Abstract
BACKGROUND:
Carbapenems show excellent activity against resistant uropathogens, and they are the antibiotics of choice for urinary tract infections (UTIs). The choice of carbapenem prescription is strongly influenced by antimicrobial susceptibility testing (AST) report. With the publication of recent AST guidelines by the European Committee on AST (EUCAST), we were curious to evaluate the difference in results between Clinical and Laboratory Standards Institute (CLSI) and the EUCAST guidelines for the interpretation of carbapenems.
METHODS:
During a period of 1 year, midstream urine specimens received in the laboratory were cultured by conventional techniques and 2932 of them grew significant colony counts of Escherichia coli. Out of them, 501 E. coli isolates which were resistant to at least six first-line antibiotics were further subjected to second-line antimicrobials imipenem and meropenem, reported by E-tests (bioMerieux, France). The E-test results were interpreted by both CLSI 2016 and EUCAST 6.0 (2016) guidelines. Weighted kappa was used to determine absolute agreement, and McNemar's Chi-square test was used to test the difference in proportions of susceptibility between two methods, respectively.
RESULTS:
Taking CLSI guidelines as a gold standard, there was 100% sensitivity in a susceptible category by the EUCAST guidelines for both the carbapenems. Weighted kappa showed good and moderate agreement between them for imipenem and meropenem, respectively. However, McNemar Chi-square test in the nonsusceptible category between the two tests was 9.38% and 33.03% for imipenem and meropenem, respectively, and they were highly significant (P < 0.001).
CONCLUSIONS:
A laboratory can follow EUCAST guidelines as well and the guidelines are more useful in urinary concentrated antibiotics such as carbapenems. Further other antibiotics need to be evaluated by both these guidelines.
Key words: Carbapenem, Clinical and Laboratory Standards Institute, European Committee on antimicrobial susceptibility testing, interpretation
Introduction
Carbapenems are the most extensively used antimicrobial agents for Gram-negative infections in tertiary care centers. Since carbapenems show excellent activity against uropathogens, they are the antimicrobial agents of choice for complicated urinary tract infections (UTIs), especially those due to extended spectrum beta-lactamase producing Escherichia coli, the most common uropathogen. The choice of carbapenem prescription is strongly influenced by the antimicrobial susceptibility testing (AST) report.
Clinical and Laboratory Standards Institute (CLSI) is a volunteer-driven, membership-supported standards development organization, which updates guidelines on AST annually. However, these guidelines are expensive, not available free of cost, and may not be exactly applicable to clinical strains from countries with epidemiology that differs widely from the USA.
European Committee on AST (EUCAST) was set to develop clinical breakpoints better suited for the epidemiology of Europe. Epidemiological cutoff value was determined over the years 2002–2009 and collated with minimum inhibitory concentration data from international surveillance programs such as SENTRY, Norwegian Surveillance System for Antimicrobial Drug Resistance and Meropenem Yearly Susceptibility Test Information Collection. The EUCAST guidelines 1.0 were released in December 2009 and claimed to have better rationale behind clinical breakpoints for guiding in vitro susceptibility testing with annual revisions. These guidelines and their subsequent annual updates are available free of cost online on the EUCAST website.
Our laboratory has been using CLSI guidelines for AST and interpretation since many years. However, with the increasing number of countries accepting EUCAST guidelines worldwide, we were curious to evaluate the difference in results between CLSI and EUCAST guidelines for the interpretation of in vitro AST by disc diffusion method, used routinely in our laboratory. Hence, we planned to determine the level of agreement between CLSI and EUCAST guidelines for interpretation of the results of carbapenems (imipenem and meropenem) susceptibility.
