Abstract
BACKGROUND:
Preeclampsia (PE) is a systemic pregnancy-related disorder characterized by hypertension, proteinuria, and edema. Free radicals seem to play an important role in the induction of endothelial dysfunction in PE.
AIM:
The aim of the present study was to investigate serum levels of nitric oxide (NO), peroxynitrite (ONOO−), paraoxonase (PON-1), malondialdehyde (MDA), and lipid profile in preeclamptic patients compared to the women with normal pregnancy.
MATERIALS AND METHODS:
A total of 68 pregnant women were recruited. They were divided into two groups - Group A, 40 women were a newly diagnosed with PE and Group B, 28 women with normal pregnancy. Anthropometric measurements including body mass index and blood pressure in accordance with biochemical measurements including NO, ONOO−, PON-1, MDA, and lipid profile were done for preeclamptic pregnant women compared to the controls.
RESULTS:
Pregnant women with pre-eclampsia illustrated insignificant differences in the age (31.22±2.87) compared to the age of control P > 0.05. There were significant changes in the body mass index (BMI), type of delivery and smoking status of pregnant women with pre-eclampsia compared to the control P < 0.05. Both systolic and diastolic blood pressures were high in pregnant women with pre-eclampsia compared to the control P < 0.01. PON-1 and NO serum levels were significantly decreased (P < 0.01) while ONOO− and MDA serum levels were significantly increased in PE compared to the women with normal pregnancy.
CONCLUSIONS:
This study concluded that PE is associated with the augmentation of oxidative stress and reduction of endogenous antioxidant capacity regarding PON-1.
Key words: Antioxidant capacity, hypertension, nitric oxide, paraoxonase, peroxynitrite, preeclampsia, proteinuria
Introduction
Preeclampsia (PE) is a systemic pregnancy-related disorder characterized by hypertension, proteinuria, edema and impaired organ functions including pulmonary edema, thrombocytopenia and impaired liver functions in the second half of pregnancy. If untreated PE will be developed into eclampsia which is characterized by the occurrence of seizure.[1] The international incidence of PE is 5%–7% of all pregnancies causing maternal and/or neonatal morbidity and mortality. Risk factors for the incidence of PE include older age, obesity, diabetes mellitus, and previous hypertension. PE may start at the postpartum period. PE is routinely screened during prenatal care; so, high blood pressure more than 140 mmHg systolic and/or 90 mmHg diastolic at two divided time with proteinuria after 20 weeks of pregnancy is regarded as diagnostic criteria.[2]
The pathogenesis of PE is started during trophoblast development and invasion that result in neovascularization and placental dysfunction which consequently causes the release of humoral substances into the maternal circulation. These substances causing a maternal organ injury which per SE leads to the clinical manifestation of PE.[3]
Lipid peroxidation and oxidative stress have been regarded as the main factors responsible for the generation of free radicals by a poorly perfuse placenta which leads to platelets and leukocytes adhesion to the vascular endothelium causing vasoconstriction and augmentation of peripheral vascular resistance.[4] Furthermore, placental vasoconstriction leads to a reduction of uteroplacental circulation that trigger the further release of inflammatory cytokines and anti-angiogenic factors which participate in the generation of a vicious cycle in the development of vascular endothelial dysfunction.[5]
Free radicals seem to play an important role in the induction of endothelial dysfunction in PE. During normal pregnancy, elevated estrogen levels activate production of nitric oxide (NO) from vascular endothelium which is responsible for the vasorelaxation and anticoagulant effects through up-regulation of cAMP, and NO synthase is activated during normal pregnancy to maintain vascular tone and organ perfusions.[6]
During PE, free radicals and reactive oxygen species (ROS) that are released from placenta cause inhibition the function and expression of NO synthase. Scavenging of NO induces the production of peroxynitrite (ONOO−) as an end-product which itself leads to lipid peroxidation and endothelial dysfunction.[7]
