers. Thromb. Haemost. 2006;95:22–28. [PubMed] [Google Scholar]
- 10.Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor. Rev. 2005;16:581–592. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Lukacs-Kornek V, Engel D, Tacke F, Kurts C. The role of chemokines and their receptors in dendritic cell biology. Front. Biosci. 2008;13:2238–2252. doi: 10.2741/2838. [DOI] [PubMed] [Google Scholar]
- 13.Bachelerie F, et al. An atypical addition to the chemokine receptor nomenclature: IUPHAR Review 15. Br. J. Pharmacol. 2015;172:3945–3949. doi: 10.1111/bph.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–336. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 16.Bonecchi R, Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front. Immunol. 2016;7:224. doi: 10.3389/fimmu.2016.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 18.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Yun TJ, et al. Indoleamine 2,3-dioxygenase-expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell. Metab. 2016;23:852–866. doi: 10.1016/j.cmet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Sozzani S, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 21.Nakano H, Lyons-Cohen MR, Whitehead GS, Nakano K, Cook DN. Distinct functions of CXCR4, CCR2, and CX3CR1 direct dendritic cell precursors from the bone marrow to the lung. J. Leukoc. Biol. 2017;101:1143–1153. doi: 10.1189/jlb.1A0616-285R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassone L, et al. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood. 2010;116:4870–4873. doi: 10.1182/blood-2010-03-272096. [DOI] [PubMed] [Google Scholar]
- 23.Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front. Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun A, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 25.Lian J, Luster AD. Chemokine-guided cell positioning in the lymph node orchestrates the generation of adaptive immune responses. Curr. Opin. Cell. Biol. 2015;36:1–6. doi: 10.1016/j.ceb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int. Immunol. 2010;22:839–849. doi: 10.1093/intimm/dxq435. [DOI] [PubMed] [Google Scholar]
- 27.Vaahtomeri K, et al. Locally triggered release of the chemokine CCL21 promotes dendritic cell transmigration across lymphatic endothelia. Cell Rep. 2017;19:902–909. doi: 10.1016/j.celrep.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber M, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 29.Tal O, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MartIn-Fontecha A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Prete A, et al. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumann K, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Bryce SA, et al. ACKR4 on stromal cells scavenges CCL19 to enable CCR7-dependent trafficking of APCs from inflamed skin to lymph nodes. J. Immunol. 2016;196:3341–3353. doi: 10.4049/jimmunol.1501542. [DOI] [PubMed] [Google Scholar]
- 35.Ulvmar MH, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 36.Leventhal DS, et al. Dendritic cells coordinate the development and homeostasis of organ-specific regulatory T cells. Immunity. 2016;44:847–859. doi: 10.1016/j.immuni.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J. Cell. Sci. 2013;126:5259–5270. doi: 10.1242/jcs.135343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabashima K, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am. J. Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stutte S, et al. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc. Natl Acad. Sci. Usa. 2010;107:8736–8741. doi: 10.1073/pnas.0906126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruland C, et al. Chemokine CCL17 is expressed by dendritic cells in the CNS during experimental autoimmune encephalomyelitis and promotes pathogenesis of disease. Brain Behav Immun. 2017;66:382–393. doi: 10.1016/j.bbi.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J. Leukoc. Biol. 2004;76:185–194. doi: 10.1189/jlb.1003479. [DOI] [PubMed] [Google Scholar]
- 42.Gouwy M, et al. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology. 2014;219:218–229. doi: 10.1016/j.imbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Sebastiani S, Danelon G, Gerber B, Uguccioni M. CCL22-induced responses are powerfully enhanced by synergy inducing chemokines via CCR4: evidence for the involvement of first beta-strand of chemokine. Eur. J. Immunol. 2005;35:746–756. doi: 10.1002/eji.200525800. [DOI] [PubMed] [Google Scholar]
- 44.Panzer U, Uguccioni M. Prostaglandin E2 modulates the functional responsiveness of human monocytes to chemokines. Eur. J. Immunol. 2004;34:3682–3689. doi: 10.1002/eji.200425226. [DOI] [PubMed] [Google Scholar]