Methods
The prospective hospital-based study was conducted in the Department of Microbiology in a tertiary care and referral center from September 1st, 2016 to August 31st, 2017. Mid-stream urine specimens from suspected UTIs were cultured on HiCrome UTI Agar (HiMedia, Mumbai, India). E. coli strains with significant colony counts (>10[5] CFU/ml) were included in the study for AST. AST was carried out on Mueller-Hinton agar (Becton Dickinson, Maryland, USA) using a lawn of McFarland 0.5 in normal saline. Each isolate was subjected to a first-line antimicrobial panel consisting of eight antibiotics such as ceftazidime, ceftriaxone, ertapenem, gentamicin, ciprofloxacin, nitrofurantoin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole by disc diffusion as per the CLSI guidelines. Any E. coli isolate which was found to be resistant to at least six of these agents was further tested for carbapenem (imipenem and meropenem) susceptibility by E-test (bioMerieux, France) and the results were interpreted as susceptible (S), intermediate susceptible (IS) and resistant (R) following CLSI 2016 and EUCAST 6.0 (2016) guidelines and compared[1,2] and subsequently by their 2017 versions.[3,4] ATCC E. coli 25922 was used as the quality control reference strain.
Statistical analysis
The data were analyzed using software MedCalc. Sensitivity, Specificity, false-positive rate (FPR), false-negative rate (FNR), positive predictive value (PPV), NPV, likelihood ratio positive (LR+), likelihood ratio negative (LR−) and overall accuracy of the diagnostic test was assessed. Weighted kappa was calculated to find the level of absolute agreement between two methods. McNemar's Chi-square test was used to test the difference in proportions of susceptibility between two methods. A value of P < 0.05 was considered as statistically significant.
Results
Over the 1-year period from September 1st, 2016 to August 31st, 2017, the laboratory received 26,656 mid-stream urine specimens for bacterial culture. Of these, 2932 specimens grew significant E. coli colonies. On AST, 501 E. coli isolates were resistant to at least six of eight first-line agents and subjected to second-line AST with imipenem and meropenem by E-test.
Imipenem
Comparative evaluation of imipenem susceptibility results by the two guidelines was represented as 3 × 3 table [Table 1] and 2 × 2 table [Table 2]
Table 1.
The comparative evaluation of imipenem susceptibility results by the two guidelines (n=501)
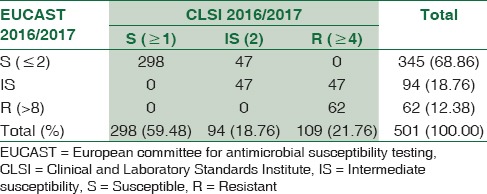
Table 2.
Imipenem results expressed as Susceptible (S) and Non-susceptible (NS = IS + R) by the two guidelines (n=501)
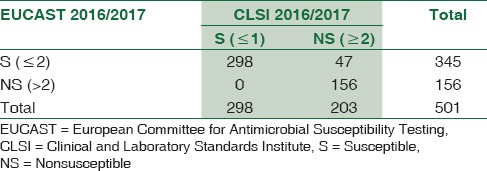
Agreement between the results of susceptible (S) = 100%
Agreement between the results of intermediate susceptibility (IS) = 50%
Agreement between the results of resistant (R) = 56.88%
Weighted kappa agreement score = 64.5% (52.4%-70.3%)
Interpretation of kappa = Good agreement.
The comparison of EUCAST 2016 guidelines for the purpose of prediction of susceptibility as compared to the CLSI 2016 guidelines (Gold standard), is as follows:
Sensitivity (true positive rate [TPR] = 100%, Specificity (TNR) = 76.85%,
FNR = 0%, FPR = 23.15%
PPV = 86.38%, NPV = 100%,
LR+ = 4.32 LR− = 0%
Overall Accuracy = 90.62%
Mc Nemar Chi-square test showed that the difference in prediction of nonsusceptible category between the results of the two tests was 9.38% (11.93%–8.83%), which was highly significant (P < 0.001).
Meropenem
Comparative evaluation of meropenem susceptibility results by the two guidelines were represented as 3 × 3 table [Table 3] and 2 × 2 table [Table 4].
Table 3.