At the molecular level, hypoxic placenta stimulates liberation of syncytiotrophoblast microparticle that stimulates the generation of the damage-associated molecular pattern which triggers the assembly of proinflammatory cytokines and ROS. In addition, advanced glycation end-products are augmented during PE which induces placental ROS interaction.[8]
On the other hand, lipid peroxidation is increased in PE that expressed as elevated malondialdehyde (MDA) serum levels which is a marker of lipid peroxidation. Therefore, high lipid peroxidation and elevated lipid profile in preeclamptic women might be the cause of increased risk of coronary artery disease in PE.[9]
Indeed, it has been reported that endogenous anti-oxidant capacity is reduced in PE; thus, glutathione, superoxide dismutase, and other antioxidant potentials are significantly reduced. Furthermore, Vitamin E which is known to reduce ONOO− is reduced in PE; whereas, ascorbic serum levels are severely declining in patients with PE. Moreover, a high ascorbate serum level is dangerous since it may be oxidized into free radicals causing oxidative stress and endothelial dysfunction.[10]
Furthermore, paraoxonase (PON-1), also known as arylesterase, is an enzyme found in high-density lipoprotein (HDL) and released from the liver. It protects HDL and low-density lipoprotein (LDL) from oxidation by free radicals. PON-1 has the ability to hydrolyze oxidized phospholipid and hydroperoxides. Serum levels of PON-1 may be reduced during PE and oxidative stress induced-disorders.[11]
Therefore, the aim of the present study was to investigate serum levels of NO, ONOO−, PON-1, and lipid profile in preeclamptic patients compared to the women with normal pregnancy.
Materials and Methods
The existing study was done in the Department of Clinical Pharmacology and Therapeutics, College of Medicine, Al-Mustansiriyah University in collaboration with the Department of Gynecology and Obstetrics, Baghdad Teaching Hospital, Baghdad, Iraq, from June to September 2016. The study was approved by the Scientific and Ethical Committee in College of Medicine, Al-Mustansiriyah University.
Study design
In this cross-sectional study, a total number of 68 pregnant women with ages ranging from 28 to 37 years were recruited from consultant unit in the Department of Gynecology and Obstetrics, Baghdad Teaching Hospital according to the American college of obstetrician and gynecologist recommendations. The pregnant women were divided into:
Group A: 40 women were a well diagnosed with PE
Group B: 28 women with normal pregnancy.
Women in both groups matching the age and body weight and written informed permission were attained from all recruited pregnant women according to the Ethical Committee of Medical Faculty College of Medicine, Al-Mustansiriyah University. A full history was taken for the parity and pregnancy-induced complications.
Inclusion criteria
Clinically diagnosed women with PE (BP > 140/90 plus gestational age >20 weeks).
Exclusion criteria
Any women with previous metabolic diseases, cardiovascular disorders, endocrine disorders, hypertension, diabetes mellitus, obesity, psychiatric, and mental disorders were excluded from this study.
Anthropometric measurements
Blood pressure was recorded at the supine position from the left arm by automated digital sphygmomanometer. Mild PE was defined when systolic/diastolic BP (DBP) was 140/90 while severe PE was defined when systolic/DBP was 160/110. BP measurements were taken on occasion 4 h apart.
All recruited preeclamptic women had mild PE.
Weight and height measurements were done by digital calculator and tape measure, respectively.
Body mass index (BMI) was estimated by specific equation, BMI = Weight (kg)/Height (m2).
Gestational age was estimated by ultrasonographic monitoring, examination, and dating from the 1st day of last menses.
Biochemical measurements
Ten milliliter of venous blood was drained from each pregnant woman, 6 mL stored in a plain tube for routine investigations, and 4 mL put into an ethylenediaminetetraacetic acid tube for estimation of oxidative markers.
Triglyceride, total cholesterol, and HDL were evaluated by colorimetric kits. LDL was calculated according to Friedewald et al.'s method.[12]
Very LDL (VLDL) = TG/5, cardiac risk ratio (CCR) = TC/HDL, atherogenic index (AI) = log (TG/HDL), and atherogenic coefficient (AC) = TC-HDL/HDL.