- 45.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 2012;91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sozzani S, et al. Synergism between platelet activating factor and C-C chemokines for arachidonate release in human monocytes. Biochem. Biophys. Res. Commun. 1994;199:761–766. doi: 10.1006/bbrc.1994.1294. [DOI] [PubMed] [Google Scholar]
- 47.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 48.Krug A, et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 2002;169:6079–6083. doi: 10.4049/jimmunol.169.11.6079. [DOI] [PubMed] [Google Scholar]
- 49.Bai Z, et al. CXC chemokine ligand 12 promotes CCR7-dependent naive T cell trafficking to lymph nodes and Peyer’s patches. J. Immunol. 2009;182:1287–1295. doi: 10.4049/jimmunol.182.3.1287. [DOI] [PubMed] [Google Scholar]
- 50.Umemoto E, et al. Constitutive plasmacytoid dendritic cell migration to the splenic white pulp is cooperatively regulated by CCR7- and CXCR4-mediated signaling. J. Immunol. 2012;189:191–199. doi: 10.4049/jimmunol.1200802. [DOI] [PubMed] [Google Scholar]
- 51.Cecchinato V, D’Agostino G, Raeli L, Uguccioni M. Chemokine interaction with synergy-inducing molecules: fine tuning modulation of cell trafficking. J. Leukoc. Biol. 2016;99:851–855. doi: 10.1189/jlb.1MR1015-457R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellado M, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. Embo. J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majumdar R, Sixt M, Parent CA. New paradigms in the establishment and maintenance of gradients during directed cell migration. Curr. Opin. Cell. Biol. 2014;30:33–40. doi: 10.1016/j.ceb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sozzani S, Del Prete A. Chemokines as relay signals in human dendritic cell migration: serum amyloid A kicks off chemotaxis. Eur. J. Immunol. 2015;45:40–43. doi: 10.1002/eji.201445305. [DOI] [PubMed] [Google Scholar]
- 55.Chou RC, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen K, et al. Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J. Biol. Chem. 2013;288:16262–16273. doi: 10.1074/jbc.M113.450635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salogni L, et al. Activin A induces dendritic cell migration through the polarized release of CXC chemokine ligands 12 and 14. Blood. 2009;113:5848–5856. doi: 10.1182/blood-2008-12-194597. [DOI] [PubMed] [Google Scholar]
- 59.Gouwy M, et al. Serum amyloid A chemoattracts immature dendritic cells and indirectly provokes monocyte chemotaxis by induction of cooperating CC and CXC chemokines. Eur. J. Immunol. 2015;45:101–112. doi: 10.1002/eji.201444818. [DOI] [PubMed] [Google Scholar]
- 60.Hjorto GM, et al. Differential CCR7 targeting in dendritic cells by three naturally occurring CC-chemokines. Front. Immunol. 2016;7:568. doi: 10.3389/fimmu.2016.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bachelerie F, et al. International union of basic and clinical pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachelerie F, et al. New nomenclature for atypical chemokine receptors. Nat. Immunol. 2014;15:207–208. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- 63.McKimmie CS, et al. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood. 2013;121:3768–3777. doi: 10.1182/blood-2012-04-425314. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, et al. Cutting edge: the silent chemokine receptor D6 is required for generating T-cell responses that mediate experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:17–21. doi: 10.4049/jimmunol.177.1.17. [DOI] [PubMed] [Google Scholar]
- 65.Hansell CA, et al. The atypical chemokine receptor ACKR2 suppresses Th17 responses to protein autoantigens. Immunol. Cell. Biol. 2015;93:167–176. doi: 10.1038/icb.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Prete A, Bonecchi R, Vecchi A, Mantovani A, Sozzani S. CCRL2, a fringe member of the atypical chemoattractant receptor family. Eur. J. Immunol. 2013;43:1418–1422. doi: 10.1002/eji.201243179. [DOI] [PubMed] [Google Scholar]
- 67.Otero K, et al. Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood. 2010;116:2942–2949. doi: 10.1182/blood-2009-12-259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Del Prete A, et al. The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood. 2017;130:1223–1234. doi: 10.1182/blood-2017-04-777680. [DOI] [PubMed] [Google Scholar]
- 69.Monnier J, et al. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J. Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalvo-Feo S, et al. Endothelial cell-derived chemerin promotes dendritic cell transmigration. J. Immunol. 2014;192:2366–2373. doi: 10.4049/jimmunol.1302028. [DOI] [PubMed] [Google Scholar]