The comparative evaluation of meropenem susceptibility results by the two guidelines (n=501)
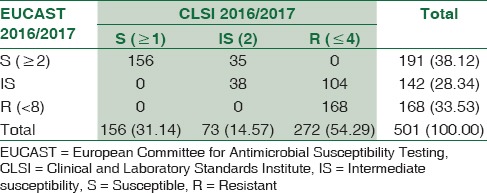
Table 4.
The comparative evaluation of meropenem susceptibility results by the two guidelines (n=501)
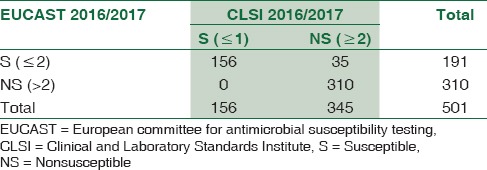
Agreement between the results of susceptibles (S) = 100%
Agreement between the results of intermediate susceptibility (IS) = 52.05%
Agreement between the results of resistant = 61.76%
Weighted kappa agreement = 57.8% (58.7%–63.4%)
Interpretation of kappa = moderate agreement
The comparison of EUCAST 2016 guidelines for prediction of susceptibility as compared to the CLSI 2016 (Gold standard) guidelines is as follows:
Sensitivity (TPR) = 100%, Specificity (TNR) = 89.86%,
FNR = 0% FPR = 10.14%
PPV = 81.68% NPV = 100%,
LR+ = 9.86 LR− = 0%
Overall Accuracy = 93.01%
Mc Nemar Chi-square test showed that the difference in prediction of nonsusceptible category between the results of the two tests was 33.03% (31.93%–37.83%) and the difference was statistically significant (P < 0.001).
Discussion
Our institution has been following the CLSI guidelines (along with annual updates) for the interpretation of AST. With the recent availability of EUCAST guidelines and their rapid acceptance by many countries, led us to evaluate the guidelines recommended by the two agencies.
Among the 501 urinary strains of E. coli tested for carbapenems over 1 year, there was good agreement between the results of susceptibility interpreted by the two guidelines.
For imipenem, 298/501 (59.48%) and 345/501 (68.86%) E. coli isolates tested susceptible, using CLSI 2016 and EUCAST 6.0 (2016) guidelines, respectively. A study from Turkey based on Imipenem disc diffusion also showed similar results.[5] The IS category remained unchanged irrespective of the guideline used (97/501 = 19.36%). The number of absolute resistant strains estimated by CLSI exceeded those by EUCAST. The kappa value (0.645) showed good agreement between the two guidelines.
For meropenem, 156/501 (31.14%) and 191/501 (38.12%) E. coli isolates tested susceptible, using CLSI 2016 and EUCAST 2016 guidelines, respectively. The number of strains categorized as IS by the EUCAST guidelines 142/501 (28.34%) was double those allotted by CLSI 73/501 (14.56%). Here too, the number of absolute resistant strains estimated by CLSI exceeded those by EUCAST. The kappa value (0.578) showed a moderate agreement between the two guidelines.
The real discrepancy between the antibiograms reported by the two methods in our study lies in the reporting of the IS category. The cutoff for susceptible strains is different for both guidelines; 1 μg/ml for CLSI and 2 μg/ml for the EUCAST. Both have kept a margin of two dilutions for their respective resistant cutoffs; 4 μg/ml for CLSI and 8 μg/ml for EUCAST.