Serum uric acid level was calculated by a specific enzymatic method using the chemical analyzer as mg/dl. Proteinuria was estimated by dipstick method (protein dipstick grading).
Oxidative markers
Serum levels malondialdehyde
A marker of lipid peroxidation was estimated by ELISA kit method (Shanghai Yehua Technology Co. Ltd., China) expressed as μmol/L.
Assessment of peroxynitrite
A marker of oxidative stress was estimated by ELISA kit method (ONOO− Assay Kit AAT, Bioquest) expressed in μmol/L.
Assessment of nitric oxide
A marker of endothelial function was estimated by ELISA kit method (NO Assay Kit, Colorimetric, ab65328, USA) expressed in μmol/L.
Assessment of PON-1 activity by Human PON-1 (PicoKine Kit, USA) expressed as U/L.
All kit procedures were done according to the kit instructions.
Statistical analysis
SPSS for Windows version 21.0 (IBM Corp, Armonk, NY, USA) software was used for statistical analysis. Data are expressed as mean ± standard deviation (SD) Unpaired Student's t-test was used for estimating the level of significant regarding (P < 0.05) as the lower limit of significance.
Results
Results of the present study illustrated that the ages in both pregnant women with pre-eclampsia and normal control were insignificantly differed P > 0.05 while the type of deliveries was significantly different, in PE most of the deliveries were cesarian section (65%) compared to normal vaginal delivery (35%). There were insignificant differences regarding the race and gestational age (P > 0.05). Most of the preeclamptic patients were on antihypertensive medications: 95% on methyldopa and 5% on labetalol. Indeed, 15% of preeclamptic patients were smokers compared to 10.71% women with normal pregnancy P < 0.01 [Table 1].
Table 1.
Demographic characteristics of the present study
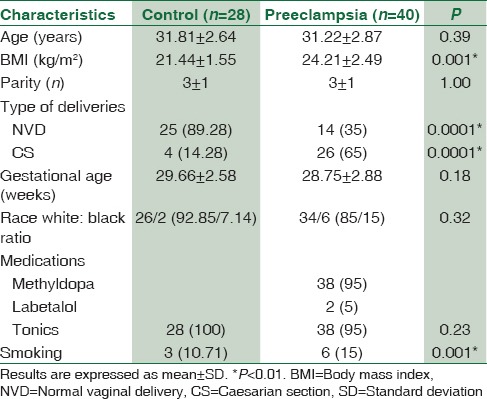
Blood pressure changes were highly different in preeclamptic patients compared to the normal pregnancy; systolic blood pressure was high in PE (103.22 ± 11.62 mmHg) compared to the control (103.22±11.62 mmHg) (P < 0.01). Also diastolic, mean and pulse pressures were high in PE compared to the control [Table 2].
Table 2.
Blood pressure in pregnant women with preeclampsia
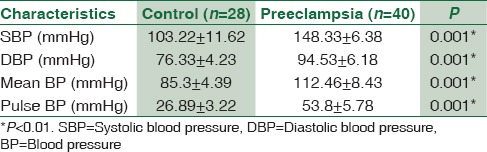
Moreover, there was a significant elevation in the levels of triglyceride, total cholesterol, and LDL with a significant reduction in HDL compared to the control (26.65±3.38 mg/dL, P < 0.01), but VLDL differed in preeclamptic women but to a lesser extent to the control (provide values) (P < 0.05). Indeed, AI, AC, and CCR were high in PE compared to the control (P < 0.01). Proteinuria was high in preeclamptic women compared to the control P < 0.01 [Table 3].
Table 3.