- 71.Sozzani S, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J. Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 72.Chen K, et al. The formylpeptide receptor 2 (Fpr2) and its endogenous ligand cathelin-related antimicrobial peptide (CRAMP) promote dendritic cell maturation. J. Biol. Chem. 2014;289:17553–17563. doi: 10.1074/jbc.M113.535674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 74.Morelli A, Larregina A, Chuluyan I, Kolkowski E, Fainboim L. Expression and modulation of C5a receptor (CD88) on skin dendritic cells. Chemotactic effect of C5a on skin migratory dendritic cells. Immunology. 1996;89:126–134. doi: 10.1046/j.1365-2567.1996.d01-701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gutzmer R, et al. Human plasmacytoid dendritic cells express receptors for anaphylatoxins C3a and C5a and are chemoattracted to C3a and C5a. J. Invest. Dermatol. 2006;126:2422–2429. doi: 10.1038/sj.jid.5700416. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, et al. Complement C1q chemoattracts human dendritic cells and enhances migration of mature dendritic cells to CCL19 via activation of AKT and MAPK pathways. Mol. Immunol. 2008;46:242–249. doi: 10.1016/j.molimm.2008.08.279. [DOI] [PubMed] [Google Scholar]
- 77.Vegh Z, Kew RR, Gruber BL, Ghebrehiwet B. Chemotaxis of human monocyte-derived dendritic cells to complement component C1q is mediated by the receptors gC1qR and cC1qR. Mol. Immunol. 2006;43:1402–1407. doi: 10.1016/j.molimm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 78.Idzko M, et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.v100.3.925. [DOI] [PubMed] [Google Scholar]
- 79.Ring S, et al. Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J. Immunol. 2015;194:3735–3744. doi: 10.4049/jimmunol.1401434. [DOI] [PubMed] [Google Scholar]
- 80.Li X, et al. Plasmin triggers chemotaxis of monocyte-derived dendritic cells through an Akt2-dependent pathway and promotes a T-helper type-1 response. Arterioscler. Thromb. Vasc. Biol. 2010;30:582–590. doi: 10.1161/ATVBAHA.109.202044. [DOI] [PubMed] [Google Scholar]
- 81.Sozzani S, et al. Human monocyte-derived and CD34 + cell-derived dendritic cells express functional receptors for platelet activating factor. FEBS Lett. 1997;418:98–100. doi: 10.1016/s0014-5793(97)01358-6. [DOI] [PubMed] [Google Scholar]
- 82.Angeli V, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Robbiani DF, et al. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 84.Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- 85.Sawada Y, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015;212:1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gatto D, et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat. Immunol. 2013;14:446–453. doi: 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- 87.Czeloth N, et al. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J. Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 88.Lamana A, et al. CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J. Invest. Dermatol. 2011;131:1503–1512. doi: 10.1038/jid.2011.54. [DOI] [PubMed] [Google Scholar]
- 89.Wittamer V, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vermi W, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Palma G, et al. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011;79:1228–1235. doi: 10.1038/ki.2011.32. [DOI] [PubMed] [Google Scholar]
- 92.Parolini S, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 93.Albanesi C, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skrzeczynska-Moncznik J, et al. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem. Biophys. Res. Commun. 2009;380:323–327. doi: 10.1016/j.bbrc.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seeger P, Musso T, Sozzani S. The TGF-beta superfamily in dendritic cell biology. Cytokine Growth Factor. Rev. 2015;26:647–657. doi: 10.1016/j.cytogfr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Gutzmer R, et al. Human dendritic cells express the IL-18R and are chemoattracted to IL-18. J. Immunol. 2003;171:6363–6371. doi: 10.4049/jimmunol.171.12.6363. [DOI] [PubMed] [Google Scholar]
- 97.Kaser A, et al. Interleukin-18 attracts plasmacytoid dendritic cells (DC2s) and promotes Th1 induction by DC2s through IL-18 receptor expression. Blood. 2004;103:648–655. doi: 10.1182/blood-2002-07-2322. [DOI] [PubMed] [Google Scholar]
- 98.Teijeira A, Russo E, Halin C. Taking the lymphatic route: dendritic cell migration to draining lymph nodes. Semin. Immunopathol. 2014;36:261–274. doi: 10.1007/s00281-013-0410-8. [DOI] [PubMed] [Google Scholar]
- 99.Weinstock M, Rosenblatt J, Avigan D. Dendritic cell therapies for hematologic malignancies. Mol. Ther. Methods Clin. Dev. 2017;5:66–75. doi: 10.1016/j.omtm.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seyfizadeh N, Muthuswamy R, Mitchell DA, Nierkens S. Migration of dendritic cells to the lymph nodes and its enhancement to drive anti-tumor responses. Crit. Rev. Oncol. Hematol. 2016;107:100–110. doi: 10.1016/j.critrevonc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Fiorina P, et al. Characterization of donor dendritic cells and enhancement of dendritic cell efflux with CC-chemokine ligand 21: a novel strategy to prolong islet allograft survival. Diabetes. 2007;56:912–920. doi: 10.2337/db06-1445. [DOI] [PubMed] [Google Scholar]