The intermediate category acts as a buffer between the in vitro allotment of susceptible and resistant categories to tide over errors of media preparation, the accuracy of inoculum preparation and subjective variations in reporting. In vivo, such drugs, if concentrated at the site of infection, usually prove clinically effective. Finally, they may be considered for combination therapy with a drug that has a different mechanism of action with the susceptible result in the antibiogram.[6,7]
The intermediate category is defined as uncertain therapeutic success for the individual species/drug combination tested by the EUCAST and is intended for compounds for which dosing can be increased. CLSI defines the intermediate category as a lower response rate than for susceptible isolates, but clinical efficacy, if the drug accumulates at the site of infection. The intermediate category represents the “grey zone” regarding therapeutic success; it also helps to prevent serious categorization errors resulting from imprecision of inhibition zone readings.[8]
Carbapenems are concentrated in the urinary tract, and there is a high chance that they would prove effective in UTI even if the laboratory reports them under the IS category. They are also frequently used in combination with either colistin or amikacin for complicated UTI in our setup. Therefore, substituting CLSI guidelines with EUCAST for the reporting of carbapenems-imipenem and meropenem in UTI, will most likely not affect the results in a laboratory like ours. Similar conclusions were drawn by a Kenyan study.[9]
There were a greater number of susceptible strains reported by EUCAST than CLSI, both for imipenem (68.86% vs. 59.48%) and meropenem (38.12% vs. 31.14%). The study findings were similar to a study where the susceptibilities were identical for both imipenem and meropenem.[10] Interpretation by the EUCAST guidelines seems to have provided the clinician with carbapenems as a viable treatment option in UTI, while CLSI guidelines have reported greater number of resistant strains. In vitro results of strains reported resistant by CLSI need to be further evaluated with the clinical outcome of patients following carbapenem therapy based on EUCAST guidelines. However, evaluation of clinical response was beyond the scope of this observational study. There is a need for conducting such studies in the future to truly understand the implication of switching over from old established guidelines to newer ones.
To conclude, there was 100% agreement between the results of the susceptible category by both guidelines, whereas EUCAST did not miss out any carbapenem resistance, as evident by an NPV of 100%. Therefore, if our laboratory were to switch over from CLSI to EUCAST interpretive standards, there would be clear agreement between the two agencies on susceptible strains and no carbapenem-resistant strain would be missed. Given the free availability of EUCAST guidelines and the epidemiological similarity of European isolates with south-east Asian strains regarding carbapenem resistance,[11] the results suggest that a laboratory like ours may use either of the two guidelines, without there being any significant change in AST reporting patterns.
Further other antibiotics need to be evaluated by both these methods.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.CLSI. CLSI Supplement M100S. 26th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 2.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 6.0. 2016. [Last accessed on 2017 Nov 05]. Available from: http://www.eucast.org .
- 3.CLSI. CLSI Supplement M100. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 4.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.0. 2017. [Last accessed on 2017 Nov 05]. Available from: http://www.eucast.org .
- 5.Süzük S, Kaşkatepe B, Avcıküçük H, Aksaray S, Başustaoǧlu A. [The comparison of antibiotic susceptibilities of uropathogenic Escherichia coli isolates in transition from CLSI to EUCAST] Mikrobiyol Bul. 2015;49:494–501. doi: 10.5578/mb.10106. [DOI] [PubMed] [Google Scholar]
- 6.Mackie TJ, Collee JG, McCartney JE. Mackie & McCartney Practical Medical Microbiology. New York: Churchill Livingstone; 1996. [Google Scholar]
- 7.Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, Richter S, et al., editors. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015. [Google Scholar]
- 8.Hombach M, Böttger EC, Roos M. The critical influence of the intermediate category on interpretation errors in revised EUCAST and CLSI antimicrobial susceptibility testing guidelines. Clin Microbiol Infect. 2013;19:E59–71. doi: 10.1111/1469-0691.12090. [DOI] [PubMed] [Google Scholar]
- 9.Wolfensberger A, Sax H, Weber R, Zbinden R, Kuster SP, Hombach M, et al. Change of antibiotic susceptibility testing guidelines from CLSI to EUCAST: Influence on cumulative hospital antibiograms. PLoS One. 2013;8:e79130. doi: 10.1371/journal.pone.0079130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassim A, Omuse G, Premji Z, Revathi G. Comparison of clinical laboratory standards institute and European committee on antimicrobial susceptibility testing guidelines for the interpretation of antibiotic susceptibility at a university teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann Clin Microbiol Antimicrob. 2016;15:21. doi: 10.1186/s12941-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in gram-negative bacteria. Biomed Res Int 2014. 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]