Lipid profile, renal indices, and metabolic variables in the pregnant women with preeclampsia
Regarding lipid peroxidation and oxidative profile, MDA serum levels were high in preeclampsia (9.33 ± 2.38 μmol/L) compared to the control (l 6.22 ± 1.62 μmol/L) (t = 6.003, 95% confidence interval [CI] 2.0756–4.1444, P < 0.001) [Figure 1]. Paraoxonase U/L (PON-1) serum levels were low in PE 194.53 ± 66.18 U/L compared to the control (376.33 ± 40.23 U/L) (t = −12.942, 95% CI - 209.8466–53.7534, P < 0.001) [Figure 2]. NO levels were low in PE (14.46 ± 3.43 μmol/L) compared to the control (44.3 ± 8.39 μmol/L) (t=-20.255, 95% CI - 32.7814–26.8986, P < 0.001) [Figure 3], while peroxynitrite levels were high in PE (5.8 ± 1.78 μmol/L) compared to the control (1.89 ± 0.22 μmol/L) (t = 11.536, 95% CI 3.2333–4.5867, P < 0.001) [Figure 4].
Figure 1.
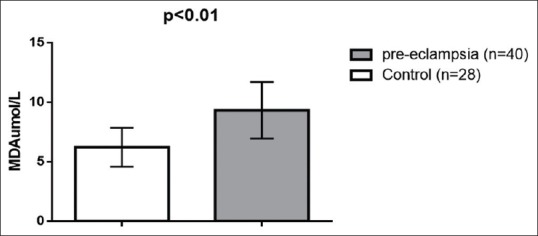
Malondialdehyde serum levels in patients with preeclampsia compared to the control
Figure 2.
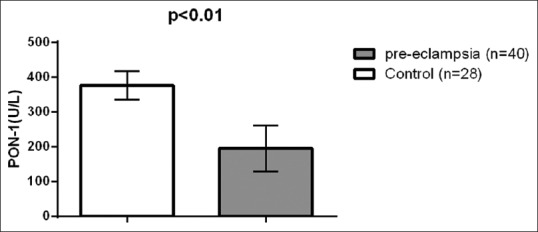
Paraoxonase serum levels in patients with preeclampsia compared to the control
Figure 3.
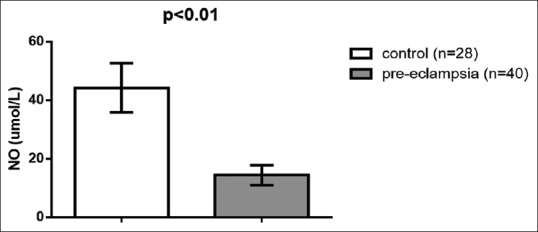
Nitric oxide serum levels in patients with preeclampsia compared to the control
Figure 4.
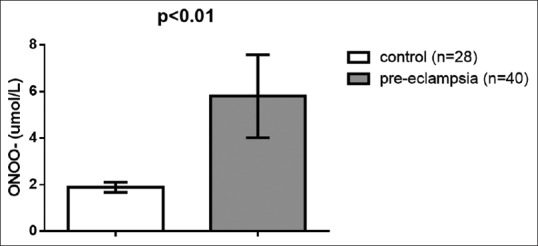
Peroxynitrite serum levels in patients with preeclampsia compared to the control
Discussion
The present study illustrated that PE was linked with parity and overweight as documented by Taylor et al. study which showed that ethnicity, lifestyle, parity, and BMI might affect the occurrence of PE. Parity is linked with pregnancy induced-hypertension. Parity increases systolic blood pressure, but it decreases DBP even with high BMI after 3–4 delivery by an unknown mechanism. Recently, race plays a minor role in the incidence of PE as reported by Zamora-Kapoor et al. study.[13] In addition, 15% of preeclamptic patients were smokers which in turn may affect the occurrence of PE; since smoking reduces the risk of PE. In addition, nonsmokers both before and during pregnancy have a superior risk of PE compared to smoker patients. The protective role of smoking is related to the carbon monoxide, which plays an important role in the prevention of hypertension and proteinuria. Indeed, smoking provokes the expression of vascular endothelial growth factor which controls the survival and differentiation of cytotrophoblast during uterine invasion.[14]
Nonsmokers both before and during pregnancy have a greater risk of PE/eclampsia compared to smokers. A dose-response relationship was evident between the number of cigarettes smoked per day during pregnancy and the risk of PE/eclampsia. Furthermore, discontinuance of smoking during pregnancy did not increase the risk of PE/eclampsia.