- 102.Ziegler E, et al. CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking. J. Am. Soc. Nephrol. 2006;17:2521–2532. doi: 10.1681/ASN.2005070782. [DOI] [PubMed] [Google Scholar]
- 103.Del Prete A, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. Embo. J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.See P, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356:6342. doi: 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rose CE, Jr, et al. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell. Mol. Immunol. 2010;7:361–374. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha + conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J. Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 107.Le Borgne M, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8 + T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Cook DN, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 109.Leon B, et al. Regulation of T(H)2 development by CXCR5 + dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bradford BM, Reizis B, Mabbott NA. Oral prion disease pathogenesis is impeded in the specific absence of CXCR5-expressing dendritic cells. J. Virol. 2017;91:10. doi: 10.1128/JVI.00124-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorner BG, et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 112.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohta T, et al. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci. Rep. 2016;6:23505. doi: 10.1038/srep23505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swiecki M, et al. Microbiota induces tonic CCL2 systemic levels that control pDC trafficking in steady state. Mucosal Immunol. 2017;10:936–945. doi: 10.1038/mi.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sawai CM, et al. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J. Exp. Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sisirak V, et al. CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood. 2011;118:5130–5140. doi: 10.1182/blood-2010-07-295626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goubier A, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mizuno S, et al. CCR9 + plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol. Lett. 2012;146:64–69. doi: 10.1016/j.imlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 119.Hadeiba H, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kohara H, et al. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110:4153–4160. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 121.Seth S, et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J. Immunol. 2011;186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 122.Yoneyama H, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 123.Vanbervliet B, et al. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur. J. Immunol. 2002;32:231–242. doi: 10.1002/1521-4141(200201)32:1<231::AID-IMMU231>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 124.Dieu MC, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cavarelli M, Foglieni C, Rescigno M, Scarlatti G. R5 HIV-1 envelope attracts dendritic cells to cross the human intestinal epithelium and sample luminal virions via engagement of the CCR5. EMBO Mol. Med. 2013;5:776–794. doi: 10.1002/emmm.201202232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c + CD141 + cells as homologues of mouse CD8 + dendritic cells. J. Exp. Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen SC, et al. Expression of chemokine receptor CXCR3 by lymphocytes and plasmacytoid dendritic cells in human psoriatic lesions. Arch. Dermatol. Res. 2010;302:113–123. doi: 10.1007/s00403-009-0966-2. [DOI] [PubMed] [Google Scholar]
- 128.Sato K, et al. CC chemokine receptors, CCR-1 and CCR-3, are potentially involved in antigen-presenting cell function of human peripheral blood monocyte-derived dendritic cells. Blood. 1999;93:34–42. [PubMed] [Google Scholar]
- 129.Beaulieu S, et al. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J. Immunol. 2002;169:2925–2936. doi: 10.4049/jimmunol.169.6.2925. [DOI] [PubMed] [Google Scholar]
- 130.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 131.Migeotte I, et al. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J. Exp. Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu C, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J. Clin. Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]