The present study certainly illustrated that all preeclamptic women are associated with SBB >140 Hgmm and DBP >90 Hgmm so they regarded as mild PE. Consequently, mean arterial pressure and pulse pressure was elevated; these findings are in coincidence with Peres et al. study.[15]
In our study, preeclamptic women revealed dyslipidemic status which is characterized by elevated serum levels of total cholesterol, triglyceride, VLDL, and LDL with a significant reduction in HDL compared to the women with normal pregnancy. Accordingly, these changes led to augmentation of AI and CCR as reported by different studies which showed that elevated lipid profile in PE affect the incidence of coronary artery disease.[16] In addition, findings of our study are more consistent with Baumfeld et al.'s study that exposed a negative correlation between HDL serum levels and severity of PE.[17]
Profile of renal function in the present study was exemplified by proteinuria and elevated serum uric acid in concern with normal blood urea and serum creatinine compared to the control as supported by Chung and To study.[18]
Serum uric acid is regarded as an impact factor on the severity of PE and perinatal outcomes. On the other hand, Chen et al. disclosed that uric acid serum levels were not increased in women who developed PE and is not regarded as a predictive biomarker in the development of PE.[19] As well Laughon et al.'s study demonstrated that hyperuricemia is often linked with PE but is regarded as a poor predictor of fetal and maternal complications in women with PE.[20]
The present study also confirmed the significant role of oxidative stress in the pathogenesis of PE as revealed by reduction of NO and PON-1 and increment in the levels of MDA and ONOO−. These findings are inconsistent with Bharadwaj et al., cohort study which showed that all oxidative stress biomarkers and total antioxidant status were high in preeclamptic patients compared to the normal pregnancy.[21] Furthermore, increased levels of oxidative derivatives and reduced antioxidant potential in PE may donate to the endothelial dysfunction. MDA serum levels which reflect lipid peroxidation process were increased in PE. Demir et al. study demonstrated that HDL, PON-1, and ApoA1 were lower in patients with PE compared with controls; since oxidized lipoproteins could participate significant roles in the progression of PE.[22] PON-1 is an antioxidant enzyme proceeds as a protective factor against oxidative stress and LDL oxidation. Genc et al. study documented low PON-1 serum levels compared to the controls,[23] which is coincided with our findings. On contrary, Yaghmaei et al. study found high PON-1 activity in PE,[24] whereas Sarandöl et al. study disclosed the insignificant difference in PON-1 activity in mild-to-severe PE compared to the normal pregnancy.[25] It has been shown that PON-1 is linked with HDL and protects HDL from oxidation due to the ability of PON-1 to hydrolyze oxidized phospholipid. Therefore, reduction of PON-1 in the present study was related to the reduction in HDL serum levels which is decreased in PE. Likewise, increased oxidative stress and lipid peroxidation in our study may be a potential factor in the reduction of PON-1 in PE; since PON-1 activity is blocked in the presence of lipid peroxides.[26]
Regarding the oxidative profile in PE, the present study showed low NO and high ONOO− compared to the controls. This finding is supported by Osol et al., a study which confirmed that abnormal NO signaling pathway leads to low NO levels which consequently leads to vasoconstriction and PE. Under the physiological condition, NO is produced by endothelial NO synthase which expressed in the endothelium and contributes to vasodilatation and prevention of tissue damage. NO is also produced by inducible NO synthase which is not expressed in the endothelium. It is provoked by inflammatory cytokines that cause excessive NO production which leads to endothelial dysfunction.[27]
In PE-free radicals induced by ischemic placenta interacts with NO leading to a reduction of NO bioavailability and induction of ONOO− formation from NO. On the other hand, ONOO− serum levels were high in our study due to scavenging of NO by free radicals. ONOO− leads to endothelial dysfunction due to interfering with endothelial prostaglandin, MAP kinase and NF-kB causing vasoconstriction and augmentation of peripheral vascular resistance.[28]
Moreover, in the present study, 95% of preeclamptic patients were on methyldopa compared to 5% on labetalol. Gomes et al. study confirmed the significant anti-oxidant effect of labetalol through reduction of ONOO− and induction of vascular NO.[29] Alpha-methyldopa is a centrally sympatholytic drug which has no anti-oxidant effect, but it reduces placental leptin secretion. It is possible that the leptin released from the ischemic placenta is responsible for activation of maternal sympathetic outflow causing hypertension.[30] Unfortunately, serum leptin was not assessed in the present study.
Limitations
The main limitation of the present study was a comparatively small sample size of the groups, but our study was a cross-sectional study designed to evaluate the oxidative stress and PON-1 levels in preeclamptic pregnant women compared to the control. This is regarded as a base for our future studies for a prospective study to assess the effect of anti-hypertensive agents used during PE on the oxidative stress and PON-1 levels in preeclamptic pregnant women. The placental function was not determined in view of placental hormones. Even though, this study provided evidence of low PON-1 in PE which there is a controversy about it.
Conclusions
This study concluded that PE is associated with the augmentation of oxidative stress and reduction of endogenous antioxidant capacity regarding PON-1.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to all postgraduate technicians and medical students for their technical support during sample analysis. Great thanks to the Department of Obstetrics and Gynecology in Baghdad Medical City for their cooperation and support. Special thanks to Professor Dr. Sadiq M. Al-Hamash, the head of Al-Mustansiriyah University for his enormous support for the scientific research.
References
- 1.Hansson SR, Nääv Š, Erlandsson L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front Physiol. 2014;5:516. doi: 10.3389/fphys.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navaratnam K, Alfirevic A, Jorgensen A, Alfirevic Z. Aspirin non-responsiveness in pregnant women at high-risk of pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2018;221:144–50. doi: 10.1016/j.ejogrb.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 3.Schneider H. Placental dysfunction as a key element in the pathogenesis of preeclampsia. Dev Period Med. 2017;21:309–16. doi: 10.34763/devperiodmed.20172104.309316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kuraishy HM, Al-Gareeb AI. Eustress and malondialdehyde (MDA): Role of Panax ginseng: Randomized placebo controlled study. Iran J Psychiatry. 2017;12:194–200. [PMC free article] [PubMed] [Google Scholar]
- 5.de Lucca L, Rodrigues F, Jantsch LB, Kober H, Neme WS, Gallarreta FM, et al. Delta-aminolevulinate dehydratase activity and oxidative stress markers in preeclampsia. Biomed Pharmacother. 2016;84:224–9. doi: 10.1016/j.biopha.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Motta-Mejia C, Kandzija N, Zhang W, Mhlomi V, Cerdeira AS, Burdujan A, et al. Placental vesicles carry active endothelial nitric oxide synthase and their activity is reduced in preeclampsia. Hypertension. 2017;70:372–81. doi: 10.1161/HYPERTENSIONAHA.117.09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Gao Q, Jiang L, Feng X, Zhu X, Fan X, et al. The NOX2-derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia. Hypertens Res. 2017;40:457–64. doi: 10.1038/hr.2016.180. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AK, Holzgreve W, Hahn S. Decrease in lipid levels of syncytiotrophoblast micro-particles reduced their potential to inhibit endothelial cell proliferation. Arch Gynecol Obstet. 2008;277:115–9. doi: 10.1007/s00404-007-0425-2. [DOI] [PubMed] [Google Scholar]
- 9.Nasifah I, Soeharto S, Nooryanto M. Effects of anti-lipid peroxidation of Punica granatum fruit extract in endothelial cells induced by plasma of severe pre-eclamptic patients. J Ayurveda Integr Med. 2017;8:215–7. doi: 10.1016/j.jaim.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins AV. Endogenous anti-oxidants in pregnancy and preeclampsia. Aust N Z J Obstet Gynaecol. 2006;46:77–83. doi: 10.1111/j.1479-828X.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 11.Isbilen E, Yilmaz H, Arzu Ergen H, Unlucerci Y, Isbir T, Gurdol F, et al. Association of paraoxonase 55 and 192 gene polymorphisms on serum homocysteine concentrations in preeclampsia. Folia Biol (Praha) 2009;55:35–40. [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Zamora-Kapoor A, Nelson LA, Buchwald DS, Walker LR, Mueller BA. Pre-eclampsia in American Indians/Alaska natives and whites: The significance of body mass index. Matern Child Health J. 2016;20:2233–8. doi: 10.1007/s10995-016-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Liu CX, Gong TT, Wu QJ, Wu L. Cigarette smoking during pregnancy and preeclampsia risk: A systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6:43667–78. doi: 10.18632/oncotarget.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peres GM, Mariana M, Cairrão E. Pre-eclampsia and eclampsia: An update on the pharmacological treatment applied in Portugal. J Cardiovasc Dev Dis. 2018;5 doi: 10.3390/jcdd5010003. pii: E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6 doi: 10.3390/diseases6010009. pii: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumfeld Y, Novack L, Wiznitzer A, Sheiner E, Henkin Y, Sherf M, et al. Pre-conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 2015;10:e0139164. doi: 10.1371/journal.pone.0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung WH, To WW. Outcome of pregnancy with new onset proteinuria and progression to pre-eclampsia: A retrospective analysis. Pregnancy Hypertens. 2017 doi: 10.1016/j.preghy.2017.11.007. pii: S2210-7789 (17) 30129-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Lau S, Tong M, Wei J, Shen F, Zhao J, et al. Serum uric acid may not be involved in the development of preeclampsia. J Hum Hypertens. 2016;30:136–40. doi: 10.1038/jhh.2015.47. [DOI] [PubMed] [Google Scholar]
- 20.Laughon SK, Catov J, Powers RW, Roberts JM, Gandley RE. First trimester uric acid and adverse pregnancy outcomes. Am J Hypertens. 2011;24:489–95. doi: 10.1038/ajh.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharadwaj SK, Vishnu Bhat B, Vickneswaran V, Adhisivam B, Bobby Z, Habeebullah S, et al. Oxidative stress, antioxidant status and neurodevelopmental outcome in neonates born to pre-eclamptic mothers. Indian J Pediatr. 2018;85:351–57. doi: 10.1007/s12098-017-2560-5. [DOI] [PubMed] [Google Scholar]
- 22.Demir B, Demir S, Atamer Y, Guven S, Atamer A, Kocyigit Y, et al. Serum levels of lipids, lipoproteins and paraoxonase activity in pre-eclampsia. J Int Med Res. 2011;39:1427–31. doi: 10.1177/147323001103900430. [DOI] [PubMed] [Google Scholar]
- 23.Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, et al. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet. 2011;284:1367–73. doi: 10.1007/s00404-011-1865-2. [DOI] [PubMed] [Google Scholar]
- 24.Yaghmaei M, Hashemi M, Azarian A, Moazeni-Roodi A, Mokhtari M, Naghavai A, et al. Association of L55M and Q192R polymorphisms of paraoxonase-1 gene with preeclampsia. Arch Med Res. 2011;42:324–8. doi: 10.1016/j.arcmed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Sarandöl E, Safak O, Dirican M, Uncu G. Oxidizability of apolipoprotein B-containing lipoproteins and serum paraoxonase/arylesterase activities in preeclampsia. Clin Biochem. 2004;37:990–6. doi: 10.1016/j.clinbiochem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Park H, Lee HY, Ahn YM, Ha EH, Suh SH, et al. Paraoxonase gene polymorphism, serum lipid, and oxidized low-density lipoprotein in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2007;133:47–52. doi: 10.1016/j.ejogrb.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–54. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–75. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 29.Gomes A, Costa D, Lima JL, Fernandes E. Antioxidant activity of beta-blockers: An effect mediated by scavenging reactive oxygen and nitrogen species? Bioorg Med Chem. 2006;14:4568–77. doi: 10.1016/j.bmc.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Sucak A, Kanat-Pektas M, Gungor T, Mollamahmutoglu L. Leptin levels and antihypertensive treatment in preeclampsia. Singapore Med J. 2010;51:39–43. [PubMed] [Google Scholar]


